Abstract
Staphylococcus (S.) aureus is a common infectious agent of bovine chronic mastitis, a disease that is difficult to eradicate. The abilities of Staphylococci to be internalized and form a biofilm can contribute to host immunological defence evasion that subsequently impairs antimicrobial therapy. The invasive capability of six S. aureus field isolates with different biofilm-forming profiles was compared in vitro using a bovine mammary epithelial cell line. This was further confirmed in primary cell cultures using fluorescent rRNA probes against S. aureus. The results suggest that S. aureus invasion levels are not related to biofilm formation.
Keywords: biofilm, bovine mammary epithelial cells, bovine subclinical mastitis, invasion, Staphylococci
Bovine mastitis is a disease characterised by mammary gland inflammation, usually caused by intramammary infections. This condition is difficult to eradicate and responsible for severe economic losses for dairy producers. Staphylococcus (S.) aureus is a recognised mastitis pathogen often detected in dairy herds [5]. Internalization is an important step in staphylococcal mastitis pathogenesis [9]. In vitro studies have shown that Staphylococci are able to adhere to and invade bovine mammary epithelium [1,2,6,7,10].
Biofilm formation is a potential virulence factor [2,9]. During intramammary infection bacterial clusters may develop within the udder [9], and biofilm structures may facilitate Staphylococci adherence and colonization of the mammary gland epithelium [9]. In other bacterial genera, the ability to form biofilm appears to be associated with invasiveness [4,11]. In S. aureus, this relationship has not yet been clarified although it has been shown that capsule and other exoproducts may inhibit internalization [10]. This study compared the invasiveness of field isolates with different biofilm-forming profiles to determine the presence of these two virulence factors and their relationship in the isolates.
Six S. aureus isolates from a collection of subclinical mastitis isolates from dairy cows in Portuguese commercial dairy farms were used [12]. Isolates were chosen on the basis of their biofilm-forming ability. Three isolates (C607, C978, L145) were biofilm-positive as determined by optical density (OD), culturing in Congo red agar (CRA), and fluorescence in situ hybridization (FISH). The other three (D43, D8927.2, Z990) were biofilm-negative [12]. Phenotypic expression of biofilm in CRA was determined by observation of black (biofilm-positive) or red (biofilm-negative) colonies produced by the isolates after incubation (18 h, 37℃) in an agar medium to which sterilized Congo red stain (Sigma, USA) was added [12]. Quantification of biofilm formation by OD determination was performed using bacterial cultures grown in tryptic soya broth (TSB; Oxoid, UK) for 18 h at 37℃ as previously described [12].
A fluorescent 16S rRNA oligonucleotide probe, Sta (5'-TCCTCCATATCTCTGCGC-3'; Escherichia coli 697), specific for Staphylococcus spp. was used for FISH (MWG-Biotech, Germany) [12]. Ten µL of overnight bacterial suspensions in TSB were placed into each well of 10-well Teflon slides (Heinz Herenz, Germany) and incubated in a humid chamber for 24 h at 37℃, to allow biofilm formation. Afterwards, FISH was performed. S. epidermidis ATCC 35984 and ATCC12228 (LGC Standards, Spain) were used as positive and negative controls for the biofilm formation assay.
Invasion assays were performed using a bovine mammary epithelial (BME) cell line provided by Prof. Christian Burvenich (Ghent University, Belgium). Cells were grown in 24-well plates (Nunc, Denmark) in an atmosphere of 5% CO2 at 37℃ for 3 to 4 days in the following media: 40% Ham's F-12 (Invitrogen, USA), 30% RPMI 1640 (Gibco, USA), 20% NCTC 135 (Invitrogen, USA), 10% fetal calf serum (Invitrogen, USA), 0.1% lactose (Sigma, USA), 0.1% lactalbumin hydrolysate (Sigma, USA), 1.2 mM GSH (Sigma, USA), 10 µg/mL L-ascorbic acid (Sigma, USA), 1 µg/mL hydrocortisone (Sigma, USA), 1 µg/mL insulin (Sigma, USA) and 2.5 µg/mL amphotericin B (Gibco, USA). Bacterial cultures grown on Columbia agar plates (BioMérieux, France) at 37℃ for 18 h were suspended in Hank's balanced salt solution (HBSS; Gibco, USA) at a concentration adjusted to 106 colony forming units (CFU) per mL by OD based on standard curves previously determined. One mL of the bacterial suspensions from each isolate and control strains were used to infect confluent BME monolayers of approximately 2 × 105 cells per well after removing cell growth media. Each bacterial strain was evaluated in three independent assays.
Co-cultures with bacterial cells were performed as previously described, with a few modifications. Cells and bacteria were incubated at 37℃ in 5% CO2, 95% air for 30 min. Non-adherent bacteria were removed by washing five times with 1 mL HBSS. To eliminate adherent extracellular bacteria, BME were treated with 5 µg/mL lysostaphin (Sigma, USA) for 2 h in an atmosphere of 5% CO2 at 37℃ [2,10]. Cells were washed three times with 1 mL HBSS. Killing of extracellular bacteria was monitored by culturing the supernatants from the last washing in plate count agar (PCA; Difco, USA). BME monolayers were detached with trypsin-EDTA (0.1%/0.04%) for 10 min in an atmosphere of 5% CO2 at 37℃ and lysed with 0.1% Trinton-X (VWR, USA) in distilled water (vol/vol) [1,2]. Ten-fold dilutions of BME lysates were then plated in duplicate onto PCA and incubated for 18 h at 37℃ [1]. The number of CFU was determined by colony counting. Threshold for bacterial invasion was adjusted above the highest count obtained in the last wash in order to reduce the bias of remaining extracellular bacteria.
Invasion ability was confirmed in primary cultures of bovine mammary epithelial cells with fluorescent probes. Epithelial cells isolation was performed as previously described [8] with a few modifications. When monolayers were confluent, any remaining fibroblasts were eliminated by selective trypsinization and epithelial cells were then detached by mild trypsinization with 0.03% trypsin/0.02% EDTA in PBS [10]. Cells were vigorously pipetted to break up aggregates, resuspended in growth medium, and grown on glass coverslips placed inside 6-well tissue culture plates. When epithelial cells covered the glass slides in confluent monolayers, the slides were transferred to a new 6-well plate and incubated with 1 mL suspensions of the invasive S. aureus strains (C607, C978, D43) and ones containing the control S. epidermidis ATCC 35984. After a 30 min incubation at 37℃, the monolayers were washed three times with 1 mL PBS, fixed with ice-cold 95% ethanol for 10 min at 4℃, and again washed three times with 1 mL PBS. Cells were incubated with 1 mL of 0.01 mg/mL lysostaphin at room temperature for 30 min in an atmosphere of 5% CO2 at 37℃ to eliminate adherent extracellular bacteria. The period of lysostaphin treatment of epithelial monolayers was reduced so that it did not interfere with the FISH. The remaining lysostaphin solution and S. aureus were removed by washing three times with 1 mL PBS. Cell samples were serially dehydrated through a graded alcohol series (50%, 80%, and 96%; 3 min each) [1,2], followed by the addition of 250 µL of hybridisation buffer (0.9 M NaCl, 20 mM Tris-HCl, pH 7.2, 0.01% SDS) containing 5 ng/µL of the Sta probe [12]. After incubation (3 h, 45℃), non-hybridised probe was removed by washing three times with 1 mL PBS.
BME cells were characterised by immunostaining with monoclonal antibodies against cytokeratin and vimentin (k8.13 and VIM13.2; Sigma, USA) diluted 1:20 in PBS. A negative control was included by replacing the monoclonal antibodies with PBS. After a 30 min incubation at 37℃, cell monolayers were washed three times with 1 mL PBS, and incubated for 30 min at 37℃ with 100 µL of FITC-labelled antiserum raised against mouse immunoglobulin (Sigma, USA) diluted 1:120 in PBS. Slides were then washed three times with 1 mL PBS, mounted in Vectashield Mounting Medium with DAPI (Vector, USA) and visualized by fluorescent microscopy with a Leica DMR microscope (Leica Microsystems, Germany).
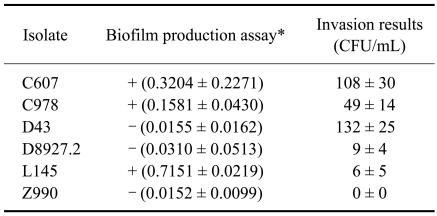
Strains showed different levels of invasion (Table 1). Two biofilm-positive isolates (C607 and C978) showed a high invasive ability into BME cells. The third biofilm-positive isolate (L145) was found to have a low level of invasive ability. Among the biofilm-negative isolates, one (D43) showed a high invasive ability into BME cells, one (D8927.2) had a low level of invasiveness, and the other (Z990) did not invade the BME cells. In the assay confirming invasiveness, intracellular S. aureus were observed in all primary cell cultures.
Table 1.
Biofilm-forming and bovine mammary epithelial invasive abilities of Staphylococcus (S.) aureus subclinical mastitis isolates
*Quantification of biofilm formation by optical density (OD) determination: values of OD570 >0.1 were considered positive (+) and OD570 <0.1 negative (-) [12]. OD570 positive control (S. epidermidis ATCC35984) = 1.2022 ± 0.5123, 34 ± 36 CFU/mL; OD570 negative control (S. epidermidis ATCC12228) = 0.0946 ± 0.0230, 0 ± 0 CFU/mL. CFU: colony forming unit.
The ability of S. aureus to invade bovine mammary epithelial cells has already been demonstrated [5] and our results are in accordance with those of Hensen et al. [9]. Results from other studies suggest that different strains have different internalization mechanisms, and that epithelium invasion is not essential for bacterial intramammary infection establishment or persistence [1,2]. S. aureus mastitis pathogenesis is very complex and influenced by several factors, namely the ability for bacteria to adhere to host cells, to spread within the host tissues, and to evade the host immune system [9,10]. Differences in invasion may be explained by the ability to produce capsule or adhesins [10]. The strains that show a faster adherence rate, which might influence bovine udder colonization, are the most likely to avoid bacteria removal by milk flow [10].
In our study, two out of the three biofilm-positive isolates showed a high invasive ability, while among the biofilm-negative isolates, only one was able to invade BME cells. These results suggest that biofilm-forming ability and invasive capacity are not necessarily related to each other. Nevertheless, further studies should be performed using a higher number of isolates to confirm these results.
Invasive isolates were located inside the epithelial cells, as detected by a positive hybridisation signal from a specific fluorescent probe labelled. These results support the study by Almeida et al. [1] that found no significant differences in internalization between line cultures and primary cells. However, other authors have reported differences in S. aureus invasive ability due to the growth medium, growth phase, and the origin of mammary epithelial cells [10]. In conclusion, our results show that S. aureus mastitis isolates are internalized by bovine mammary epithelial cells and that invasion capacity is strain-dependent. Data also suggest that biofilm-forming ability does not influence the invasion capacity of the S. aureus mastitis isolates analysed in this study.
Acknowledgments
This work was supported by the project POCTI/CA/1995/95/2003 from the "Fundação para a Ciência e Tecnologia" and by CIISA (Faculty of Veterinary Medicine, Technical University of Lisbon, Portugal). We would like to thank Dr. Ana Caria at Santacarnes, Portugal, for allowing the collection of the mammary glands.
References
- 1.Almeida RA, Matthews KR, Cifrian E, Guidry AJ, Oliver SP. Staphylococcus aureus invasion of bovine mammary epithelial cells. J Dairy Sci. 1996;79:1021–1026. doi: 10.3168/jds.S0022-0302(96)76454-8. [DOI] [PubMed] [Google Scholar]
- 2.Almeida RA, Oliver SP. Interaction of coagulase-negative Staphylococcus species with bovine mammary epithelial cells. Microb Pathog. 2001;31:205–212. doi: 10.1006/mpat.2001.0465. [DOI] [PubMed] [Google Scholar]
- 3.Barkema HW, Schukken YH, Zadoks RN. Invited Review: The role of cow, pathogen, and treatment regimen in the therapeutic success of bovine Staphylococcus aureus mastitis. J Dairy Sci. 2006;89:1877–1895. doi: 10.3168/jds.S0022-0302(06)72256-1. [DOI] [PubMed] [Google Scholar]
- 4.Berlutti F, Morea C, Battistoni A, Sarli S, Cipriani P, Superti F, Ammendolia MG, Valenti P. Iron availability influences aggregation, biofilm, adhesion and invasion of Pseudomonas aeruginosa and Burkholderia cenocepacia. Int J Immunopathol Pharmacol. 2005;18:661–670. doi: 10.1177/039463200501800407. [DOI] [PubMed] [Google Scholar]
- 5.Bradley AJ, Leach KA, Breen JE, Green LE, Green MJ. Survey of the incidence and aetiology of mastitis on dairy farms in England and Wales. Vet Rec. 2007;160:253–257. doi: 10.1136/vr.160.8.253. [DOI] [PubMed] [Google Scholar]
- 6.Dziewanowska K, Patti JM, Deobald CF, Bayles KW, Trumble WR, Bohach GA. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect Immun. 1999;67:4673–4678. doi: 10.1128/iai.67.9.4673-4678.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferens WA, Bohach GA. Persistence of Staphylococcus aureus on mucosal membranes: superantigens and internalization by host cells. J Lab Clin Med. 2000;135:225–230. doi: 10.1067/mlc.2000.105179. [DOI] [PubMed] [Google Scholar]
- 8.Fitzpatrick JL, Mayer SJ, Vilela C, Bland PW, Stokes CR. Cytokine-induced major histocompatibility complex class II antigens on cultured bovine mammary gland epithelial cells. J Dairy Sci. 1994;77:2940–2948. doi: 10.3168/jds.S0022-0302(94)77235-0. [DOI] [PubMed] [Google Scholar]
- 9.Hensen SM, Pavičić MJAMP, Lohuis JACM, de Hoog JAM, Poutrel B. Location of Staphylococcus aureus within the experimentally infected bovine udder and the expression of capsular polysaccharide type 5 in situ. J Dairy Sci. 2000;83:1966–1975. doi: 10.3168/jds.S0022-0302(00)75073-9. [DOI] [PubMed] [Google Scholar]
- 10.Hensen SM, Pavičić MJAMP, Lohuis JACM, Poutrel B. Use of bovine primary mammary epithelial cells for the comparison of adherence and invasion ability of Staphylococcus aureus strains. J Dairy Sci. 2000;83:418–429. doi: 10.3168/jds.S0022-0302(00)74898-3. [DOI] [PubMed] [Google Scholar]
- 11.Latasa C, Roux A, Toledo-Arana A, Ghigo JM, Gamazo C, Penadés JR, Lasa I. BapA, a large secreted protein required for biofilm formation and host colonization of Salmonella enterica serovar Enteritidis. Mol Microbiol. 2005;58:1322–1339. doi: 10.1111/j.1365-2958.2005.04907.x. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira M, Bexiga R, Nunes SF, Carneiro C, Cavaco LM, Bernardo F, Vilela CL. Biofilm-forming ability profiling of Staphylococcus aureus and Staphylococcus epidermidis mastitis isolates. Vet Microbiol. 2006;118:133–140. doi: 10.1016/j.vetmic.2006.07.008. [DOI] [PubMed] [Google Scholar]