Abstract
Objective
To determine if oral administration of a cyclooxygenase-2 (COX2) inhibitor affects oocyte nuclear maturation and fertilization in non-human primates.
Design
Laboratory research study.
Setting
Medical school.
Animals
Adult female cynomolgus monkeys (Macaca fascicularis).
Interventions
Monkeys received gonadotropins to stimulate multiple follicular development. An ovulatory dose of human chorionic gonadotropin (hCG) was administered either alone or concomitant with oral celecoxib, a COX2 inhibitor; oocytes were retrieved 36 hours later and exposed to sperm in vitro.
Main Outcome Measures
Oocytes were assessed for nuclear status at retrieval, resumption of meiosis in vitro, and success of in vitro fertilization.
Results
Treatment with hCG alone yielded oocytes which were primarily at the meiosis II (MII) stage of nuclear maturation (72.9%); few oocytes were obtained at the germinal vesicle (GV) and germinal vesicle break down (GVBD) stages. Treatment with hCG and celecoxib yielded fewer mature (MII) oocytes (35.6%) and more oocytes at less mature stages when compared to oocytes from monkeys treated with hCG alone. The majority (68.3±15.9%) of MII oocytes from monkeys treated with hCG alone fertilized in vitro, compared with only 11.0±5.9% of MII oocytes from monkeys treated with hCG and celecoxib.
Conclusions
Oral administration of a COX2 inhibitor reduced the rate of oocyte nuclear maturation and the success of in vitro fertilization. Drugs of this class may block multiple essential steps in female reproduction and be effective contraceptives for women.
Keywords: Monkey, Prostaglandin, Contraception, Ovary
INTRODUCTION
Cyclooxygenase-2 (COX2) expressed by the ovarian follicle is necessary for successful ovulation. Studies in several mammalian species confirm that the ovulatory surge of luteinizing hormone (LH) induces COX2 expression by granulosa cells of the periovulatory follicle (1–3). Essential ovarian processes, including cumulus expansion and follicle rupture, require prostaglandin E2 (PGE2) produced via COX2 activity (4; 5). However, other eicosanoids produced via COX2 may also be required for normal fertility. Mice lacking COX2 expression are sub-fertile, with defects in oocyte release, oocyte nuclear maturation, and fertilization (6; 7), suggesting a link between COX2 activity and these essential reproductive processes.
Inhibitors of COX2 can prevent ovulation. Administration of either general COX inhibitors or inhibitors selective for COX2 reduce ovulation rates in rodents, domestic animals, and monkeys (4; 8–10). Perhaps more interesting is the observation that COX inhibitors can cause reversible infertility in women (11). In prospective experiments, oral administration of COX2 inhibitors to women around the time of the midcycle LH surge prevented or delayed follicular collapse as assessed by ultrasound, indicating failure of follicle rupture (12; 13). Oral administration of the COX2 inhibitor meloxicam caused failure of oocyte release in monkeys, with trapped oocytes found within the majority of follicles several days after the ovulatory LH surge (14).
The studies presented here use the monkey as a model for human reproductive processes to determine if COX2 inhibitors reduce rates of oocyte nuclear maturation and fertilization in addition to preventing oocyte release. If so, then COX2 inhibitors may be effective contraceptives for women.
MATERIALS AND METHODS
Animals
Oocytes were obtained from adult female cynomolgus macaques (Macaca fascicularis) at Eastern Virginia Medical School (EVMS). All animal protocols were approved by the EVMS Animal Care and Use Committee and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Adult females 4–8 years of age with regular menstrual cycles were checked daily for menstruation; the first day of menstruation was designated day 1 of the menstrual cycle (15). Blood samples were obtained under ketamine chemical restraint (10 mg/kg body weight) by femoral or saphenous venipuncture, and serum was stored at −20C.
Controlled Ovulation Stimulation
A controlled ovarian stimulation model developed for the collection of multiple oocytes was used as previously described (15). Recombinant human FSH (r-hFSH, 90 IU/day, Schering-Plough Corp., now Merck & Co., Inc., Whitehouse Station, NJ) was administered for 6–8 days, followed by administration of r-hFSH plus r-hLH (Serono, 60 IU/day) for 2–3 days to stimulate the growth of multiple preovulatory follicles. A GnRH antagonist (Ganirelix (30 µg/kg body weight; Schering-Plough) or Antide (0.5 mg/kg body weight; Serono)) was also administered daily to prevent an endogenous ovulatory LH surge. Adequate follicular development was monitored by serum estradiol levels and ultrasonography (16). In some animals, follicular aspiration was performed 36 hours after administration of 1000 IU r-hCG (Serono). Additional animals were treated with hCG and the COX2 inhibitor celecoxib (Celebrex; Pfizer, New York, NY; 32 mg orally every 12 hours) for 36 hours prior to follicle aspiration (17). At aseptic surgery, each follicle was pierced with a 22-gauge needle, and the aspirated contents of all follicles larger than 4 mm in diameter were pooled. In some experiments, only one ovary was aspirated. For data in Figure 1, both ovaries were aspirated in 83% of monkeys treated with hCG only and 33% of hCG+celecoxib treated monkeys. For data in Figure 2, both ovaries were aspirated in 33% of monkeys treated with hCG only and 50% of hCG+celecoxib treated monkeys. Recovery ranged from 3–37 oocytes/monkey.
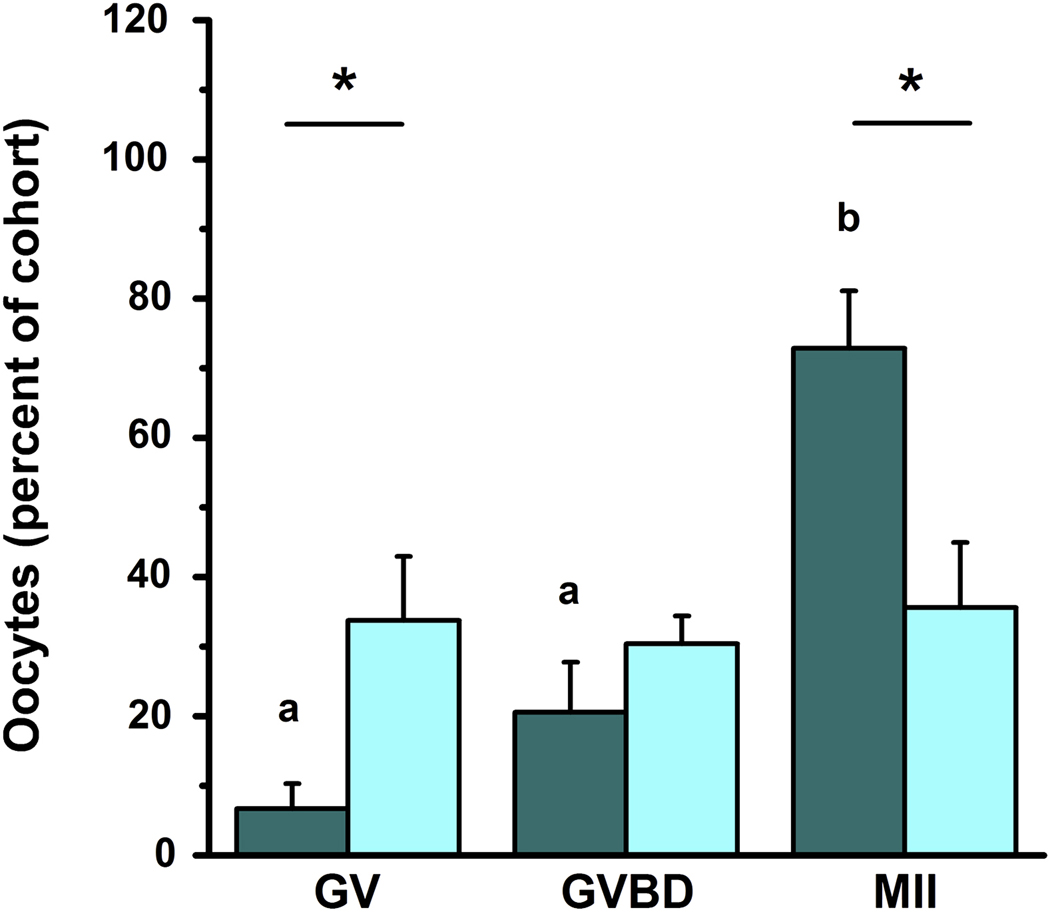
Figure 1.
Celecoxib treatment reduces oocyte nuclear maturation. Oocytes were obtained from monkeys experiencing controlled ovarian stimulation after treatment for 36 hours with either hCG alone (dark bars; n=12 monkeys) or hCG and the COX2 inhibitor celecoxib (light bars; n=6 monkeys). Oocytes were assessed for nuclear status (germinal vesicle intact (GV), germinal vesicle break down (GVBD), or presence of a single polar body (MII)) by light microscopy. Data are expressed as the percent of total cohort of oocytes which are at the indicated stage. Within each class of oocytes, differences between treatment groups are indicated with an asterisk (*) as determined by unpaired t-test, p<0.05. For oocytes from animals receiving hCG alone, b>a as determined by ANOVA and Newman-Keuls’ test, p<0.05.
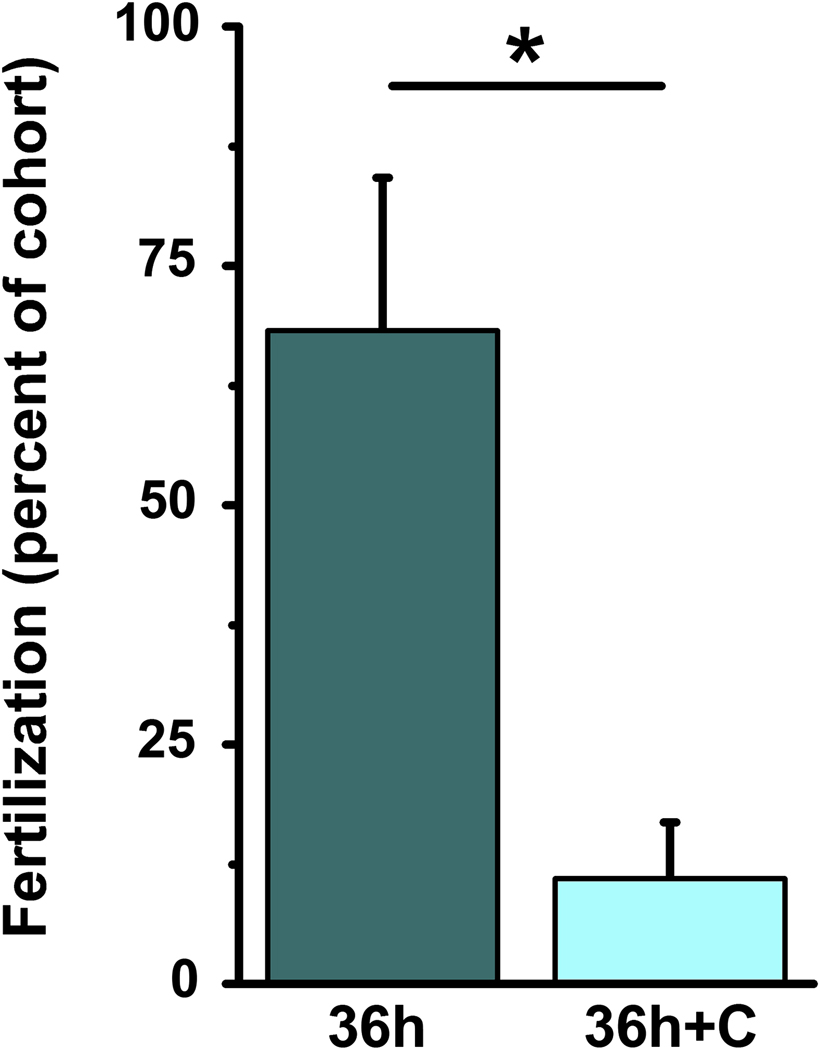
Figure 2.
Celecoxib treatment reduces in vitro fertilization. Oocytes were obtained from monkeys experiencing controlled ovarian stimulation after treatment for 36 hours with either hCG only (36h; dark bar; n=3 monkeys) or hCG and the COX2 inhibitor celecoxib (36h+C; light bar; n=4 monkeys). Oocytes at the MII stage of nuclear maturation at the time of insemination were assessed for fertilization as determined by presence of a second polar body. Data are expressed as percent of total cohort of oocytes. Difference between treatment groups is indicated with an asterisk (*) as determined by unpaired t-test, p<0.05.
In Vitro Maturation
Aspirates were subjected to centrifugation, and the pelleted cells were resuspended in TALP media containing HEPES (16). Cells were maintained at 37C while oocytes were mechanically removed. Granulosa cells were removed with Stripper tips (MidAtlantic Diagnostics, Mount Laurel, NJ). Oocytes were placed in 100 µl drops of TALP medium under oil and assessed for nuclear maturation and oocyte quality within 3 hours of follicle aspiration. Nuclear maturation was assessed as germinal vesicle intact (GV), germinal vesicle break down (GVBD), or having produced a single polar body and progressed to metaphase of the second meiotic cell division (MII) as previously described (18). Oocytes were placed in a humidified incubator (37C, 5% O2, 95% CO2). In vitro fertilization was performed within 2 hours of initial assessment of nuclear status; nuclear status was assessed again at the time of sperm addition.
Sperm Collection
Adult male cynomolgus monkeys were housed at the California National Primate Research Center (CNPRC) as described previously (19). All animal procedures were approved by the Institutional Animal Use and Care Administrative Advisory Committee at the University of California at Davis. Experiments were conducted in accordance with the NIH Guide for Care and Use of Laboratory Animals. Two adult males were trained to chair restraint, and semen was collected by direct penile stimulation with a Grass 6 stimulator (Grass Medical Instruments, Quincy, Massachusetts) equipped with electrocardiogram pad electrodes (30–50 V, 20 ms duration, 18 pulses s−1) (20). Samples were allowed to liquefy for 30 minutes before processing.
The sperm cryopreservation method used was identical to that previously published for rhesus monkey sperm (21). In brief, a Styrofoam box was filled with a depth of 4 cm liquid nitrogen, and a 0.4–1 cm thick Styrofoam "boat" was floated on top for 10 minutes. Straws were then placed on the "boat" for 10 minutes before being plunged into liquid nitrogen. The extender used was TEST-20% yolk–3% glycerol. The average cooling rate from −10°C to −70°C was about 220°C min−1.
In Vitro Fertilization
Semen from cynomolgus macaques was thawed, washed, and activated as previously described (22; 23). Motility was 32±5%; acquisition of hypermotility was near 100% of motile sperm. Hypermotile sperm (10,000 per oocyte) were added to each drop of media. About 18 hours later, oocytes were moved to fresh drops of TALP media and were observed for nuclear status and fertilization twice each day for the next 2 days. Fertilization was assessed by observation of a second polar body; presence of male and female pronuclei were not recorded.
Data Analysis
The number of oocytes at a specific stage of nuclear maturation or fertilization was expressed as a percentage of the total cohort obtained from each individual animal. Comparisons between treatment groups were performed using an unpaired 2-tailed student’s t-test; comparisons within each treatment group were performed using analysis of variance (ANOVA) with one repeated measure, followed by post hoc analysis with Newman-Keuls’ test when appropriate. These statistical analyses were performed using StatPak version 4.12 software (Northwest Analytical, Portland, OR). The numbers of healthy and poor quality oocytes were compared using a chi-squared test (24). Post-hoc power analyses determined that data in Figure 1 (GV, GVBD, and MII stage oocytes) and Figure 2 (fertilization) were powered at levels of 90%, 10%, 85%, and 99%, respectively, assuming a 2-tailed unpaired test with α=0.05 (StatMate, Graphpad, La Jolla, CA). Data are presented as mean + standard error of the mean (SEM). For all experiments, significance was assumed at p<0.05.
RESULTS
Oocytes of large preovulatory follicles are typically at a germinal vesicle (GV) intact stage of nuclear maturation; oocytes resume meiosis in response to an ovulatory dose of gonadotropin, experience GV breakdown (GVBD), arrest at the MII stage of maturation, and await fertilization. Oral administration of the COX2 inhibitor celecoxib reduced the percentage of oocytes that resumed meiosis in vivo (Figure 1). In the absence of celecoxib treatment, the majority of oocytes obtained 36 hours after hCG administration were at the MII stage of nuclear maturation (72.9%), while lower percentages of oocytes were at the GVBD (20.6%) and GV (6.7%) intact stages. Administration of celecoxib with hCG for 36 hours before follicle aspiration yielded oocytes distributed among the MII (35.6%), GVBD (30.4%), and GV (33.8%) stages; there were no significant differences between the percentage of oocytes obtained at each of these three stages of nuclear maturation. Administration of hCG and celecoxib significantly increased the percentage of oocytes recovered at the GV stage and reduced the percentage of oocytes obtained at the MII stage when compared with oocytes obtained after treatment with hCG only.
Oral celecoxib administration did not alter the number of oocytes retrieved or oocyte quality. Similar numbers of oocytes were obtained from animals treated with hCG (5.4 oocytes per ovary) and animals receiving hCG and celecoxib (6.1 oocytes per ovary). Oocytes with dark or grainy cytoplasm, one large or multiple cytoplasmic vacuoles, or a large perivitelline space were classified as poor quality. Percentages of poor quality oocytes recovered from animals treated with hCG alone (7%) and hCG with celecoxib (20%) did not differ significantly.
Oocytes were monitored for continued nuclear maturation in vitro. In these experiments, 63% of oocytes from monkeys treated with hCG alone and obtained at the GVBD stage matured to the MII stage within 18 hours in vitro, similar to a previous report (25). In contrast, none of the very few oocytes recovered at the GV intact stage progressed to more mature stages in vitro. Among oocytes from monkeys treated with hCG and celecoxib, 47% of those recovered at the GVBD stage matured to the MII stage during the subsequent 18 hours in vitro. Of oocytes from monkeys treated with hCG and celecoxib, 53% of those recovered at the GV-intact stage matured to GVBD within 48 hours in vitro; however, none of these oocytes reached the MII stage in vitro.
Oocytes obtained from monkeys treated with hCG alone or hCG and celecoxib were exposed to monkey sperm in vitro and assessed for presence of a second polar body as an indicator of fertilization. 56% of oocytes obtained from monkeys treated with hCG alone fertilized in vitro. Oocytes recovered from these animals at GVBD and MII stages fertilized with similar efficiency (50% vs. 58%), while oocytes recovered at the GV stage did not mature to MII and so could not fertilize. Oocytes from monkeys treated with hCG and celecoxib rarely fertilized successfully. Very low rates of fertilization were observed for oocytes obtained at the GV (0%), GVBD (6%), and MII (15%) stages of nuclear maturation at follicle aspiration. Of the oocytes which reached the MII stage at the time of insemination, the fertilization rate was higher in oocytes from monkeys treated with hCG alone when compared with oocytes from monkeys treated with hCG and celecoxib (Figure 2).
DISCUSSION
This report is the first to demonstrate that inhibition of COX2 may reduce fertility by affecting oocyte maturation and fertilization. Exposure to the COX2 inhibitor celecoxib, the resulting altered intrafollicular milieu (15; 26), and the downstream effects of COX2 inhibition on the oocyte limited resumption of meiosis and decreased progression to the MII stage. More interestingly, reduced COX2 activity led to alterations of the oocyte prior to the anticipated time of oocyte release which subsequently compromised fertilization. These findings suggest that products of COX2 directly or indirectly influence the ability of oocytes to complete nuclear maturation and prepare for fertilization. COX2 inhibitors block oocyte release at ovulation in monkeys, presumably due to failure of both cumulus expansion and rupture of the follicle wall (14). Therefore, COX2 inhibitors may prevent production of an embryo by acting at multiple essential prefertilization steps in female reproduction.
While cyclooxygenases can catalyze the rate-limiting step in the production of many eicosanoids, COX2 is thought to produce primarily thromboxanes and prostaglandins (PGs). Mice lacking expression of COX2 demonstrate severe fertility deficits (6; 7), so studies focused on identification of receptors for the specific COX2 products essential for reproduction. Mice lacking expression of the PGE receptors EP1 (27), EP3 (28), and EP4 (29) as well as receptors for thromboxane A2 (30) and prostacyclin (31) have no reproductive deficits. In contrast, mice lacking expression of EP2 have reduced fertility (5; 32). Oocytes of mice lacking expression of the PGF2α (FP) receptor ovulate and fertilize normally, but overall fertility is compromised by defects during implantation (33). In vivo treatment of monkeys with the COX2 inhibitor celecoxib reduced monkey follicular fluid levels of PGE2 and PGF2α (15; 26); follicular production of other eicosanoids are likely reduced as well (34). While PGE2 action is clearly responsible for follicle rupture and cumulus expansion in rodents, domestic animals, and monkeys (4; 8; 10), the specific COX2 product responsible for alterations in oocyte maturation remains to be identified.
The present study is the first to demonstrate that in vivo administration of a COX2 inhibitor reduces the percentage of oocytes which undergo nuclear maturation in response to an ovulatory dose of gonadotropin. In a previous study, some oocytes which failed to be released from periovulatory follicles after oral administration of the COX2 selective inhibitor meloxicam appeared to be arrested at the GV stage (14). Administration of high doses of the COX inhibitor indomethacin to mice at the time of hCG administration also delayed oocyte nuclear maturation (35; 36). Administration of lower doses of indomethacin yielded primarily MII oocytes, even when follicle rupture failed (37), suggesting that follicle rupture is more sensitive to inhibition of COX2 than is oocyte maturation. Although MII stage oocytes were rarely recovered from mice lacking COX2 expression after administration of an ovulatory dose of gonadotropin in vivo, oocytes from COX2 null mice were capable of resuming meiosis in vitro (6; 38). PGE2 injections nearly restored resumption of meiosis in COX2 knockout mice (7), suggesting that PGE2 is a key COX2 derived product involved in mediating the ovulatory gonadotropin signal to the mouse oocyte. While the specific COX2 product needed for proper oocyte maturation and the cell type(s) upon which this COX2 product acts remain to be identified, COX2 activity is likely required for normal resumption and/or progression of meiosis when the oocyte is in the context of a periovulatory follicle.
Exposure to the COX2 inhibitor celecoxib during the periovulatory interval reduced fertilization rates of monkey oocytes that achieved the MII stage of nuclear maturation, suggesting that COX2 activity is required for proper maturation of the oocyte in advance of fertilization. Studies in rodents provide a complex view of the impact of COX inhibition on subsequent fertilization. Oocytes obtained from mice treated in vivo with the COX inhibitor indomethacin fertilized at the same rate as oocytes from vehicle-treated animals, suggesting little or no impact of COX2 products on oocyte preparation for fertilization (37). However, oocytes from COX2 knockout mice have reduced rates of in vitro fertilization and in vitro development to blastocyst. In addition, injection of COX2 knockout mice with PGE2 restored the percentage of oocytes which fertilized and went on to form blastocysts (7). EP2 knockout mice have pronounced defects in fertilization in vivo, but oocytes fertilize at a normal rate in vitro, suggesting that the defect may be secondary to PGE2-dependent cumulus cell function (5). It is likely that COX2 derived PGE2 is required for normal fertilization in vivo, additional COX2 products may also be involved in preparation of the oocyte for fertilization.
This report presents data from non-human primates suggesting that COX2 inhibitors adversely affect oocyte maturation and preparation of the oocyte for fertilization. COX products are required for oocyte release in non-human primates (4; 14) and may also be required for additional reproductive processes leading to implantation. Earlier studies concluded that fertilization was less efficient in vivo and in vitro in the presence of general COX inhibitors and specifically implicate prostaglandins of the E series (PGE1 and PGE2) in the process of sperm-oocyte interaction (37; 39; 40). PGI2 produced within the oviduct also appears to promote early embryo development and improve implantation rates in mice (41). The role of COX2-derived eicosanoids in fertilization and early embryo development in primates, including monkeys and women, is currently unknown. However, the ability of COX2 inhibitors to reduce rates of oocyte release, oocyte nuclear maturation, and fertilization while maintaining normal serum ovarian steroid hormone levels suggests that drugs of this class may be effective contraceptives for women.
Acknowledgements
The Authors would like to thank Kim Hester for her role in monkey training and handling. Recombinant human FSH and Ganirelix were generously provided by Schering-Plough Corporation, now Merck & Co., Inc., Whitehouse Station, NJ, USA. Serono Reproductive Biology Institute, Rockland, MA generously provided recombinant human LH and Antide. This research was supported by grant funding from Virginia’s Commonwealth Health Research Board (DMD), NICHD (HD054691 to DMD), and NCRR (RR13439 to CAV and RR00169 to the California National Primate Research Center).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sirois J, Dore M. The late induction of prostaglandin G/H synthase-2 in equine preovulatory follicles supports its role as a determinant of the ovulatory process. Endocrinology. 1997;138:4427–4434. doi: 10.1210/endo.138.10.5462. [DOI] [PubMed] [Google Scholar]
- 2.Wong WYL, DeWitt DL, Smith WL, Richards JS. Rapid induction of prostaglandin endoperoxide synthase in rat preovulatory follicles by luteinizing hormone and cAMP is blocked by inhibitors of transcription and translation. Molecular Endocrinology. 1989;3:1714–1723. doi: 10.1210/mend-3-11-1714. [DOI] [PubMed] [Google Scholar]
- 3.Duffy DM, Stouffer RL. The ovulatory gonadotrophin surge stimulates cyclooxygenase expression and prostaglandin production by the monkey follicle. Molecular Human Reproduction. 2001;7:731–739. doi: 10.1093/molehr/7.8.731. [DOI] [PubMed] [Google Scholar]
- 4.Duffy DM, Stouffer RL. Follicular administration of a cyclooxygenase inhibitor can prevent oocyte release without alteration of normal luteal function in rhesus monkeys. Human Reproduction. 2002;17:2825–2831. doi: 10.1093/humrep/17.11.2825. [DOI] [PubMed] [Google Scholar]
- 5.Hizaki H, Segi E, Sugimoto Y, Hirose M, Saji T, Ushikubi F, et al. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP 2. Proceedings of the National Academy of Sciences, USA. 1999;96:10501–10506. doi: 10.1073/pnas.96.18.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, et al. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi T, Morrow JD, Wang H, Dey SK. Cyclooxygenase-2 derived prostaglandin E2 directs oocyte maturation by differentially influencing multiple signaling pathways. The Journal of Biological Chemistry. 2006;281:37117–37129. doi: 10.1074/jbc.M608202200. [DOI] [PubMed] [Google Scholar]
- 8.Janson PO, Brannstrom M, Holmes PV, Sogn J. Studies on the mechansim of ovulation using the model of the isolated ovary. Annals of the New York Academy of Sciences. 1988:22–29. doi: 10.1111/j.1749-6632.1988.tb22238.x. [DOI] [PubMed] [Google Scholar]
- 9.Wallach EE, Bronson R, Hamada Y, Wright KH, Stevens VC. Effectiveness of prostaglandin F2α in restoration of hMG-hCG induced ovulation in indomethacin-treated rhesus monkeys. Prostaglandins. 1975;10:129–138. doi: 10.1016/0090-6980(75)90099-4. [DOI] [PubMed] [Google Scholar]
- 10.Peters MW, Pursley JR, Smith GW. Inhibition of intrafollicular PGE2 synthesis and ovulation following ultrasound-mediated intrafollicular injection of the selective cyclooxygenase-2 inhibitor NS-398 in cattle. Journal of Animal Science. 2004;82:1656–1662. doi: 10.2527/2004.8261656x. [DOI] [PubMed] [Google Scholar]
- 11.Killick S, Elstein M. Pharmacologic production of luteinized unruptured follicles by prostagladin synthetase inhibitors. Fertility and Sterility. 1987;47:773–777. doi: 10.1016/s0015-0282(16)59163-8. [DOI] [PubMed] [Google Scholar]
- 12.Pall M, Friden BE, Brannstrom M. Induction of delayed follicular rupture in the human by the selective COX-2 inhibitor rofecoxib: A randomized double-blind study. Human Reproduction. 2001;16:1323. doi: 10.1093/humrep/16.7.1323. [DOI] [PubMed] [Google Scholar]
- 13.Bata MS, Al-Ramahi M, Salhab AS, Gharaibeh MN, Schwartz J. Delay of ovulation by meloxicam in healthy cycling volunteers: a placebo-controlled, double-blind, crossover study. Journal of Clinical Pharmacology. 2006;46:925–932. doi: 10.1177/0091270006289483. [DOI] [PubMed] [Google Scholar]
- 14.Hester KE, Harper MJK, Duffy DM. Oral administration of the cyclooxygenase-2 (COX-2) inhibitor meloxicam blocks ovulation in non-human primates when administered to simulate emergency contraception. Human Reproduction. 2010;25:360–367. doi: 10.1093/humrep/dep424. [DOI] [PubMed] [Google Scholar]
- 15.Duffy DM, Dozier BL, Seachord CL. Prostaglandin dehydrogenase (PGDH) and prostaglandin levels in periovulatory follicles: Implications for control of primate ovulation by PGE2. Journal of Clinical Endocrinology and Metabolism. 2005;90:1021–1027. doi: 10.1210/jc.2004-1229. [DOI] [PubMed] [Google Scholar]
- 16.Wolf DP, Alexander M, Zelinski-Wooten MB, Stouffer RL. Maturity and fertility of rhesus monkey oocytes collected at different intervals after an ovulatory stimulus (human chorionic gonadotropin) in in vitro fertilization cycles. Molecular Reproduction and Development. 1996;43:76–81. doi: 10.1002/(SICI)1098-2795(199601)43:1<76::AID-MRD10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Seachord CL, VandeVoort CA, Duffy DM. Adipose-differentiation related protein: a gonadotropin- and prostaglandin-regulated protein in primate ovulatory follicles. Biology of Reproduction. 2005;72:1305–1314. doi: 10.1095/biolreprod.104.037523. [DOI] [PubMed] [Google Scholar]
- 18.Yin H, Duffy DM, Gosden RG. Comparative maturation of cynomolgus monkey oocytes in vivo and in vitro. Reproductive Biology and Endocrinology. 2006;4:14. doi: 10.1186/1477-7827-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nyholt de Prada JK, VandeVoort CA. Growth hormone and in vitro maturation of rhesus macaque oocytes and subsequent embryo development. Journal of Assisted Reproduction and Genetics. 2008;25:145–158. doi: 10.1007/s10815-008-9208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarason RL, VandeVoort CA, Mader DR, Overstreet JW. The use of nonmetal electrodes in electroejaculation of restrained by unanesthetized macaques. Journal of Medical Primatology. 1991;20:122–125. [PubMed] [Google Scholar]
- 21.Dong Q, Rodenburg SE, Huang C, VandeVoort CA. Effect of prefreezing conditions on semen cryopreservation of rhesus monkey. Theriogenology. 2008;70:61–66. doi: 10.1016/j.theriogenology.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong Q, Correa LC, VandeVoort CA. Rhesus monkey sperm cryopreservation with TEST-yolk extender in the absence of permeable cryoprotectant. Cryobiology. 2009;58:20–27. doi: 10.1016/j.cryobiol.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schramm RD, Paprocki AM, VandeVoort CA. Causes of developmental failure of in-vitro matured rhesus monkey oocytes: Impairment in embryonic genome activation. Human Reproduction. 2003;18:826–833. doi: 10.1093/humrep/deg144. [DOI] [PubMed] [Google Scholar]
- 24.Schefler WC. Statistics for the Biological Sciences. Second edn. Reading, MA: Addison-Wesley; 1979. [Google Scholar]
- 25.Morgan PM, Boatman DE, Bavister BD. Relationships between follicular fluid steroid hormone concentrations, oocyte maturity, in vitro fertilization and embryonic development in the rhesus monkey. Molecular Reproduction and Development. 1990;27:145–151. doi: 10.1002/mrd.1080270209. [DOI] [PubMed] [Google Scholar]
- 26.Dozier BL, Watanabe K, Duffy DM. Two pathways for prostaglandin F2α synthesis by the primate periovulatory follicle. Reproduction. 2008;136:53–63. doi: 10.1530/REP-07-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuoka Y, Furuyashiki T, Haruhiko B, Ushikubi F, Tanaka Y, Kobayashi T, et al. Impaired adrenocorticotropic hormone response to bacterial endotoxin in mice deficient in prostaglandin E receptor EP1 and EP3 subtypes. Proceedings of the National Academy of Sciences, USA. 2003;7:4132–4137. doi: 10.1073/pnas.0633341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ushikubi F, Segi E, Sugimoto Y, Murata T, Matsuoka T, Kobayashi T, et al. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature. 1998;395:281–284. doi: 10.1038/26233. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen T, Camenisch T, Snouwaert JN, Hicks E, Coffman TM, Anderson PAW, et al. The prostaglandin receptor EP4 triggers remodelling of the cardiovascular system at birth. Nature. 1997;390:78–81. doi: 10.1038/36342. [DOI] [PubMed] [Google Scholar]
- 30.Thomas DW, Mannon RB, Mannon PJ, Latour A, Oliver JA, Hoffman M, et al. Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2. Journal of Clinical Investigation. 1998;102:1994–2001. doi: 10.1172/JCI5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murata M, Ushikubi F, Matsuda T, Hirata M, Yamasaki A, Sugimoto Y, et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. 1997;388:678–682. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- 32.Patwardhan VV, Lanthier A. Prostaglandins PGE and PGF in human ovarian follicles: Endogenous contents and in vitro formation by theca and granulosa cells. Acta Endocrinologica. 1981;97:543–550. doi: 10.1530/acta.0.0970543. [DOI] [PubMed] [Google Scholar]
- 33.Sugimoto Y, Yamasaki A, Segi E, Tsuboi K, Aze Y, Nishimura T, et al. Failure of parturition in mice lacking the prostaglandin F receptor. Science. 1997;277:681–683. doi: 10.1126/science.277.5326.681. [DOI] [PubMed] [Google Scholar]
- 34.Murdoch WJ, Hansen TR, McPherson LA. A review-role of eicosanoids in vertebrate ovulation. Prostaglandins. 1993;46:85–115. doi: 10.1016/0090-6980(93)90037-8. [DOI] [PubMed] [Google Scholar]
- 35.Downs SM, Longo FJ. Effects of indomethacin on preovulatory follicles in immature, superovulated mice. American Journal of Anatomy. 1982;164:265–274. doi: 10.1002/aja.1001640307. [DOI] [PubMed] [Google Scholar]
- 36.Downs SM, Longo FJ. Prostaglandins and preovulatory follicular maturation in mice. Journal of Experimental Zoology. 1983;228:99–108. doi: 10.1002/jez.1402280111. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi S, Noda Y, Matsumoto H, Mori T. Fertilizability of unovulated mature eggs following indomethacin administration in mice. Gamete Research. 1987;18:291–299. doi: 10.1002/mrd.1120180403. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto H, Ma W-G, Smalley W, Trzaskos J, Breyer RM, Dey SK. Diversification of cyclooxygenase-2-derived prostaglandins in ovulation and implantation. Biology of Reproduction. 2001;64:1557–1565. doi: 10.1095/biolreprod64.5.1557. [DOI] [PubMed] [Google Scholar]
- 39.Viggiano JM, Herrero MB, Cebral E, Boquet MG, de Gimeno MF. Prostaglandin synthesis by cumulus-oocyte complexes: effects of in vitro fertilization in mice. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 1995;53:261–265. doi: 10.1016/0952-3278(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 40.Joyce CL, Nuzzo NA, Wilson L, Jr, Zaneveld LJD. Evidence for a role of cyclooxygenase (prostaglandin synthetase) and prostaglandins in the sperm acrosome reaction and fertilization. Journal of Andrology. 1987;8:74–82. doi: 10.1002/j.1939-4640.1987.tb00953.x. [DOI] [PubMed] [Google Scholar]
- 41.Huang J-C, Wun W-SA, Goldsby JS, Wun IC, Falconi SM, Wu KK. Prostacyclin enhances embryo hatching but not sperm motility. Human Reproduction. 2003;18:2582–2589. doi: 10.1093/humrep/deg490. [DOI] [PubMed] [Google Scholar]