Abstract
Mucoadhesion is commonly defined as the adhesion between two materials, at least one of which is a mucosal surface. Over the past few decades, mucosal drug delivery has received a great deal of attention. Mucoadhesive dosage forms may be designed to enable prolonged retention at the site of application, providing a controlled rate of drug release for improved therapeutic outcome. Application of dosage forms to mucosal surfaces may be of benefit to drug molecules not amenable to the oral route, such as those that undergo acid degradation or extensive first-pass metabolism. The mucoadhesive ability of a dosage form is dependent upon a variety of factors, including the nature of the mucosal tissue and the physicochemical properties of the polymeric formulation. This review article aims to provide an overview of the various aspects of mucoadhesion, mucoadhesive materials, factors affecting mucoadhesion, evaluating methods, and finally various mucoadhesive drug delivery systems (buccal, nasal, ocular, gastro, vaginal, and rectal).
Keywords: Mucoadhesion, mucoadhesive drug delivery systems, mucoadhesive materials
In the last two decades, mucoadhesion has shown renewed interest for prolonging the residence time of mucoadhesive dosage forms through various mucosal routes in drug delivery applications. Mucoadhesive-based topical and local systems have shown enhanced bioavailability. Mucoadhesive drug delivery gives rapid absorption and good bioavailability due to its considerable surface area and high blood flow. Drug delivery across the mucosa bypasses the first-pass hepatic metabolism and avoiding the degradation of gastrointestinal enzymes. Thus mucosal drug delivery system could be of value in delivering a growing number of high-molecular-weight sensitive molecules such as peptide and oligonucleotides. In this review, the aim is to provide detailed understanding of mucoadhesion, bioadhesion of polymer, and techniques for the determination of mucoadhesion; finally most common routes of mucoadhesive administration will be presented along with examples of formulation studied.
Bioadhesion and Mucoadhesion
The term bioadhesion can be defined as the state in which two materials, at least one biological in nature, are held together for an extended period of time by interfacial forces.[1] In biological systems, bioadhesion can be classified into 3 types:
Type 1, adhesion between two biological phases, for example, platelet aggregation and wound healing.
Type 2, adhesion of a biological phase to an artificial substrate, for example, cell adhesion to culture dishes and biofilm formation on prosthetic devices and inserts.
Type 3, adhesion of an artificial material to a biological substrate, for example, adhesion of synthetic hydrogels to soft tissues[2] and adhesion of sealants to dental enamel.
For drug delivery purposes, the term bioadhesion implies attachment of a drug carrier system to a specified biological location. The biological surface can be epithelial tissue or the mucus coat on the surface of a tissue. If adhesive attachment is to a mucus coat, the phenomenon is referred to as mucoadhesion. Leung and Robinson[3] described mucoadhesion as the interaction between a mucin surface and a synthetic or natural polymer. Mucoadhesion should not be confused with bioadhesion; in bioadhesion, the polymer is attached to the biological membrane and if the substrate is mucus membrane the term mucoadhesion is used.
Theories of Mucoadhesion
Various theories exist to explain at least some of the experimental observations made during the bioadhesion process. Unfortunately, each theoretical model can only explain a limited number of the diverse range of interactions that constitute the bioadhesive bond.[4] However, four main theories can be distinguished.
Wetting Theory of Mucoadhesion
The wetting theory is perhaps the oldest established theory of adhesion. It is best applied to liquid or low-viscosity bioadhesives. It explains adhesion as an embedding process, whereby adhesive agents penetrate into surface irregularities of the substrate and ultimately harden, producing many adhesive anchors. Free movement of the adhesive on the surface of the substrate means that it must overcome any surface tension effects present at the interface.[5] The wetting theory calculates the contact angle and the thermodynamic work of adhesion.
The work done is related to the surface tension of both the adhesive and the substrate, as given by Dupre’s equation;[6]
| (1) |
where ωA is the specific thermodynamic work of adhesion and γb, γτ, and γbt represent, respectively, the surface tensions of the bioadhesive polymer, the substrate, and the interfacial tension. The adhesive work done is a sum of the surface tensions of the two adherent phases, less the interfacial tensions apparent between both phases.[7] Figure 1 shows a drop of liquid bioadhesive spreading over a soft-tissue surface.
Figure 1.
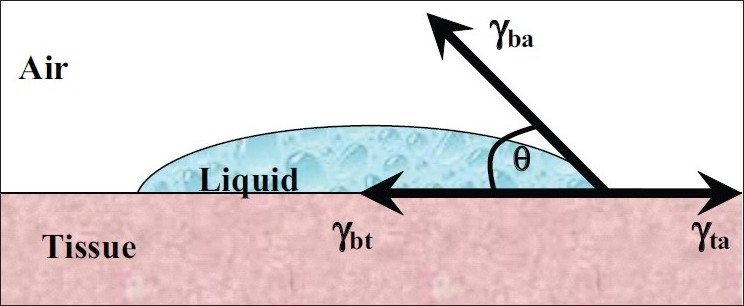
A liquid bioadhesive spreading over a typical soft tissue surface
Horizontal resolution of the forces gives the Young equation:
| (2) |
where θ is the angle of contact, γbt is the surface tension between the tissue and polymer, γba is the surface tension between polymer and air, and γta is the surface tension between tissue and air. Equation 3 states that if the angle of contact,θ, is greater than zero, the wetting will be incomplete. If the vector γta greatly exceeds γbt + γba, that is:
| (3) |
then θ will approach zero and wetting will be complete. If a bioadhesive material is to successfully adhere to a biological surface, it must first dispel barrier substances and then spontaneously spread across the underlying substrate, either tissue or mucus. The spreading coefficient, Sb, can be defined as shown in Equation 4:
| (4) |
which states that bioadhesion is successful if Sb is positive, thereby setting the criteria for the surface tension vectors; in other words, bioadhesion is favored by large values of γta or by small values of γbt and γba.[7]
Electrostatic Theory of Mucoadhesion
According to electrostatic theory, transfer of electrons occurs across the adhesive interface and adhering surface. This results in the establishment of the electrical double layer at the interface and a series of attractive forces responsible for maintaining contact between the two layers.[8]
Diffusion Theory of Mucoadhesion
Diffusion theory describes that polymeric chains from the bioadhesive interpenetrate into glycoprotein mucin chains and reach a sufficient depth within the opposite matrix to allow formation of a semipermanent bond.[9] The process can be visualized from the point of initial contact. The existence of concentration gradients will drive the polymer chains of the bioadhesive into the mucus network and the glycoprotein mucin chains into the bioadhesive matrix until an equilibrium penetration depth is achieved as shown in Figure 2.
Figure 2.
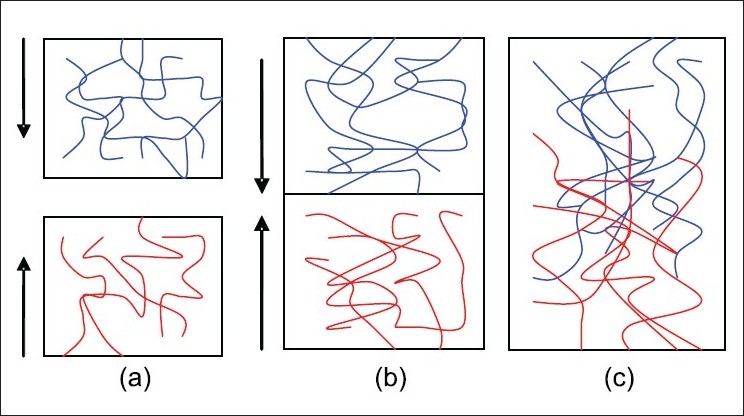
(a) Schematic representation of the diffusion theory of bioadhesion. Blue polymer layer and red mucus layer before contact; (b) Upon contact; (c) The interface becomes diffuse after contact for a period of time
The exact depth needed for good bioadhesive bonds is unclear, but is estimated to be in the range of 0.2–0.5 μm.[10] The mean diffusional depth of the bioadhesive polymer segments, s, may be represented by Equation 5:
| (5) |
where D is the diffusion coefficient and t is the contact time. Duchene[11] adapted Equation 5 to give Equation 6, which can be used to determine the time, t, to bioadhesion of a particular polymer:
| (6) |
in which l represents the interpenetrating depth and Db the diffusion coefficient of a bioadhesive through the substrate.
Once intimate contact is achieved, the substrate and adhesive chains move along their respective concentration gradients into the opposite phases. Depth of diffusion is dependent on the diffusion coefficient of both phases. Reinhart and Peppas[12] reported that the diffusion coefficient depended on the molecular weight of the polymer strand and that it decreased with increasing cross-linking density.
Adsorption Theory of Mucoadhesion
According to the adsorption theory, after an initial contact between two surfaces, the materials adhere because of surface forces acting between the chemical structures at the two surfaces.[13] When polar molecules or groups are present, they reorientate at the interface.[7] Chemisorption can occur when adhesion is particularly strong. The theory maintains that adherence to tissue is due to the net result of one or more secondary forces (van der Waal’s forces, hydrogen bonding, and hydrophobic bonding).[14–16]
Fracture Theory of Adhesion
This theory describes the force required for the separation of two surfaces after adhesion. The fracture strength is equivalent adhesive strength through the following equation. This theory is useful for the study of bioadhesion by tensile apparatus.
| (7) |
where σ is the fracture strength, e fracture energy, E young modulus of elasticity, and L the critical crack length.[17]
Mucoadhesive Materials
Mucoadhesive polymers have numerous hydrophilic groups, such as hydroxyl, carboxyl, amide, and sulfate. These groups attach to mucus or the cell membrane by various interactions such as hydrogen bonding and hydrophobic or electrostatic interactions. These hydrophilic groups also cause polymers to swell in water and, thus, expose the maximum number of adhesive sites.[16]
An ideal polymer for a bioadhesive drug delivery system should have the following characteristics;[9,13]
The polymer and its degradation products should be nontoxic and nonabsorbable.
It should be nonirritant.
It should preferably form a strong noncovalent bond with the mucus or epithelial cell surface.
It should adhere quickly to moist tissue and possess some site specificity.
It should allow easy incorporation of the drug and offer no hindrance to its release.
The polymer must not decompose on storage or during the shelf life of the dosage form.
The cost of the polymer should not be high so that the prepared dosage form remains competitive.
Polymers that adhere to biological surfaces can be divided into three broad categories:[7,10]
Polymers that adhere through nonspecific, noncovalent interactions which are primarily electrostatic in nature
Polymers possessing hydrophilic functional groups that hydrogen bond with similar groups on biological substrates
Polymers that bind to specific receptor sites on the cell or mucus surface
The latter polymer category includes lectins and thiolated polymers. Lectins are generally defined as proteins or glycoprotein complexes of nonimmune origin that are able to bind sugars selectively in a noncovalent manner.[18] Lectins are capable of attaching themselves to carbohydrates on the mucus or epithelial cell surface and have been extensively studied, notably for drug-targeting applications.[19,20] These second-generation bioadhesives not only provide for cellular binding, but also for subsequent endo- and transcytosis. Thiolated polymers, also designated thiomers, are hydrophilic macromolecules exhibiting free thiol groups on the polymeric backbone. Due to these functional groups, various features of polyacrylates and cellulose derivatives were strongly improved.[21] The presence of thiol groups in the polymer allows the formation of stable covalents bonds with cysteine-rich subdomains of mucus glycoproteins leading to increased residence time and improved bioavailability.[22] Other advantageous mucoadhesive properties of thiolated polymers include improved tensile strength, rapid swelling, and water uptake behavior. Table 1 shows the chemical structures of several bioadhesive polymers commonly used in modern drug delivery.
Table 1.
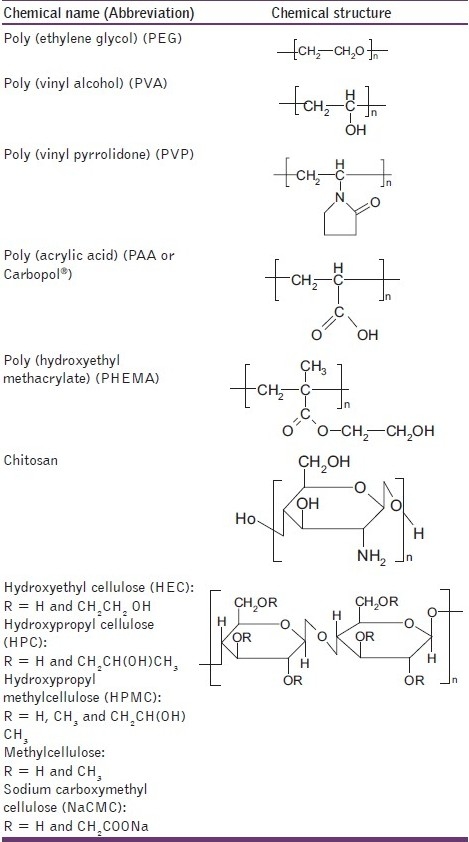
Chemical structures of some bioadhesive polymers used in drug delivery
Factors Affecting Mucoadhesion
Mucoadhesion may be affected by a number of factors, including hydrophilicity, molecular weight, cross-linking, swelling, pH, and the concentration of the active polymer.[9,13,23]
Hydrophilicity
Bioadhesive polymers possess numerous hydrophilic functional groups, such as hydroxyl and carboxyl. These groups allow hydrogen bonding with the substrate, swelling in aqueous media, thereby allowing maximal exposure of potential anchor sites. In addition, swollen polymers have the maximum distance between their chains leading to increased chain flexibility and efficient penetration of the substrate.
Molecular Weight
The interpenetration of polymer molecules is favored by low-molecular-weight polymers, whereas entanglements are favored at higher molecular weights. The optimum molecular weight for the maximum mucoadhesion depends on the type of polymer, with bioadhesive forces increasing with the molecular weight of the polymer up to 100,000. Beyond this level, there is no further gain.[24]
Cross-linking and Swelling
Cross-link density is inversely proportional to the degree of swelling.[25] The lower the cross-link density, the higher the flexibility and hydration rate; the larger the surface area of the polymer, the better the mucoadhesion. To achieve a high degree of swelling, a lightly cross-linked polymer is favored. However, if too much moisture is present and the degree of swelling is too great, a slippy mucilage results and this can be easily removed from the substrate.[26] The mucoadhesion of cross-linked polymers can be enhanced by the inclusion in the formulation of adhesion promoters, such as free polymer chains and polymers grafted onto the preformed network.[23]
Spatial Conformation
Besides molecular weight or chain length, spatial conformation of a polymer is also important. Despite a high molecular weight of 19,500,000 for dextrans, they have adhesive strength similar to that of polyethylene glycol (PEG), with a molecular weight of 200,000. The helical conformation of dextran may shield many adhesively active groups, primarily responsible for adhesion, unlike PEG polymers, which have a linear conformation.[9]
pH
The pH at the bioadhesive to substrate interface can influence the adhesion of bioadhesives possessing ionizable groups. Many bioadhesives used in drug delivery are polyanions possessing carboxylic acid functionalities. If the local pH is above the pK of the polymer, it will be largely ionized; if the pH is below the pK of the polymer, it will be largely unionized. The approximate pKa for the poly(acrylic acid) family of polymers is between 4 and 5. The maximum adhesive strength of these polymers is observed around pH 4–5 and decreases gradually above a pH of 6. A systematic investigation of the mechanisms of mucoadhesion clearly showed that the protonated carboxyl groups, rather than the ionized carboxyl groups, react with mucin molecules, presumably by the simultaneous formation of numerous hydrogen bonds.[27]
Concentration of Active Polymer
Ahuja[10] stated that there is an optimum concentration of polymer corresponding to the best mucoadhesion. In highly concentrated systems, beyond the optimum concentration the adhesive strength drops significantly. In concentrated solutions, the coiled molecules become solvent-poor and the chains available for interpenetration are not numerous. This result seems to be of interest only for more or less liquid mucoadhesive formulations. It was shown by Duchêne[11] that, for solid dosage forms such as tablets, the higher the polymer concentration, the stronger the mucoadhesion.
Drug/Excipient Concentration
Drug/excipient concentration may influence the mucoadhesion. BlancoFuente[28] studied the effect of propranolol hydrochloride to Carbopol® (a lightly cross-linked poly(acrylic acid) polymer) hydrogels adhesion. Author demonstrated increased adhesion when water was limited in the system due to an increase in the elasticity, caused by the complex formation between drug and the polymer. While in the presence of large quantities of water, the complex precipitated out, leading to a slight decrease in the adhesive character. Increasing toluidine blue O (TBO) concentration in mucoadhesive patches based on Gantrez® (poly(methylvinylether/maleic acid) significantly increased mucoadhesion to porcine cheek tissue.[29] This was attributed to increased internal cohesion within the patches due to electrostatic interactions between the cationic drug and anionic copolymer.
Other Factors Affecting Mucoadhesion
Mucoadhesion may be affected by the initial force of application.[30] Higher forces lead to enhanced interpenetration and high bioadhesive strength.[11] In addition, the greater the initial contact time between bioadhesive and substrate, the greater the swelling and interpenetration of polymer chains.[31] Physiological variables can also affect mucoadhesion. The rate of mucus turnover can be affected by disease states and also by the presence of a bioadhesive device.[32] In addition, the nature of the surface presented to the bioadhesive formulation can vary significantly depending on the body site and the presence of local or systemic disease.[31]
Techniques for the Determination of Mucoadhesion
The evaluation of bioadhesive properties is fundamental to the development of novel bioadhesive delivery systems. These tests are also important to screen large number of materials and their mechanisms. Numerous methods have been developed for studying mucoadhesion. Since no standard apparatus is available for testing bioadhesive strength, an inevitable lack of uniformity between test methods has arisen. Nevertheless, three main testing modes are recognized – tensile test, shear strength, and peel strength.
The most popular technique used for the determination of force of separation in bioadhesive testing is the application of force perpendicularly to the tissue/adhesive interface, during which a state of tensile stress is set up. But during the shear stress, the direction of the forces is reoriented so that it acts along the joint interface. In both tensile and shear modes, an equal pressure is distributed over the contact area.[33]
The peel test is based on the calculation of energy required to detach the patch from the substrate. The peel test is of limited use in most bioadhesive systems. However, it is of value when the bioadhesive system is formulated as a patch.[34]
In tensile and shear experiments, the stress is uniformly distributed over the adhesive joint, whereas in the peel strength stress is focused at the edge of the joint. Thus tensile and shear measure the mechanical properties of the system, whereas peel strength measures the resistant of the peeling force.
Review of the literature confirmed that the most common technique used for the measurement of bioadhesion test is tensile strength method. McCarron et al.[26,34,35] and Donnelly[36] have reported extensively on the use of a commercial apparatus, in the form of a texture profile analyzer [Figure 3] operating in bioadhesive test mode, to measure the force required to remove bioadhesive films from excised tissue in vitro.
Figure 3.
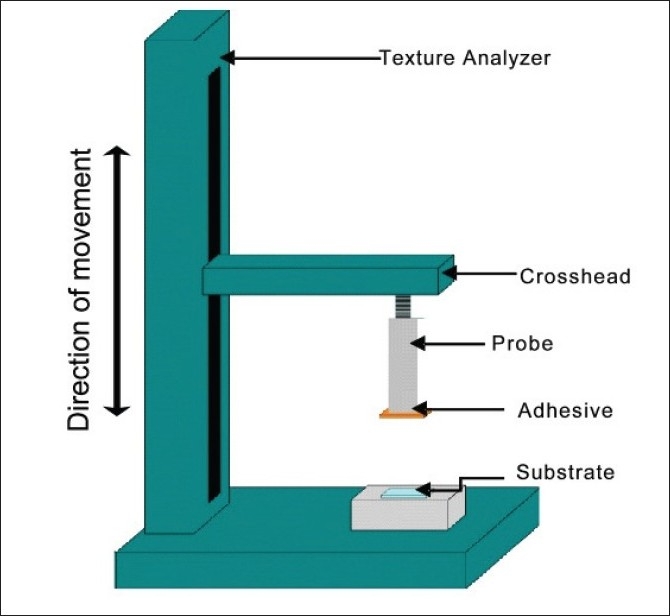
Texture profile analyzer in bioadhesion test mode
The texture analyzer, operating in tensile test mode and coupled with a sliding lower platform, was also used to determine peel strength of similar formulations [Figure 4].[34]
Figure 4.
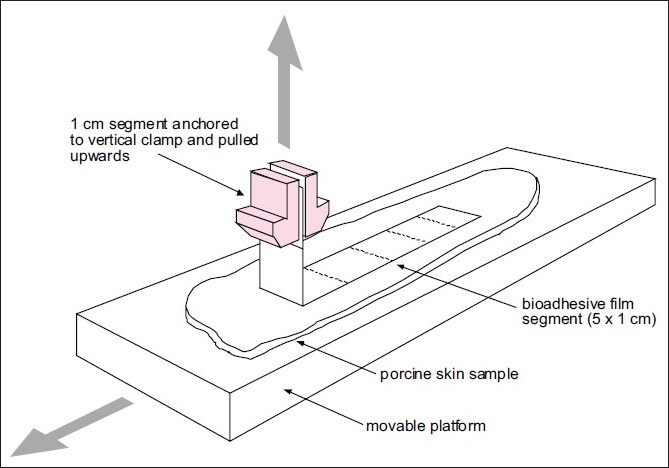
Simplified representation of a typical test set-up used to determine peel strength of bioadhesive films
Rheological techniques that study the flow and deformation of materials may be useful in predicting the mucoadhesive ability of a polymeric formulation. A simple rheological approach for polymer solutions and gels was first suggested by Hassan and Gallo.[37] In this method, rheological interaction between a polymer gel and mucin solution was determined. It was shown that a polymer gel and mucin solution mixture exhibited larger rheological responsethan the sum of the values of polymer and mucin. However, a wide variation in results is found in the literature that utilize rheological methods for mucoadhesion determination, which may be attributable to differences in mucin type and concentration,[38,39] as well as polymer concentrations.[40,39] Therefore, Hagerstrom[41] recommend that the rheological method should not be used as a stand-alone method for studying the mucoadhesive properties of the polymer gels.
In vivo aspects of mucoadhesive testing have recently been reported to monitor the mucoadhesion on tissue surface such as the GIT or the buccal cavity. However, there are only a limited number of in vivo studies reported in the literature in vitro work because of the time, cost, and ethical constrains. The most common in vivo techniques to monitor mucoadhesion include GI transit times of bioadhesive-coated particles and drug release from in situ bioadhesive devices.
Ch’ng[42] studied the in vivo transit time for bioadhesive beads in the rat GIT. A 51Cr-labeled bioadhesive was inserted at selected time intervals; the GITs were removed. The GIT of the rat was then cut into 20 equal segments and the radioactivity was measured.
Davis[43] investigated the noninvasive in vivo technique to determine the transit of mucoadhesive agent. Therefore, in this study a formulation was used containing a gamma-emitting radionuclide. The release characteristics and the position polymer could be examined by gamma scintigraphy.
In recent times, magnetic resonance imaging (MRI) is another noninvasive technique that is widely used. Christian Kremser[44] used MRI to detect the time and location of release of mucoadhesive formulation using dry Gd-DOTA powder.
Routes of Administration for Mucoadhesive-based Drug Delivery Systems
Mucosa or the mucus membrane is the moist tissue that lines organs and body cavities such as mouth, gut, rectum, genital area, nose, and eye lid. Anatomical differences of the mucus membrane at varying body locations are given in Table 2. Mucoadhesive drug delivery systems in the past have been formulated as powders, compacts, sprays, semisolids, or films. For example, compacts have been used for drug delivery to the oral cavity,[51] and powders and nanoparticles have been used to facilitate drug administration to the nasal mucosa.[52,53] Recently oral strips[54] were developed for tongue or buccal cavity. Details of the mucoadhesive dosage forms are given in Table 3. Recently, there has been a growing interest in alternative delivery system designs. Buccal films have been suggested as a means of offering greater flexibility and comfort than adhesive tablets. In addition, films may circumvent the problem of the relatively short residence time of oral gels.[77] Film-forming bioadhesive polymers used in the production of bioadhesive films include the cellulose derivatives,[77] poly(acrylic acids) such as Carbopol,®78] and Gantrez® copolymers such as poly(methylvinylether/maleic anhydride).[45]
Table 2.
Anatomical differences of the mucus membrane
| Mucus membrane | Relevant anatomical features |
|---|---|
| Buccal[45] | Buccal mucosa surface area approximately 30 cm2 |
| Comprised of three distinct layers – epithelium, basement membrane, and connective tissues | |
| Buccal mucosa, sublingual are soft palate nonkeratinized tissue, and gingival are hard palate keratinized tissue | |
| Thickness of buccal epithelium is in the range of 500–800 μm, 40–50 cells thick | |
| Mucus secreted by salivary glands, as a component of saliva, forming a 0.1–0.7 mm thick layer | |
| Turnover time for buccal epithelium 5–6 days | |
| Permeability barrier property of oral mucosa due to intercellular materials derived from membrane-coating granules | |
| Nasal[46] | Nasal cavity surface area 160 cm2 |
| Lined with mucous membrane containing columnar cells, goblet cells, and basal cells | |
| Columnar cells are covered with cilia, apart from the anterior part of the nasal cavity | |
| Both keratinized and nonkeratinized epithelial cells present depending upon location within nasal cavity | |
| Cilia responsible for mucociliary clearance | |
| Mucus secreted by the submucosal glands and the goblet cells, forming a mucus layer approximately 5–20 μm thick | |
| Nasal cavity length approximately 60 mm | |
| Nasal cavity volume approximately 20 mL | |
| Turn-over time for mucus is usually 10–15 min | |
| Ocular[47] | Cornea is composed of five layers – epithelium, Bowman’s layer, stroma, Descemet’s membrane, and endothelium |
| Epithelium consists of 5–6 layers of cells, with the cells of the basal layer being columnar, and the outermost cells flattened polygonal cells | |
| Tight junctions present between the basal cells of the corneal epithelium | |
| At the corneal margin, the conjunctiva is structurally continuous with the corneal epithelium | |
| The conjunctival tissue is permeable to molecules up to 20,000 Da, whereas the cornea is impermeable to molecules greater than 5000 Da | |
| The conjunctiva contains around 1.5 million goblet cells, which synthesize secretory mucins and peptides | |
| A volume of about 2–3 μL of mucus os secreted daily | |
| A turnover of the mucus layer occurs in approximately 15–20 h | |
| Exposed part of the eye is covered by a thin fluid layer – percorneal tear film | |
| Mucus Membrane | Relevant Anatomical Features |
| Tear film thickness is approximately 3–10 μm | |
| Vaginal[48] | Length of vagina varies from 6 to 10 cm |
| The epithelial layer consists of the lamina propia and stratified squamous epithelium | |
| A cell turnover of about 10–15 layers is estimated to be in the order of 7 days | |
| Although there are no glands in the vagina mucosa, the surface is usually covered with vaginal fluid | |
| Major components of vaginal fluid are cervical mucus and vaginal fluid from the well-vascularized mucosa | |
| The volume, viscosity, and pH of the cervical mucus vary with age and during the menstrual cycle | |
| Rectal[49] | Length approximately 15–20 cm |
| Surface area of approximately 300 cm2 | |
| Epithelium consists of a single layer of cylindrical cells and goblet cells secreting mucus | |
| Flat surface, without villi, and with three major fold, the rectal valves | |
| Approximately 3 mL of mucus with a neutral pH spread over the surface |
Table 3.
Different types of mucoadhesive dosage forms
| Delivery routes | Dosage forms |
||||
|---|---|---|---|---|---|
| Tablet | Ointment | Gel | Patch | Film | |
| Buccal | Theophylline, multiple polymers[55] | Benzyl nicotinate, multiple polymers[56] | Benzydamine, chitosan derivatives[57] | Miconazole, PVA/PVP[58] | Fentanyl, PVP[59] |
| Nasal | N/A | Mupirocin, glycerin ester[60] | Insulin, starch[61] | Insulin, chitosan/PEG[62] | Chlorpromazine, chitosan/pectin[63] |
| Ocular | Diclofenac, poly(acrylic) acid[64] | Sulphadicramide, multiple polymers[65] | Puerarin, poloxamer/carbopol[66] | Ciprofloxacin, PVA/CMC[67] | Fluorescein, HPMC[68] |
| Vaginal | Metronidazole, chitosan[69] | Terameprocol, white petroleum[70] | Amphotericin, pluronic[71] | ALA, PMVE/MA[34] | SDS, multiple polymers[72] |
| Rectal | Ramosetron, carbopol[73] | Zinc oxide, petroleum[74] | Quinine, HPMC[75] | N/A | Theophylline, pHEMA[76] |
Oral Mucoadhesive Drug Delivery Systems
Drug delivery through the oral mucosa has gained significant attention due to its convenient accessibility. The buccal and sublingual routes are considered as the most commonly used rotes. The nonkeratinized epithelium in the oral cavity, such as the soft palate, the mouth floor, the ventral side of the tongue, and the buccal mucosa, offers a relatively permeable barrier for drug transport.[79] Hydrophilic compounds and large or highly polar molecules follow paracellular transport, whereas transcellular transport through the lipid bilayer is followed by lipophilic drugs.[80] Drug delivery through the oral mucosa has proven particularly useful and offers several advantages over other drug delivery systems including bypassing hepatic first-pass metabolism, increasing the bioavailability of drugs, improved patient compliance, excellent accessibility, unidirectional drug flux, and improved barrier permeability compared, for example, to intact skin.[81,82] The oral cavity has been used as a site for local and systemic drug delivery. Local drug therapy is used to treat disease states like aphthous ulceration gingivitis, periodontal disease, and xerostoma. Different dosage designs include adhesive gels, tablets, films, patches, ointments, mouth washes, and pastes.
Until now adhesive tablets have been the most commonly used dosage forms for buccal drug delivery. Tablets can be applied to different regions of oral cavity, such as cheeks, lips, gums, and palate. Unlike conventional tablets, buccal tablets allow drinking, eating, and speaking without any major discomfort. Perioli[83] studied the influence of compression force on tablet behavior and drug release rate for mucoadhesive buccal tablets. Tablets were prepared by using hydroxyethyl cellulose (HEC) and carbopol 940 in a 1:1 ratio as matrix-forming polymers at varying compression forces. Compression forces did not significantly affect the water penetration and polymer chain stretching; however, mucoadhesion performance and drug release were influenced by compression force. Increase in compression force resulted in a decreased in vitro and in vivo drug release while giving the best in vivo mucoadhesive and hydration time. Moreover, it was observed that tablets prepared with the lowest force gave the best results, in comparison with tablets prepared with highest forces causing pain during in vivo application, needing to be detached by human volunteers.
Oral mucosal ulceration is a common condition with up to 50% of healthy adults suffering from recurrent minor mouth ulcers (aphthous stomatitis). Shermer[84] evaluated the efficacy and tolerability of a mucoadhesive patch compared with a pain-relieving oral solution for the treatment of aphthous stomatitis. The mucoadhesive patch was found to be more effective than the oral solution in terms of healing time and pain intensity after 12 and 24 h. Local adverse effects 1 h after the treatment were significantly less frequent among the mucoadhesive patch patients compared with the oral solution patients.
Donnelly[29] reported on a mucoadhesive patch containing TBO as a potential delivery system for use in photodynamic antimicrobial chemotherapy (PACT) of oropharyngeal candidiasis. Patches are prepared from aqueous blends of poly(methyl vinyl ether/maleic anhydride) and tripropyleneglycol methyl ether. The authors concluded that short application times of TBO-containing mucoadhesive patches should allow the treatment of recently acquired oropharyngeal candidiasis, caused solely by planktonic cells. Longer patch application times may be required for persistent disease where biofilms are implicated.
Periodontitis is an inflammatory disease of the oral cavity, which results in the destruction of the supporting structures of the teeth.[85] Inflammatory periodontitis disease can be treated by the combination of mechanical and intraperiondontal pocket chemotherapeutic agents.[86] Jones and Andrews[87,88] described the formulation and physicochemical characterization of syringeable semisolid, bioadhesive networks (containing tetracycline, metronidazole, or model protein drugs). Such systems may be formulated to exhibit requisitory flow properties (and hence may be easily administered into the periodontal pocket using a syringe), mucoadhesive properties (ensuring prolonged retention within the pocket), and sustained release of therapeutic agent within this environment.
Mucosal delivery of drugs via the buccal route is still very challenging in spite of extensive clinical studies. Here, we are underlining several formulations which are in clinical trials or commercial products. The 3M company has developed a buccal patch system which consists of a matrix patch containing drug, mucoadhesive polymers, and polymeric elastomers surrounded by a backing material. Their buprenorphine patch is capable of delivering the drug for a period up to 12 h, with good patient comfort reported.[89]
Oralin, a novel liquid aerosol formulation (Generex Biotechnology), has been developed and it is now in clinical phase II trials.[90] Oralin allows precise insulin dose delivery via a metered dose inhaler in the form of fine aerosolized droplets directed into the mouth. Levels of drug in the mouth are noticeably increased compared with conventional formulations. This oral aerosol formulation is rapidly absorbed through the buccal mucosal epithelium, and it provides the plasma insulin levels necessary to control postprandial glucose rise in diabetic patients. This novel, pain-free, oral insulin formulation has a number of advantages, including rapid absorption, user-friendly administration technique, precise dosing control (comparable to injection within one unit), and bolus delivery of drug. Furthermore, BioAlliance Pharma’s miconazole tablet (Lauriad®) formulation is now in clinical phase III trials, and Aphtach® (triamcinolone acetonide buccal tablets from Teijin Ltd.) are now commercially available.[90]
Nasal Mucoadhesive Drug Delivery Systems
The area of the normal human nasal mucosa is approximately 150 cm2, a highly dense vascular network and relatively permeable membrane structure; all these factors make nasal cavity interesting.[91] Drawbacks are local toxicity/irritation mucociliary clearance of 5 min, presence of proteolytic enzymes, and influence of pathological conditions (cold and allergies). Among the advantages are rapid uptake and avoiding first-pass hepatic metabolism. In addition, bioadhesive application of liquids, semisolids, and solids can significantly increase retention time.
Nasal delivery of protein and peptide therapeutics can be compromised by the brief residence time at this mucosal surface. Some bioadhesive polymers have been suggested to extend residence time and improve protein uptake across the nasal mucosa. McInnes[92] quantified nasal residence of bioadhesive formulations using gamma scintigraphy and investigated absorption of insulin. A four-way crossover study was conducted in six healthy male volunteers, comparing a conventional nasal spray solution with three lyophilized nasal insert formulations (1–3% w/w hydroxypropylmethyl cellulose, HPMC). The authors concluded that the 2% w/w HPMC lyophilized insert formulation achieved extended nasal residence, demonstrating an optimum combination of rapid adhesion without overhydration.
Coucke[93] studied viscosity-enhancing mucosal delivery systems for the induction of an adaptive immune response against viral antigen. Powder formulations based on spray-dried mixtures of starch (Amioca®) and poly(acrylic acid) (Carbopol® 974P) in different ratios were used as carriers of the viral antigen. A comparison of these formulations for intranasal delivery of heat-inactivated influenza virus combined with LTR 192G adjuvant was made in vivo in a rabbit model. The authors concluded that the use of bioadhesive carriers based on starch and poly(acrylic acid) facilitates the induction of a systemic anti-HA antibody response after intranasal vaccination with a whole virus influenza vaccine.
Functionalized mucoadhesive polymers, such as polycarbophil, hyaluronan, and amberlite resin, have been developed and the characterization and safety aspects of nasal drug products extensively studied. Recently, mucosal vaccines have been introduced in immunization to induce a systemic immune response. Addition of mucoadhesive polymer to the vaccine formulation increases the affinity for mucus membranes and may enhance the stability of the preparation. Examples of these include intranasal vaccines against influenza, diphtheria, and tetanus.[94]
Pilot studies involving the use of a nasal morphine–chitosan formulation for the treatment of breakthrough pain in 14 cancer patients suggested that this system was acceptable, well-tolerated, and may lead to rapid onset of pain relief.[95]
Tzachev[96] has compared a mucoadhesive solution (formulation of xylometazoline) with commercially available decongestatnt solution in 20 human subjects with perennial allergic rhinitis. The author concluded that the mucoadhesive formulation exhibited a significantly more prolonged clinical effect than the nonmucoadhesive product.
Ocular Mucoadhesive Drug Delivery Systems
Drug administration to the eye is a challenge because there are several mechanisms (tear production, tear flow, and blinking) that protect the eye from the harmful agents. Conventional delivery methods are not ideal. Solutions and suspensions are readily washed from the cornea and ointments alter the tear refractive index and blur vision; so it is a target to prolong the residence time by mucoadhesion.
Sensoy[97] aimed to prepare bioadhesive sulfacetamide sodium microspheres to increase residence time on the ocular surface and to enhance treatment efficacy of ocular keratitis. Microspheres were fabricated by a spray-drying method using a mixture of polymers, such as pectin, polycarbophil, and HPMC at different ratios. Author concluded that a sulfacetamide sodium–loaded polycarbophil microsphere formulation with a polymer:drug ratio of 2:1 was found to be the most suitable for ocular application and used in in vivo studies on New Zealand male rabbit eyes with keratitis caused by Pseudomonas aeruginosa and Staphylococcus aureus.
Gene transfer is considered to be a promising alternative for the treatment of several chronic diseases that affect the ocular surface. De la Fuente[98] investigated the efficacy and mechanism of action of a bioadhesive DNA nanocarrier made of hyaluronan (HA) and chitosan (CS), specifically designed for topical ophthalmic gene therapy. The author concluded that on topical administration to rabbits, the nanoparticles entered the corneal and conjunctival epithelial cells and got assimilated by the cells. More importantly, the nanoparticles provided an efficient delivery of the associated plasmid DNA inside the cells, reaching significant transfection levels.
Many clinical studies have been performed on mucoadhesive ocular dosage forms. Ocular films applied behind the eye lid were found to prolong retention time and precision of dosing. However, films were found to have a tendency to move across the surface of the eye, thus resulting in irritation, for example, from Ocusert® (Alza). It has been shown that the addition of mucoadhesive polymers to ocular films reduced film movement across the eye, minimizing ocular irritation and burning sensations.[94]
Baeyens[99] conducted a clinical study in dogs presenting with external ophthalmic diseases (conjunctivitis, superficial corneal ulcer, or keratoconjuctivitissicca) using soluble bioadhesive ophthalmic drug inserts (BODI®) in comparison with classical Tiacil® eye drops from Virbac Laboratories. The results of the clinical study showed that BODI® demonstrated an advantage over the Tiacil® by reducing the treatment to a single application and, therefore, improving patient compliance.
Mucoadhesive polymers have been incorporated into ophthalmic gels to increase gel efficacy, such as NyoGel ® (timolol, Novartis) and Pilogel® (pilocarpine hydrochloride, Alcon Laborataries).[100]
Vaginal Mucoadhesive Drug Delivery Systems
The vagina is a fibrovascular tube connecting the uterus to the outer surface of the body. The vaginal epithelium consists of a stratified squamous epithelium and lamina propia. Dosage forms used for vaginal route are solutions, gels, suspensions, suppositories, creams, and tablets and all have short residence time.[101,102,103] Bioadhesives can control the rate of drug release from, and extend the residence time of, vaginal formulations. These formulations may contain drug or, quite simply, act in conjunction with moisturizing agents as a control for vaginal dryness.
Alam[104] developed an acid-buffering bioadhesive vaginal clotrimazole (antifungal) and metronidazole (antiprotozoal and antibacterial) tablets for the treatment of genitourinary tract infections. From bioadhesion experiment and release studies, it was found that polycarbophil and sodium carboxymethyl cellulose was a good combination for an acid-buffering bioadhesive vaginal tablet. From ex vivo retention studies, it was found that the bioadhesive polymers held the tablet for more than 24 h inside the vagina. The cumulative release profile of the developed tablet was matched with a marketed conventional tablet (Infa-V®). The in vitro spreadability of the swelled tablet was comparable to the marketed gel. In the in vitro antimicrobial study, it was found that the acid-buffering bioadhesive tablet produced better antimicrobial action than marketed intravaginal drug delivery systems (Infa-V®, Candid-V®, and Canesten® 1).
Cevher[105] aimed to prepare clomiphene citrate (CLM) gel formulations for the local treatment of human papilloma virus infections. In this respect, 1% w/w CLM gels including polyacrylic acid (PAA) polymers such as Carbopol® 934P (C934P), Carbopol® 971P (C971P), Carbopol® 974P (C974P) in various concentrations, and their conjugates containing thiol groups, were prepared. Author concluded that gels containing C934P-Cys showed the highest adhesiveness and mucoadhesion. A significant decrease was observed in drug release from gel formulations as the polymer concentration increased.
Recent advances in polymeric technology have increased the potential of vaginal gels. Vaginal gels are semisolid polymeric matrices comprising small amounts of solid, dispersed in relatively large amounts of liquid and have been used in systems for microbicides, contraceptives, labor inducers, and other substances.
Several clinical trials are in underway on microbicidal gels. Microbicidal gels are intended to improve mucosal permeation rate of microbicides for the prevention of sexually transmitted diseases. A 1% tenofovir gel is being investigated in phase II clinical trials for determining the safety and acceptability of vaginal microbicides.[106]
Various clinical trials of contraceptive gels are also ongoing, with a view to determine their effectiveness. BufferGel® is in phases II and III clinical trial comparing itto the Gynol II ® marketed product.[106]
Pharmacia conducted clinical trials of the Prostin E2® suppository containing dinoprostone, and found that administration of prostaglandin E2 gel showed to be more effective in inducing labor.[106]
Janssen Pharmaceutica conducted phase III clinical trial of mucoadhesive systems based on itraconazole vaginal cream containing cyclodextrins and other ingredients. Clinical investigation indicated that application of 5 g of 2% cream was well tolerated and was found to be an effective delivery system for selective vaginal delivery.[107]
Rectal Mucoadhesive Drug Delivery Systems
The rectum is part of the colon, it is 10 cm in length, and has surface area 300 cm2. The function of the rectum is mostly concerned with removing water. Surface area without villi gives it a relatively small surface area for drug absorption.[54] Most rectal absorption of drugs is achieved by a simple diffusion process through the lipid membrane. Drugs that are liable to extensive first-pass metabolism can benefit greatly if delivered to the rectal area, especially if they are targeted to areas close to the anus. Furthermore, addition of bioadhesive polymer the migration distance in the rectum decreased.
Kim[108] aimed to develop a thermoreversible flurbiprofen liquid suppository base composed of poloxamer and sodium alginate for the improvement of rectal bioavailability of flurbiprofen. Cyclodextrin derivatives, such as alpha-, beta-, gamma-cyclodextrin, and hydroxypropyl-beta-cyclodextrin (HP-beta-CD), were used to enhance the aqueous solubility of flurbiprofen. Pharmacokinetic studies were performed after rectal administration of flurbiprofen liquid suppositories with and without HP-beta-CD or after intravenous administration of a commercially available product (Lipfen®, flurbiprofen axetil-loaded emulsion) to rats. Flurbiprofen liquid suppository containing HP-beta-CD showed an excellent bioavailability in that the AUC of flurbiprofen after its rectal administration was not significantly different from that after intravenous administration of Lipfen®. The authors concluded that HP-beta-CD could be a preferable solubility enhancer for the development of liquid suppositories containing poorly water-soluble drugs.
Cervical and Vulval Drug Delivery Systems
A novel bioadhesive cervical patch containing 5-fluorouracil for the treatment of cervical intraepithelial neoplasia (CIN) was described by Woolfson.[109] This patch was a bilaminar design, with a drug-loaded bioadhesive film cast from a gel containing 2% w/w Carbopol® 981 plasticized with 1%w/w glycerine; the casting solvent was ethanol:water 30:70. The film, which was mechanically stable on storage under ambient conditions, was bonded directly to a backing layer formed from thermally cured poly(vinyl chloride) emulsion. Release of 5-fluorouracil from the bioadhesive layer into an aqueous sink was rapid but was controlled down to an undetectable level through the backing layer. Despite the relatively hydrophilic nature of 5-fluorouracil, substantial drug release through human cervical tissue samples was observed over approximately 20 h.[110]
Donnelly[111] described the design, physicochemical characterization, and clinical evaluation of bioadhesive drug delivery systems for photodynamic therapy of difficult-to-manage vulval neoplasias and dysplasias. Aminolevulic acid (ALA) is commonly delivered to the vulva using creams or solutions, which are covered with an occlusive dressing. Such dressings are poor at staying in place at the vulva, where shear forces are high in mobile patients. To overcome the problems, the authors produced a bioadhesive patch by a novel laminating procedure. The ALA loading was 38 mg cm −2 . Patches were shown to release more ALA over 6 h than the proprietary cream (Porphin®, 20% w/w ALA). Clinically, the patch was extensively used in successful PDT of vulval intraepithelial neoplasia, lichen sclerosus, squamous hyperplasia, Paget’s disease, and vulvodynia.
Gastrointestinal Mucoadhesive Drug Delivery Systems
Oral route is undoubtedly most favored route of administration, but hepatic first-pass metabolism, degradation of drug during absorption, mucus covering GI epithilia, and high turnover of mucus are serious concerns of oral route. In recent years, the gastrointestinal tract (GIT) delivery emerged as a most important route of administration. Bioadhesive retentive system involves the use of bioadhesive polymers, which can adhere to the epithelial surface in the GIT. Using bioadhesive would be achieved increase GI transit time and increase in bioavailability.
Ahmed[112] studied gastric retention formulations (GRFs) made of naturally occurring carbohydrate polymers and containing riboflavin in vitro for swelling and dissolution characteristics as well as in fasting dogs for gastric retention. The bioavailability of riboflavin, from the GRFs was studied in fasted healthy humans and compared to an immediate release formulation. It was found that when the GRFs were dried and immersed in gastric juice, they swelled rapidly and released their drug payload in a zero-order fashion for a period of 24 h. In vivo studies in dogs showed that a rectangular shaped GRF stayed in the stomach of fasted dogs for more than 9 h, then disintegrated and reached the colon in 24 h. Considering pharmacokinetic parameters of human subjects under fasting conditions, bioavailability of riboflavin from a large size GRF was more than triple of that measured after administration of an immediate release formulation.
Salman[113] aimed to develop polymeric nanoparticulate carriers with bioadhesive properties and to evaluate their adjuvant potential for oral vaccination. Thiamine was used as a specific ligand–nanoparticle conjugate (TNP) to target specific sites within the gastrointestinal tract, namely enterocytes and Peyer’s patches. The affinity of nanoparticles to the gut mucosa was studied in orally inoculated rats. The authors concluded that thiamine-coated nanoparticles showed promise as particulate vectors for oral vaccination and immunotherapy.
Conclusion
The mucoadhesive dosage forms offer prolonged contact at the site of administration, low enzymatic activity, and patient compliance. The formulation of mucoadhesive drug delivery system depends on the selection of suitable polymer with excellent mucosal adhesive properties and biocompatibility. Now researchers are looking beyond traditional polymers, in particular next-generation mucoadhesive polymers (lectins, thiols, etc.); these polymers offer greater attachment and retention of dosage forms. However, these novel mucoadhesive formulations require much more work, to deliver clinically for the treatment of both topical and systemic diseases.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Good WR. Transdermal nitro-controlled delivery of nitroglycerin via the transdermal route. Drug Dev Ind Pharm. 1983;9:647–70. [Google Scholar]
- 2.Henriksen I, Green KL, Smart JD, Smistad G, Karlsen J. Bioadhesion of Hydrated Chitosans: An in vitro and in vivo Study. Int J Pharm. 1996;145:231–40. [Google Scholar]
- 3.Leung SH, Robinson JR. The Contribution of anionic polymer structural features related to mucoadhesion. J Control Release. 1988;5:223–31. [Google Scholar]
- 4.Longer MA, Robinson JR. Fundamental aspects of bioadhesion. Pharmacy Int. 1986;7:114–7. [Google Scholar]
- 5.McBain JW, Hopkins DG. On adhesives and adhesive action. J Phys Chem. 1925;29:188–204. [Google Scholar]
- 6.Pritchard WH. Aspects of adhesion 6. In: Alder D, editor. 3rd edition ed. London: London University Press; 1970. pp. II–23. [Google Scholar]
- 7.Wake WC. London: Applied Science Publishers; 1982. Adhesion and the Formulation of Adhesives. [Google Scholar]
- 8.Deraguin BV, Smilga VP. London: McLaren; 1969. Adhesion: Fundamentals and Practice. [Google Scholar]
- 9.Jimenez-Castellanos MR, Zia H, Rhodes CT. Mucoadhesive drug delivery systems. Drug Dev Ind Pharm. 1993;19:143–94. [Google Scholar]
- 10.Duchene D, Touchard F, Peppas NA. Pharmaceutical and medical aspects of bioadhesive systems for drug administration. Drug Dev Ind Pharm. 1988;14:283–18. [Google Scholar]
- 11.Peppas NA, Buri PA. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J Control Release. 1985;2:257–75. [Google Scholar]
- 12.Reinhart CP, Peppas NA. Solute diffusion in swollen membranes ii. influence of crosslinking on diffusion properties. J Memb Sci. 1984;18:227–39. [Google Scholar]
- 13.Ahuja A, Khar RK, Ali J. Mucoadhesive drug delivery systems. Drug Dev Ind Pharm. 1997;23:489–515. [Google Scholar]
- 14.Huntsberger JR. Mechanisms of adhesion. J Pain Technol. 1967;39:199–211. [Google Scholar]
- 15.Kinloch AJ. The science of adhesion I.Surface and interfacial aspects. J Material Sci. 1980;15:2141. [Google Scholar]
- 16.Yang X, Robinson JR. Bioadhesion in Mucosal Drug Delivery. In: Okano T, editor. Biorelated Polymers and Gels. London: Academic Press; 1998. [Google Scholar]
- 17.Gu JM, Robinson JR, Leung SH. Binding of acrylic polymers to mucin/epithelial surfaces: Structure–property relationships. Crit Rev Ther Drug Carrier Syst. 1988;5:21–67. [PubMed] [Google Scholar]
- 18.Smart JD, Nicholls TJ, Green KL, Rogers DJ, Cook JD. Lectins in Drug Delivery: a study of the acute local irritancy of the lectins from Solanum tuberosum and helix pomatia. Eur J Pharm Sci. 1999;9:93–8. doi: 10.1016/s0928-0987(99)00050-0. [DOI] [PubMed] [Google Scholar]
- 19.Naisbett B, Woodley J. The potential use of tomato lectin for oral drug delivery. Int J Pharm. 1994;107:223–30. [Google Scholar]
- 20.Nicholls TJ, Green KL, Rogers DJ, Cook JD, Wolowacz S, Smart JD. Lectins in ocular drug delivery.An investigation of lectin binding sites on the corneal and conjunctival surfaces. Int J Pharm. 1996;138:175–83. [Google Scholar]
- 21.Hornof M, Weyenberg W, Ludwig A, Bernkop-Schnurch A. A mucoadhesive ophthalmic insert based on thiolated poly(acrylic) acid: Development and in vivo evaluation in human volunteers. J Control Release. 2003;89:419–28. doi: 10.1016/s0168-3659(03)00135-4. [DOI] [PubMed] [Google Scholar]
- 22.Albrecht K, Zirm EJ, Palmberger TF, Schlocker W, Bernkop-Schnurch A. Preparation of thiomer microparticles and in vitro evaluation of parameters influencing their mucoadhesive properties. Drug Dev Ind Pharm. 2006;32:1149–57. doi: 10.1080/03639040600712334. th. [DOI] [PubMed] [Google Scholar]
- 23.Peppas NA, Little MD, Huang Y. Bioadhesive Controlled Release Systems. In: Wise DL, editor. Handbook of pharmaceutical controlled release technology. New York: Marcel Dekker; 2000. pp. 255–69. [Google Scholar]
- 24.Gurny R, Meyer JM, Peppas NA. Bioadhesive intraoral release systems: Design, testing and analysis. Biomaterials. 1984;5:336–40. doi: 10.1016/0142-9612(84)90031-0. [DOI] [PubMed] [Google Scholar]
- 25.Gudeman L, Peppas NA. Preparation and characterisation of ph- sensitive, interpenetrating networks of poly(vinyl alcohol) and poly(acrylic acid) J Appl Polym Sci. 1995;55:919–28. [Google Scholar]
- 26.McCarron PA, Woolfson AD, Donnelly RF, Andrews GP, Zawislak A, Price JH. Influence of plasticiser type and storage conditions on the properties of poly(methyl vinyl ether-co-maleic anhydride) bioadhesive films. J Appl Polym Sci. 2004;91:1576–89. [Google Scholar]
- 27.Park H, Robinson JR. Physicochemical properties of water soluble polymers important to mucin/epithelium adhesion. J Control Release. 1985;2:47–7. [Google Scholar]
- 28.Blanco Fuente H, AnguianoIgea S, OteroEspinar FJ, BlancoMendez J. In-vitro bioadhesion of carbopol hydrogels. Int J Pharm. 1996;142:169–74. [Google Scholar]
- 29.Donnelly RF, McCarron PA, Tunney MM, Woolfson AD. Potential of photodynamic therapy in treatment of fungal infections of the mouth.design and characterisation of a mucoadhesive patch containing toluidine Blue O. J Photochem Photobiol B. 2007;86:59–69. doi: 10.1016/j.jphotobiol.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Smart JD. An in vitro assessment of some mucoadhesive dosage forms. Int J Pharm. 1991;73:69–74. [Google Scholar]
- 31.Kamath KR, Park K. Mucosal Adhesive Preparations. In: Swarbrick J, Boylan JC, editors. Encyclopedia of Pharmaceutical Technology. New York: Marcel Dekker; 1992. p. 133. [Google Scholar]
- 32.Lehr CM, Poelma FG. An Estimate of turnover time of intestinal mucus gel layer in the Rat in situ Loop. Int J Pharm. 1991;70:235. [Google Scholar]
- 33.Park K, Park H. Test methods of bioadhesion, Bioadhesive drug delivery systems. In: Lenaerts V, Gurney R, editors. Florida, Boca Raton: CRC Press; 1990. [Google Scholar]
- 34.McCarron PA, Donnelly RF, Zawislak A, Woolfson AD, Price JH, McClelland R. Evaluation of a Water-soluble Bioadhesive patch for photodynamic therapy of vulval lesions. Int J Pharm. 2005;293:11–23. doi: 10.1016/j.ijpharm.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 35.McCarron PA, Donnelly RF, Zawislak A, Woolfson AD. Design and evaluation of a water-soluble bioadhesive patch formulation for cutaneous delivery of 5-aminolevulinic acid to superficial neoplastic lesions. Eur J Pharm Sci. 2006;27:268–79. doi: 10.1016/j.ejps.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Donnelly RF, McCarron PA, Zawislak AA, Woolfson AD. Design and physicochemical characterisation of a bioadhesive patch for dose-controlled topical delivery of imiquimod. Int J Pharm. 2006;307:318–25. doi: 10.1016/j.ijpharm.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 37.Hassan EE, Gallo JM. A simple rheological method for the in vitro assessment of mucin-polymer bioadhesive bond strength. Pharm Res. 1990;7:491–5. doi: 10.1023/a:1015812615635. [DOI] [PubMed] [Google Scholar]
- 38.Rossi S, Bonferoni MC, Lippoli G, Bertoni M, Ferrari F, Caramella C, et al. Influence of mucin type on polymer-mucin rheological interactions. Biometerials. 1995;16:1073–9. doi: 10.1016/0142-9612(95)98903-r. [DOI] [PubMed] [Google Scholar]
- 39.Hagerstrom H, Paulsson M, Edsman K. Evaluation of mucoadhesion for two polyelectrolyte gels in simulated physiological conditions using a rheological method. Eur J Pharm Sci. 2000;9:301–9. doi: 10.1016/s0928-0987(99)00070-6. [DOI] [PubMed] [Google Scholar]
- 40.Rossi S, Ferrari F, Bonferoni MC, Caramella C. Characterization of chitosan hydrochloride--mucin rheological interaction: Influence of polymer concentration and polymer: Mucin weight ratio. Eur J Pharm Sci. 2001;12:479–85. doi: 10.1016/s0928-0987(00)00194-9. [DOI] [PubMed] [Google Scholar]
- 41.Hagerstrom H, Edsman K. Limitations of the rheological mucoadhesion method: The effect of the choice of conditions and the rheological synergism parameter Eur J Pharm Sci. 2003;18:349–57. doi: 10.1016/s0928-0987(03)00037-x. [DOI] [PubMed] [Google Scholar]
- 42.Ch’ng HS, Park H, Kelly P, Robinson JR. Bioadhesive polymers as platforms for oral controlled drug delivery.II Synthesis and evaluation of some swelling, water-insoluble bioadhesive polymers. J Pharm Sci. 1985;74:399–05. doi: 10.1002/jps.2600740407. [DOI] [PubMed] [Google Scholar]
- 43.Davis SS. The design and evaluation of controlled release systems for the gastro-intestinal tract. J Control Release. 1985;2:27–38. [Google Scholar]
- 44.Christian K, Karin A, Melanie G, Christian W, Paul D, Andreas BS. in vivo determination of the time and location of mucoadhesive drug delivery systems disintegration in the gastrointestinal tract. Magn Reson Imaging. 2008;26:638–43. doi: 10.1016/j.mri.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Woolfson AD, McCafferty DF, Moss GP. Development and characterisation of a moisture-activated bioadhesive drug delivery system for percutaneous local anaesthesia. Int J Pharm. 1998;169:83–94. [Google Scholar]
- 46.Salamat-Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev. 2005;57:1666–91. doi: 10.1016/j.addr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Ugwoke MI, Agu RU, Verbeke N, Kinget R. Nasal mucoadhesive drug delivery: Background, applications, trends and future perspectives. Adv Drug Deliv Rev. 2005;57:1640–65. doi: 10.1016/j.addr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Ludwig A. The use of mucoadhesive polymers in ocular drug delivery. Adv Drug Deliv Rev. 2005;57:1595–639. doi: 10.1016/j.addr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Valenta C. The use of mucoadhesive polymers in vaginal delivery. Adv Drug Deliv Rev. 2005;57:1692–712. doi: 10.1016/j.addr.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Edsman K, Hagerstrom H. Pharmaceutical applications of mucoadhesion for the non-oral routes. J Pharm Pharmacol. 2005;57:3–22. doi: 10.1211/0022357055227. [DOI] [PubMed] [Google Scholar]
- 51.Ponchel G, Touchard F, Duchene D, Peppas NA. Bioadhesive analysis of controlled release systems I.Fracture and interpenetration analysis in poly(acrylic acid)-containing systems. J Control Release. 1987;5:129–41. [Google Scholar]
- 52.Nagai T, Konishi R. Buccal/gingival drug delivery systems. J Control Release. 1987;6:353–60. [Google Scholar]
- 53.Sayin B, Somavarapu S, Li XW, Thanou M, Sesardic D, Alpar HO, Senel S. Mono-N-carboxymethyl chitosan (MCC) and N-trimethyl chitosan (TMC) nanoparticles for non-invasive vaccine delivery. Int J Pharm. 2008;363:139–48. doi: 10.1016/j.ijpharm.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 54.Ango R, Kavimani S, Mullaicharam AR, Jayakar B. in vitro studies on buccal strips of glibenclamide using chitosan. Indian J Pharm Sci. 1997;59:232–5. [Google Scholar]
- 55.Boyapally H, Nukala RK, Bhujbal P, Douroumis D. Controlled release from directly compressible theophylline buccal tablets. Colloids Surf B Biointerfaces. 2010;77:227–33. doi: 10.1016/j.colsurfb.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 56.Petelin M, Pavlica Z, Bizimoska S, Sentjurc M. In vivo study of different ointments for drug delivery into oral mucosa by EPR oximetry. Int J Pharm. 2004;270:83–91. doi: 10.1016/j.ijpharm.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 57.Rossi S, Maricello M, Bonferoni MC, Ferrari F, Sandri G, Dacarro C, et al. Thermally sensitive gels based on chitosan derivatives for the treatment of oral mucositis. Eur J Pharm Biopharm. 2010;74:248–54. doi: 10.1016/j.ejpb.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 58.Nafee NA, Ismail FA, Boraie NA, Mortada LM. Mucoadhesive buccal patches of miconazole nitrate: in vitro/in vivo performance and effect of ageing. Int J Pharm. 2003;264:1–14. doi: 10.1016/s0378-5173(03)00371-5. [DOI] [PubMed] [Google Scholar]
- 59.Consuelo ID, Falson F, Guy R H, Jacques Y. Ex vivo evaluation of bioadhesive films for buccal delivery of fentanyl. J Control Release. 2007;122:135–40. doi: 10.1016/j.jconrel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 60. Nasal Ointment Abvailable from: http://www.Medicines.Org.Uk/Emc/Medicine/2027/Spc/ Jun2010.htm. Electronic Medicines Compendium [Last scited on 2010 Aug 16]
- 61.Jain AK, Khar RK, Ahmed FJ, Diwan PV. Effective insulin delivery using starch nanoparticles as a potential trans-nasal mucoadhesive carrier. Eur J Pharm Biopharm. 2008;69:426–35. doi: 10.1016/j.ejpb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 62.Wu J, Wei W, Wang LY, Su ZG, Ma GH. A thermosensitive hydrogel based on quarternized chitosan and poly(ethylene glycol) for nasal drug delivery system. Biomaterials. 2007;28:2220–32. doi: 10.1016/j.biomaterials.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 63.Luppi B, Bigucci F, Abruzzo A, Corace G, Cerchia T, Zecchi V. Freeze dried chitosan/pectin nasal inserts for antipsychotic drug delivery. Eur J Pharm Biopharm. 2010;75:381–7. doi: 10.1016/j.ejpb.2010.04.013. 0. [DOI] [PubMed] [Google Scholar]
- 64.Hornof M, Weyenberg W, Ludwig A, Bernkop-Schnurch A. Mucoadhesive ocular insert based on thiolated poly(acrylic acid): Development and in vivo evaluation in humans. J Control Release. 2003;89:419–28. doi: 10.1016/s0168-3659(03)00135-4. [DOI] [PubMed] [Google Scholar]
- 65.Grezeskowiak E. Biopharmaceutical availability of sulphadicramide from ocular ointments in vitro. Eur J Pharm Sci. 1998;6:247–53. doi: 10.1016/s0928-0987(97)10006-9. 0. [DOI] [PubMed] [Google Scholar]
- 66.Qi H, Chen W, Huang C, Li L, Chen C, Li W, et al. Development of a poloxamer analogs / carbopol based in situ gelling and mucoadhesive ophthalmic delivery system for puerain. Int j pharm. 2007;337:178–87. doi: 10.1016/j.ijpharm.2006.12.038. 0. [DOI] [PubMed] [Google Scholar]
- 67.Jain D, Carvalho E, Banerjee R. Biodegradable hybrid polymeric membranes for ocular drug delivery. Acta Biomater. 2010;6:1370–9. doi: 10.1016/j.actbio.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 68.Lux A, Maier S, Dinslay S, Suverkrup R, Diestelhorst M. A comparative bioavailability study of three conventional eye drops versus a single lyophilisate. Br J Ophthalmol. 2003;87:436–40. doi: 10.1136/bjo.87.4.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perioli L, Ambrogi V, Pagano C, Sw S, Rossi C. Fg90 Chitosan as a new polymer for metronidazole mucoadhesive tablets for vaginal administration. Int J Pharm. 2009;337:120–7. doi: 10.1016/j.ijpharm.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 70.Khanna N, Dalby R, Tan M, Arnold S, Stern J, Frazer N. Phase I/II clinical safety studies of terameprocol vaginal ointment. Gyn Oncol. 2007;107:554–62. doi: 10.1016/j.ygyno.2007.08.074. [DOI] [PubMed] [Google Scholar]
- 71.Kim Yt, Shin BK, Garripelli VK, Kim JK, Davaa E, et al. a Thermosensitive vaginal gel formulation with hpucd for the ph dependent release and solubilsation of amphotericin b. Eur J Pharm Sci. 2010;9:399–406. doi: 10.1016/j.ejps.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 72.Yoo JW, Dharmala K, Lee CH. The physicodynamic properties of mucoadhesive polymeric films developed as female controlled drug delivery system. Int J Pharm. 2006;309:139–45. doi: 10.1016/j.ijpharm.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 73.Yahagi R, Machida Y, Onishi H. Mucoadhesive suppositories of ramosetron hydrochloride utilizing carbopol. Int J Pharm. 2000;193:205–12. doi: 10.1016/s0378-5173(99)00338-5. [DOI] [PubMed] [Google Scholar]
- 74. Available from: http://www.medicines.org.uk/emc/medicine/7162/spc/Anusol+Ointment/#excipients/Jun2010.htm [Last accessed on 2010 Aug 02]
- 75.Koffi AA, Agnely F, Ponchel G, Grossiord JL. Modulation of the rheological and mucoadhesive properties of thermosensitive poloxamer-based hydrogels intended for the rectal administration of quinine. Eur J Pharm Sci. 2006;27:328–35. doi: 10.1016/j.ejps.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 76.Leede LG, Boer AG, Portzgen E, Feijen J, Breimer DD. Rate controlled rectal drug delivery in man with a hydrogel preparation. J Control Release. 1986;4:17–24. [Google Scholar]
- 77.Anders R, Merkle HP. Evaluation of laminated mucoadhesive patches for buccal drug delivery. Int J Pharm. 1989;49:231–40. [Google Scholar]
- 78.Woolfson AD, McCafferty DF, McCallion CR, McAdams ET, Anderson J. Moisture-activated, electrically-conducting bioadhesive hydrogels as interfaces for bio-electrodes: Effect of film hydration on cutaneous adherence in wet environments. J Appl Polym Sci. 1995;58:1291–6. [Google Scholar]
- 79.Leung SHS, Robinson JA. Polyanionic polymers in bioadhesive and mucoadhesive drug delivery. ACS Symposium Series. 1992;480:269–84. [Google Scholar]
- 80.Shojaei AH, Berner B, Xiaoling L. Transbuccal delivery of acyclovir: I.in vitro determination of routes of buccal transport. Pharm Res. 1998;15:1182–8. doi: 10.1023/a:1011927521627. [DOI] [PubMed] [Google Scholar]
- 81.Al-Achi A, Greenwood R. Buccal administration of human insulin in streptozocin-diabetic rats. Res Commun Chem Pathol Pharmacol. 1993;82:297–306. [PubMed] [Google Scholar]
- 82.Bouckaert S, Remon JP. In-vitro bioadhesion of a buccal, miconazole slow-release tablet. J Pharm Pharmacol. 1993;45:504–7. doi: 10.1111/j.2042-7158.1993.tb05588.x. [DOI] [PubMed] [Google Scholar]
- 83.Perioli L, Ambrogi V, Giovagnoli S, Blasi P, Mancini A, Ricci M, et al. Influence of compression force on the behaviour of mucoadhesive buccal tablets. AAPS PharmSciTech. 2008;9:274–81. doi: 10.1208/s12249-007-9029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shemer A, Amichai B, Trau H, Nathansohn N, Mizrahi B, Domb AJ. Efficacy of a mucoadhesive patch compared with an oral solution for treatment of aphthous stomatitis. Drugs R D. 2008;9:29–35. doi: 10.2165/00126839-200809010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Medlicott NJ, Rathbone MJ, Tucker IJ, Holborow DW. Delivery systems for the administration of drugs to the periodontal pocket. Adv Drug Deliv Rev. 1994;13:181–203. [Google Scholar]
- 86.Mizrahi B, Domb AJ. Mucoadhesive polymers for delivery of drugs to the oral cavity. Recent Pat Drug Deliv Formul. 2008;2:108–19. doi: 10.2174/187221108784534126. th. [DOI] [PubMed] [Google Scholar]
- 87.Andrews GP, Jones DS, Redpath JM, Woolfson AD. Characterisation of protein-containing binary polymeric gel systems designed for the treatment of periodontal disease. J Pharm Pharmacol. 2004;56:S71–2. [Google Scholar]
- 88.Jones DS, Bruschi ML, de Freitas O, Gremiao MP, Lara EH, Andrews GP. Rheological, mechanical and mucoadhesive properties of thermoresponsive, bioadhesive binary mixtures composed of poloxamer 407 and carbopol 974P designed as platforms for implantable drug delivery systems for use in the oral cavity. Int J Pharm. 2009;372:49–58. doi: 10.1016/j.ijpharm.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 89.3M, 3M buccal drug delivery system [UKICRS Newsletter. 1998 Page 7. [Google Scholar]
- 90.Modi P, Mihic M, Lewin A. Evolving role of oral insulin in the treatment of diabetes using a novel rapidmist system. Diabetes Metab Res Rev. 2002;18:38–42. doi: 10.1002/dmrr.208. [DOI] [PubMed] [Google Scholar]
- 91.Nagai T, Machida Y. Bioadhesive dosage forms for nasal administration, Bioadhesive Drug Delivery Systems. In: Lenaerts V, Gurney R, editors. Florida. Boca Raton: CRC Press; 1990. [Google Scholar]
- 92.McInnes FJ, O’Mahony B, Lindsay B, Band J, Wilson CG, Hodges LA, et al. Nasal residence of insulin containing lyophilised nasal insert formulations, using gamma scintigraphy. Eur J Pharm Sci. 2007;31:25–31. doi: 10.1016/j.ejps.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 93.Coucke D, Schotsaert M, Libert C, Pringels E, Vervaet C, Foreman P, et al. Spray-dried powders of starch and crosslinked poly(acrylic acid) as carriers for nasal delivery of inactivated influenza vaccine. Vaccine. 2009;27:1279–86. doi: 10.1016/j.vaccine.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 94.Kharenko EA, Larionova NI, Demina NB. Mucoadhesive drug delivery systems (Review) 43, 4, Pharm Chem. 2009;43:200, 8. [Google Scholar]
- 95.Pavis H, Wilcock A, Edgecombe J, Carr D, Manderson C, Church A, et al. Pilot study of nasal morphinechitosan for the relief of breakthrough pain in patients with cancer. J Pain Symp Manag. 2002;24:598–602. doi: 10.1016/s0885-3924(02)00522-5. [DOI] [PubMed] [Google Scholar]
- 96.Tzachev CT, Mandajieva M, Minkov EH, Popov TA. Comparison of the clinical efficacy of standard and mucoadhesive- based nasal decongestants. Br J Clin Pharmacol. 2002;53:107–9. doi: 10.1046/j.0306-5251.2001.01525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sensoy D, Cevher E, Sarici A, Yilmaz M, Ozdamar A, Bergisadi N. Bioadhesive sulfacetamide sodium microspheres: Evaluation of their effectiveness in the treatment of bacterial keratitis caused by Staphylococcus aureus and Pseudomonas aeruginosa in a rabbit model. Eur J Pharm Biopharm. 2009;72:487–95. doi: 10.1016/j.ejpb.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 98.de la Fuente M, Seijo B, Alonso MJ. Bioadhesive hyaluronan-chitosan nanoparticles can transport genes across the ocular mucosa and transfect ocular tissue. Gene Therapy. 2009;15:668–76. doi: 10.1038/gt.2008.16. [DOI] [PubMed] [Google Scholar]
- 99.Baeyens V, Felt-Baeyens O, Rougier S, Pheulpin S, Boisrame B, Gurnya R. Clinical evaluation of bioadhesive ophthalmic drug inserts (BODI®) for the treatment of external ocular infections in dogs. J Control Release. 2002;85:163–8. doi: 10.1016/s0168-3659(02)00284-5. [DOI] [PubMed] [Google Scholar]
- 100.Batchelor H. Formulation strategies in mucosal-adhesive drug delivery CRS Newsletter. 2005;22:4–5. [Google Scholar]
- 101.Robinson JR, Bologna WJ. Vaginal and reproductive- system treatments using a bioadhesive polymer. J Control Release. 1994;28:87–94. [Google Scholar]
- 102.O’Hagan DT, Rafferty D, Wharton S, Illum L. Intravaginal immunization in sheep using a bioadhesive microsphere antigen delivery system. Vaccine. 1993;11:660–4. doi: 10.1016/0264-410x(93)90313-m. [DOI] [PubMed] [Google Scholar]
- 103.Bonucci E, Ballanti P, Ramires PA, Richardson JL, Benedetti LM. Prevention of ovariectomy osteopenia in rats after vaginal administration of Hyaff 11 microspheres containing salmon calcitonin. Calcif Tissue Intl. 1995;56:274–9. doi: 10.1007/BF00318046. [DOI] [PubMed] [Google Scholar]
- 104.Alam MA, Ahmad FJ, Khan ZI, Khar RK, Ali M. Development and evaluation of acid-buffering bioadhesive vaginal Tablet for Mixed vaginal Infections. AAPS PharmSciTech. 2007;8:109. doi: 10.1208/pt0801015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cevher E, Taha MA, Orlu M, Araman A. Evaluation of mechanical and mucoadhesive properties of clomiphene citrate gel formulations containing carbomers and their thiolated derivatives. Drug Deliv. 2008;15:57–67. doi: 10.1080/10717540701829234. [DOI] [PubMed] [Google Scholar]
- 106. Available from: http://www.clinicaltrials.gov. [Laat accessed on 2010 Aug 02]
- 107.Francois M, Snoeckx E, Putteman P, Wouters F, Proost E De, Delaet U, et al. A mucoadhesive, cyclodextrin-based vaginal cream formulation of itraconazole. AAPS PharmSci. 2003;5:E5. doi: 10.1208/ps050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim JK, Kim MS, Park JS, Kim CK. Thermo-reversible flurbiprofen liquid suppository with HP-beta-CD as a solubility enhancer: Improvement of rectal bioavailability. J Incl Phenom Macrocycl Chem. 2009;64:265–72. [Google Scholar]
- 109.Woolfson AD, McCafferty DF, McCarron PA, Price JH. A Bioadhesive patch cervical drug delivery system for the administration of 5-fluorouracil to cervical tissue. J Control Release. 1995;35:49–58. [Google Scholar]
- 110.Woolfson AD, McCafferty DF, Moss GP. Development and characterisation of a moisture-activated bioadhesive drug delivery system for percutaneous local anaesthesia. Int J Pharm. 1998;169:83–94. [Google Scholar]
- 111.Donnelly RF, McCarron PA, Zawislak A, Woolfson AD. Photodynamic therapy of vulval neoplasias and dysplasias: Design and evaluation of bioadhesive photosensitiser delivery systems. In: Verlag M, editor. Saarbrücken: VDM Publication; 2009. pp. 78–400. [Google Scholar]
- 112.Ahmed IS, Ayres JW. Bioavailability of riboflavin from a gastric retention formulation. Int J Pharm. 2007;330:146–54. doi: 10.1016/j.ijpharm.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 113.Salman HH, Gamazo C, Agueros M, Irache JM. Bioadhesive capacity and immunoadjuvant properties of thiamine-coated nanoparticles. Vaccine. 2007;48:8123–32. doi: 10.1016/j.vaccine.2007.09.044. [DOI] [PubMed] [Google Scholar]


