Abstract
Physical illness may precipitate psychological distress among older adults. This study examines whether social support and self-efficacy moderate the associations between physical health and depression and anxiety. Predictions were tested in 222 individuals age 60 or older presenting for help with worry. Physical health was assessed through self-report (subjective) and physical diagnoses (objective). Objective physical health did not have a significant association with depression or anxiety. Worse subjective physical health was associated with increased somatic anxiety, but not with depression or worry. The relationship between subjective physical health and depressive symptoms was moderated by self-efficacy and social support. As predicted, when self-efficacy was low, physical health had its strongest negative association with depressive symptoms such that as physical health improved, depressive symptoms also improved. However, the moderation effect was not as expected for social support; at high levels of social support, worse physical health was associated with increased depressive affect.
Keywords: elderly, depression, anxiety, social support, self-efficacy, physical health
Physical health problems are the “hallmark” of older-adult depression (Diagnosis and treatment of depression in late life, 1991) and are common among older adults with anxiety (Kim, Braun, & Kunik, 2001). As many as 28% of community-dwelling older adults have health problems that interfere with their daily activities (Lindesay, 1990); and of these individuals, 12% to 42% have clinically significant symptoms of depression or anxiety (Baker, 1996; Smalbrugge, Pot, Jongenelis, Beekman, & Eefsting, 2005). These estimates are over twice what they are for physically healthy older adults (Katona, 1991), indicating that physical health problems may significantly influence the presence of anxiety and depression among older adults. Only a few decades ago, anxiety and depression were thought to be normal reactions to medical illness in older adults; but now it has been recognized that special attention should be paid to assessing and treating depression and anxiety in older adults with physical illnesses (Williamson et al., 2000). To adequately assess and treat depression and anxiety in older adults, it is important to explore factors contributing to their development, such as physical health, self-efficacy and social support.
The association of physical illness with depression and anxiety has been supported across studies examining a variety of health problems (Lenze et al., 2001). Longitudinal studies indicate that physical illness leads to increased depressive (Wallace & O'Hara, 1992) and anxiety (De Beurs, Beekman, Deeg, Dyck, & Tilburg, 2000) symptoms. Depression and anxiety are related to both objective and subjective measures of physical health, including self-rated global health (De Beurs et al., 2000), self-reported disease-severity ratings (Lindesay, 1990), and number of co-morbid medical conditions (Bosma et al., 2004); and functional impairment is more strongly associated with distress than presence or absence of a disease (Lenze et al., 2001). Several theoretical models with research support, such as the transactional model of stress (Lazarus & Folkman, 1984), the integrative theory of depression (Lewinsohn, Hoberman, Teri, & Hautzinger, 1985), and the helplessness/hopelessness model (Alloy et al., 1990), suggest why certain older adults are at higher risk for developing depression or anxiety when physical health problems occur. These models suggest that stressors, such as physical illness, cause significant distress if the individual does not have coping strategies to react successfully. Particularly relevant coping mechanisms for older adults may be self-efficacy and social support.
Self-efficacy, the belief that one has the capability to be successful, even in adverse situations (Bandura, 1977), may become more important in maintaining good mental health as individuals transition into older adulthood (Welch & West, 1995). High self-efficacy in the presence of physical health stressors may be particularly important in protecting older adults from depression because of the frequency and expectations of health problems (Davis-Berman, 1990). An older adult with high self-efficacy may accept limitations caused by physical illness but compensate by capitalizing on alternative strengths (Blazer, 2002), thus continuing efforts to complete activities successfully. This coping style may help maintain a sense of control (Bandura, 1977) and protect against depression and anxiety (Lewinsohn et al., 1985). General self-efficacy may be an even stronger predictor of depression than the presence of physical illness (Jang, Haley, Small, & Mortimer, 2002). Both general and illness-specific self-efficacy (e.g., belief that one has the power to cope with kidney failure) have been found to moderate the relationship between physical illness and depression in Asian samples, such that high self-efficacy protects against the negative effects of physical illness on depression (Chou & Chi, 2001; Takaki et al., 2003).
The primary focus at retirement shifts from work to social networks (Isaacowitz, 2005); but, at the same time, there are more impediments than previously to maintaining social ties as individuals age (e.g., others' deaths and fewer opportunities to meet new people, Mollenkopf et al., 1997). Social support may protect older adults from the stress of physical health problems by acting as a “buffer” and preventing development of depressive and anxiety symptoms (Blazer, 2003). Depression in older adults with physical health stressors is consistently predicted by low social support (e.g., Oxman, Freeman, Manheimer, & Stukel, 1994), even when other significant predictors of depression, such as past history of depression, recent loss, comorbid chronic somatic illness, and functional impairment, are taken into account (Parashos et al., 2002; Sherman et al., 2006). However, among individuals with high social support, physical illnesses are less likely to precipitate the development of anxiety or depressive symptoms (Jang et al., 2002; Majercsik & Haller, 2004).
There are several forms of social support. Friendship, is uniquely important in older-adult mental health (Horowitz, Reinhardt, Boerner, & Travis, 2003) because of its distinctive qualities. Friendships are chosen; while family is not (Friedman, 1993). Choosing friends increases sense of control (Alloy et al., 1990), and being chosen as a friend increases positive thoughts about oneself (Alloy et al., 1990). Individuals generally maintain friendships only with those with whom they have a primarily supportive relationship (Friedman, 1993). Lastly, friendships are emotionally rewarding; there is lowered perceived dependence than in family relationships (Friedman, 1993).
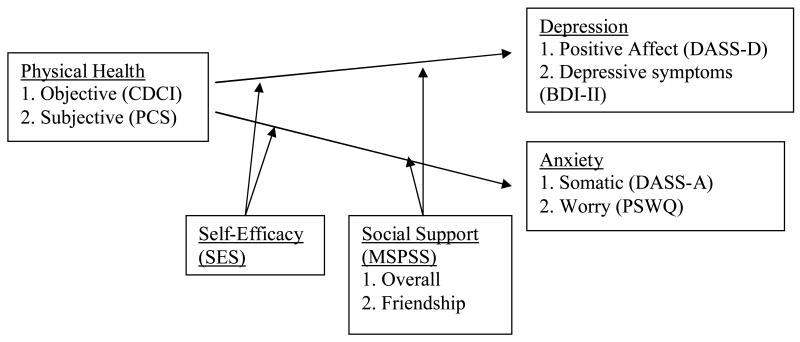
This study examined the effects of social support and self-efficacy on the relationship between physical health, depression, and anxiety in a sample of older adults. We tested four hypotheses (Figure 1). First, physical health was predicted to be significantly correlated with depression and anxiety. A self-report measure of severity of physical-health problems (subjective physical health) was predicted to be more robustly associated with depression and anxiety than severity of disease diagnoses (objective physical health). Second and third, self-efficacy and global perceived social support were predicted to moderate these relationships such that the associations between physical health and depression and anxiety would be weakest among individuals high in self-efficacy and social support. Fourth, social support from friendships, in particular, was predicted to moderate the effects of physical health on depression and anxiety.
Figure 1.
Hypothesized relationships.
Methods
Participants
Participants were all originally recruited for a randomized clinical trial for late-life (age 60 or older) generalized anxiety disorder (GAD). The data used for the present study were collected prior to beginning the treatment phase of the trial. Participants were recruited from Kelsey-Seybold Clinic (KSC), a large multi-specialty medical organization (primary recruitment source) and various clinics within Baylor College of Medicine. Both organizations are located in the greater Houston, Texas, area. Recruitment letters were sent to patients age 60 and older treated at both clinic sites. These letters included study contact information to enable patients to call the research staff if they were interested in participating. Additional participants were either referred to the study by their primary care providers or self-referred from brochures available in their provider's waiting room.
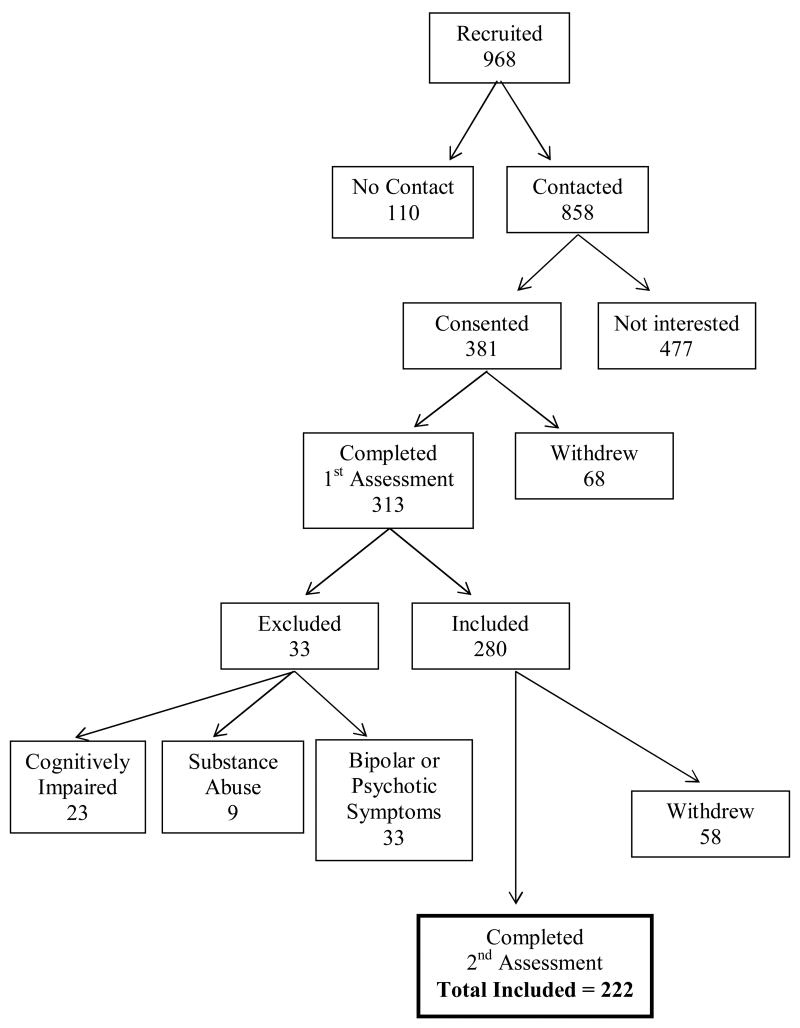
Initial recruitment resulted in 968 potential participants and the final sample consisted of 222 participants (see Figure 2 for details on the number of participants excluded in each step of the selection process) with a mean age of 67.5 (SD = 6.08). Some potential participants were unable to be contacted or did not sign consent to participate in the study. After consenting, additional participants withdrew before completing the first assessment, which included the Mini-Mental State Exam (MMSE, Folstein, Folstein, & McHugh, 1975) and the Structured Clinical Interview for Diagnosis (SCID, Spitzer, Williams, Gibbon, & First, 1992). Based on this assessment, participants were excluded for the following reasons: cognitive impairment (MMSE < 24), substance abuse, or current bipolar or psychotic symptoms. The remaining participants that completed the second assessment, which consisted of self-report instruments, were included in the final study sample.
Figure 2.
Participant-selection process.
The current sample included both those with a primary mental health diagnosis of GAD (n = 134, 60%) that were included in the clinical trial and those excluded from the clinical trial. In the total study sample of 222, anxiety disorders besides GAD (n = 17, 8%), mood disorders (n = 29, 13%), and other mental health diagnoses (n = 9, 4%) were also common as primary mental health diagnoses. Of those with primary GAD diagnoses, 60 (45%) had secondary mood diagnoses; and 34 (25%) had another anxiety disorder as a secondary diagnosis.
Approximately 2 weeks after completing the MMSE and the SCID, self-report measures were administered via telephone interview. The validity and reliability of telephone-administered self-report measures are supported by research with a subgroup of this sample (Senior et al., 2007). Physical health diagnoses were collected from administrative databases for the year prior to completion of self-report measures. However, this information was available only for the participants receiving medical care at KSC (n = 191).
Measures
Comparison of previous studies suggests that inconsistent results (Lenze et al., 2001) may have occurred because the studies measured different dimensions of physical health, depression, or anxiety. Thus, in this study, physical health, depression, and anxiety were measured using two scales to assess different aspects of each construct.
Physical Health
Two measures of physical health were used: a subjective self-report measure of global physical health and an objective comorbidity index representing the severity of physical health diagnoses.
Short-Form Health Survey (SF-12)
The SF-12 (Ware, Kosinski, & Keller, 1996) is a 12-item self-report measure assessing the perceived severity and functional impact of health problems. Item-response formats vary. For example, the first question asks: “In general, would you say your health is:” with five possible responses ranging from excellent to very good. The second set of questions asks: “The following questions are about activities you might do during a typical day. Does your health limit you in these activities? If so, how much?” such as “climbing several flights of stairs” with three possible responses ranging from “yes, limited a lot” to “no, not limited at all.” Composite scores for mental and physical functioning are calculated using complex algorithms and are standardized to have a range of 0 to 100, with a mean score of 50, and a standard deviation of 10. The Physical Composite Score (PCS) provides a summary score with higher scores indicating better overall physical functioning. Test-retest reliability and criterion validity of the PCS are strong (Ware et al., 1996). This measure has been used previously in geriatric populations (Yochim, Kerkar, & Lichtenberg, 2006). In this sample, internal consistency was satisfactory (α = .78).
Charlson/Deyo Comorbidity Index (CDCI)
Scores for the CDCI (Charlson, Pompei, Ales, & MacKenzie, 1987; Deyo, Cherkin, & Ciol, 1992), originally designed to predict 10-year mortality (Charlson et al., 1987), are calculated by adding together weighted severity ratings of comorbid physical diseases. Scores typically range from 0 (no physical diseases predicting mortality) to 3 (several physical diseases or one severe physical disease). Deyo et al. (1992) revised this index for use with the International Statistical Classification of Diseases and Related Health Problems, 9th Revision, Clinical Modification (ICD-9-CM). The CDCI is the most widely used index to measure risk of health-related outcomes in scientific research (Southern, Quan, & Ghali, 2004). It predicts postoperative complications, mortality, blood transfusion, discharge to nursing home, length of hospital stay, and hospital discharge, even after controlling for age (Deyo et al., 1992). The mean and distribution of CDCI scores in the current study sample were similar to those reported in a public healthcare sample of older adults from British Columbia (Schneeweiss et al., 2004), with 61% scoring 0, 26% scoring 1, 11% scoring 2, and only 3% scoring 3 or above.
Depression and Anxiety
For depression, assessment included a clinical measure of depressive symptoms and a measure of positive affect. To assess anxiety, participants completed a measure of somatic anxiety and a measure of worry. Low positive affect and somatic anxiety were measured because they are unique and distinguishing features of depression and anxiety (Antony, Bieling, Cox, Enns, & Swinson, 1998) even though depressive and anxiety symptoms are often clustered together, especially in older adults (Lenze et al., 2001). To present findings that are both clinically relevant and comparable to previous research, nonspecific measures of depressive symptoms and worry were included as well.
Beck Depression Inventory – II (BDI-II)
The BDI-II (Beck, Steer, & Brown, 1996) is a 21-item self-report measure of the severity of depressive symptoms. Scores range from 0 to 63 with higher scores indicating more symptoms indicative of depression. The internal consistency, factor structure, and validity of the BDI-II have received ample support. Coefficient alphas are typically at or above .90 (Beck et al., 1996), similar to what they were in this sample (α = .87). The BDI-II has also been used in studies examining depression among older adults (Bourland et al., 2000).
Penn State Worry Questionnaire (PSWQ)
The PSWQ (Meyer, Miller, Metzger, & Borkovec, 1990) is a 16-item self-report questionnaire for measuring the severity of worry. Participants rate statements such as, “My worries overwhelm me” and “I find it easy to dismiss worrisome thoughts” on a Likert scale from 1, “Not at all typical of me,” to 5, “Very typical of me.” Total scores range from 16 to 80 with higher scores indicating more severe problems with worry. There is support for its internal consistency and convergent and discriminant validity in older-adult populations (Stanley, Novy, Bourland, Beck, & Averill, 2001). The internal consistency in this sample was good (α = .90).
Depression, Anxiety, and Stress Scales- 21 (DASS)
The DASS (Antony et al., 1998; Lovibond & Lovibond, 1995) consists of 21 items broken into scales independently assessing depression, anxiety and stress. Each factor is measured by seven items on which participants rate the extent to which they experienced the symptoms described in that item over the past week on a 4-point Likert scale from 0, “Did not apply to me at all,” to 3, “Applied to me very much or most of the time.” The two subscales used in this study measure low positive affect (DASS-D) and somatic anxiety (DASS-A, Brown, Chorpita, Korotitsch, & Barlow, 1997). The DASS-D subscale includes items such as, “I felt I wasn't worth much as a person,” and “I couldn't seem to experience any positive feeling at all.” The DASS-A includes items such as, “I felt I was close to panic,” and “I was aware of dryness of my mouth.” Scores range from 0 to 21 on each subscale. High scores indicate low positive affect (increased depression) and high somatic anxiety. Evidence in clinical samples supports the external, construct and discriminant validity of the DASS, as well as the test-retest and internal reliability (Brown et al., 1997). Internal consistency was similar to what has been found in other studies (DASS-D: α = .87, DASS-A: α = .70).
Proposed Moderators
To test moderation hypotheses, self-efficacy and perceived social support were measured with one scale each.
Self-Efficacy Scale (SES)
The SES (Sherer, 1982) is a 23-item measure of self-efficacy not linked with specific situations or behavior. The general self-efficacy subscale containing 17 items was used for the present study. Participants indicate on a scale of 1, “agree strongly,” to 5, “disagree strongly,” how they feel about statements such as, “If I can't do a job the first time, I keep trying until I can,” and “I am a self-reliant person.” Scores range from 17 to 85, with high scores indicating high self-efficacy. Evidence supports its internal reliability and criterion validity (Sherer, 1982). It has been used with older adults with good internal consistency for the general scale (Bosma et al., 2004) that is comparable to that of the present study (α = .84).
Multidimensional Scale of Perceived Social Support (MSPSS)
The MSPSS (Zimet, Dahlem, Zimet, & Farley, 1988) has 12 items measuring perceived social support. Participants are asked to what extent they agree each item is true of them on a Likert scale from 1, “very strongly disagree,” to 7, “very strongly agree.” All MSPSS items may be added to calculate total perceived social support, which ranges from 12 to 84. The MSPSS also allows for the calculation of specific social support subscales (family, friends, and significant others) by adding the responses to four items in each relationship category with subscale scores ranging from 4 to 28. Higher scores on both the total scale and the subscales indicate higher levels of perceived social support. Items measuring family support include, “My family really tries to help me,” and “I can talk about problems with my family.” Friendship items include, “My friends really try to help me,” and “I can count on my friends when things go wrong.” Significant other items include, “There is a special person who is around when I am in need,” and “I have a special person who is a real source of comfort to me.” This study used both the overall scale (MSPSS) to measure total perceived social support and the friendship subscale (MSPSS-FR) to specifically measure perceived level of support from friends. The MSPSS has demonstrated adequate internal consistency, test-retest reliability, factorial validity, and construct validity in younger (Zimet et al., 1988) and older samples (Oxman et al., 1994). In this study, the internal consistencies of the subscales were adequate (α = .91 to .93).
Analyses
Demographic variables (age, gender, education, ethnicity, and income) were examined for impact on all dependent measures (DASS-D, BDI-II, DASS-A, PSWQ). Any demographic variable that was significantly (p < .05) associated with a dependent variable (DV) was controlled for in all subsequent analyses.
The four hypotheses were examined through two statistical methods. First, Pearson product-moment correlations were used to examine the relationship between physical health and depression and anxiety (Table 1). Though bivariate correlations provide easily interpretable data regarding the strength and direction of the relationship between two variables, they do not provide information about the shared variance between two variables after variance attributable to other predictors is accounted for. Thus, the relationship between physical health and depression and anxiety was examined further with multiple regression, including the hypothesized moderators, self-efficacy and social support. The hypothesized interactions were tested through hierarchical regression (Tables 2, 3, and 4). The variance attributable to the interaction terms is reflected in the difference in the model the R2's from Step 1 to Step 2. When interaction terms are added to regression equations, multicollinearity, or high correlations between predictors, may cause difficulties obtaining valid estimates of the variance attributable to the interaction term (Tabachnick & Fidell, 2001). Thus, all independent variables included in the interaction terms were centered (the mean was subtracted from each data point such that the new mean was 0 for both variables) to reduce the impact of multicollinearity and to enhance the interpretability of the results. A Bonferroni correction was used to set alpha to .025 because hypotheses were tested with two measures of each construct (depression and anxiety).
Table 1. Means, standard deviations, and intercorrelations between variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | |||||||||||
| 2. BDI-II | -.13* | ||||||||||
| 3. DASS-D | -.14* | .76** | |||||||||
| 4. PSWQ | -.12* | .53** | .52** | ||||||||
| 5. DASS-A | -.02 | .47** | .44** | .34** | |||||||
| 6. PCS | -.31** | -.03 | .11 | .08 | -.20** | ||||||
| 7. CDCI | .24** | -.09 | -.14 | -.18* | -.03 | .01 | |||||
| 8. MSPSS | .16* | -.30** | -.40** | -.17** | -.07 | -.09 | .06 | ||||
| 9. MSPSSFA | .20** | -.39** | -.48** | -.25** | -.12 | -.12 | .10 | .85** | |||
| 10. MSPSSFR | .09 | -.15* | -.24** | -.08 | -.03 | -.08 | -.01 | .81** | .52** | ||
| 11. SES | -.02 | -.42** | -.37** | -.25** | -.15* | .05 | .04 | .20** | -.21** | .13* | |
| Mean | 67.5 | 15.0 | 4.46 | 51.8 | 3.31 | 43.7 | .61 | 5.29 | 5.31 | 5.24 | 60.9 |
| SD | 6.08 | 8.78 | 4.17 | 12.13 | 3.24 | 8.70 | 1.04 | 1.26 | 1.62 | 1.61 | 9.47 |
Note. N = 222 for all except CDCI where N = 191;
p < .01;
p < .05. BDI-II = Depressive symptoms. DASS-D = Higher scores indicate lower positive affect; DASS-A = Somatic Anxiety. PSWQ = Worry. PCS = Higher scores indicate better perceived physical health. CDCI = Higher scores indicate worse objective physical health. MSPSS = Total perceived social support. MSPSSFA = Perceived family support; MSPSSFR = Perceived friend support; SES = Self-efficacy.
Table 2.
Self-efficacy and physical health interaction regression models predicting depressive symptoms, positive affect, worry, and somatic anxiety (N = 222).
| Dependent Variable | Independent Variables | B | SE B | β | Model R2 |
|---|---|---|---|---|---|
| Depressive Symptoms (BDI-II) | |||||
| Step 1 | .20 | ||||
| Age | -.23 | .09 | -.16* | ||
| Ethnicity | .20 | 1.15 | .01 | ||
| Physical health (PCS) | -.06 | .06 | -.06 | ||
| Self-efficacy (SES) | -.39 | .06 | -.42** | ||
| Step 2 | .23 | ||||
| Age | -.21 | .09 | -.15* | ||
| Ethnicity | -.16 | 1.15 | -.01 | ||
| Physical health (PCS) | -.03 | .06 | -.04 | ||
| Self-efficacy (SES) | -.39 | .06 | -.42** | ||
| Interaction of PCS and SES | -.02 | .01 | .16** | ||
| Positive Affect (DASS-D) | |||||
| Step 1 | .17 | ||||
| Age | -.09 | .04 | -.13 | ||
| Ethnicity | .38 | .56 | .04 | ||
| Physical health (PCS) | .04 | .03 | .09 | ||
| Self-efficacy (SES) | -.17 | .03 | -.38** | ||
| Step 2 | .18 | ||||
| Age | -.08 | .04 | -.12 | ||
| Ethnicity | .28 | .56 | .03 | ||
| Physical health (PCS) | .05 | .03 | .10 | ||
| Self-efficacy (SES) | -.17 | .03 | -.37** | ||
| Interaction of PCS and SES | .00 | .00 | .10 | ||
| Worry (PSWQ) | |||||
| Step 1 | .08 | ||||
| Age | -.23 | .14 | -.11 | ||
| Ethnicity | .33 | 1.71 | .01 | ||
| Physical health (PCS) | .07 | .10 | .05 | ||
| Self-efficacy (SES) | -.33 | .08 | -.26** | ||
| Step 2 | .08 | ||||
| Age | -.22 | .14 | -.11 | ||
| Ethnicity | .27 | 1.73 | .01 | ||
| Physical health (PCS) | .07 | .10 | .05 | ||
| Self-efficacy (SES) | -.33 | .08 | -.26** | ||
| Interaction of PCS and SES | .00 | .01 | .02 | ||
| Somatic Anxiety (DASS-A) | |||||
| Step 1 | .11 | ||||
| Age | -.04 | .04 | -.074 | ||
| Ethnicity | --1.5 | .45 | -.22** | ||
| Physical health (PCS) | -.08 | .03 | -.22** | ||
| Self-efficacy (SES) | -.05 | .02 | -.16* | ||
| Step 2 | .11 | ||||
| Age | -.04 | .04 | -.07 | ||
| Ethnicity | -1.51 | .45 | -.22** | ||
| Physical health (PCS) | -.08 | .03 | -.21** | ||
| Self-efficacy (SES) | -.05 | .02 | -.16* | ||
| Interaction of PCS and SES | .00 | .00 | .03 | ||
p < .01;
p < .025; PCS and SES were centered.
Table 3.
Social Support and physical health interaction regression models predicting depressive symptoms, positive affect, worry, and somatic anxiety (N = 222).
| Dependent Variable | Independent Variables | B | SE B | β | Model R2 |
|---|---|---|---|---|---|
| Depressive Symptoms (BDI-II) | |||||
| Step 1 | .11 | ||||
| Age | -.17 | .10 | -.11 | ||
| Ethnicity | .52 | 1.22 | .03 | ||
| Physical health (PCS) | -.10 | .07 | -.10 | ||
| Social support (MSPSS) | -1.86 | .41 | -.30** | ||
| Step 2 | .12 | ||||
| Age | -.19 | .10 | -.13 | ||
| Ethnicity | .62 | 1.22 | .03 | ||
| Physical health (PCS) | -.11 | .07 | -.11 | ||
| Social support (MSPSS) | -1.86 | .42 | -.30** | ||
| Interaction of PCS and MSPSS | -.07 | .05 | -.09 | ||
| Positive Affect (DASS-D) | |||||
| Step 1 | .17 | ||||
| Age | -.05 | .05 | -.07 | ||
| Ethnicity | .43 | .56 | .05 | ||
| Physical health (PCS) | .02 | .03 | .05 | ||
| Social support (MSPSS) | -1.14 | .19 | -.38** | ||
| Step 2 | .20 | ||||
| Age | -.06 | .04 | -.09 | ||
| Ethnicity | .52 | .55 | .06 | ||
| Physical health (PCS) | .01 | .03 | .02 | ||
| Social support (MSPSS) | -1.14 | .19 | -.38** | ||
| Interaction of PCS and MSPSS | -.06 | .02 | -.17** | ||
| Worry (PSWQ) | |||||
| Step 1 | .04 | ||||
| Age | -..18 | .14 | -.09 | ||
| Ethnicity | .65 | 1.75 | .02 | ||
| Physical health (PCS) | .04 | .10 | .03 | ||
| Social support (MSPSS) | -1.35 | .60 | -.15 | ||
| Step 2 | .06 | ||||
| Age | -.22 | .14 | -.11 | ||
| Ethnicity | .89 | 1.7 | .03 | ||
| Physical health (PCS) | .01 | .10 | .00 | ||
| Social support (MSPSS) | -1.34 | .59 | -.15* | ||
| Interaction of PCS and MSPSS | -.15 | .07 | -.16* | ||
| Somatic Anxiety (DASS-A) | |||||
| Step 1 | .10 | ||||
| Age | -.03 | .04 | -.06 | ||
| Ethnicity | -1.43 | .45 | -.21** | ||
| Physical health (PCS) | -.09 | .03 | -.23** | ||
| Social support (MSPSS) | -.23 | .15 | -.10 | ||
| Step 2 | .10 | ||||
| Age | -.03 | .04 | -.06 | ||
| Ethnicity | -1.44 | .45 | -.21** | ||
| Physical health (PCS) | -.08 | .03 | -.23** | ||
| Social support (MSPSS) | -.23 | .16 | -.10 | ||
| Interaction of PCS and MSPSS | .01 | .02 | .03 | ||
p < .01;
p < .025; PCS and MSPSS were centered.
Table 4.
Friendship and physical health interaction regression models predicting depressive symptoms, positive affect, worry, and somatic anxiety (N = 222).
| Dependent Variable | Independent Variables | B | SE B | β | Model R2 |
|---|---|---|---|---|---|
| Depressive Symptoms (BDI-II) | |||||
| Step 1 | .05 | ||||
| Age | -.22 | .10 | -.15 | ||
| Ethnicity | 1.11 | 1.26 | .06 | ||
| Physical health (PCS) | -.09 | .07 | -.09 | ||
| Friendship Support (MSPSS-FR) | -.86 | .36 | -.16* | ||
| Step 2 | .07 | ||||
| Age | -.24 | .10 | -.17* | ||
| Ethnicity | 1.31 | 1.25 | .07 | ||
| Physical health (PCS) | -.11 | .07 | -.11 | ||
| Friendship Support (MSPSS-FR) | -.86 | .36 | -.16* | ||
| Interaction of PCS and MSPSS-FR | -.09 | .04 | -.14 | ||
| Positive Affect (DASS-D) | |||||
| Step 1 | .08 | ||||
| Age | -.08 | .05 | -.11 | ||
| Ethnicity | .81 | .59 | .09 | ||
| Physical health (PCS) | .02 | .03 | .05 | ||
| Friendship Support (MSPSS-FR) | -.61 | .17 | -.23** | ||
| Step 2 | .12 | ||||
| Age | -.09 | .05 | -.13 | ||
| Ethnicity | .95 | .58 | .11 | ||
| Physical health (PCS) | .01 | .03 | .02 | ||
| Friendship Support (MSPSS-FR) | -.62 | .17 | -.24** | ||
| Interaction of PCS and MSPSS-FR | -.06 | .02 | -.20** | ||
| Worry (PSWQ) | |||||
| Step 1 | .02 | ||||
| Age | -.22 | .14 | -.11 | ||
| Ethnicity | 1.07 | 1.76 | .04 | ||
| Physical health (PCS) | .04 | .10 | .03 | ||
| Friendship Support (MSPSS-FR) | -.59 | .51 | -.08 | ||
| Step 2 | .06 | ||||
| Age | -.26 | .14 | -.13 | ||
| Ethnicity | 1.44 | 1.74 | .06 | ||
| Physical health (PCS) | .01 | .10 | .00 | ||
| Friendship Support (MSPSS-FR) | -.60 | .50 | -.08 | ||
| Interaction of PCS and MSPSS-FR | -.17 | .06 | -.18** | ||
| Somatic Anxiety (DASS-A) | |||||
| Step 1 | .09 | ||||
| Age | -.04 | .04 | -.07 | ||
| Ethnicity | -1.37 | .45 | -.20** | ||
| Physical health (PCS) | -.09 | .03 | -.23** | ||
| Friendship Support (MSPSS-FR) | -.07 | .13 | -.04 | ||
| Step 2 | .09 | ||||
| Age | -.04 | .04 | -.07 | ||
| Ethnicity | -1.36 | .46 | -.19** | ||
| Physical health (PCS) | -.09 | .03 | -.23** | ||
| Friendship Support (MSPSS-FR) | -.07 | .13 | -.04 | ||
| Interaction of PCS and MSPSS-FR | -.00 | .02 | -.01 | ||
p < .01;
p < .025; PCS and MSPSS-FR were centered.
Analyses were first performed without controlling for anxiety or depression because the variability to be accounted for once other symptoms are controlled may be severely limited by high comorbidity in older-adult samples. However, to examine whether significant results occurred due to a high correlation between anxiety and depression, analyses that produced statistically significant results were then repeated, including somatic anxiety (DASS-A) as a covariate when depression (DASS-D or BDI-II) was the DV and positive affect (DASS-D) as a covariate when anxiety (DASS-A or PSWQ) was the DV. The DASS scales were used as control variables because they assess anxiety and depression independently of each other (Lovibond & Lovibond, 1995).
Results
Demographics
The final sample of 222 participants were primarily women (164, 74%). Ethnic distribution was 68% Caucasian (n = 152), 21% African American (n = 47), 7% Hispanic (n = 16), and 3% other (n = 7). Most participants were married (129, 58%), but many were widowed (34, 15%) or divorced (48, 22%). On average, participants were well educated (education level M = 15.8, SD = 2.89) and had a high income level (40% reported an income above $50,000). Most were retired (122, 55%) or not working outside the home (18, 8%), but some were employed full-time (49, 22%) or part-time (32, 14%).
Of the demographic variables, only age and ethnicity were associated with depression or anxiety. Lower age was associated with increased depression (DASS-D: r = -.14, p < .05; BDI-II: r = -.13, p < .05). Ethnic minority status (non-Caucasian) was associated with increased somatic anxiety (DASS-A: t[220] = 3.05, p < .01). Thus, age and ethnicity (with a dichotomous variable indicating whether the participant was Caucasian or non-Caucasian) were included as covariates in all analyses.
Bivariate Relationships (Table 1)
Objective physical health (CDCI) did not correlate with any measure of depression or anxiety (Table 1), and it did not moderate any other relationships. Thus, objective physical health will not be discussed further.
After controlling for age and ethnicity, subjective physical health scores were still significantly correlated with somatic anxiety (DASS-A: pr = -.22, p < .01) such that worse physical health was related to higher somatic anxiety. This correlation remained significant after controlling for positive affect (pr = -.28, p < .01). Subjective physical health was unrelated to either measure of depression (controlling for age and ethnicity: BDI-II: pr = -.08, p = .24; DASS-D: pr = .06, p = .35) or worry (PSWQ: pr = .04, p = .60).
Self-Efficacy and Physical Health Interaction
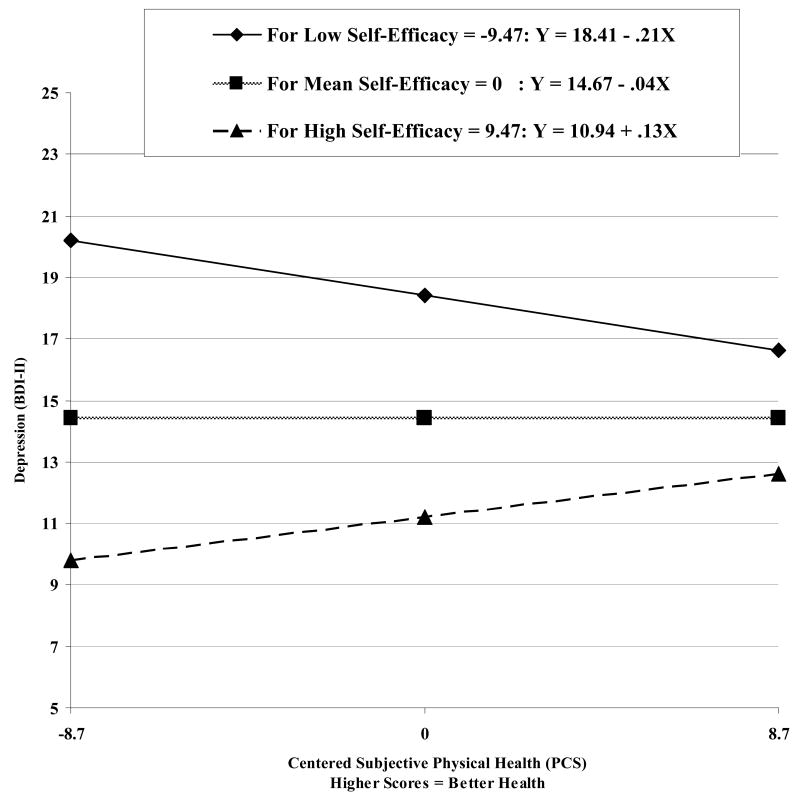
Results of the regression models, before controlling for positive affect or somatic anxiety, are displayed in Table 2. The interaction between subjective physical health and self-efficacy contributed a significant amount of variance to the regression equation predicting depressive symptoms (BDI-II: F[1, 216] = 7.32, p < .01; Figure 3), even after controlling for somatic anxiety (F[1, 215] = 7.83, p < .01). The interaction of subjective physical health and self-efficacy did not significantly predict positive affect (DASS-D: F[1, 216] = 2.17, p = .14) or either dimension of anxiety (PSWQ: F[1, 216] = .10, p = .75; DASS-A: F[1, 216] = .24, p = .63).
Figure 3.
Relation between subjective physical health (PCS) and depressive symptoms (BDI-II) at different levels of self-efficacy.
Social Support and Physical Health Interaction
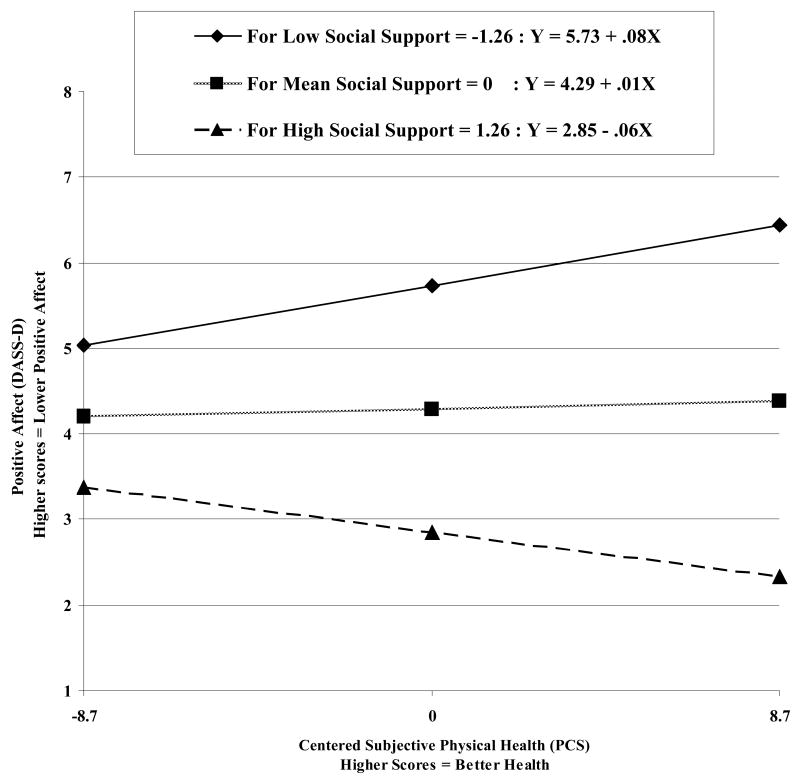
Results of the regression models, before controlling for positive affect or somatic anxiety, are displayed in Table 3. The interaction of social support and subjective physical health significantly predicted positive affect (DASS-D: F[1, 216] = 7.27, p < .01; Figure 4), even after controlling for somatic anxiety (F[1, 215] = 11.63, p < .01). The interaction of social support and subjective physical health also significantly predicted worry (PSWQ: F[1, 216] = 5.41, p = .02), but not after controlling for positive affect (F[1, 215] = 1.33, p = .25). The interaction of social support and subjective physical health did not significantly predict overall depressive symptoms (BDI-II: F[1, 216] = 2.08, p = .15) or somatic anxiety (DASS-A: F[1, 216] = .23, p = .63).
Figure 4.
Relation between subjective physical health (PCS) and positive affect (DASS-D) at different levels of social support.
Friendship and Physical Health Interaction
Results of the regression models, before controlling for family support, positive affect or somatic anxiety, are displayed in Table 4. The interaction of social support from friends and subjective physical health significantly predicted positive affect (DASS-D: F[1, 216] = 9.76, p < .01; Figure 5), even after controlling for family support and somatic anxiety (F[1, 213] = 8.43, p < .01). The interaction of social support from friends and subjective physical health also significantly predicted worry (PSWQ: F[1, 216] = 7.37, p < .01), but not after controlling for family support and somatic anxiety (F[1, 213] = 1.55, p = .21). The interaction between friendship and subjective physical health did not significantly predict depressive symptoms (BDI-II: F[1, 216] = 4.48, p = .04) or somatic anxiety (F[1, 216] = .04, p = .83).
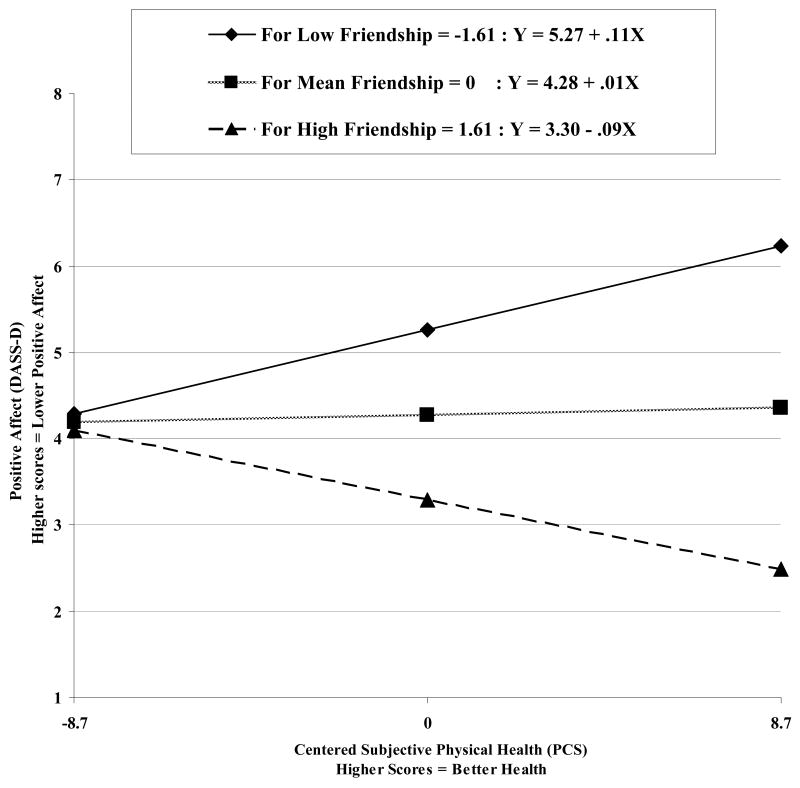
Figure 5.
Relation between subjective physical health (PCS) and positive affect (DASS-D) at different levels of friendship.
Discussion
This study examined the relationship between physical health and several dimensions of depression and anxiety among older adults, including the possible moderating effects of self-efficacy and social support. Although significant results were found for subjective physical health, objective physical health (CDCI) was not associated with anxiety or depression. As hypothesized, self-efficacy was related to decreased depression and anxiety and moderated the relationship between subjective physical health and depressive symptoms (BDI-II). Also as hypothesized, social support was significantly associated with positive affect, depressive symptoms, and worry. Social support also moderated the relationship between physical health and positive affect, but the effect of this moderation was not as expected.
The finding that objective physical health (CDCI) was not associated with anxiety or depression is in concordance with previous findings that the CDCI is not predictive of depression (Bayliss, Ellis, Steiner, & Main, 2005). CDCI scores may not reflect functional impairment from physical illness, and this may hinder their ability to predict depression or anxiety (Lenze et al., 2001). Alternatively, the limited variability of CDCI scores may have restricted the predictive ability of this measure.
As expected, subjective physical health was significantly associated with somatic anxiety, even when positive affect was controlled, which supports the hypothesis that physical health problems have a specific impact on anxiety (Beekman et al., 2000). Poor physical health may be associated with higher somatic anxiety through loss of perceived control (Lenze et al., 2001) or hypervigilance about internal signs and symptoms (Sallis & Lichstein, 1982). Worry was unrelated to subjective physical health. However, these results may have been affected by the high levels of worry in the current sample as participants were recruited for an intervention to decrease worry. In this sample in which most distressed individuals had a primary anxiety diagnosis, physical health problems may have their negative psychological effects primarily through somatic symptoms of anxiety.
Consistent with previous research (Chou & Chi, 2001), self-efficacy was related to decreased depression and anxiety and moderated the relationship between subjective physical health and depressive symptoms (BDI-II). As expected, when self-efficacy was low, worse physical health was related to increased depressive symptoms (see Figure 3). Surprisingly, among those with higher self-efficacy, as physical health worsened, depressive symptoms improved. One possible explanation is that those with high self-efficacy use active coping mechanisms in response to problems (Cassidy & Burnside, 1996) and, as a result of active coping and positive mentality, mood may actually be higher in the presence of stressors (Ben-Zur, 2002). Second, a positive cognitive response to stressors may correspond to reduced reporting of depressive symptoms.
Social support was significantly associated with positive affect, depressive symptoms, and worry, but not with somatic anxiety. This is consistent with the literature indicating a strong relationship between decreased social support and depression among older adults (Blazer, 2003) and a lack of extensive evidence for this same relationship between social support and anxiety (with a few notable exceptions, Beekman et al., 2000; Majercsik & Haller, 2004). Social support also moderated the relationship between physical health and positive affect, but the effect of this moderation was not as expected (Figure 4). When social support was high, better physical health was related to increased positive affect (indicating decreased depressive mood). When social support was low, better physical health was related to decreased positive affect (indicating increased depressive mood). Although social support moderated the relationship between physical health and worry, the interaction was not significant after controlling for positive affect. Thus, it will not be considered further.
Friendship was associated with positive affect and depressive symptoms but was unrelated to anxiety in bivariate analyses. Again, although friendship moderated the relationship between subjective physical health and worry, the interaction was not significant after controlling for family support and positive affect. Friendship consistently moderated the relationship between physical health and positive affect in the same manner that overall social support moderated the relationship (Figure 5). These findings contradict previous research indicating that friendship and social support are especially important in preventing depression among physically ill individuals (Parashos et al., 2002) as it was among individuals with high social support that worsening physical health seemed to have the most harmful effect on depressive mood and among those with low social support, worsening physical health actually seemed to be associated with improved mood. However, research typically focuses on individuals with severe chronic illnesses (Oxman et al., 1994). In a relatively healthy older-adult sample such as the participants in the present study, the absence of social support, particularly friends, may be more salient and detrimental to psychological functioning when physical health is good. In addition, among those with low social support, physical illness may temporarily increase others' attentiveness to them and thus increase positive affect (Grant, Patterson, & Yager, 1988), but it may not have this same moderating effect on all depressive symptoms.
This study has several limitations. First, the sample was disproportionately female and well-educated and had better overall objective physical health than the general older-adult population (Schneeweiss et al., 2004). Mean PCS scores were between the ranges for patients with minor medical problems and severe physical conditions (Ware et al., 1996). Second, recruitment of participants who presented for help with worry might have limited both generalizability and interpretation of the results, even though depressive symptoms were common in the sample. Third, several measures in this study may have limited the ability to test the proposed hypotheses. The CDCI may not adequately represent the severity of physical health problems for the prediction of psychological distress. Although the depression scales both displayed adequate internal consistency, the internal consistency of the scale used to measure somatic anxiety was lower. All scales except the CDCI were self-report. Lastly, the cross-sectional nature of the data precludes conclusions of directionality.
Despite these limitations, this study makes a significant contribution to the knowledge base about the relationship between physical and mental health among older adults. It tested predictions using both objective and subjective measures of physical health. It also examined psychological distress with two dimensions of anxiety (somatic anxiety and worry) and depression (low positive affect and depressive symptoms). Although anxiety is very common among older adults, there is a dearth of research on anxiety in the elderly population (Lenze et al., 2001). This study provides evidence that subjective physical health problems are associated with somatic anxiety independent of depression. It is unclear whether this association is caused by heightened somatic anxiety in physically ill individuals or because the assessment instrument (DASS-A) was labeling physical health symptoms as somatic anxiety. Most likely, this association was caused by a mixture of the two explanations. Thus, it is recommended that anxiety be assessed both among physically healthy and ill older adults, keeping in mind that older adults are more likely to report somatic anxiety than worry (Bryant, Jackson, & Ames, 2008). As self-efficacy and social support were consistently correlated with depression, low levels of either of these constructs indicate that further assessment and possibly intervention for depression should be pursued. Cognitive-Behavioral or Interpersonal Therapies for treatment of depression have the strongest evidence base among older adults and have particular strengths addressing these constructs (American Psychiatric Association, 2000).
Results of this study also suggest several avenues for future research. First, null results for most analyses performed using the CDCI highlight the necessity of a more sensitive objective index that can be used in psychological research to determine the severity and functional impact of physical disease. Second, other potential moderators of the impact of physical illness on psychological functioning should be explored, as this type of research may help identify at-risk older adults and determine potential avenues for intervention with older adults experiencing physical health stressors. Third, research using both healthy and severely physically ill samples might assist in understanding how social support and, in particular, friendship, moderate the relationship between physical health and depression. Lastly, longitudinal data are required to explore the directionality of relationships. Cross-sectional studies such as this can begin to illuminate the processes underlying the development of anxiety and depression among older adults and lay important groundwork for more resource-consuming longitudinal studies.
Acknowledgments
While conducting this research, Amber Paukert received support as a graduate student at the University of Houston, a psychology intern at Baylor College of Medicine, and a postdoctoral research fellow (TPP 61-000) for the Department of Veterans Affairs, Veterans Health administration, Health Services Research and Development Service (HSR&D) in Seattle, WA. This work was also supported in part by the Houston VA HSR&D Center of Excellence (HFP90-020), the Veterans Affairs South Central Mental Illness Research, Education and Clinical Center, and a grant from the National Institute of Mental Health (NIMH) (R01-MH53932) for $1,826,539 from 2003-2008 to the last author.
Footnotes
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
References
- Alloy LB, Kelly KA, Mineka S, Clements CM, Maser JD, Cloninger CR. Comorbidity of anxiety and depressive disorders: A helplessness-hopelessness perspective. In: Maser JD, Cloninger CR, editors. Comorbidity of Mood and Anxiety Disorders. Washington DC: American Psychiatric Association; 1990. pp. 499–543. [Google Scholar]
- American Psychiatric Association Practice guidelines for the treatment of patients with major depressive disorder (revision) American Journal of Psychiatry. 2000;157:1–49. [PubMed] [Google Scholar]
- Antony MM, Bieling PJ, Cox BJ, Enns MW, Swinson RP. Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychological Assessment. 1998;10:176–181. [Google Scholar]
- Baker FM. An overview of depression in the elderly: A US perspective. Journal of the National Medical Association. 1996;88:178–184. [PMC free article] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- Bayliss EA, Ellis JL, Steiner JF, Main DS. Initial validation of an instrument to identify barriers to self-management for persons with co-morbidities. Chronic Illness. 2005;1:315–320. doi: 10.1177/17423953050010040101. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Beekman ATF, de Beurs E, van Balkom AJLM, Deeg DJH, van Dyck R, van Tilburg W. Anxiety and depression in later life: Co-occurrence and communality of risk factors. American Journal of Psychiatry. 2000;157:89–95. doi: 10.1176/ajp.157.1.89. [DOI] [PubMed] [Google Scholar]
- Ben-Zur H. Monitoring/blunting and social support: Associations with coping and affect. International Journal of Stress Management. 2002;9:357–373. [Google Scholar]
- Blazer DG. Self-efficacy and depression in late life: A primary prevention proposal. Aging & Mental Health. 2002;6:315–324. doi: 10.1080/1360786021000006938. [DOI] [PubMed] [Google Scholar]
- Blazer DG. Depression in late life: Review and commentary. Journals of Gerontology: Series A: Biological Sciences and Medical Sciences. 2003:249–265. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- Bosma H, Sanderman R, Scaf-Klomp W, Van Eijk JTM, Ormel J, Kempen GIJM. Demographic, health-related and psychosocial predictors of changes in depressive symptoms and anxiety in late middle-aged and older persons with fall-related injuries. Psychology & Health. 2004;19:103–115. [Google Scholar]
- Bourland SL, Stanley MA, Snyder AG, Novy DM, Beck JG, Averill PM, Swann AC. Quality of life in older adults with generalized anxiety disorder. Aging & Mental Health. 2000;4:315–323. [Google Scholar]
- Brown TA, Chorpita BF, Korotitsch W, Barlow DH. Psychometric properties of the Depression Anxiety Stress Scales (DASS) in clinical samples. Behaviour Research and Therapy. 1997;35:79–89. doi: 10.1016/s0005-7967(96)00068-x. [DOI] [PubMed] [Google Scholar]
- Bryant C, Jackson H, Ames D. The prevalence of anxiety in older adults: Methodological issues and a review of the literature. Journal of Affective Disorders. 2008;109:233–250. doi: 10.1016/j.jad.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Cassidy T, Burnside E. Cognitive appraisal, vulnerability and coping: An integrative analysis of appraisal and coping mechanisms. Counselling Psychology Quarterly. 1996;9:261–279. [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal Of Chronic Diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Chou KL, Chi I. Social comparison in Chinese older adults. Aging & Mental Health. 2001;5:242–252. doi: 10.1080/13607860120065032. [DOI] [PubMed] [Google Scholar]
- Davis-Berman J. Physical self-efficacy, perceived physical status, and depressive symptomatology in older adults. Journal of Psychology: Interdisciplinary and Applied. 1990;124:207–215. doi: 10.1080/00223980.1990.10543217. [DOI] [PubMed] [Google Scholar]
- De Beurs E, Beekman ATF, Deeg DJH, Dyck RV, Tilburg WV. Predictors of change in anxiety symptoms of older persons: Results from the Longitudinal Aging Study Amsterdam. Psychological Medicine. 2000;30:515–527. doi: 10.1017/s0033291799001956. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal Of Clinical Epidemiology. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- Diagnosis and treatment of depression in late life. NIH Consensus Statement. 1991;9:1–27. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friedman MM. Social support sources and psychological well-being in older women with heart disease. Research in Nursing & Health. 1993;16:405–413. doi: 10.1002/nur.4770160604. [DOI] [PubMed] [Google Scholar]
- Grant I, Patterson TL, Yager J. Social supports in relation to physical health and symptoms of depression in the elderly. American Journal of Psychiatry. 1988;145:1254–1258. doi: 10.1176/ajp.145.10.1254. [DOI] [PubMed] [Google Scholar]
- Horowitz A, Reinhardt JP, Boerner K, Travis LA. The influence of health, social support quality and rehabilitation on depression among disabled elders. Aging & Mental Health. 2003;7:342–350. doi: 10.1080/1360786031000150739. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM. Correlates of well-being in adulthood and old age: A tale of two optimisms. Journal of Research in Personality. 2005;39:224–244. [Google Scholar]
- Jang Y, Haley WE, Small BJ, Mortimer JA. The role of mastery and social resources in the associations between disability and depression in later life. The Gerontologist. 2002;42:807–813. doi: 10.1093/geront/42.6.807. [DOI] [PubMed] [Google Scholar]
- Katona CLE. Psychiatry of old age: Depression in old age. Reviews in Clinical Gerontology. 1991;1:371–384. [Google Scholar]
- Kim HFS, Braun U, Kunik ME. Anxiety and depression in medically ill older adults. Journal of Clinical Geropsychology. 2001;7:117–130. [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. New York: Springer Publishing Company; 1984. [Google Scholar]
- Lenze EJ, Rogers JC, Martire LM, Mulsant BH, Rollman BL, Dew MA, Schulz R, Reynolds CF. The association of late-life depression and anxiety with physical disability: A review of the literature and prospectus for future research. American Journal of Geriatric Psychiatry. 2001;9:113–135. [PubMed] [Google Scholar]
- Lewinsohn PM, Hoberman H, Teri L, Hautzinger M. An integrative theory of depression. In: Reiss S, Bootzin RR, editors. Theoretical Issues in Behavior Therapy. San Diego, CA: Academic Press; 1985. pp. 331–359. [Google Scholar]
- Lindesay J. The Guy's/Age Concern Survey: Physical health and psychiatric disorder in an urban elderly community. International Journal of Geriatric Psychiatry. 1990;5:171–178. [Google Scholar]
- Lovibond PF, Lovibond SH. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behaviour Research and Therapy. 1995;33:335–343. doi: 10.1016/0005-7967(94)00075-u. [DOI] [PubMed] [Google Scholar]
- Majercsik E, Haller J. Interactions between anxiety, social support, health status and buspirone efficacy in elderly patients. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2004;28:1161–1169. doi: 10.1016/j.pnpbp.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Mollenkopf H, Marcellini F, Ruoppila I, Flaschenträger P, Gagliardi C, Spazzafumo L. Outdoor mobility and social relationships of elderly people. Archives of Gerontology and Geriatrics. 1997;24:295–310. doi: 10.1016/s0167-4943(97)00781-4. [DOI] [PubMed] [Google Scholar]
- Oxman TE, Freeman DH, Manheimer ED, Stukel T. Social support and depression after cardiac surgery in elderly patients. American Journal of Geriatric Psychiatry. 1994;2:309–323. doi: 10.1097/00019442-199402040-00006. [DOI] [PubMed] [Google Scholar]
- Parashos IA, Stamouli S, Rogakou E, Theodotou R, Nikas I, Mougias A. Recognition of depressive symptoms in the elderly: What can help the patient and the doctor. Depression and Anxiety. 2002;15:111–116. doi: 10.1002/da.10013. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Lichstein KL. Analysis and management of geriatric anxiety. International Journal of Aging & Human Development. 1982;15:197–211. doi: 10.2190/57pt-bcem-hg6q-2mh1. [DOI] [PubMed] [Google Scholar]
- Schneeweiss S, Wang PS, Avorn J, Maclure M, Levin R, Glynn RJ. Consistency of performance ranking of comorbidity adjustment scores in Canadian and U.S. utilization data. Journal Of General Internal Medicine: Official Journal of the Society for Research and Education in Primary Care Internal Medicine. 2004;19:444–450. doi: 10.1111/j.1525-1497.2004.30109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior AC, Kunik ME, Rhoades HM, Novy DM, Wilson NL, Stanley MA. Utility of telephone assessments in an older adult population. Psychology and Aging. 2007;22:392–397. doi: 10.1037/0882-7974.22.2.392. [DOI] [PubMed] [Google Scholar]
- Sherer M. The Self-efficacy Scale: Construction and validation. Psychological Reports. 1982;51:663–671. [Google Scholar]
- Sherman AM, Shumaker SA, Rejeski WJ, Morgan T, Applegate WB, Ettinger W. Social support, social integration, and health-related quality of life over time: Results from the Fitness and Arthritis in Seniors Trial (FAST) Psychology & Health. 2006;21:463–480. [Google Scholar]
- Smalbrugge M, Pot AM, Jongenelis K, Beekman ATF, Eefsting JA. Prevalence and correlates of anxiety among nursing home patients. Journal of Affective Disorders. 2005;88:145–153. doi: 10.1016/j.jad.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Southern DA, Quan H, Ghali WA. Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Medical Care. 2004;42:355–360. doi: 10.1097/01.mlr.0000118861.56848.ee. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III--R (SCID): I. History, rationale, and description. Archives of General Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Stanley MA, Novy DM, Bourland SL, Beck JG, Averill PM. Assessing older adults with generalized anxiety: A replication and extension. Behaviour Research and Therapy. 2001;39:221–235. doi: 10.1016/s0005-7967(00)00030-9. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 4th. Boston, MA: Allyn and Bacon; 2001. p. 151. [Google Scholar]
- Takaki J, Nishi T, Shimoyama H, Inada T, Matsuyama N, Kumano H, Kuboki T. Interactions among a stressor, self-efficacy, coping with stress, depression, and anxiety in maintenance hemodialysis patients. Behavioral Medicine. 2003;29:107–112. doi: 10.1080/08964280309596063. [DOI] [PubMed] [Google Scholar]
- Wallace J, O'Hara MW. Increases in depressive symptomatology in the rural elderly: Results from a cross-sectional and longitudinal study. Journal of Abnormal Psychology. 1992;101:398–404. doi: 10.1037//0021-843x.101.3.398. [DOI] [PubMed] [Google Scholar]
- Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Welch DC, West RL. Self-efficacy and mastery: Its application to issues of environmental control, cognition, and aging. Developmental Review. 1995;15:150–171. [Google Scholar]
- Williamson GM, Shaffer DR, Parmelee PA, Williamson GM, Shaffer DR, Parmelee PA. Physical illness and depression in older adults: A handbook of theory, research, and practice. Dordrecht Netherlands: Kluwer Academic Publishers; 2000. [Google Scholar]
- Yochim BP, Kerkar SP, Lichtenberg PA. Cerebrovascular risk factors, activity limitations, and depressed mood in African American older adults. Psychology and Aging. 2006;21:186–189. doi: 10.1037/0882-7974.21.1.186. [DOI] [PubMed] [Google Scholar]
- Zimet GD, Dahlem NW, Zimet SG, Farley GK. The Multidimensional Scale of Perceived Social Support. Journal of Personality Assessment. 1988;52:30–41. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]