Abstract
Women with triple negative breast cancer (TNBC) have a worse prognosis compared with other breast cancer subtypes. Hormonal or Herceptin-based therapies were found to be ineffective because of the loss of target receptors such as ER, PR and HER-2 amplification. Conventional chemo- and/ or radiation therapy also seems to have limited efficacy in TNBC patients. We studied the effects of cisplatin plus TRAIL on one normal and two TNBC cells in vitro. The in vitro studies indicate that cisplatin plus TRAIL significantly enhanced cell death in TNBC cell lines CRL2335 and MDA-MB-468 by ~60–70% compared to ~ 10–15% in CRL8799 normal breast cell line. Treatment with cisplatin/TRAIL also inhibited the expression of EGFR, p63, survivin, Bcl-2 and Bcl-xL in TNBC cells. Specific inhibition of EGFR and/or p63 protein in TNBC cells by siRNA does not increase TRAIL-induced apoptosis. However, inhibition of survivin by siRNA enhances TRAIL-induced apoptosis. These observations suggested the possibility that Survivin played an important role in cisplatin plus TRAIL-induced apoptosis in TNBC cells. In vivo experiments, treatment of mice with cisplatin plus TRAIL resulted in a significant inhibition of CRL2335 xenograft tumors compared to untreated control tumors. Taken together the data suggests that cisplatin plus TRAIL treatment have the potential of providing a new strategy for improving the therapeutic outcome in TNBC patients.
Keywords: Triple-negative breast cancer, Caspase-3, cisplatin, TRAIL, Apoptosis
Introduction
Breast cancer continues to be a major health problem worldwide despite recent advances in its diagnosis and treatment. Based on gene expression profiles breast cancer can be the classification of into five main groups: (i) luminal A, (ii) luminal B (both are ER+), (iii) basal-like, (iv) HER2 and (v) normal breast-like tumors (1). At present, most treatment in breast cancer is targeted to either to ER or HER-2. Triple-negative breast cancer (TNBC) is characterized by the absence of three receptors, viz., estrogen receptor (ER), Progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2): hence the term “triple-negative”. In ~60% of cases, these tumor cells express the receptor for epidermal growth factor (EGFR) (2–4) and may also contain mutations in p53 gene (5–7). The disease is diagnosed more frequently in younger and pre-menopausal women (6, 8–11). It was previously shown that mutations in BRCA1 gene leads to error-prone DNA repair resulting in genomic instability and predisposition to carcinogenesis. In TNBC patient’s loss of ER, PR and HER-2 prevents targeted therapy. In general TNBC tumors are refractory to commonly used chemotherapeutic agents, as a result it leads to relatively poor prognosis (1, 12). Some reports have shown that BRCA1-associated TNBC tumor are sensitivity to cisplatin chemotherapy (13). The use of platinum complexes for the breast cancer therapy is an emerging new treatment modality. Platinum (Pt) drugs including cisplatin and carboplatin are widely used as anticancer drugs. Cisplatin is currently used to treat tumors of the head, neck, lungs, and ovarian cancer (14–16). However, therapeutic efficiency of cisplatin in TNBC is moderate. Therefore, it is important to identify new treatment modalities for TNBC patients.
Tumor necrosis-factor-related apoptosis-inducing-ligand (TRAIL, a member of tumor necrosis factor family of death-receptor-ligands) is reported to have a potential use in cancer therapy because of its ability to selectively kill cancer cells without affecting normal cells (17). TRAIL is shown to bind with death receptor 4 (DR4) and death receptor 5 (DR5) leading to the formation of death-inducing signaling complex (DISC) and Fas-associated protein with death domain (FADD). In turn, DISC and FADD recruits caspase-8 (or caspase-10) which plays an important role in inducing apoptosis either by direct activation of downstream effector caspases (caspase-3, caspase-6 and caspase-7) or by cleaving apoptotic molecules (Bcl-2 and Bcl-xL) resulting in further activation of caspase-9 complex (18). Studies in animals have also shown that TRAIL regresses cancer xenografts without affecting normal tissues (19).
In this study, we evaluated the molecular mechanism by which cisplatin sensitizes TNBC cell lines to TRAIL-induced apoptosis. Data shows that cisplatin and TRAIL down regulates anti-apoptotic proteins, survivin, Bcl-2, Bcl-xL and induces activation of caspase-3,-8 and -9 and increases apoptosis. However, in normal breast cell lines combination of cisplatin/TRAIL had a minimal effect on anti-apoptotic proteins and shows a marginal increase in apoptosis in comparison to TNBC cells.
Materials and Methods
Cell lines and reagents
The human breast carcinoma cell line CRL8799, CRL2335 and MDA-MB-468 cells were obtained from the American Type Culture Collection and maintained in according to ATCC recommended culture media. All cells obtained from ATCC were immediately expanded and frozen down such that all cell lines could be restarted every 3 to 4 months from a frozen vial of the same batch of cells and no additional authentication was done in our laboratory. DR4/TRAILR1 monoclonal antibody and DR5/TRAILR2 polyclonal antibody was purchased from Imgenex (San Diego, CA). Monoclonal antibodies of PARP, Bid, Actin, caspase-8 were purchased from BD Biosciences (San Diego, CA). Caspase-3, and Caspase-9 antibodies and Survivin siRNA were purchased from Cell Signaling Technology (Danvers, MA). Anti-Glyceraldehyde-3-phosphate dehydrogenase (G3PDH) rabbit polyclonal was obtained from Trevigen (Gaithersburg, MD). TRAIL and Caspase-3 inhibitor was purchased from R&D systems (Minneapolis, MN). Cell Death Detection ELISAPLUS was purchased from Roche (Indianapolis, IN). Reagents for protein concentration analysis and protein gel electrophoresis were obtained from Bio-Rad (Hercules, CA). p63 siRNA was obtained from Santa Cruz biotechnology, Inc, (Santa Cruz, CA). EGFR and random siRNA were obtained from Millipore (USA). All other chemicals, unless otherwise specified, were obtained from Sigma in the highest suitable purities.
MTT assay
In brief, 5 × 104 cells were added in 96-well tissue culture plates. After 24h, cells were treated with TRAIL (10 ng/ml), cisplatin (10 ug/ml), or combination of TRAIL plus cisplatin for another 24h. Following treatments, 100 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (1 mg/ml) was added into each sample and incubated for 3h under 5% CO2 and 37°C. The cell viability was measured by MTT, which is converted by succinate dehydrogenase in mitochondria of viable cells to yield a purple formazan dye. The formazan dye was dissolved in dimethyl sulfoxide (DMSO) and measured by absorption at a wavelength of 550 nm using Benchmark® microplate reader from Bio-Rad.
Western immunoblot analysis
Cells were grown in 6 well plates, to near confluence in the presence or absence of various treatments. Cells were lysed and Western blotting was performed as described previously (20) using a standard protocol. In brief: Cell extracts were obtained by lysing the cells in RIPA buffer (20 mM Hepes, 100 mM NaCl, 0.1% SDS, 1% Nonidet P-40, 1% deoxycholate, 1 mM Na3VO4, 1 mM EGTA, 50 mM NaF, 10% glycerol, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 1x protease inhibitor mixture). Samples containing 100 μg of total protein were electrophoresed on 10 % or 15% SDS-polyacrylamide gels and transferred on to PVDF membrane by electroblotting. Membranes were probed with antibodies as indicated, followed by HRP-conjugated mouse or rabbit secondary antibodies (Amersham). Anti-G3PDH was used for loading controls.
RNA interference assay
Cells were plated in 6-well tissue culture plates at a density of 3 × 105/well in medium containing 10% FBS. After 24h, cells were transfected with 100 pmol of siRNA’s from EGFR, and/or p63 or Survivin or random siRNA with scrambled sequence was used as control. Lipofectamine transfection reagent was used to transfect sequence according to the manufacturer’s instructions. After 48h of transfection, cells were treated with or without TRAIL for additional 24h. Cells were then harvested for Western blot analysis.
Apoptosis assay
Cells were treated with cisplatin, TRAIL or combination of cisplatin plus TRAIL for 16 h. Cells were harvested and stained with FITC Annexin V and propidium iodide (PI) to identify early apoptotic cells. Apoptosis was determined using FITC Annexin V Apoptosis Detection Kit (BD Biosciences) according to the manufacturer’s instructions.
Crystal violet staining
Crystal violet stain binds to the nuclei of the cells and gives a violet color (an OD595 reading) that is proportional to surviving cell. In brief, 5 × 105 cells were plated in 12 well tissue culture plates. After 24h, cells were treated with TRAIL, cisplatin, or combination of TRAIL plus cisplatin for another 24h. Following treatments, the medium was removed and cells were washed with PBS, fixed and stained with 0.2% crystal violet in 10% phosphate-buffered formaldehyde for 30 seconds. Excess crystal violet solution was removed and cells were washed 3 times with PBS. The pictures were taken after the plates completely dried.
Results
Cisplatin plus TRAIL enhanced cell death in TNBC cell lines without significantly affecting normal breast cells
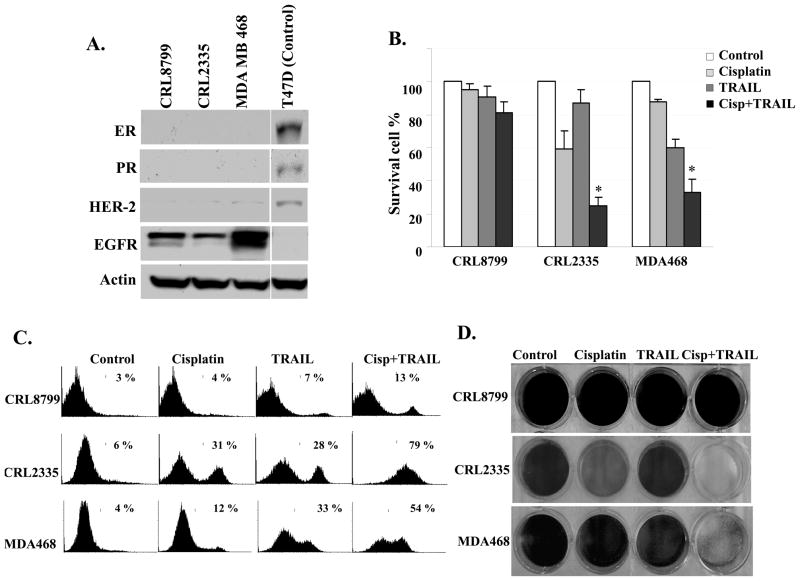
We used three cell line, two triple-negative breast cancer (TNBC) cell lines, CRL2335 and MDA-MB-468 cells and an immortalized normal breast cell line CRL8799 to determine the effect of cisplatin, TRAIL, or combination of cisplatin plus TRAIL on cell death. Western blot analysis indicated the absence of ER, PR and HER-2 amplification in both CRL2335 and MDA-MB-468 TNBC cells and thus confirmed the triple negative status of the cells and the cell lines over express EGFR (Figure 1A). HER-2 expressing T47D cells lysate was used as positive control to determine ER, PR and HER2 immunoreactivity of antibodies. MTT data indicates that cisplatin or TRAIL treatment induced ~10–30% cell death in TNBC cell lines. However, combination of cisplatin plus TRAIL enhanced cell death to ~60–70% in TNBC cells (Fig. 1B). In contrast, cisplatin plus TRAIL treatment of normal CRL8799 cells had minimal (~10 –15%) effect on cell death (Fig. 1B). The FITC Annexin V and PI staining indicated that the proportion of cells in apoptosis in CRL2335 and MDA-MB-468 TNBC cells treated with cisplatin plus TRAIL was significantly higher (~79% and ~54% respectively) as compared with those treated with cisplatin or TRAIL alone. Similar treatment of normal CRL8789 cells resulted in a moderate increase in apoptotic cells (~13%) (Figure 1C). These observations were further confirmed in cells stained with crystal violet (Figure 1D). Thus, taken together, the results suggested that cisplatin plus TRAIL treatment significantly enhanced cell death in both CRL-2335 and MDA-MB-468 TNBC cell lines while it had a minimal or moderate effect on normal CRL8799 cells.
Figure 1. Cisplatin plus TRAIL treatment resulted in a significantly enhanced cell death in CRL-2335 and MDA-MB-468 TNBC without affecting CRL8799 normal breast cells.
(A) Whole-cell extracts were prepared and analyzed by Western blotting using antibodies against the estrogen receptor (ER), progesterone receptor (PR), HER-2 and EGFR. Equal protein loading was evaluated by Actin. (B) Cells were treated with cisplatin (10 μg/ml), TRAIL (10 ng/ml) or cisplatin (10 μg/ml) plus TRAIL(10 ng/ml) for 24 h. Cell viability was then analyzed by MTT method. Cisplatin plus TRAIL treatment significantly inhibited CRL 2335 (P < 0.001) and MDA-MB-468 (P< 0.005) cells. (C) Cells were treated with cisplatin, TRAIL or cisplatin plus TRAIL and washed with PBS. Cells were stained with Annexin V-FITC and PI. Cells undergoing apoptosis were determined using FITC Annexin V apoptosis detection kit. (D) Cells were treated with cisplatin, TRAIL or a combination of cisplatin plus TRAIL for 24 h. Following treatments, the medium was removed and cells were washed with PBS, fixed and stained with crystal violet.
Cisplatin plus TRAIL treatment down regulated the expression of EGFR and P63 proteins
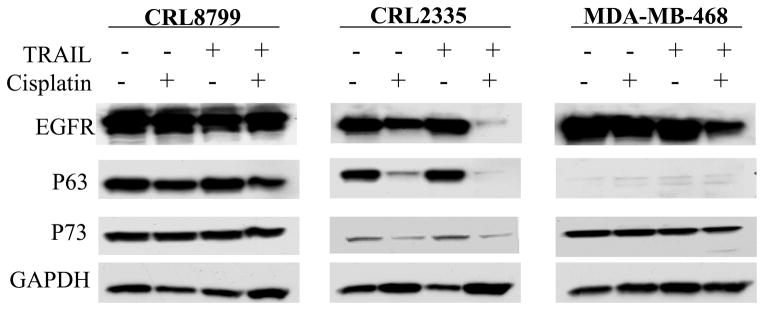
Gobson et al (21, 22) reported that EGFR played a dual role in apoptosis: increased expression gave resistance while decreased expression promoted TRAIL-induced apoptosis. Leong et al (23) observed that the P53 family member ΔNP63α isoform promoted the survival of primary breast cancer cells by binding to TAP73 and thus inhibiting its pro-apoptotic activity. The effect of cisplatin, TRAIL or cisplatin plus TRAIL on EGFR protein and ΔNP63 was tested in CRL2335 and MDA-MB-468 TNBC cells as well as in CRL8799 cells in order to understand the mechanism involved in cell death. The data in Figure 2 indicated that in normal CRL8799 cells treatment with cisplatin, TRAIL or combination of cisplatin plus TRAIL had a minimal effect on both EGFR and p63 proteins compared to untreated control cells. In comparison, CRL2335 TNBC cells expressed both EGFR and P63 proteins, 24 hour treatment with cisplatin resulted in a significant reduction while similar treatment with TRAIL had a minimal effect on these proteins. In contrast, cisplatin plus TRAIL treatment resulted in a significant suppression of both proteins. On the other hand, MDA-MB-468 TNBC cells expressed higher level of EGFR and negligible level of P63 protein. Treatments with cisplatin, TRAIL and cisplatin plus TRAIL for 24 hours resulted in a significant reduction in the expression of EGFR. Taken together, these data suggested that cisplatin plus TRAIL treatment had a significant effect in reducing EGFR and/or P63 protein levels in TNBC cells while similar treatment had a minimal effect in CRL8799 normal breast cancer cells.
Figure 2.
Expression of EGFR, P63 and P73 in CRL2335 and MDA-MB-468 TNBC cells and CRL8799 normal breast cancer cells treated with cisplatin, TRAIL or cisplatin plus TRAIL (10 μg/ml, 10 ng/ml and 10 μg/ml+10 ng/ml, respectively) for 24 h. Whole cell extracts were prepared and analyzed for EGFR, P63, P73 by Western blotting. Equal protein loading was compared with that of GAPDH.
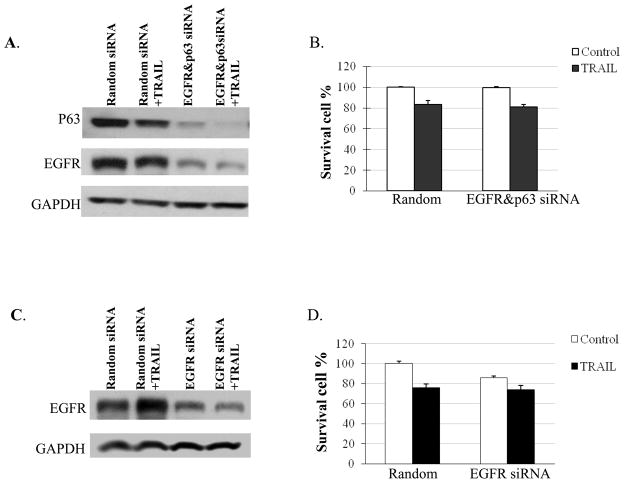
The expression of EGFR and P63 protein in CRL-2335 TNBC cells were knocked down using EGFR and/or P63 siRNA to determine whether inhibition of these proteins is essential for TRAIL-induced apoptosis. The results presented in Figure 3A, and 3B suggested that the inhibition of both proteins by siRNA did not increase cell death: the percentages of surviving cells were not significantly different than in control cells treated with random siRNA (Figure 3B). Similar results were obtained in MDA-MB-468 TNBC cells when EGFR expression was knocked down using EGFR siRNA (which expressed EGFR but negligible levels of P63 protein as shown in Figure 2) and treated with TRAIL: there was no increase cell death and the percentage of surviving cells were not significantly different than in control cells treated with random siRNA (Figure 3D). These results indicated that inhibition of EGFR and/or P63 may not be sufficient to increase TRAIL-induced apoptosis in TNBC cells. Perhaps, combined treatment with cisplatin may be affecting other ‘death’ proteins to enhance TRAIL-induced apoptosis.
Figure 3.
Effect of siRNA knockdown of EGFR and/or P63 in TNBC cells. CRL2335 TNBC cells were transfected with both EGFR siRNA and P63 siRNA and MDA-MB-468 cells were transfected with EGFR siRNA alone or random siRNA. After 48h of transfection, cells were treated with or without TRAIL for additional 24h. Whole cell extracts were prepared and analyzed for EGFR, P63 by Western blotting (A, C). Equal protein loading was compared with that of GAPDH. (B, D) Under similar conditions cell viability was assessed using trypan blue exclusion method.
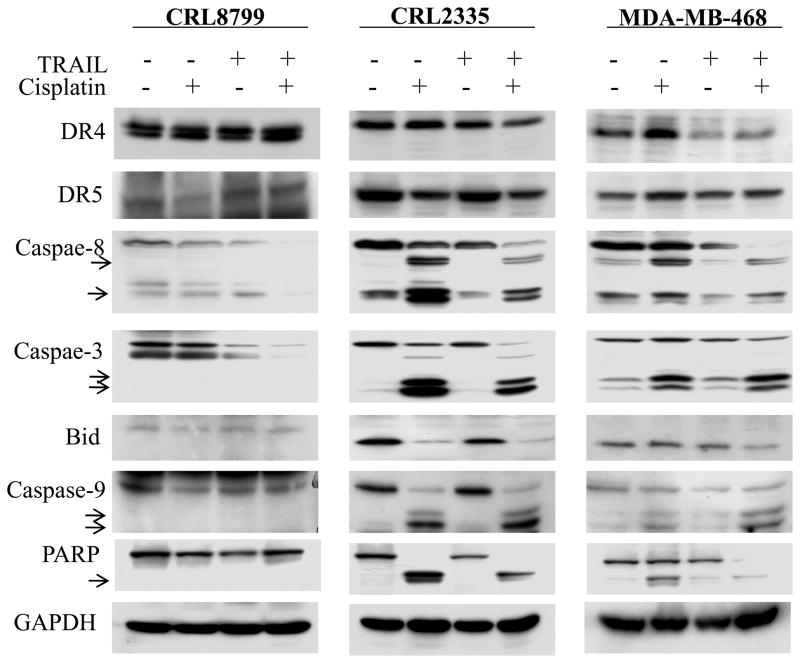
Cisplatin and TRAIL enhanced caspase activation and suppresses anti-apoptotic proteins in TNBC cells without effecting normal cells
Caspases are reported to play an important role in TRAIL-induced apoptosis (24). Therefore, the effect of cisplatin, TRAIL and cisplatin plus TRAIL treatment on activation of caspases -8, -3 and -9, Bid and cleavage of poly (ADP-ribose) polymerase (PARP) was investigated in CRL8799 normal cells and CRL2335 and MDA-MB-468 TNBC cells. The data in Figure 4 indicated that the activities of caspases -8, -3 and -9 were increased in TNBC cells by cisplatin treatment leading to enhanced Bid and PARP cleavage. However, the maximum effect was observed when the same cells were treated with cisplatin plus TRAIL. In contrast, similar treatments in CRL8799 normal cells had a minimal effect on activity of caspases -8, -3 and -9, Bid and PARP cleavage.
Figure 4.
CRL8799 normal breast cancer cells, CRL2335, MDA-MB-468 TNBC cells were treated with cisplatin, TRAIL or cisplatin plus TRAIL as shown in Fig. 2. Whole cell extracts were prepared and analyzed by Western blotting. Equal protein loading was compared with that of GAPDH. Cisplatin plus TRAIL treatment increased the activation of caspases -8, -3 and -9 in TNBC cells and had no effect on CRL8799 normal cells.
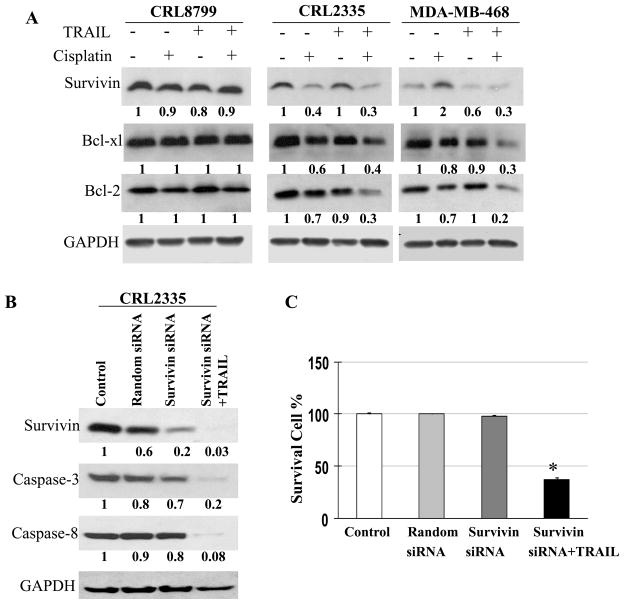
The effect of cisplatin, TRAIL, cisplatin plus TRAIL treatment was also examined on anti-apoptotic protein Survivin (25), Bcl-xL and Bcl-2 in CRL8799 normal cells and CRL2335, MDA-MB-468 TNBC cells. The results in Figure 5A showed maximum inhibition of Survivin as well as Bcl-xl and Bcl-2 in TNBC cells treated with cisplatin plus TRAIL compared to cisplatin or TRAIL alone. These observations provided support to the data presented in Figure 1B where CRL2335 and MDA-MB-468 TNBC cells treated with cisplatin plus TRAIL showed significantly decreased percentage of surviving cells. It is interesting to note that similar treatments had no effect or, at best, a minimal effect in CRL8799 normal cells.
Figure 5.
CRL8799 normal breast cancer cells, CRL2335, MDA-MB-468 TNBC cells were treated with cisplatin, TRAIL or cisplatin plus TRAIL as shown in Fig.2 . Whole cell extracts were prepared and examined the expression of Survivin, Bcl-2 and Bcl-xl by Western blotting (Fig. 5A). Equal protein loading was compared with that of GAPDH. (B) Effect of Survivin knockdown on TRAIL-induced cell death. CRL2335 TNBC cells were transfected with either Survivin siRNA or random siRNA. After 48h of transfection, cells were treated with or without TRAIL for additional 24h. Whole cell extracts were prepared and the expression of Survivin, caspases -3 and -8 were examined by Western blotting. Equal protein loading was compared with that of GAPDH. (C) Under similar conditions cell viability was also assessed using trypan blue exclusion method.
Previous reports have suggested that Survivin significantly reduces apoptosis by inhibiting caspases (26–28). If down regulation of Survivin sensitizes CRL-2335 TNBC cells to TRAIL-induced apoptosis were examined by inhibiting Survivin by siRNA in the presence of TRAIL. Indeed, CRL2335 TNBC cells treated with Survivin siRNA and TRAIL exhibited higher activation of caspases -3 and -8 (Figure 5B) with a concomitant and significant increase TRAIL-induced cell death (Figure 5C) in comparison with the cells treated with random siRNA. These results provide support that Survivin plays an important role in TRAIL-induced apoptosis in TNBC cells. It is interesting to note that random siRNA treatment had no effect on cell survival in CRL8799 normal cells.
Efficacy of Cisplatin plus TRAIL Treatment on the growth of TNBC cells in Vivo
The inhibition of CRL2335 TNBC cell xenografts in mice treated with cisplatin plus TRAIL was assessed in vivo in mice. The results in Figure 6 indicated that there was a gradual regression of tumor xenografts as indicated by reduction in tumor size/volume in mice treated with cisplatin plus TRAIL as compared with control mice which were injected with vehicle. Also, the mice tolerated cisplatin plus TRAIL treatment with no signs of toxicity or weight loss during the observation period (data not shown). These findings suggest that cisplatin plus TRAIL therapy enhanced antitumor effect in vivo and, these observations were consistent with results obtained in vitro.
Figure 6.
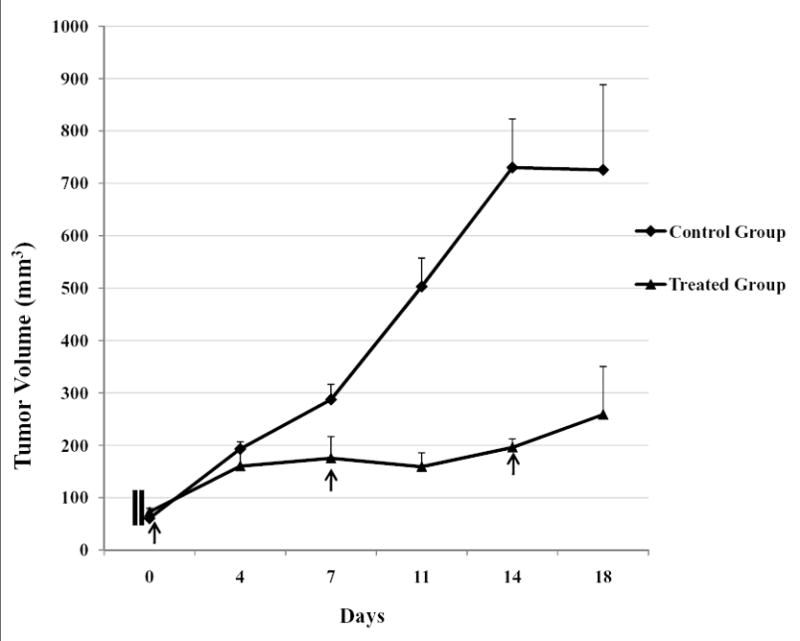
Regression of CRL2335 xenografts in mice treated in vivo with cisplatin plus TRAIL.
Six scid mice were injected with 5×105 CRL2335 TNBC cells. When the tumors were palpable and ~50mm3 in size, 3 mice were treated with cisplatin plus TRAIL (4 mg/kg+15 mg/kg, respectively, intra-peritoneal injection, once a week for 3 weeks). The other 3 animals were used as controls and injected with vehicle (1x PBS). Day 0 indicates the first injection. All mice were healthy and active. Treated mice exhibited no significant weight loss after two injections. Tumor volume was determined at the indicated times after the onset of treatment. Data are mean +/- SE. The difference between treated and control group was P<0.05.
Discussion
Triple-negative Breast cancer (TNBC) is the most aggressive and difficult to treat form of cancer. TNBC patients do not benefit from hormonal or herceptin-based therapies due to loss of target receptors such as ER, PR and HER-2. The results from the present investigations clearly demonstrated that CRL2335 and MDA-MB-468 TNBC cells with cisplatin plus TRAIL exhibited significantly enhanced apoptosis (~ 60–70% cell death) as compared to untreated cells. Also, similar treatment had a minimal effect on CRL8799 normal breast cancer cells (~10–15% cell death) (Figure 1). In addition, the results from the current study also provided evidence that cisplatin exerted its influence on TRAIL-induced TNBC cell death by simultaneous activation of caspases and inhibition of anti-apoptotic protein Survivin. Furthermore, suppression of Survivin appears to have played a major role in TRAIL-induced cell death in TNBC cells. These observations gain support from Altier (25) report that increased Survivin expression enhanced tumor resistance to various apoptotic stimuli, primarily through caspase-dependent mechanism and antagonizing Survivin-induced apoptosis in tumor cells. Bcl-2 and Bcl-xl reside within the mitochondrial membrane where they act by inhibiting adaptor molecules needed for the activation of effector caspases.
The results from the present study have suggested that treatment with cisplatin alone induced ~20–30% and ~ 8–12% cell death in CRL2335 and MDB-MB-468 TNBC cells which express high and low levels of P63, respectively (Figure 1&2). These observations are consistent with recently published clinical (pathological assessment) data where cisplatin treatment was shown to have a significantly higher rate of remission in P63-positive tumors as compared with P63-negative tumors (29). Furthermore, Leong et al (23) have reported that cisplatin treatment of breast cancer cells which express both P63 and P73 activated c-Abl-dependent phosphorylation of P73 and dissociation of P63/P73 protein complex leading to P73-dependent transcription of pro-apoptotic Bcl-2 family members and thus, significantly enhanced apoptosis. These observations suggested that cisplatin in very effective in P63-positive TNBC patients. However, only a small percent of tumors are P63 positive in TNBC patients (30). The results from the present investigation have shown that the combination of cisplatin plus TRAIL is effective in both P63-positive and P63-negative TNBC cells.
Approximately 60% of TNBC cells show expression of EGFR and, and drugs that target EGFR could be beneficial to treat TNBC patients. Although Carey et al (31) reported that cetuximan, a chimeric monoclonal antibody which targets EGFR, elicited little or no response from patients with advanced TNBC, the results from phase II clinical trial using cetuximab+carboplatin suggested favorable response (18%) and overall therapeutic gain (27%) in 102 patients with advanced TNBC (32). The observations from previous phase II clinical trials have also indicated an overall response rate of ~ 32% when unselected breast cancer patients were treated with cisplatin plus carboplatin (33–35). In more recent studies, platinum-based chemotherapy increased the response rate in TNBC tumors, with a trend towards improved survival in TNBC patients (36). A number of phase III clinical trials are in progress in which advanced TNBC patients were randomized and treated first with carboplatin or docetaxel, with cross-over on progression (37). A non-randomized phase II study using cisplatin to treat TNBC patients is also under way in the USA (38).
In conclusion the results from the present investigations suggested that treatment of CRL2335 and MDA-MB-468 TNBC cells with cisplatin can enhance TRAIL-induced apoptosis by activating caspases along with simultaneous reduction of anti-apoptotic proteins such as Survivin, Bcl-xL and Bcl-2 expression. Comparatively, similar treatment of immortalized CRL8799 normal breast cells had a minimal effect. Individually, cisplatin and TRIAL are approved by FDA to treat other forms of cancer. Since TNBC patients do not benefit from hormonal or herceptin-based therapies as well as other such as surgery, chemo- and radiation-therapy, cisplatin plus TRAIL treatment is suggested as a ‘potential’ new cancer treatment strategy in TNBC patients.
Acknowledgments
This work was supported in part by Department of Pathology, WSU and the National Institute of Health Grant RO1 CA 118089 (to K.B.R)
The abbreviations used are
- TRAIL
Tumor necrosis factor-related apoptosis-inducing ligand
- DR4
Death receptor 4
- DR5
Death receptor 5
- PARP
poly(ADP-ribose) polymerase
- FADD
Fas-associated protein with death domain
- Cas-3
Caspase-3
- EGFR
Epidermal growth factor receptor
- ER
Estrogen receptor
- PR
Progesterone receptor
Footnotes
Disclosure of potential conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–74. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 3.Reis-Filho JS, Milanezi F, Steele D, Savage K, et al. Metaplastic breast carcinomas are basal-like tumours. Histopathology. 2006;49:10–21. doi: 10.1111/j.1365-2559.2006.02467.x. [DOI] [PubMed] [Google Scholar]
- 4.Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008;52:108–18. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 5.Calza S, Hall P, Auer G, et al. Intrinsic molecular signature of breast cancer in a population-based cohort of 412 patients. Breast Cancer Res. 2006;8:R34. doi: 10.1186/bcr1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. Jama. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 7.Kim MJ, Ro JY, Ahn SH, Kim HH, Kim SB, Gong G. Clinicopathologic significance of the basal-like subtype of breast cancer: a comparison with hormone receptor and Her2/neu-overexpressing phenotypes. Hum Pathol. 2006;37:1217–26. doi: 10.1016/j.humpath.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 9.Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–7. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 10.Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 11.Tischkowitz M, Brunet JS, Begin LR, et al. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. BMC Cancer. 2007;7:134. doi: 10.1186/1471-2407-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005;23:7350–60. doi: 10.1200/JCO.2005.03.3845. [DOI] [PubMed] [Google Scholar]
- 13.Tassone P, Tagliaferri P, Perricelli A, et al. BRCA1 expression modulates chemosensitivity of BRCA1-defective HCC1937 human breast cancer cells. Br J Cancer. 2003;88:1285–91. doi: 10.1038/sj.bjc.6600859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahsan A, Hiniker SM, Ramanand SG, et al. Role of epidermal growth factor receptor degradation in cisplatin-induced cytotoxicity in head and neck cancer. Cancer Res. 2010;70:2862–9. doi: 10.1158/0008-5472.CAN-09-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seetharamu N, Kim E, Hochster H, Martin F, Muggia F. Phase II study of liposomal cisplatin (SPI-77) in platinum-sensitive recurrences of ovarian cancer. Anticancer Res. 2010;30:541–5. [PubMed] [Google Scholar]
- 16.William WN, Jr, Kies MS, Fossella FV, et al. Phase 2 study of carboplatin, docetaxel, and bevacizumab as frontline treatment for advanced nonsmall-cell lung cancer. Cancer. 2010;116:2401–08. doi: 10.1002/cncr.24996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelley SK, Ashkenazi A. Targeting death receptors in cancer with Apo2L/TRAIL. Curr Opin Pharmacol. 2004;4:333–9. doi: 10.1016/j.coph.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Herr I, Debatin KM. Cellular stress response and apoptosis in cancer therapy. Blood. 2001;98:2603–14. doi: 10.1182/blood.v98.9.2603. [DOI] [PubMed] [Google Scholar]
- 19.Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–63. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 20.Nabha SM, Glaros S, Hong M, et al. Upregulation of PKC-delta contributes to antiestrogen resistance in mammary tumor cells. Oncogene. 2005;24:3166–76. doi: 10.1038/sj.onc.1208502. [DOI] [PubMed] [Google Scholar]
- 21.Gibson EM, Henson ES, Haney N, Villanueva J, Gibson SB. Epidermal growth factor protects epithelial-derived cells from tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by inhibiting cytochrome c release. Cancer Res. 2002;62:488–96. [PubMed] [Google Scholar]
- 22.Gibson S, Tu S, Oyer R, Anderson SM, Johnson GL. Epidermal growth factor protects epithelial cells against Fas-induced apoptosis. Requirement for Akt activation. J Biol Chem. 1999;274:17612–8. doi: 10.1074/jbc.274.25.17612. [DOI] [PubMed] [Google Scholar]
- 23.Leong CO, Vidnovic N, DeYoung MP, Sgroi D, Ellisen LW. The p63/p73 network mediates chemosensitivity to cisplatin in a biologically defined subset of primary breast cancers. J Clin Invest. 2007;117:1370–80. doi: 10.1172/JCI30866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin J, Zhang Z, Zeng S, et al. TRAIL-induced apoptosis proceeding from caspase-3-dependent and -independent pathways in distinct HeLa cells. Biochem Biophys Res Commun. 2006;346:1136–41. doi: 10.1016/j.bbrc.2006.05.209. [DOI] [PubMed] [Google Scholar]
- 25.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 26.Dohi T, Beltrami E, Wall NR, Plescia J, Altieri DC. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Invest. 2004;114:1117–27. doi: 10.1172/JCI22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin S, Sung BJ, Cho YS, et al. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry. 2001;40:1117–23. doi: 10.1021/bi001603q. [DOI] [PubMed] [Google Scholar]
- 28.Tamm I, Wang Y, Sausville ES, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315–20. [PubMed] [Google Scholar]
- 29.Rocca A, Viale G, Gelber RD, et al. Pathologic complete remission rate after cisplatin-based primary chemotherapy in breast cancer: correlation with p63 expression. Cancer Chemother Pharmacol. 2008;61:965–71. doi: 10.1007/s00280-007-0551-3. [DOI] [PubMed] [Google Scholar]
- 30.Jumppanen M, Gruvberger-Saal S, Kauraniemi P, et al. Basal-like phenotype is not associated with patient survival in estrogen-receptor-negative breast cancers. Breast Cancer Res. 2007;9:R16. doi: 10.1186/bcr1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carey LA, Mayer E, Marcom P. TBCRC 001:EGFR inhibition with cetuximab in metastatic triple negative (basal-like) breast cancer (abstract 307) Breast cancer Res Treat. 2007;106 (suppl 1):S32. [Google Scholar]
- 32.Carey LA, Rugo HS, Marcom PK, et al. TBCRC 001: EGFR inhibition with cetuximab added to carboplatin in metastatic triple-negative (basal-like) breast cancer (abstract 1009) J Clin Oncol. 2008;26 (15S):43s. [Google Scholar]
- 33.Kolaric K, Roth A. Phase II clinical trial of cis-dichlorodiammine platinum (cis-DDP) for antitumorigenic activity in previously untreated patients with metastatic breast cancer. Cancer Chemother Pharmacol. 1983;11:108–12. doi: 10.1007/BF00254257. [DOI] [PubMed] [Google Scholar]
- 34.Sledge GW, Jr, Loehrer PJ, Sr, Roth BJ, Einhorn LH. Cisplatin as first-line therapy for metastatic breast cancer. J Clin Oncol. 1988;6:1811–4. doi: 10.1200/JCO.1988.6.12.1811. [DOI] [PubMed] [Google Scholar]
- 35.Smith IE, Talbot DC. Cisplatin and its analogues in the treatment of advanced breast cancer: a review. Br J Cancer. 1992;65:787–93. doi: 10.1038/bjc.1992.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sirohi B, Arnedos M, Popat S, et al. Platinum-based chemotherapy in triple-negative breast cancer. Ann Oncol. 2008;19:1847–52. doi: 10.1093/annonc/mdn395. [DOI] [PubMed] [Google Scholar]
- 37.Triple negative trial: a randomised phase III trial of carboplatin compared to docetaxel for patients with advanced oestrogen receptor-progesterone receptor-human epidermal growth factor receptor two-breast cancer. 2007 http://www.controlled-trials.com/ISRCTN97330056.
- 38.ClinicalTrials.gov. Cisplatin as first line therapy for triple-negative metastatic breast cancer and evaluation of p63/p73 as a biomarker response. 2007 http://www.clinicaltrials.gov/ct2/show/NCT00483223?term=NCT00483223&rank=1.