Abstract
Intermediate filaments, including nestin and glial fibrillary acidic protein (GFAP), are important for the brain to accommodate neural activities and changes during development. The present study examined the temporal changes of nestin and GFAP protein levels in the postnatal development of the mouse hippocampus. Mouse hippocampi were sampled on postnatal day (PND) 1, 3, 6, 18, and 48. Western blot analysis showed that nestin expression was high at PND 1 and markedly decreased until PND 18. Conversely, GFAP expression was acutely increased in the early phase of postnatal development. Nestin immunoreactivity was localized mainly in the processes of ramified cells at PND 1, but expression subsequently decreased. In contrast, GFAP was evident mainly in the marginal cells of the hippocampus at PND 1, but immunoreactivity revealed satellite, radial, or ramified shapes of the cells from PND 6-48. This study demonstrates that the opposing pattern of nestin and GFAP expressions in mouse hippocampus during postnatal development occur in the early development stage (PND 1-18), suggesting that the opposing change of nestin and GFAP in early postnatal development is important for neural differentiation and positioning in the mouse hippocampus.
Keywords: GFAP, hippocampus, mice, nestin, postnatal development
Introduction
Intermediate filaments (IFs) are a family of related proteins that share common structural and sequence features. IFs have an average diameter of 10 nm, which is between that of actin (microfilaments) and microtubules [7]. These proteins give mechanical stability to cells and tissues, and play an important role in mechanotransduction and neurogenesis [2,15]. IFs are subcategorized into six types based on similarities in amino acid sequence and protein structure [16]. IF gene expression changes occur during key steps in the differentiation of cell types in the mammalian central nervous system (CNS) [17,28].
Nestin is a class III IF, which also include vimentin and glial fibrillary acidic protein (GFAP). Nestin is more closely related structurally to class IV IFs, such as neurofilaments and α-internexin [12,16,18]. The nestin protein is highly localized in the proliferative regions of embryonic or developing CNS, peripheral nervous system, and myogenic and other tissues, and is also predominately localized in radial glial cells that are recognized as neural precursors [9,27,28]. Nestin is also expressed by many types of cells during development although its expression is usually transient and does not persist into adulthood [16,23]. GFAP is a class III IF protein that is found in glial cells such as astrocytes [22] and other cell types such as Leydig cells in the testis and satellite cells in the liver [4]. It is functionally involved in many cellular processes and communications, and the blood-brain barrier [5].
Upregulation of IFs after CNS injury may result in gliosis and formation of a glial scar [6,11,19]. Interestingly, the expression of nestin in hippocampal astrocytes after injury deceases depending on age [1]. Even though a previous study reported on nestin and GFAP changes in the proliferative regions of CNS of developing brain [24], semi-quantitatively determined changes of nestin and GFAP changes in the developing hippocampus are required to validate the role of nestin and GFAP in the developing hippocampus. In this study, biochemical and immunohistochemical approaches were applied to investigate the temporal profiles of nestin and GFAP expression in developing mouse hippocampi.
Materials and Methods
Animals
ICR adult mice (Orient Bio, Korea) of both genders were maintained and mated in our laboratory. Brain tissues (seven samples at each time point) from two or three litters were sampled at 1, 3, 6, 18, and 48 days after birth (post-natal day, PND). All animal experiments followed a protocol approved by the Committee for Animal Experimentation at Chonnam National University, Korea.
Western blotting
Mice were sacrificed and hippocampi were dissected at PND 1, 3, 6, 18, and 48 (n = 4 mice/group). The hippocampus from each mouse was individually immersed quickly in buffer H (50 mM β-glycerophosphate, 1.5 mM EGTA, 0.1 mM Na3VO4, 1 mM dithiothreitol, 10 µg/mL aprotinin, 2 µg/mL pepstatin, 10 µg/mL leupeptin; 1 mM phenylmethanesulfonylfluoride, pH 7.4), and sonicated for 10 sec. Sodium dodecyl sulfate sample buffer (×4) was added to each homogenized sample, and the samples were heated to 100℃ for 10 min. The samples were then separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The resolved proteins were transferred to a nitrocellulose membrane, which was then blocked with 5% skim milk in phosphate-buffered saline with 0.1% Tween 20 (PBS-T, pH 7.4) for 30 min at room temperature. The membrane was then incubated with primary antibody against mouse nestin (1 : 1,000 dilution; Chemicon International, USA) or anti-mouse GFAP (1 : 10,000 dilution; Sigma-Aldrich, USA) in PBS-T overnight at 4℃. After extensive washing with PBS-T and incubation with horseradish peroxidase-conjugated anti-mouse antibody (1 : 10,000 dilution; Pierce, USA), signals were visualized using a chemiluminescence kit (SuperSignal West Pico; Pierce, USA). For normalization purposes, the membranes were stripped and re-probed with an antibody against β-actin (1 : 20,000; Sigma-Aldrich, USA). Several exposure times were used to obtain signals in the linear range. The bands were quantified using Scion Image Beta 4.0.2 for Windows XP software (Scion, USA).
Immunohistochemistry
Mice were sacrificed and the brains dissected at PND 1, 3, 6, 18, and 48 (n = 3 mice/group). The samples were fixed in 4% paraformaldehyde in PBS before being embedded in paraffin wax. Coronal sections (5 µm in thickness) were cut and then deparaffinized, hydrated, and incubated with monoclonal anti-nestin antibody (1 : 100 dilution; Chemicon International, USA) and rabbit anti-GFAP antibody (1 : 500; Dakocytomation, Denmark). Primary antibody binding was detected with biotinylated horse anti-mouse or goat anti-rabbit IgG (Vector Elite kit; Vector, USA). Immunoreactivity was observed using an avidin-biotin peroxidase complex (Vector Elite kit; Vector, USA). The peroxidase reaction was developed using a diaminobenzidine substrate kit (Vector, USA). As a control, the primary antibodies were omitted for a few test sections in each experiment. The sections were counterstained with hematoxylin before being mounted on slides.
Statistical analysis
The data is reported as the mean ± SE. The data was analyzed using one-way ANOVA followed by a Student-Newman-Keuls post hoc test for multiple comparisons. In all cases, a p value < 0.05 was considered significant.
Results
Temporal expression pattern of nestin and GFAP during postnatal development of mouse hippocampi
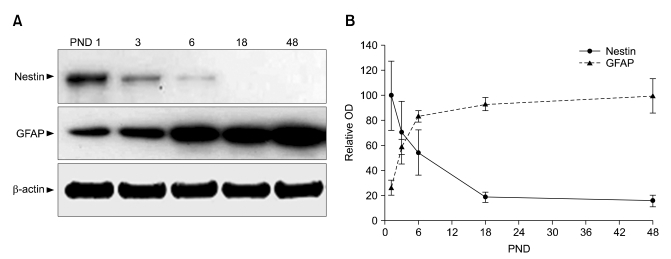
Nestin and GFAP protein expression in mouse hippocampi during postnatal development were semi-quantitatively analyzed on PND 1, 3, 6, 18, and 48 by Western blotting. The level of nestin expression in hippocampi peaked on PND 1 and sharply decreased until PND 18 (p < 0.05; Fig. 1). Nestin expression was rarely detected after PND 18. In contrast, starting from a low level at PND 1, hippocampal GFAP expression abruptly increased (3.2-fold; p < 0.01) until PND 6, then stabilized but increased progressively until PND 48 (Fig. 1).
Fig. 1.
Temporal expression of nestin and glial fibrillary acidic protein (GFAP) in mouse hippocampus during postnatal development. (A) Representative photographs of the Western blots for nestin, GFAP, and β-actin. The arrowheads indicate the positions of nestin (~215 kDa), GFAP (~53 kDa), and β-actin (~45 kDa). (B) Bar graphs showing the results of densitometric data analysis (mean ± SE, n = 4 mice/group). The value indicating nestin and GFAP expression levels from the hippocampus at postnatal day (PND) 1 and 48, respectively, was arbitrarily defined as 1 (B, bar graphs).
Immunohistochemical analysis of nestin and GFAP in mice hippocampi
At PND 1, nestin immunoreactivity was localized in all hippocampal regions, including the cornu ammonis (CA) 1, CA3, and dentate gyrus (DG), with a dense pattern of radial fibers in ramified cells (Figs. 2A-C). Some nestin immunoreactivity was localized in blood vessels (Figs. 2B and C, denoted by arrows). At PND 6 (Figs. 2D-F), DG immunoreactivity was similar to that observed at PND 1 (Fig. 2E), but a reduction of nestin-positive radial fibers was evident in the CA1 (Fig. 2F). At PND 48 (Figs. 2G-I), a few nestin-positive cells were found in the DG (Fig. 2H), but there was no nestin immunoreactivity in the CA1 (Fig. 2I).
Fig. 2.
Nestin immunostaining at PND 1 (A, B, C), 6 (D, E, F), and 48 (G, H , I) in the dentate gyrus (DG) and cornu ammonis (CA) 1 of mouse hippocampus. Nestin expression was strongly detected at PND 1. Arrows indicate nestin-positive blood vessels. Left panels show low magnification views of unilateral hippocampus. Middle panels present higher magnification views of DG. Right panels show higher magnification views of CA1. Scale bars = 250 µm (A, D, G); 20 µm (B, E, H); 25 µm (C, F, I).
Few GFAP-positive elements were detected at PND 1 in the hippocampus (Figs. 3A-C) although GFAP immunoreactivity was found in the margin cells of the DG (Fig. 3B). At PND 6, GFAP-positive immunoreactivity appeared in radial fibers of ramified cells in the DG and astrocytes in the CA1 (Figs. 3D-F); many positive cells had the appearance of typical mature astrocytes, and were satellite, radial, or ramified shaped (Figs. 3E and F). At PND 48, GFAP immunopositivity was strongly evident in all areas of the adult hippocampus including the CA1, CA3, and DG (Figs. 3G-I). The majority of GFAP positive-cells displayed the typical astrocytic morphology (Figs. 3H and I).
Fig. 3.
GFAP immunostaining at PND 1 (A, B, C), 6 (D, E, F) and 48 (G, H, I) in the DG and CA1 of mouse hippocampus. Left panels present low magnification views of unilateral hippocampus. Middle panels show higher magnification views of DG. Right panel present higher magnification view of CA1. Scale bars = 200 µm (A, D, G); 25 µm (B, C, E, F, H, I).
Discussion
This study demonstrated the progressive changes with opposing patterns of nestin and GFAP expression in mouse hippocampi during postnatal development. While nestin protein expression acutely decreased, GFAP immunoreactivity sharply increased in the early phase of postnatal development.
There is consensus that the process of hippocampal neurogenesis continues throughout mammalian life, including in humans [13]. The hippocampus is the most vulnerable brain region (especially multipotent progenitor cells in the DG), and cell replacement follows cell death induced by various effectors [14,21,26]. Changes in IF gene expression occur at key steps in cell differentiation in the mammalian CNS [17,28]. Cells expressing nestin show characteristic features of progenitor cells that include multipotency, high proliferation, limited self-renewal capability, and limited regeneration capacity [25]. In the adult CNS, nestin expression is most closely associated with a stem/progenitor cell populations, and proliferation and migration of these nestin-positive cells appear to be reactivated in response to injury during tissue regeneration [6,11,19,25]. Multipotentl hippocampal cells frequently display extending processes and representing an early stage of cell differentiation [27]. In this study, nestin immunoreactivity was localized in all hippocampal regions at PND 1 and 6 with a dense pattern of radial fibers in ramified cells. Some nestin immunoreactivity was localized in blood vessels. Therefore, substantial distribution of nestin-containing cells has provided evidence of neural differentiation.
Nestin immunoreactivity has been demonstrated in various cells of neuroepithelial origin [16,27]. Nestin appears prenatally, and marks several immature cells of neuroepithelial origin such as neuroepithelial columnar cells, radial glia, multipotent stem cells, and bipotent glial/neuronal progenitor cells [8,10,16]. In this study, nestin immunopositivity was present in the mouse hippocampus as early as PND 1. In contrast to the temporal expression of nestin, the timing of the appearance of GFAP-immunopositive structures in the hippocampus resulted in a pattern of increasing expression in the early phase of postnatal development (i.e., the radial fibers of ramified cells). Their processes were not previously evident in GFAP-immunostained structures [3,12]. However, we observed that in the immature hippocampus, some nestin-positive staining had an arborization pattern, which proved the astrocytic nature of the cells. Such arborization is not found in neuroblasts or early precursors of oligodendrocytes [3,8,27]. A previous study indicated that nestin expression seems to be an early event in the differentiation of glial progenitors along an astrocyte pathway in the immature brain [27]. These observations support the idea that GFAP progressively replaces nestin as these cells differentiate. Since GFAP is present only within astrocytes in the CNS, its expression is considered to be a hallmark of astrocytic differentiation [20]. Therefore, an increase in both nestin and GFAP may be necessary for stabilization of major astrocyte processes during the early phase of astrocyte differentiation [27]. However, many questions concerning the functional role and transcriptional regulation of nestin and GFAP expression in various cells and tissues remain unanswered. Furthermore, the precise role of nestin and GFAP in the developing hippocampus is not presently clear.
A previous study showed the distribution pattern of nestin-containing neural precursors in the postnatal hippocampus and provided evidence of their differentiation fate to neurons and astrocytes [24]. The authors determined that nestin-containing cells are decreased in the hippocampus during postnatal developing by evaluating the intensity of nestin in an immunohistochemical analysis. However, this method, which scored nestin staining intensity as high (+++), moderate (++), low (+), or negative (-), was limited in its quantitative capability. In the current study, we assessed semi-quantitative changes in nestin and GFAP expression in the hippocampus during postnatal development using Western blot analysis. Moreover, we compared the temporal change in GFAP expression with that of nestin in the hippocampus during PND 1-48. The previous study performed an immunohistochemical comparison between nestin and GFAP only at 60 days after birth.
In conclusion, this study confirmed the progressive changes with opposing pattern of nestin and GFAP expression in mouse hippocampus during postnatal development, suggesting that the opposing changes in nestin and GFAP expression during the early stage of postnatal development is important for neural differentiation and positioning in mouse hippocampus.
Acknowledgments
This work was supported by a National Research Foundation of Korea Grant funded by the Korean Government (2009-0068372), a Grant of the Korean Ministry of Education, Science and Technology (the Regional Core Research Program/Biohousing Research Institute) and the Biohousing Research Center.
References
- 1.Abdel-Rahman A, Rao MS, Shetty AK. Nestin expression in hippocampal astrocytes after injury depends on the age of the hippocampus. Glia. 2004;47:299–313. doi: 10.1002/glia.20047. [DOI] [PubMed] [Google Scholar]
- 2.Bennett GS. Changes in intermediate filament composition during neurogenesis. Curr Top Dev Biol. 1987;21:151–183. doi: 10.1016/s0070-2153(08)60136-2. [DOI] [PubMed] [Google Scholar]
- 3.Compston A, Zajicek J, Sussman J, Webb A, Hall G, Muir D, Shaw C, Wood A, Scolding N. Glial lineages and myelination in the central nervous system. J Anat. 1997;190:161–200. doi: 10.1046/j.1469-7580.1997.19020161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidoff MS, Middendorff R, Köfüncü E, Müller D, Jezek D, Holstein AF. Leydig cells of the human testis possess astrocyte and oligodendrocyte marker molecules. Acta Histochem. 2002;104:39–49. doi: 10.1078/0065-1281-00630. [DOI] [PubMed] [Google Scholar]
- 5.Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969-2000) Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- 6.Frisén J, Johansson CB, Török C, Risling M, Lendahl U. Rapid, widespread, and longlasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. J Cell Biol. 1995;131:453–464. doi: 10.1083/jcb.131.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- 8.Gallo V, Armstrong RC. Developmental and growth factor-induced regulation of nestin in oligodendrocyte lineage cells. J Neurosci. 1995;15:394–406. doi: 10.1523/JNEUROSCI.15-01-00394.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gates MA, Thomas LB, Howard EM, Laywell ED, Sajin B, Faissner A, Götz B, Silver J, Steindler DA. Cell and molecular analysis of the developing and adult mouse subventricular zone of the cerebral hemispheres. J Comp Neurol. 1995;361:249–266. doi: 10.1002/cne.903610205. [DOI] [PubMed] [Google Scholar]
- 10.Holmin S, Almqvist P, Lendahl U, Mathiesen T. Adult nestin-expressing subependymal cells differentiate to astrocytes in response to brain injury. Eur J Neurosci. 1997;9:65–75. doi: 10.1111/j.1460-9568.1997.tb01354.x. [DOI] [PubMed] [Google Scholar]
- 11.Jin JK, Jeong BH, Na YJ, Kim YS, Carp RI, Wie MB, Moon C, Shin T. Increased expression of the embryonic intermediate filament, nestin, in the brains of scrapie-infected mice. Neurosci Lett. 2004;367:254–258. doi: 10.1016/j.neulet.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 12.Kálmán M, Ajtai BM. A comparison of intermediate filament markers for presumptive astroglia in the developing rat neocortex: immunostaining against nestin reveals more detail, than GFAP or vimentin. Int J Dev Neurosci. 2001;19:101–108. doi: 10.1016/s0736-5748(00)00058-7. [DOI] [PubMed] [Google Scholar]
- 13.Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci USA. 1997;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JS, Lee HJ, Kim JC, Kang SS, Bae CS, Shin T, Jin JK, Kim SH, Wang H, Moon C. Transient impairment of hippocampus-dependent learning and memory in relatively low-dose of acute radiation syndrome is associated with inhibition of hippocampal neurogenesis. J Radiat Res (Tokyo) 2008;49:517–526. doi: 10.1269/jrr.08020. [DOI] [PubMed] [Google Scholar]
- 15.Kreplak L, Fudge D. Biomechanical properties of intermediate filaments: from tissues to single filaments and back. Bioessays. 2007;29:26–35. doi: 10.1002/bies.20514. [DOI] [PubMed] [Google Scholar]
- 16.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 17.Menet V, Giménez Y, Ribotta M, Sandillon F, Privat A. GFAP null astrocytes are a favorable substrate for neuronal survival and neurite growth. Glia. 2000;31:267–272. doi: 10.1002/1098-1136(200009)31:3<267::aid-glia80>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 18.Miyaguchi K. Ultrastructure of intermediate filaments of nestin- and vimentin-immunoreactive astrocytes in organotypic slice cultures of hippocampus. J Struct Biol. 1997;120:61–68. doi: 10.1006/jsbi.1997.3900. [DOI] [PubMed] [Google Scholar]
- 19.Moon C, Ahn M, Kim S, Jin JK, Sim KB, Kim HM, Lee MY, Shin T. Temporal patterns of the embryonic intermediate filaments nestin and vimentin expression in the cerebral cortex of adult rats after cryoinjury. Brain Res. 2004;1028:238–242. doi: 10.1016/j.brainres.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 20.Rutka JT, Murakami M, Dirks PB, Hubbard SL, Becker LE, Fukuyama K, Jung S, Tsugu A, Matsuzawa K. Role of glial filaments in cells and tumors of glial origin: a review. J Neurosurg. 1997;87:420–430. doi: 10.3171/jns.1997.87.3.0420. [DOI] [PubMed] [Google Scholar]
- 21.Seo HS, Yang M, Song MS, Kim JS, Kim SH, Kim JC, Kim H, Shin T, Wang H, Moon C. Toluene inhibits hippocampal neurogenesis in adult mice. Pharmacol Biochem Behav. 2010;94:588–594. doi: 10.1016/j.pbb.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Tardy M, Fages C, Le Prince G, Rolland B, Nunez J. Regulation of the glial fibrillary acidic protein (GFAP) and of its encoding mRNA in the developing brain and in cultured astrocytes. Adv Exp Med Biol. 1990;265:41–52. doi: 10.1007/978-1-4757-5876-4_4. [DOI] [PubMed] [Google Scholar]
- 23.Wegner M, Stolt CC. From stem cells to neurons and glia: a Soxist's view of neural development. Trends Neurosci. 2005;28:583–588. doi: 10.1016/j.tins.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Wei LC, Shi M, Chen LW, Cao R, Zhang P, Chan YS. Nestin-containing cells express glial fibrillary acidic protein in the proliferative regions of central nervous system of postnatal developing and adult mice. Brain Res Dev Brain Res. 2002;139:9–17. doi: 10.1016/s0165-3806(02)00509-6. [DOI] [PubMed] [Google Scholar]
- 25.Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov KV, Tarasova Y, Wersto RP, Boheler KR, Wobus AM. Nestin expression--a property of multi-lineage progenitor cells? Cell Mol Life Sci. 2004;61:2510–2522. doi: 10.1007/s00018-004-4144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang M, Kim JS, Song MS, Kim SH, Kang SS, Bae CS, Kim JC, Wang H, Shin T, Moon C. Cyclophosphamide impairs hippocampus-dependent learning and memory in adult mice: Possible involvement of hippocampal neurogenesis in chemotherapy-induced memory deficits. Neurobiol Learn Mem. 2010;93:487–494. doi: 10.1016/j.nlm.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Zerlin M, Levison SW, Goldman JE. Early patterns of migration, morphogenesis, and intermediate filament expression of subventricular zone cells in the postnatal rat forebrain. J Neurosci. 1995;15:7238–7249. doi: 10.1523/JNEUROSCI.15-11-07238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmerman L, Parr B, Lendahl U, Cunningham M, McKay R, Gavin B, Mann J, Vassileva G, McMahon A. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12:11–24. doi: 10.1016/0896-6273(94)90148-1. [DOI] [PubMed] [Google Scholar]