Abstract
Objective
This study aimed to examine the effects of salt loading, with or without simultaneous angiotensin receptor blocker (ARB) treatment, on the systemic and tissue renin-angiotensin system (RAS) in spontaneously hypertensive rats (SHRs).
Method
Evaluation was performed early (4 weeks) in the course of salt loading in order to examine initial mediating events of cardiovascular and renal damage produced by salt excess. Four groups of rats were studied. Group 1 received regular rat chow (normal-salt diet); group 2 received normal-salt diet and an ARB (losartan, 30 mg/kg per day); group 3 received high-salt (8%) chow; and group 4 received high-salt diet and losartan.
Results
High-salt diet increased systolic pressure to 193 ± 1 mmHg compared to 180 ± 2 in normal-salt diet group. Losartan reduced SBP in SHRs fed normal-salt diet but did not reduce SBP in the SHRs fed high-salt diet (192 ± 2 mmHg). High-salt diet markedly increased urinary protein excretion from 27 ± 4 to 64 ± 13 mg/day and this increase was ameliorated by losartan (40 ± 9 mg/day). In SHRs on high-salt diet, plasma angiotensin II concentration increased three to four-fold, whereas urinary angiotensinogen excretion increased 10-fold; and these changes were significantly reduced by losartan. High-salt diet accelerated glomerular injury and interstitial fibrosis in SHRs which were reduced by losartan.
Conclusion
These results demonstrate that the activity of RAS was either not suppressed or, even augmented, after 4 weeks of salt loading despite high salt intake and increased SBP. The data suggest that an augmented intrarenal RAS during high-salt diet may contribute to the development of renal injury in this experimental model.
Keywords: heart angiotensin, kidney angiotensin, plasma angiotensin concentration, renin–angiotensin system, salt overload, urinary angiotensinogen excretion
Introduction
The association of dietary salt with hypertensive cardiovascular and renal disease is well recognized, but there are still many controversies regarding the role of salt in the pathogenesis of hypertension and cardiovascular and renal injury [1–5]. Studies in humans and animals have demonstrated that high salt intake in the presence of hypertension induces extensive target organ damage with the cardiovascular system and kidneys inevitably involved [2,4,6,7]. Specifically, excessive salt intake exerts severe detrimental effects on cardiovascular and renal structure and function in spontaneously hypertensive rats (SHRs) and normotensive Wistar–Kyoto (WKY) rats [8–11]. Clearly the increased arterial pressure associated with excess salt intake was partially responsible for inflicting target organ damage [2,6–11]. However, the available data suggest strongly that salt overload exerts adverse structural and functional cardiovascular and renal effects through pressure-independent mechanisms [10–12]. Moreover, recent results also suggest that the renin–angiotensin system (RAS) is involved in mediating the adverse effects of salt, since RAS blockade prevented or ameliorated salt-induced cardiovascular and renal injury [13,14]. Nevertheless, there are no studies directly examining the changes in the intrarenal RAS components in SHRs fed a high-salt diet. Accordingly, the present study was designed to examine more directly the activity of the systemic and local tissue (heart, kidneys, adrenals) RAS in SHRs given high-salt diet with or without simultaneous angiotensin II receptor blockade (ARB).
Thus, the objective of the present study was to examine the role of RAS in mediating salt-overload-induced cardiovascular and renal damage. To this end, we examined the components of the RAS in the blood, urine, and myocardial and renal tissue after 4 weeks of salt loading. In previous studies on the effects of salt overload, we used male, 8-week old SHRs that were given high-salt (8%) diet for 8 weeks and were studied at 16 weeks of age [13,14]. The cardiovascular and renal damage in these salt-loaded SHRs was extensive and this, by itself, may have affected the activity of the RAS which would, in turn, make identification and interpretation of the early mediating events difficult. Therefore, we reduced the duration of the high-salt diet intake. One previous study as well as the results of our pilot experiment demonstrated that the activity of RAS was suppressed after 2 weeks of salt loading indicating a physiologically appropriate response with this period of salt loading [15]. We, therefore, extended the period of high-salt diet to 4 weeks of salt loading (at the age of 12 weeks). At this point target organ damage was not as severe as previously observed after 8 weeks of high-salt diet, although it was clearly evident as indicated by increased left-ventricular mass and proteinuria.
Material and methods
Animals
Seven-week-old male SHRs were purchased from Harlan Laboratories (Indianapolis, IN), and were maintained in a temperature and humidity-controlled room with a 12-h light/dark cycle. All rats were handled in accordance with National Institute of Health guidelines; and the protocol was approved by the Institutional Animal Care and Use Committee.
Experimental protocol
At 8 weeks of age, rats weighing 180–200 g were divided randomly into four groups, with eight rats in each group. The first (control) group received no treatment and was given a standard diet, containing 0.6% NaCl, with tap water ad libitum. The second group was given standard diet and an ARB (losartan, 30 mg/kg per day by gavage). The third and fourth groups were given rat chow containing 8% NaCl; and the fourth group also received losartan treatment. All diets were obtained from Harlan-Teklad (Madison, Wisconsin, USA). We chose an 8% NaCl diet, since the majority of published studies examining salt-induced renal and cardiovascular damage use this concentration of salt in food [2,6–8,11–14,16]. This enabled us to compare the present data with the results of previous studies.
Rats received their respective treatments for 4 weeks. Body weight was measured weekly in all rats. During the last week all rats were housed in metabolic cages for 24-h urine collections. While in metabolic cages, rats were given tap water and respective chow ad libitum. Systolic pressure was measured by a tail-cuff technique using the Coda 6 System (Kent Scientific) [16] and was recorded at the beginning of the experiment and during the final week of salt loading. At the end of this treatment period the conscious rats were decapitated and samples collected for measurement of plasma renin activity (PRA) and plasma, heart, kidney and adrenal angiotensin II (AII) levels. Trunk blood was collected and the organs were immediately removed, quickly weighed, and homogenized in methanol. The time delay between decapitation and homogenization of the organs did not exceed 1 min.
Collection and extraction of blood, kidney, and urine samples
Blood was collected in chilled tubes containing a mixed inhibitor solution (final concentration: 5 mmol/l EDTA, 20 μmol/l pepstatin-A, 10 μmol/l phenylmethylsulfonyl fluoride, 20 μmol/l enalaprilat, and 1.25 mmol/l 1,10-phenanthroline). After centrifugation at 4°C for 10 min at 1000 g, plasma was separated and applied to phenyl-bonded solid-phase extraction columns (Bond-Elut; Varian) that had been prewashed with 90% methanol followed by water. After sample application, each solid-phase extraction column was washed sequentially with water, hexane, and chloroform. The water removed salts and other polar substances from the column. The hexane and chloroform eluted contaminating lipid and hydrophobic material from the column but did not affect the recovery of AII [17]. AII was eluted from the solid-phase extraction column with 90% methanol. The eluants were collected and evaporated to dryness under vacuum [15,17]. For AII measurements, each organ was immersed in cold methanol (100%), minced, and homogenized with a Polytron tissue homogenizer immediately after harvesting and weighing. The homogenates were centrifuged and the supernatants from the kidney homogenates were dried overnight in a vacuum centrifuge. The dried residue was reconstituted in 1 ml radio-immuno assay (RIA) buffer. These samples were extracted and evaporated as described above for plasma.
Measurement of angiotensin II by radioimmunoassay
The reconstituted plasma, heart, kidney, and adrenal fractions were incubated with rabbit anti-AII antisera (Peninsula Laboratories) and 125I-radiolabeled AII (Perkin Elmer Life and Analytical Sciences) for 48 h at 4°C. Bound and free AII were separated by dextran-coated charcoal, and the supernatants were counted by a computer-linked gamma counter for 3 min. Results are reported in femto-moles (fmol) per gram of organ weight or fmol per milliliter of plasma. The sensitivity of the AII assay was 1.39 fmol. For the AII assays, the specific binding was 78.952%, and nonspecific binding was 2.8% [17].
Plasma renin activity assays
For PRA determinations, trunk blood was collected in chilled tubes containing EDTA (5 mmol/l). Plasma was separated and stored at −20°C until assayed with a commercially available Gamma Coat Plasma Renin Activity RIA kit (DiaSorin) [18].
Measurements of urinary angiotensinogen
Urinary concentrations of angiotensinogen (AGT) were evaluated by ELISA using commercially available kits (IBL) [19] and urinary excretion rates were calculated from the volumes collected.
Renal expression of angiotensinogen mRNA
Total RNA was extracted from kidney (tissue weight 150–200 mg) using an RNeasy Midi Kit (Qiagen, Chatsworth, California, USA). For determination of AGT mRNA, real-time quantitative reverse transcription-PCR (RT-PCR) was then performed as described previously [20,21].
Histological examination of the kidney
Kidney sections were immersed in zinc-saturated formalin (Anatech, Battle Creek, Michigan, USA) for tissue fixation. The extent of glomerular injury was evaluated quantitatively by an automatic image analysis to each glomerulus using periodic acid-Schiff (PAS)-stained sections (Mass Histology Service, Worcester, Massachusetts, USA) as described previously [21].
The extent of interstitial fibrosis was evaluated quantitatively by an automatic image analysis to renal cortex occupied by interstitial tissue staining positively for collagen in Masson’s trichrome-stained sections (Mass Histology) as described previously [21].
Statistical analysis
All values are expressed as means ± SEM. The differences between different groups were analyzed using ANOVA followed by a Bonferroni post-hoc test for multi-group comparisons [22]. All tests were two-sided, and a value of P less than 0.05 was considered to be of statistical significance.
Results
Body weight, arterial pressure, and urinary execretory variables
After 4 weeks, SHRs fed high-salt diet had a significantly lower body weight as compared with SHRs fed normal-salt diet and treatment with the ARB ameliorated mass loss (Table 1). SBP was significantly (P < 0.05) higher in rats on the high-salt diet than in rats on normal-salt diet (Table 1). Losartan decreased SBP in rats on normal-salt diet, but failed to do so in rats on high-salt diet (Table 1). Daily urinary output was significantly (P < 0.05) greater in rats on high-salt diet than in those on normal-salt diet, reflecting the increased water intake in rats on high-salt diet (Table 1). Urinary sodium excretion was greatly increased in rats on high-salt diet. There were no differences between the groups given the same diet (Table 1). Urinary protein excretion was significantly (P < 0.05) increased in rats on high-salt diet and this increase was ameliorated by losartan (Table 1).
Table 1.
Body weight, systolic arterial pressure (SAP), urinary volume (UV), urinary sodium excretion (uNa), and urinary protein excretion (UP) after 4 weeks of salt loading
| Normal-salt diet (n = 8) | Normal-salt diet + losartan (n = 8) | High-salt diet (n = 8) | High-salt diet + losartan (n = 8) | |
|---|---|---|---|---|
| Body weight (g) | 298 ± 4 | 301 ± 4 | 273 ± 3* | 284 ± 5 |
| SAP (mmHg) | 180 ± 2 | 141 ± 3*,# | 193 ± 1* | 192 ± 2* |
| UV (ml/24 h) | 15 ± 1 | 16 ± 1 | 65 ± 4* | 49 ± 5* |
| uNa (mmol/24 h) | 1.7 ± 0.2 | 1.9 ± 0.3 | 17.9 ± 1.6* | 18.4 ± 1.7* |
| UP (mg/24 h) | 27 ± 4 | 26 ± 4 | 63 ± 8* | 42 ± 5* |
Values are mean ± 1 SEM.
P < 0.05 when compared to NS;
P < 0.05 compared to HS and losartan.
Plasma renin activity and plasma angiotensin II concentration
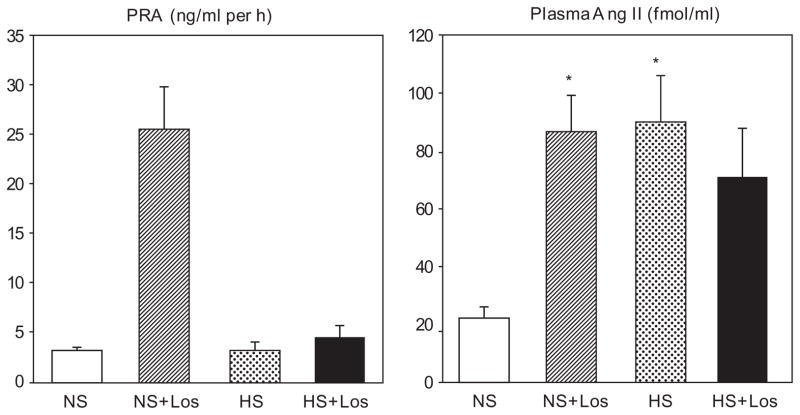
Plasma renin activity values were similar in the SHRs receiving normal-salt and high-salt diets (Fig. 1). Losartan greatly (P < 0.05) increased PRA in rats on normal-salt diet, but not in those on high-salt diet (Fig. 1). Plasma AII concentration was significantly (P < 0.05) higher in SHRs fed high-salt diet than in rats receiving a normal-salt diet (Fig. 1). Losartan increased (P < 0.05) plasma AII in rats receiving a normal-salt diet, but did not affect it in rats on high-salt diet (Fig. 1).
Fig. 1.
Plasma renin activity (PRA) and plasma angiotensin II (Ang II) concentration in SHRs on regular-salt diet (NS); regular-salt diet and losartan (30mg/kg per day) (NS + Los); high-salt diet (HS); and high-salt diet and losartan (HS + Los). *P < 0.05 when compared to NS.
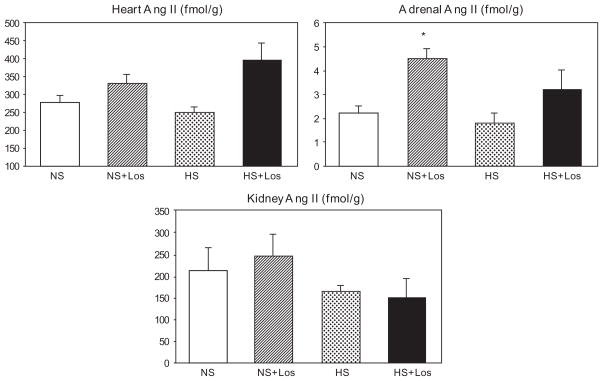
Heart, kidney, and adrenal angiotensin II concentration
No differences in heart, kidney, and adrenal AII contents were found between rats given normal-salt diet and those on a high-salt diet (Fig. 2). Losartan increased adrenal AII in rats on normal-salt chow. Importantly, high-salt diet failed to suppress the myocardial, renal, and adrenal AII contents.
Fig. 2.
Concentration of angiotensin II (Ang II) in the heart, adrenals and kidney in SHRs on regular-salt diet (NS); regular-salt diet and losartan (30 mg/kg/day) (NS + Los); high-salt diet (HS); and high-salt diet and losartan (HS + Los). *P < 0.05 when compared to NS.
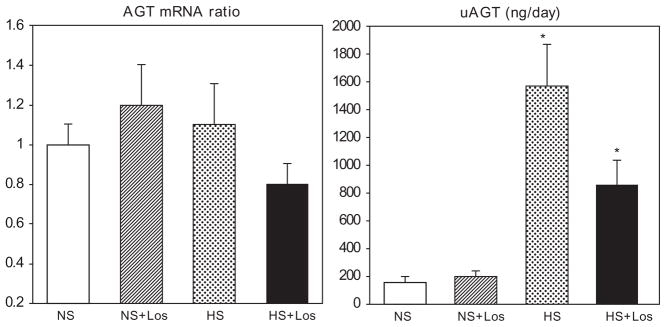
Renal angiotensinogen mRNA and urinary angiotensinogen excretion
No differences in renal AGT mRNA expression among the groups were found and neither a high-salt diet nor losartan altered renal AGT mRNA levels (Fig. 3). However, urinary AGT (uAGT) was markedly increased about eight-fold in rats fed high-salt diet and this increase was ameliorated in rats fed high-salt diet treated with losartan (Fig. 3).
Fig. 3.
Renal expression of angiotensinogen mRNA (AGT mRNA) and urinary excretion of angiotensinogen (uAGT) in SHRs on regular-salt diet (NS); regular-salt diet and losartan (30 mg/kg/day) (NS + Los); high-salt diet (HS); and high-salt diet and losartan (HS + Los). *P < 0.05 when compared to NS, #P < 0.05 when compared to HS.
Pathohistological renal changes
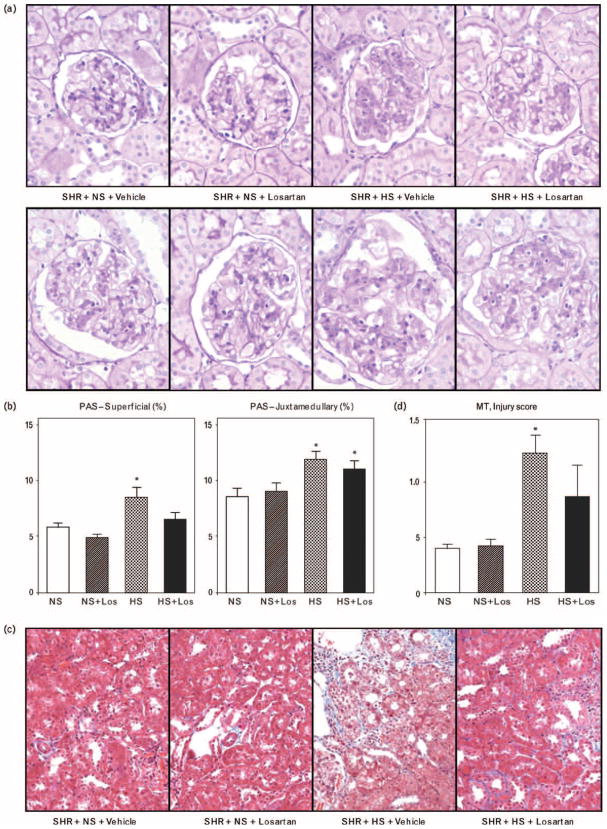
Glomerular injury
In SHRs on high-salt diet, hyalinization and increased cellularity appeared in both superficial (those in the outer 1/3 of cortex) and juxtamedullary glomeruli (those in the inner 1/3 of the cortex adjoining the medulla) (Fig. 4a) and the PAS-positive area increased significantly (P < 0.05) in animals fed a high-salt diet. PAS-positive areas were increased to a greater extent in juxtamedullary glomeruli than in superficial glomeruli and losartan treatment reduced the degree of injury in superficial and to a lesser degree in juxtamedulary glomeruli (Fig. 4b).
Fig. 4.
(a) Glomerular injury in superficial (upper panel) and juxtamedullary (lower panel) glomeruli. (b) Injury scores in superficial (upper panel) and juxtamedullary (lower panel) glomeruli, in SHRs on: regular-salt diet (NS); regular-salt diet and losartan (30 mg/kg per day) (NS + Los); high-salt diet (HS); and high-salt diet and losartan (HS + Los). *P < 0.05 when compared to NS, #P < 0.05 when compared to HS. (c) Tubulointerstitial fibrotic changes. (d) Tubulointerstitial injury scores in SHRs on regular-salt diet (NS); regular-salt diet and losartan (30 mg/kg per day) (NS + Los); high-salt diet (HS); and high-salt diet and losartan (HS + Los). *P < 0.05 when compared to NS.
Tubulointerstitial injury
Interstitial fibrosis was apparent in SHRs fed on high-salt diet and tubulointerstitial injury scores using Masson’s trichrome-stained sections were significantly greater in SHRs on high-salt diet (Fig. 4c and d). Losartan ameliorated this interstitial injury in rats fed high-salt diet.
Discussion
The findings from this study provide further evidence that salt loading aggravates cardiovascular and renal injury in SHRs [8–11] and are consistent with previous findings by other investigators [2,6,7]. We also found that arterial pressure was not reduced in rats on high-salt diet treated with losartan, which confirms our previous findings [13,14,23] and reports from others [6]. Furthermore, the failure of losartan to lower blood pressure in rats on high-salt diet provided the opportunity to evaluate the specific effects of AT1 receptor blockade on RAS components independently of arterial pressure changes. These results confirm our previous findings that an increased urinary protein excretion occurs after 4 weeks of salt loading in salt-loaded otherwise untreated SHRs [10].
The present data indicate that in the SHRs fed a high-salt diet the activity of systemic and tissue RAS was either not suppressed or even augmented after 4 weeks of salt loading. This inappropriately ‘normal’ or increased RAS activity may be, at least in part, responsible for cardiovascular and renal injury exacerbated by salt overload. Apparently, RAS activity is altered during the course of prolonged salt loading in SHRs. In a pilot study we observed that, after 2 weeks on high-salt diet, PRA and plasma angiotensin I concentrations were lower, suggesting decreased activity and generally similar responses to those from normal rats [15]. Similarly, studies with 2 and 4% salt in food reported suppression of PRA after 10 days or 4 weeks of salt loading [24,25]. However, after 4 weeks on high-salt diet, neither PRA nor heart, kidney or adrenal AII content was suppressed in SHRs in the present study. Furthermore, plasma AII concentrations and uAGT excretion rates were greatly increased, suggesting an augmented intrarenal RAS activity, thus reversing the normal inhibition of the RAS caused by increased salt intake. The observed increase in urinary AGT excretion in the SHRs given salt excess is perhaps the most novel finding as it has not been reported previously in SHRs. Similar to our results, an increase in plasma angiotensin II and AGT concentration and a decrease in PRA were found in SHRs given a high-salt load for 4 weeks suggesting that hypertension in the SHRs is associated with the loss of normal inhibitory effect of high dietary sodium on the RAS [26]. However, the status of the intrarenal RAS was not assessed in that study [26]. A recent study also reported that a high-salt diet fed to Cyp1a1-Ren2 transgenic rats exacerbated the course of hypertension and led to further increases in plasma and kidney AII levels [27]. It is also possible that increased aldosterone production may be involved. Opposing that view are the results of our recent study indicating that the mineralocorticoid receptor blocker eplerenone is far less effective in preventing adverse cardiovascular and renal effects of salt overload than angiotensin II type 1 receptor blockers or angiotensin-converting enzyme inhibitors [23]. It was also reported that plasma aldosterone is appropriately decreased by a high-salt diet in SHRs, although in that study rats were older than SHRs used in our studies [26]. However, we can not exclude the possibility that inappropriately elevated aldosterone level participated in mediating adverse effects of salt excess. Finally, we found that losartan treatment induced an increase in plasma renin activity in rats on regular salt diet but not in rats given salt excess. Similarly, it has been reported that losartan in combination with high salt increased plasma renin concentration much less than losartan in combination with low salt [24]. It is not clear why blockade of AT1 receptors under conditions of high salt intake fails to stimulate renin release and increase PRA. It is known that increased blood pressure suppresses PRA and since losartan treatment to SHRs on high-salt diet failed to significantly reduce the very high blood pressure in this group these results suggest that the inhibitory effects of the elevated arterial pressure are more powerful than the stimulatory effect of AT1 receptor blockade. It is note-worthy that in the SHRs fed a normal-salt diet, losartan did reduce blood pressure significantly so that there was a reduced inhibitory effect mediated by blood pressure in addition to the stimulatory effect of AT1 receptor blockade.
It is established that all components of the RAS are present within the tubular network of the kidney [28,29]. AGT is present in proximal tubular cells [20,30] and renin is produced by collecting duct cells [28,31–33], whereas angiotensin-converting enzyme is present in vascular endothelium and tubular epithelium [29]. The uAGT results after 4 weeks of salt loading indicate that there was marked activation of the intra-tubular RAS. Urinary protein excretion doubled after 4 weeks on the high-salt diet; however, at the same time, uAGT increased 10-fold, a finding that suggests increased tubular AGT synthesis. However, intrarenal AGT mRNA was not increased suggesting that part of the increased uAGT might have originated from the circulation via increased permeability by the glomerular capillary membrane. Yet, whether increased uAGT resulted from increased tubular synthesis or from glomerular leakage, it would still be expected to promote increased intratubular AII generation which, in turn, would result in functional and morphological derangements as well as augmented sodium reabsorption [34–36]. It has been demonstrated that enhanced intrarenal AGT contributes to early renal injury in SHRs even on normal salt [21], but the high-salt diets markedly exacerbated the renal injury.
The present findings are consistent with and support our notion that activation of the local renal RAS mediates structural and functional derangements of the kidney induced by salt overload. Similar to our findings, an increased urinary AGT and renin excretion has been found in mice given a high-salt diet [37]. Increased expression of angiotensin II type 1 receptor may also be implicated. Alternatively and/or concurrently, augmented (pro)renin synthesis and (pro)renin receptor expression may be implicated in the pathogenesis of organ damage, independently of AII [32,38]. Also, an association of augmented uAGT and activation of the intrarenal RAS was recently reported in experimental animals as well as in clinical studies [39,40]. It is possible that increased oxidative stress may be responsible for the activation of a local RAS in the kidney. Salt excess has been shown to increase oxidative stress in various experimental models [41–43]; and elevated reactive oxygen species are associated with augmentation of intrarenal AGT and renal injury [44,45]. Pathogenetic linkage between RAS and oxidative stress in chronic kidney disease was extensively discussed recently by Nistala et al. [46]. Our data demonstrating a protective effect of angiotensin II receptor blockade in the augmented renal injury occurring in the SHRs fed high-salt diet are consistent with this notion [13].
Our data further support the thesis that increased salt intake in SHRs elevated arterial pressure (albeit modestly) and that AII receptor blockade by losartan did not ameliorate the pressure rise. However, it did reduce the structural and functional effects of local AII generation [13,14]. This finding suggests that the blood pressure increase, per se, was not primarily mediated by the augmented RAS but through some other pressor mechanisms, possibly by salt excess-related volume expansion.
Finally, the data from this study do not point out the specific mechanism by which salt excess activates RAS, as best evidenced by increased plasma AII concentration [26]. Increased AGT production, increased prorenin activation via (pro)renin receptor, and increased activity of other AII-producing enzymes such as chymase may be involved.
In conclusion, the present data demonstrate that after 4 weeks of excessive salt intake, activity of RAS was either not suppressed or even augmented despite high salt intake and increased arterial pressure. This inappropriately ‘normal’ or increased RAS activity may then mediate structural and functional cardiovascular and renal injury of salt overload.
Perspectives
Numerous clinical and experimental studies have clearly demonstrated that excessive salt intake causes adverse structural and functional effects in the cardiovascular system and kidneys. However, the mechanism of salt-induced injury is still not known. Excessive salt intake is often associated with an increase in arterial pressure and, consequently, increases in arterial pressure may partially mediate salt-related adverse effects. However, the extent of the relationship between salt and arterial pressure strongly suggests that the structural and functional effects of salt overload are direct, whereas the co-incidental increases in pressure may also contribute. Considering the fact that excessive salt intake is very common and that pathophysiological consequences of salt overload are not fully appreciated, it is important to obtain a more complete understanding of the mechanisms of salt-related tissue injury.
Acknowledgments
The research work was supported, in part, by a grant-in-aid from the Investigator-Initiated Studies Program of Merck & Co., Inc. Additional support provided by NHLBI grant R01HL26371, by NIDDK grant R01DK072408, and by NCRR COBRE grant P20RR017659.
Abbreviations
- ARB
angiotensin receptor blocker
- PRA
plasma renin activity
- RAS
renin–angiotensin system
- SHRs
spontaneously hypertensive rats
- uAGT
urinary angiotensinogen
- WKY
Wistar–Kyoto rats
Footnotes
There are no conflicts of interest.
References
- 1.Ambard L, Beaujard E. Causes de l’hypertension arterielle. Arch Gen Med. 1904;1:520–533. [Google Scholar]
- 2.Leenen FH, Yuan B. Dietary-sodium-induced cardiac remodeling in spontaneously hypertensive rat versus Wistar-Kyoto rat. J Hypertens. 1998;16:885–892. doi: 10.1097/00004872-199816060-00020. [DOI] [PubMed] [Google Scholar]
- 3.Meneely GR, Dahl LK. Electrolytes in hypertension: the effect of sodium chloride. The evidence from animal and human studies. Med Clin North Am. 1961;45:271–283. doi: 10.1016/s0025-7125(16)33891-3. [DOI] [PubMed] [Google Scholar]
- 4.Mimran A, du Cailar G. Dietary sodium: the dark horse amongst cardiovascular and renal risk factors. Nephrol Dial Transplant. 2008;23:2138–2141. doi: 10.1093/ndt/gfn160. [DOI] [PubMed] [Google Scholar]
- 5.Tuomilehto J, Jousilahti P, Rastenyte D, Moltchanov V, Tanskanen A, Pietinen P, Nissinen A. Urinary sodium excretion and cardiovascular mortality in Finland: a prospective study. Lancet. 2001;357:848–851. doi: 10.1016/S0140-6736(00)04199-4. [DOI] [PubMed] [Google Scholar]
- 6.Mercier N, Labat C, Louis H, Cattan V, Benetos A, Safar ME, Lacolley P. Sodium, arterial stiffness, and cardiovascular mortality in hypertensive rats. Am J Hypertens. 2007;20:319–325. doi: 10.1016/j.amjhyper.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Yu CM, Burrell LM, Black MJ, Wu LL, Dilley RJ, Cooper ME, Johnston CI. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation. 1988;98:2621–2628. doi: 10.1161/01.cir.98.23.2621. [DOI] [PubMed] [Google Scholar]
- 8.Ahn J, Varagic J, Slama M, Susic D, Frohlich ED. Cardiac structural and functional responses to salt loading in SHR. Am J Physiol. 2004;287:H767–H772. doi: 10.1152/ajpheart.00047.2004. [DOI] [PubMed] [Google Scholar]
- 9.Frohlich ED, Chien Y, Sesoko S, Pegram B. Relationship between dietary sodium intake, hemodynamics, and cardiac mass in SHR and WKY rats. Am J Physiol. 1994;264:R30–R34. doi: 10.1152/ajpregu.1993.264.1.R30. [DOI] [PubMed] [Google Scholar]
- 10.Matavelli LC, Zhou X, Varagic J, Susic D, Frohlich ED. Salt loading produces severe renal hemodynamic dysfunction independent of arterial pressure in spontaneously hypertensive rats. Am J Physiol. 2007;292:H814–H819. doi: 10.1152/ajpheart.00671.2006. [DOI] [PubMed] [Google Scholar]
- 11.Varagic J, Frohlich ED, Diez J, Susic D, Ahn J, Gonzales A, Lopez B. Myocardial fibrosis, impaired coronary hemodynamics, and biventricular dysfunction in salt-loaded SHR. Am J Physiol. 2006;290:H1503–H1509. doi: 10.1152/ajpheart.00970.2005. [DOI] [PubMed] [Google Scholar]
- 12.Maitland K, Bridges L, Davis WP, Loscalzo J, Pointer MA. Different effects of angiotensin receptor blockade on end-organ damage in salt-dependent and salt-independent hypertension. Circulation. 2006;114:905–910. doi: 10.1161/CIRCULATIONAHA.106.622316. [DOI] [PubMed] [Google Scholar]
- 13.Susic D, Zhou X, Frohlich ED. Angiotensin blockade prevents salt-induced injury of the renal circulation in spontaneously hypertensive rats. Am J Nephrol. 2009;29:639–645. doi: 10.1159/000195633. [DOI] [PubMed] [Google Scholar]
- 14.Varagic J, Frohlich ED, Susic D, Ahn J, Matavelli L, López B, Díez J. AT1 receptor antagonism attenuates target organ effects of salt excess in SHRs without affecting pressure. Am J Physiol. 2008;294:H853–H858. doi: 10.1152/ajpheart.00737.2007. [DOI] [PubMed] [Google Scholar]
- 15.Fox J, Guan S, Hymel AA, Navar LG. Dietary Na and ACE inhibition effects on renal tissue angiotensin I and II and ACE activity in rats. Am J Physiol. 1992;262:F902–F909. doi: 10.1152/ajprenal.1992.262.5.F902. [DOI] [PubMed] [Google Scholar]
- 16.Aukes AM, Vitullo L, Zeeman GG, Cipolla MJ. Pregnancy prevents hypertensive remodeling and decreases myogenic reactivity in posterior cerebral arteries from Dahl salt-sensitive rats: a role in eclampsia? Am J Physiol. 2007;292:H1071–H1076. doi: 10.1152/ajpheart.00980.2006. [DOI] [PubMed] [Google Scholar]
- 17.Shao W, Seth DM, Navar GL. Augmentation of endogenous intrarenal angiotensin II levels in Val5-ANGII-infused rats. Am J Physiol. 2009;296:F1067–F1071. doi: 10.1152/ajprenal.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Von Thun AM, Vari RC, El-Dahr SS, Navar LG. Augmentation of intrarenal angiotensin II levels by chronic angiotensin II infusion. Am J Physiol. 1994;266:F120–F128. doi: 10.1152/ajprenal.1994.266.1.F120. [DOI] [PubMed] [Google Scholar]
- 19.Kobori H, Katsurada A, Miyata K, Ohashi N, Satou R, Saito T, et al. Determination of plasma and urinary angiotensinogen levels in rodents by newly developed ELISA. Am J Physiol Renal Physiol. 2008;294:F1257–F1263. doi: 10.1152/ajprenal.00588.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol. 2005;16:2073–2080. doi: 10.1681/ASN.2004080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armitage P, Berry G. Statistical methods in medical research. 3. Oxford, UK: Blackwell Scientific; 1994. [Google Scholar]
- 23.Susic D, Varagic J, Frohlich ED. Cardiovascular effects of inhibition of renin-angiotensin-aldosterone system components in hypertensive rats given salt excess. Am J Physiol Heart Circ Physiol. 2010;288:H1177–H1181. doi: 10.1152/ajpheart.00866.2009. [DOI] [PubMed] [Google Scholar]
- 24.Richer-Giudicelli C, Domergue V, Gonzalez MF, Messadi E, Azizi M, Giudicelli JF, Menard J. Haemodynamic effects of dual blockade of the rennin-angiotensin sustem in spontaneously hypertensive rats: influence of salt. J Hypertens. 2004;22:459–462. doi: 10.1097/00004872-200403000-00026. [DOI] [PubMed] [Google Scholar]
- 25.Gahnem F, Camargo MJ, von Lutterotti, Laragh JH, Sealyye JE. Angiotensinogen depletion by high rennin levels in hypertensive rats: no evidence for tonic stimulation of angiotensinogen by angiotensin II. J Hypertens. 1995;13:91–96. [PubMed] [Google Scholar]
- 26.Hodge G, Ye VZC, Duggan KA. Dysregulation of angiotensin II synthesis is associated with salt sensitivity in the spontaneous hypertensive rats. Acta Physiol Scand. 2002;174:209–215. doi: 10.1046/j.1365-201x.2002.00937.x. [DOI] [PubMed] [Google Scholar]
- 27.Huskova Z, Vanourkova Z, Erbanova H, Thumova M, Opocensky M, Mullins JJ, et al. Inappropriately high circulating and intrarenal angiotensin II levels during dietary salt loading exacerbate hypertension in Cyp 1a1-Ren-2 transgenic rat. J Hypertens. 2010;28:495–509. doi: 10.1097/HJH.0b013e3283345d69. [DOI] [PubMed] [Google Scholar]
- 28.Prieto MC, Navar LG. Collecting duct renin a critical link in angiotensin II: dependent hypertension. In: Frohlich ED, Re R, editors. The local renin-angiotensin aldosterone system. New York: Springer; 2009. pp. 133–141. [Google Scholar]
- 29.Siragy HM. Angiotensin II compartmentalization within the kidney: effects of salt diet and blood pressure alterations. Curr Opin Nephrol Hypertens. 2006;15:50–53. doi: 10.1097/01.mnh.0000196148.42460.4f. [DOI] [PubMed] [Google Scholar]
- 30.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peti-Peterdi J, Fintha A, Fuson AL, Tousson A, Chow RH. Real-time imaging of renin release in vitro. Am J Physiol Renal Physiol. 2004;287:F329–F335. doi: 10.1152/ajprenal.00420.2003. [DOI] [PubMed] [Google Scholar]
- 32.Peti-Peterdi J, Kang JJ, Toma I. Activation of the renal renin-angiotensin system in diabetes: new concepts. Nephrol Dial Transplant. 2008;23:3047–3049. doi: 10.1093/ndt/gfn377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertension. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzales-Villalobos RA, Navar LG. Intratrenal angiotensin II augmentation in hypertension. In: Frohlich ED, Re R, editors. The local renin-angiotensin aldosterone system. New York: Springer; 2009. pp. 121–131. [Google Scholar]
- 35.Kobori H, Ozawa Y, Satou R, Katsurada A, Miyata K, Ohashi N, et al. Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol. 2007;293:F938–F945. doi: 10.1152/ajprenal.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sachetelli S, Liu Q, Zhang SL, Liu F, Hsieh TJ, Brezniceanu ML, et al. RAS blockade decreases blood pressure and proteinuria in transgenic mice overexpressing rat angiotensinogen gene in the kidney. Kidney Int. 2006;69:1016–1023. doi: 10.1038/sj.ki.5000210. [DOI] [PubMed] [Google Scholar]
- 37.Lentelme P, Rohrwasser A, Gociman B, Hillas E, Cheng T, Petty G, et al. Effects of Dietary sodium and genetic background on angiotensinogen and renin in mouse. Hypertension. 2006;39:1007–1014. doi: 10.1161/01.hyp.0000016177.20565.a0. [DOI] [PubMed] [Google Scholar]
- 38.Melnyk RA, Tam J, Bole Y, Kennedy BP, Percival MD. Renin and prorenin activate pathways implicated in organ damage in human mesangial cells independent of angiotensin II production. Am J Nephrol. 2009;30:232–243. doi: 10.1159/000220260. [DOI] [PubMed] [Google Scholar]
- 39.Cohen JA, Lindsey SH, Pirro NT, Brosnihan KB, Gallagher PE, Chappell MC. Influence of estrogen depletion and salt loading on renal angiotensinogen expression in the mRen(2) Lewis strain. Am J Physiol Renal Physiol. 2010;299:F35–F42. doi: 10.1152/ajprenal.00138.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishiyama A, Konishi Y, Ohashi N, Morikawa T, Urushihara M, Maeda I, et al. Urinary angiotensinogen reflects the activity of intrarenal renin-angiotensin system in patients with IgA nephropathy. Nephrol Dial Transplant. 2010 doi: 10.1093/ndt/gfq371. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim-Mitsuyama S, Yamamoto E, Tanaka T, Zhan Y, Izumiya Y, Ioroi T, et al. Critical role of angiotensin II in excess salt-induced brain oxidative stress in stroke-prone spontaneously hypertensive rats. Stroke. 2009;36:1083–1088. doi: 10.1161/01.STR.0000163084.16505.e3. [DOI] [PubMed] [Google Scholar]
- 42.Matsui H, Ando K, Kawarazaki H, Nagae A, Fujita M, Shimosawa T, et al. Salt excess causes left ventricular diastolic dysfunction in rats with metabolic disorder. Hypertension. 2008;52:287–294. doi: 10.1161/HYPERTENSIONAHA.108.111815. [DOI] [PubMed] [Google Scholar]
- 43.Tian Z, Liu Y, Usa K, Mladinov D, Fang Y, Ding X, et al. Novel role of fumarate metabolism in Dahl-salt sensitive hypertension. Hypertension. 2009;54:255–260. doi: 10.1161/HYPERTENSIONAHA.109.129528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohashi N, Katsurada A, Miyate K, Satou R, Saito T, Urushihara M, Kobori H. Role of activated intrarenal reactive oxygen species and renin-angiotensin system in IgA nephropathy mice. Clin Exp Pharmacol Physiol. 2009;36:750–755. doi: 10.1111/j.1440-1681.2009.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzaki Y, Ozawa Y, Kobori H. Intrarenal oxidative stress and augmented angiotensinogen are precedent to renal injury in Zucker diabetic fatty rats. Int J Biol Sci. 2006;3:40–46. doi: 10.7150/ijbs.3.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nistala R, Wei Y, Sowers JR, Whaley-Connell A. Renin-angiotensin-aldosterone system-mediated redox effects in chronic kidney disease. Transl Res. 2009;153:102–113. doi: 10.1016/j.trsl.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]