Abstract
Mechanical forces associated with blood flow play important roles in the acute control of vascular tone, the regulation of arterial structure and remodeling, and the localization of atherosclerotic lesions. Major regulation of the blood vessel responses occurs by the action of hemodynamic shear stresses on the endothelium. The transmission of hemodynamic forces throughout the endothelium and the mechanotransduction mechanisms that lead to biophysical, biochemical, and gene regulatory responses of endothelial cells to hemodynamic shear stresses are reviewed.
I. INTRODUCTION
The mechanical environment of mammalian cells is defined by complex interactions between gravitational forces, local forces generated by air and fluid pressure and movement, and intracellular tension arising from the organization of cytoskeletal elements. With the exception of the blood, the cellular components of vertebrate tissues develop tension by physical interactions with extracellular matrix and neighboring cells, the fundamental importance of which is reflected in adhesion-dependent expression of normal cell differentiation, growth, and function. Simple responses to external forces in unicellular organisms have evolved in mammals into sophisticated acute sensory systems and adaptive responses to sustained changes of the mechanical environment. In the mammalian cardiovascular system, unique responses to fluid forces are present in the endothelium.
The endothelium is located between flowing blood and the vascular wall. Cells lining the arterial circulation are exposed to fluid forces of much greater magnitude than those experienced by other mammalian tissues. Consequently, mechanically related responses controlled by the endothelium have evolved as part of normal vascular physiology, most notably in the control of vascular tone where mechanisms responsible for the transmission and transduction of hemodynamic information from the blood to the underlying vessel wall reside in the endothelium. Hemodynamic forces also play an important role in vascular pathologies, particularly in relation to the localization of atherosclerotic lesions.
The principal functions of endothelium are the maintenance of anticoagulant properties, the physiological control of lumen diameter, the regulation of vascular permeability, and the pathological consequences associated with acute inflammation, wound healing, and cardiovascular disorders such as the focal localization of atherosclerosis. In all of these processes, hemodynamic factors (defined as mechanical forces in the flowing blood) influence endothelial biology either by the direct action of shear stress and stretch forces on the endothelium itself or by indirect modification of the local concentrations of chemicals and agonists at the endothelial surface, thereby influencing association between these molecules and their endothelial receptors. In reality, the mechanisms are not mutually exclusive; direct forces acting on surface enzymes and receptors may modify enzyme-substrate and agonist-receptor interactions at the same time that the surface concentration of agonist is being influenced by convective and diffusive transport. A major challenge to researchers in this field is identification of the mechanism(s) by which flow forces in the blood are detected and converted in the endothelial cells into a sequence of biological responses. Despite a multiplicity of flow-sensitive mechanisms, it is plausible that discrete flow sensors or mechanoreceptors exist in the endothelial cell, intervention with which would have important consequences for many vascular events.
The main objectives of this review are 1) to document the current state of knowledge concerning endothelial responses to mechanical forces related to blood flow, 2) to propose response mechanisms that accommodate sometimes inconsistent or contradictory data, and 3) to suggest future directions of research into endothelial mechanotransduction. The review is focused principally on hemodynamic shear stress-related mechanisms.
II. FLOW IN THE ARTERIAL CIRCULATION
The flow characteristics operating on the arterial side of the circulation (large elastic and muscular arteries through smaller arteries, arterioles, and precapillary vessels) generate a wide range of hemodynamic stresses that vary greatly in magnitude, frequency, and direction at the endothelial surface (77, 100, 109, 167, 169, 177, 267). The mechanics of blood flow are more complex than classical Poiseuille fluid dynamics that apply principally to steady flow in a rigid tube of circular cross section. In the arterial circulation, blood flow is pulsatile, blood is a non-Newtonian fluid (although near-Newtonian characteristics are sometimes valid), and the vessel is a compliant tube of changing cross-sectional shape and area with many side branches and bifurcations. However, with the application of the principles of fluid dynamics, with the use of experimental models of steady and pulsatile flow in rigid tubes and fixed arteries, and with the development of more accurate in vivo measurements of blood flow, some reasonable estimates of flow characteristics, and hence hemodynamic forces, can be made.
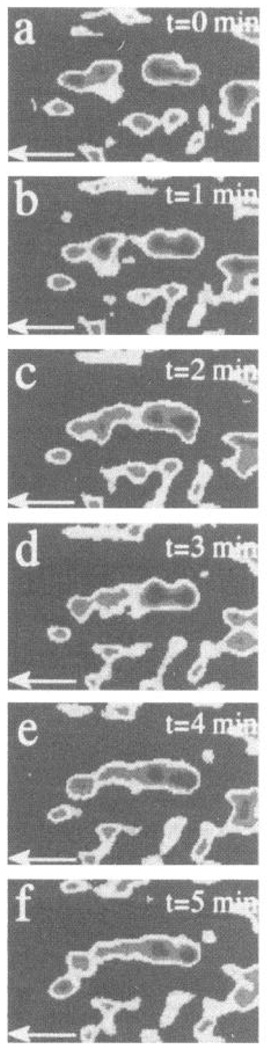
Forces acting on an artery due to blood flow can be resolved into two principal vectors. One is perpendicular to the wall and represents blood pressure, and the other acts parallel to the wall to create a frictional force, shear stress, at the surface of the endothelium (77, 109, 254). Although all of the vessel wall, including the endothelium, smooth muscle cells, and the extracellular matrix (collagen, elastin, proteoglycans), is subjected to stretch as a consequence of pulsatile pressure, the shear stress is received principally at the endothelial surface. There is slow transmural flow around smooth muscle cells that produces very low levels of shear stress, but this force is considered to be insignificant when compared with tensile stress in these cells. In large arteries, the mean wall shear stress is typically in the range of 20–40 dyn/cm2 in regions of uniform geometry and away from branch vessels. Because blood flow is pulsatile in these vessels, however, the velocity profile varies with the cardiac cycle to produce a range of shear stress and shear stress gradients. Furthermore, at curvatures in the arterial wall such as the aortic arch, and at bifurcations and near branches, the steady laminar flow is disrupted to create regions of separated flow that include recirculation sites (vortices) that may appear/disappear or elongate/shorten with the cardiac cycle (Fig. 1). Extremely complex forms of disturbed laminar and occasionally nonlaminar flow occur in large arteries. As discussed in section XII, such regions are often associated with a predisposition for atherosclerosis. In addition to these primary flow characteristics, lesser fluid motions can occur perpendicular to the principal flow direction (77). Such secondary flows, although of low velocities, modify the profile of the primary flow and thereby influence the shear stress acting at the vessel wall.
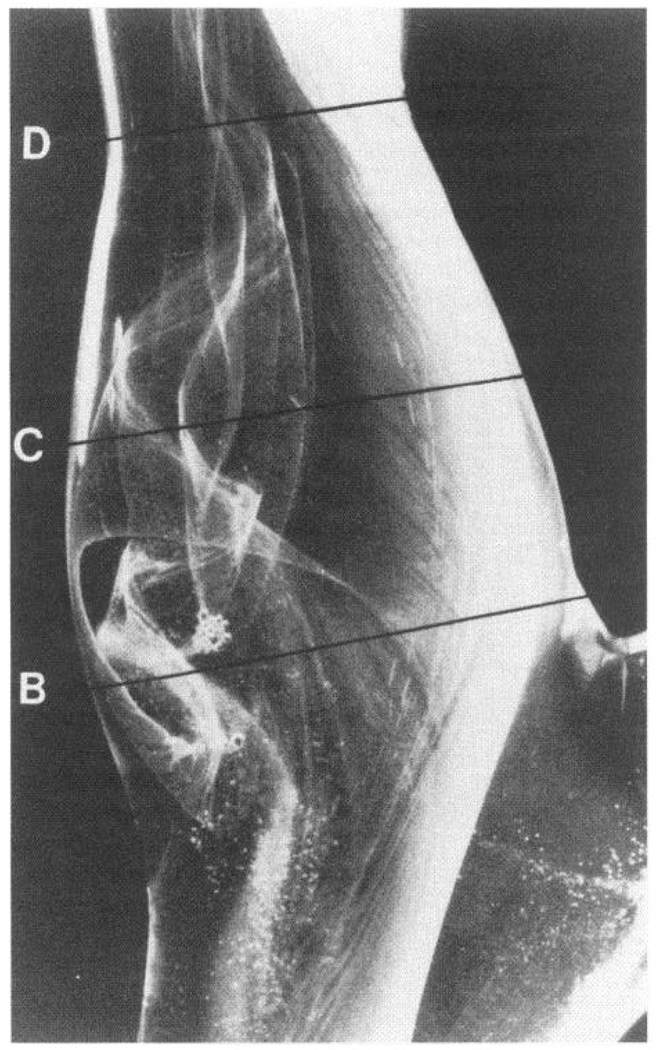
FIG. 1.
Glass model of human carotid sinus showing complex flow patterns visualized by hydrogen bubbles. In the sinus itself (B, C), there is separation of laminar flow profile to create a zone of recirculation that reattaches to main flow downstream (D). Vortices appear and disappear during pulsatile flow cycles. Note also helical pattern in main flow and absence of flow separation on opposite wall to sinus. Carotid artery atherosclerosis is common in region of flow separation. [From Zarins et al. (404).]
Models of arterial geometry and flow have been constructed that allow shear stress measurements to be estimated in vivo. Often, the morphology of endothelial cells lining real arteries accurately reflects the direction and magnitude of shear stress predicted from the models. Endothelial morphology is influenced by the prevailing hemodynamic conditions because the cells are malleable to the flow forces in vivo and in vitro; their alignment generally reflects the mean directional shear stress (78, 193, 194, 397). This is also true at regions of disturbed flow where the endothelial morphology changes abruptly over short distances (1–2 endothelial cells) from elongated alignment in the direction of flow to a configuration without preferred orientation (Fig. 2). The latter morphology is associated with disturbed flow regions of large arteries where the shear stress fluctuates in magnitude and changes direction during the cardiac cycle. From a large number of fluid dynamics studies combined with measurements and observations in real arteries, the range of shear stress in the arterial circulation in such regions has been estimated to vary from negative values, through zero values at the edges of flow separation regions, and up to values of ~40–50 dyn/cm2 (177, 178). During episodes of increased cardiac output or hypertension, these values may increase considerably; transients in excess of 100 dyn/cm2 have been recorded (77, 255). Much of the investigation of mechanotransduction of hemodynamic forces has been conducted in vitro, where control of the mechanical environment is more accessible than in vivo. The range of shear stresses examined in vitro has typically been 0– 100 dyn/cm2 mainly in steady unidirectional laminar flow, although more attention to the effects of pulsatile flow has recently been in evidence (133, 134).
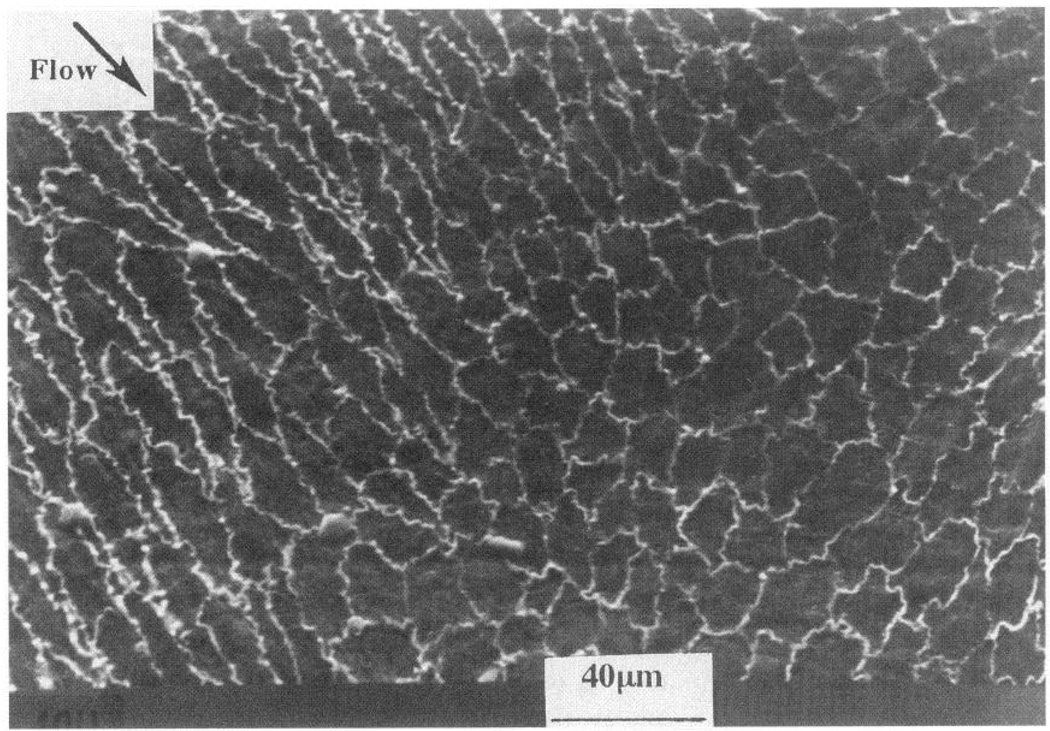
FIG. 2.
Outlines of endothelial cells in primate thoracic aorta adjacent to an intercostal branch artery. A region of predicted flow separation is shown in which shapes of cells change abruptly from elongated to polygonal (without preferred orientation). Polygonal morphology is believed to be caused by flow separation and vortices that subject endothelial surface to shear stress in several different directions during cardiac cycle. Adjacent cells are subjected to markedly different shear stress profiles. Gradients of shear stress are steepest on cells located at edge of flow separation region. Scanning electron micrograph is shown after silver staining.
III. MORPHOLOGICAL RESPONSES TO SHEAR STRESS
Since the early observations of silver-stained endothelial cells in the light microscope (1, 290), many investigators have documented a relationship between endothelial cell shape and orientation and the direction of blood flow (67, 90, 116, 187, 193, 194, 253, 268, 299). The cells align in the direction of flow. Flow separation, vortices, or other disturbances are reflected in the morphology, often as a loss of cell orientation (Fig. 2). The same is true in vitro (78, 85, 194, 300). The first demonstration that endothelial cellular morphology could be changed by flow was conducted in vivo by Flaherty et al. (90), who resected an arterial patch at 90° to its original orientation and observed that the endothelium subsequently realigned with the flow. A similar demonstration in vitro was first conducted by Dewey et al. (78), who induced cell alignment by applying unidirectional shear stress in steady flow to polygonal confluent endothelial monolayers.
In contrast, chaotic (turbulent) flow failed to align the cells (68), presumably because the direction, magnitude, and frequency of the forces were unpredictable. Cell shape and orientation are determined by the cytoskeleton, since it rearranges in response to flow. A prominent change occurs in the distribution of filamentous actin (F-actin) which in polygonal cells is organized as a dense band in the periphery of the cell. Upon exposure to shear stress, F-actin rearranges to create bundles of stress fibers (97, 169, 170, 225, 274, 390, 394). There is also evidence for the induction of F-actin (97). Attachment sites of stress fibers to the cell periphery (and associated extracellular molecules) also redistribute in response to flow (70, 64, 388, 389) as discussed in more detail in section V. The nature of the extracellular matrix appears to influence the rate of cytoskeleton reorganization (275). Endothelial morphological changes in flow have been reviewed extensively elsewhere (254, 255, 343).
IV. HEMODYNAMIC FORCE INTERACTIONS WITH THE ENDOTHELIUM
A. Forces and Cell Tension
1. Stress
The forces acting on, and within, a cell can be described in terms of stress. Stress is a force per unit area that has both normal components (tension and compression) and shear components. Stresses acting on the luminal surface of an endothelial cell cause internal stresses that are transmitted to abluminal attachment sites or to neighboring cells within the endothelial monolayer. Blood pressure acts normal to the cell surface creating a compressive stress within the cell, while the frictional force of flowing blood generates a shear stress acting tangentially to the cell surface. Furthermore, distension of the blood vessel due to the pressure pulse transmits tensile stress to the cell via its contacts with the extracellular matrix. Cell deformation in response to applied shear stress is expressed as strain and depends on the mechanical and structural properties of the cell. Tensile strain (stretch) represents a change in length per unit (original) length. In addition, torsional stresses may be imposed across the entire cell if its mechanical properties behave like a solid (103, 125), and osmotic pressure is under cellular control and must be balanced against externally applied hydrostatic pressure (385, 386). Endothelial cells are capable of altering their structure and mechanical properties resulting in the generation of intracellular stress, e.g., cytoskeletal reorganization in response to flow. Tension developed by the cytoskeleton is required for expression of differentiated properties of the endothelium. These mechanical terms, derived for inert materials, must be considered in the light of the active cellular metabolism of living endothelial cells.
2. Force transmission
Anchorage-dependent cells, including endothelial cells, exist in a state of tension associated with the maintenance of cell shape. Such tension is generated when the cytoskeleton interacts with other regions of the cell, particularly at sites of adhesion to the subendothelial matrix (35), nucleus (286), and neighboring cells (361). When external forces are loaded onto the cell during flow, the internal cellular tension changes to equalize the external force. Therefore, direct shear stress-induced mechanotransduction in endothelial cells may occur by 1) local displacement of sensors at the cell surface, 2) force transmission via cytoskeletal elements to distribute the force throughout the cell, followed by 3) force transduction of the transmitted mechanical stress at mechanotransduction sites remote from the externally applied stress, or more likely a combination of these mechanisms (Fig. 3). Thus the cell is always under tension and responds to change of tension (347). Flow imposes additional changes of cell tension that lead to morphological and functional responses.
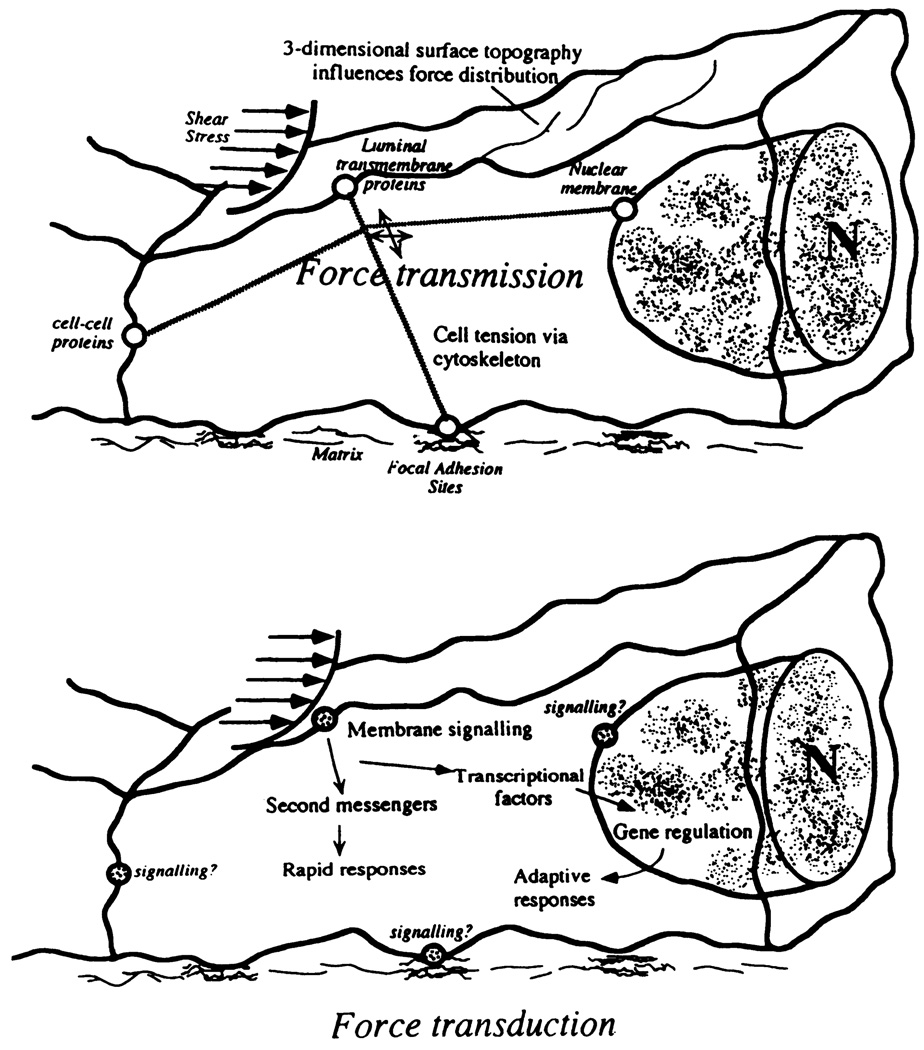
FIG. 3.
Separation of concepts of force transmission and force transduction. Cytoskeleton transfers stress to different regions of endothelial cell where mechanotransduction may occur. [From Davies and Barbee (61).]
The prominent reorganization of F-actin stress fibers, intermediate filaments, and microtubules to external forces (169, 389) implicates the cytoskeleton as a principal force transmission element in endothelial cells. Actin filaments are of particular interest in this regard because their association with transmembrane integrins appears to be the principal mechanism of the transmission of twisting forces across the plasma membrane (outside to inside; Ref. 383). Furthermore, the filaments also appear to be required for transduction because their disruption changes a number of primary and secondary responses in the endothelial cell. Consistent with this view, there is a change of stretch-activated ion channel activity in response to membrane deformation when actin filament structures are disrupted (318), and endothelial cell shape change, realignment to flow, focal adhesion remodeling, and gene regulation (243) are all inhibited by drugs that interfere with microfilament turnover.
B. Topography of the Luminal Endothelial Surface
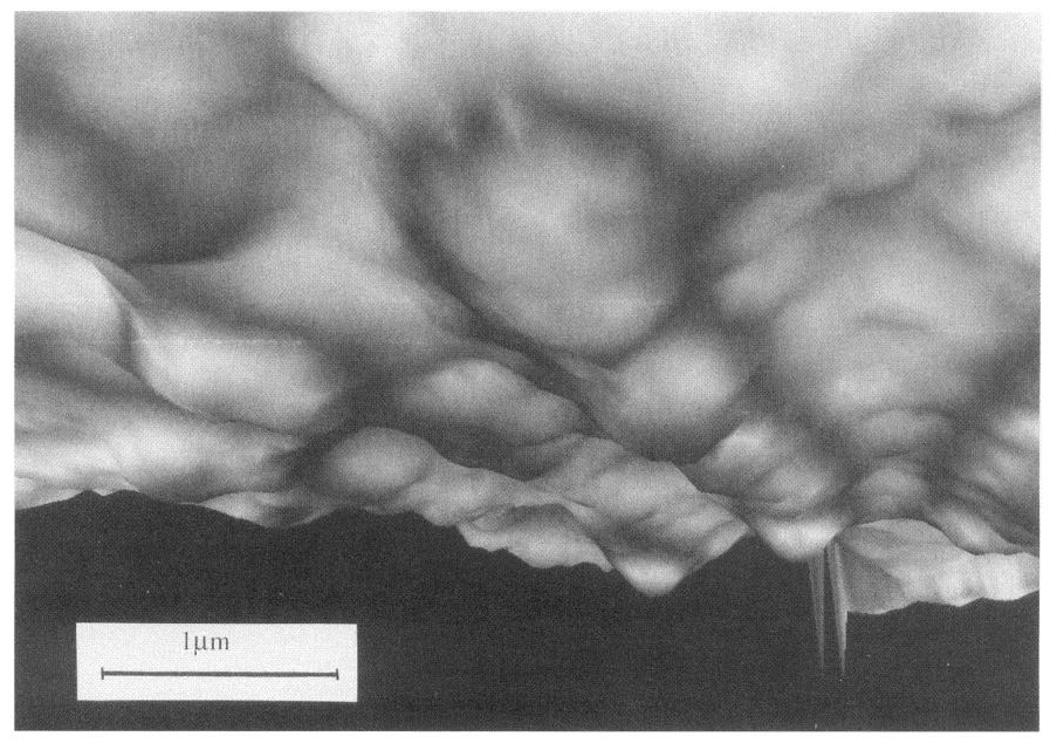
Hemodynamic force-cell interactions begin at the cell surface. In vitro experiments in which the applied forces were carefully controlled have demonstrated that endothelial behavior depends not only on the magnitude of shear stress but also on temporal and spatial variations of shear stress. Cell geometry, particularly surface topography, influences the magnitude and localization of hemodynamic forces acting at the endothelial surface and consequently affects the transmission of such forces across the plasma membrane and throughout the cell as outlined in Figure 3. However, efforts to relate molecular-scale transduction events to the physical stimuli at the cell surface have been frustrated by an insufficient characterization of the detailed distribution of fluid forces acting on the endothelium. Analyses of flow forces on a subcellular scale are needed to address the mechanisms of force transmission and its transduction into cellular responses. Knowledge of the flow characteristics in arteries provides a macroscopic profile of the flow and predicted shear stresses acting near the endothelial surface. However, the detailed real-flow behavior very close to the cell surface actually defines the cell responses. Only recently have estimates of its magnitude and spatial characteristics at a subcellular level been addressed. This was first attempted as a theoretical exercise by Satcher et al. (322), who modeled the endothelial monolayer as a wavy surface and used computational methods to estimate the influence of the waviness on local flow forces. Recently, it has been possible to image the geometry of living endothelial cells in tissue culture. Sakurai et al. (320) approximated the discontinuous contours of a single endothelial cell using differential interference contrast microscopy, an approach that was subsequently improved upon by the use of fluorescence exclusion combined with confocal microscopy (395). They estimated the shear stress to be considerably higher over the nuclear bulge, implying steep gradients of shear stress. However, the first images of the continuous geometry of the luminal surface of living endothelial cells at high resolution in real time were obtained by Barbee et al. (15) using atomic force microscopy (AFM; see Ref. 184 for a review of AFM). They reported major changes in the surface topography of cells aligned by flow compared with no-flow controls. The studies revealed significant changes in the height of the undulating endothelial monolayer when cells were aligned with flow; essentially a streamlining of the surface occurred (Fig. 4). In addition, there were localized changes of cell topography within individual endothelial cells that have implications for the local forces acting on different parts of the cell. The endothelial cell surface waviness (<6 µm) allows laminar flow to be considered as quasi-steady near the wall even in pulsatile arterial flow. From models, approximations, and actual measurements of the cell surface geometry, computational methods can be employed to study the flow very close to the cell surface to calculate shear stress distribution.
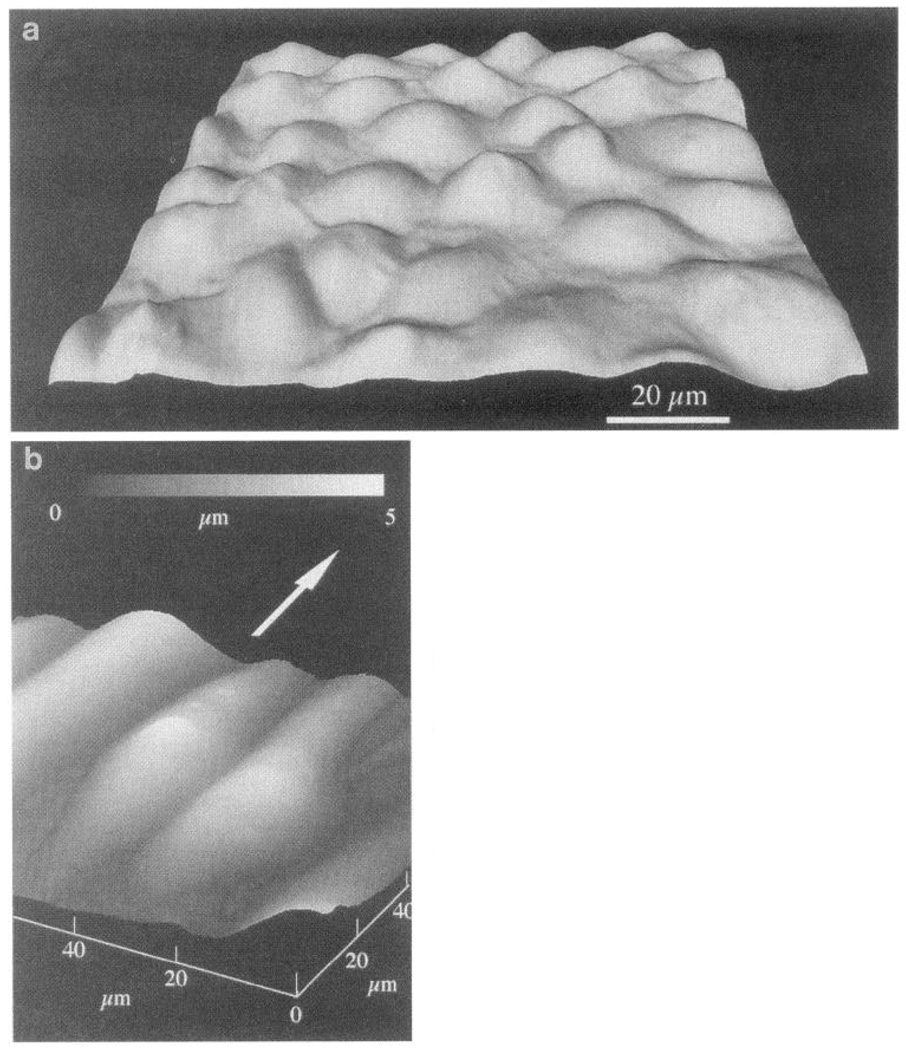
FIG. 4.
Atomic force microscope images of confluent bovine arotic endothelial cells. a: cultured under no-flow conditions. b: after 24-h exposure to 12-dyn/cm2 shear stress in steady laminar flow. Flattened profiles of aligned cells give rise to smaller spatial variations of shear stress and stress gradients at cell surface. Arrow indicates direction of flow. Gray micrometer scale in b indicates height of cell surface referenced to lowest point (at junctions between cells). (Images courtesy of Dr. Kenneth Barbee, University of Chicago, Chicago, IL.)
Spectral element analyses have been conducted to simulate flows over endothelial surface geometries defined by AFM of living endothelial cells (16). The distribution of shear stress on the cell surface was then calculated. Flow perturbations due to the undulating surface produced cell-scale variations of shear stress magnitude and, hence, large shear stress gradients. Reorganization of the endothelial surface in response to prolonged exposure to steady flow resulted in significant reductions in the peak shear stresses and shear stress gradients compared with no-flow control cells (15, 16, 64). Whereas previously the stress acting on the cell surface was assumed to be spatially uniform and equal to the average wall shear stress determined by macroscopic flow dynamics, the new studies demonstrated that there are microscopic departures from a flat boundary due to the presence of the endothelial cells. These, in turn, caused a localized perturbation of the macroscopic flow field. From the point of view of the endothelial cell, the shear stress at the luminal surface will vary within the macroscopic flow field as a function of the surface geometry. Thus cells in certain morphological configurations are likely to be exposed to much lower shear stress gradients than neighboring cells. The consequences of this may be a resetting of the threshold levels necessary to elicit bioresponses in the flow field. For example, in aligned cells there was approximately a 40% decrease in the average shear stress gradients compared with no-flow control cells. A detailed analysis of the upstream/downstream symmetry of the cell surface in vitro has yet to be made; differences may reflect sensitivity to shear stress. In this regard, altered agonist sensitivity of endothelial cells in situ has been reported when the flow direction was altered (291). Whether this phenomenon is a reflection of the endothelial microgeometry awaits the completion of AFM imaging of endothelial cells in situ.
C. Cytoskeletal Elements Enable Force Transmission Throughout the Cell
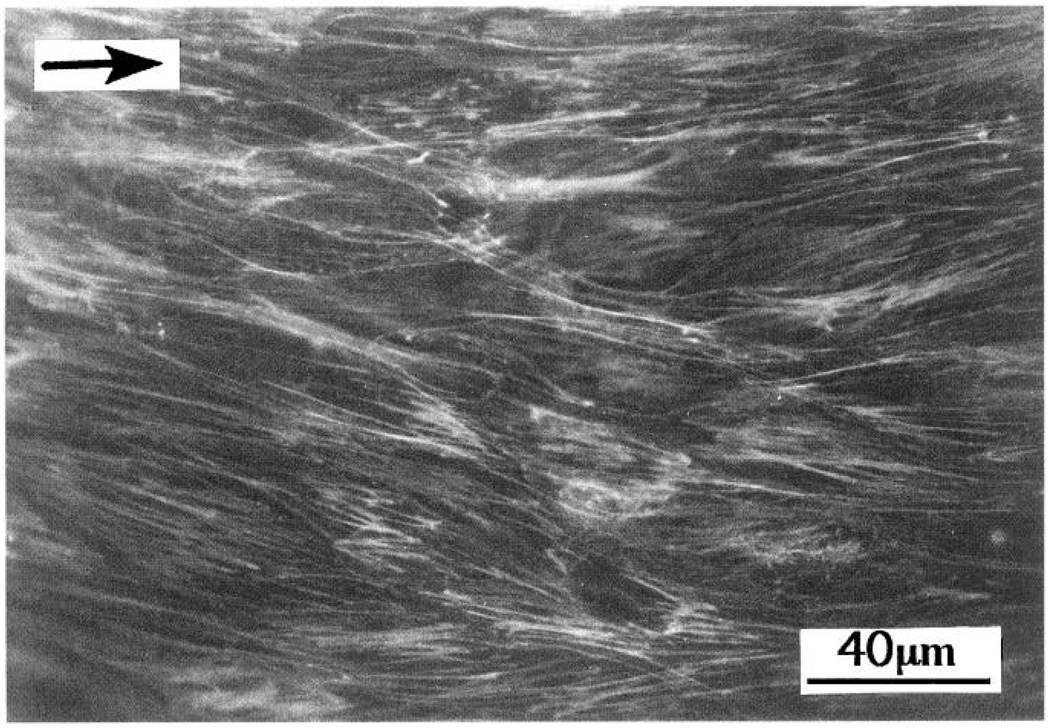
Prolonged exposure of endothelial cells to steady flow results in their realignment in the direction of flow, a process driven by reorganization of the cytoskeleton as demonstrated both in vitro (78, 388) and in situ (188, 390). Most observations have recordzed reorganization of actin microfilaments that change from a “banding pattern” around the cell periphery to a series of long, almost parallel fibers in the long axis of the aligned cell (Fig. 5). Although much attention is focused on F-actin because of its association with membrane proteins at the luminal and abluminal surfaces, the additional involvement of microtubules and vimentin-rich intermediate filaments (IF) should not be overlooked; both associate with plasma membrane proteins (356) and undergo alignment with unidirectional shear stress (F. W. Flitney, R. Goldman, and P. F. Davies; unpublished data). Unlike actin and tubulin, IF proteins are insoluble in the cytosol, and there are no soluble pools available for polymerization into filaments (59). Reorganization of endothelial IF during flow may therefore occur as a passive association of IF with F-actin that is undergoing remodeling through cycles of polymerization/depolymerization. Intermediate filaments also interact with microtubules via fibrous bridges (59). The dissolution of microtubules by colchicine results in clumping of IF. Thus there exist physical connections between these three important structural protein families that determine the shape of anchorage-dependent cells, and it is probable that a coordinated rearrangement occurs in endothelial cells subjected to flow. Until recently, only a limited appreciation of the spatial arrangements of the cytoskeleton was available from two-dimensional transmission electron micrographs. However, the development of three-dimensional reconstruction techniques from digitally imaged optical sections (4, 41, 242) and confocal imaging techniques (393) has now become economically feasible for less-specialized laboratories. Deconvolution techniques have recently been used to reconstruct endothelial cytoskeletal components from optical sections of fluorescence emissions collected in digitized form (A. Robotewskyj, M. L. Griem, J. Chen, and P. F. Davies; unpublished data). With the use of this approach, double (or triple) fluorescence labeling of cytoskel et al elements should reveal some of the physical relationships between them and how they change when mechanical stress is applied to the cell.
FIG. 5.
F-actin stress fiber distribution in aligned confluent endothelial cells. NBD-phallacidin stain was used. Arrow shows flow direction. [From Davies et al. (70). Reproduced from The Journal of Clinical Investigation, 1993, vol. 91, p. 2640–2652 by copyright permission of The Society for Clinical Investigation.]
One of the most striking consequences of endothelial realignment by flow is increased resistance of the cell surface to deformation. This was first noted by Sato et al. (323) as decreased membrane deformability on micropipette aspiration of the cell surface after exposure to shear stress. Rather than stiffening of the membrane itself, however, it is the cortical cytoskeletal elements just beneath the membrane surface and attached to it that rearrange to a more rigid configuration. Using AFM, Barbee et al. (15) noted topographic features of aligned endothelial cells that included longitudinal ridges apparently caused by the presence of cytoskeletal structures underlying the plasma membrane which was depressed by the force of the stylus in regions unsupported by cytoskeleton. In recent experiments (16) using lower spring-constant cantilevers (thus reducing the imaging forces), these features were absent unless the force was deliberately increased. It can be concluded that bundles of cytoskeletal filaments form realigned arrays just below the luminal plasma membrane after exposure to flow, resulting in decreased deformability of the surface region. Also contributing to cell-surface rigidity are ankyrin-like and spectrin-like proteins that serve as anchors at the cell membrane for other cytoskeletal proteins (59); it is not known whether these rearrange in response to mechanical forces.
Remuzzi et al. (300) filmed changes in the shape of flow-aligned endothelial cells after removal from flow. Cells remained aligned for several hours followed by periods of rapid relaxation to a polygonal orientation that occurred within discrete regions of the monolayer. Because relaxation was not temporally or spatially uniform, there may be regions of the monolayer where groups of adjacent cells shared mechanical tension. Immunofluorescence micrographs give the impression that stress fibers form a continuum between aligned cells, reinforcing the idea of intercellular tension. The detailed mechanics of these phenomena, which have not been adequately analyzed, deserve further investigation because they define the spatial distribution of intracellular (and possibly intercellular) tension.
During flow, the position of cellular organelles may also change. For example, the Golgi apparatus and microtubule organizing centers have been shown to undergo temporary redistribution (47, 226). The consequences of such rearrangements for cell function are unclear.
D. Signal Filtering and Adaptation to Mechanical Stimuli
Most information about endothelial mechanotransduction and the wide range of cellular responses that result have been obtained from steady flow experiments in vitro. Endothelial cells in arteries, however, are subjected to hemodynamic forces that might be expected to constantly stimulate the cells. This does not usually happen. For example, platelet-derived growth factor (PDGF) B chain mRNA, although greatly stimulated in vitro by shear stress, is found at low levels in endothelial cells freshly scraped from an artery (17). Intracellular calcium ([Ca2+]i) levels appear to be at baseline levels in the endothelium of excised arterioles despite steady flow; when the arteriolar flow rate increases, [Ca2+]i is stimulated (89). Consequently, there must be mechanisms to cope with overstimulation by the normal mechanical environment. Several mechanisms (which are probably interactive) include adaptation and filtering of the signal.
1. Adaptation
Sustained stimulation leads to feedback inhibition of mechanotransduction. Examples include desensitization of shear-sensitive ion channel activity (271), adjustment of cell-surface adenine nucleotide concentrations (82, 83), and intracellular renormalization of calcium levels by activation of calcium pumps to either store calcium in the endoplasmic reticulum or pump it out of the cell (18). One of the first demonstrations of adaptation was a return of flow-stimulated pinocytosis rates to no-flow levels despite the continuation of flow (66). When flow was later stopped, pinocytosis was again transiently stimulated as the cells readapted to no-flow conditions. Cell alignment under flow and its reversal several hours after cessation of flow also represent adaptation and readaptation events (300). Streamlining of the cell surface to reduce the shear stress gradients (15, 16) may be a mechanism of turning off or reducing the force stimulus. Product inhibition of enzyme activities common in ligand-mediated intracellular signaling is another example of adaptation that may also occur in mechanical signaling.
2. Filtering of the signal
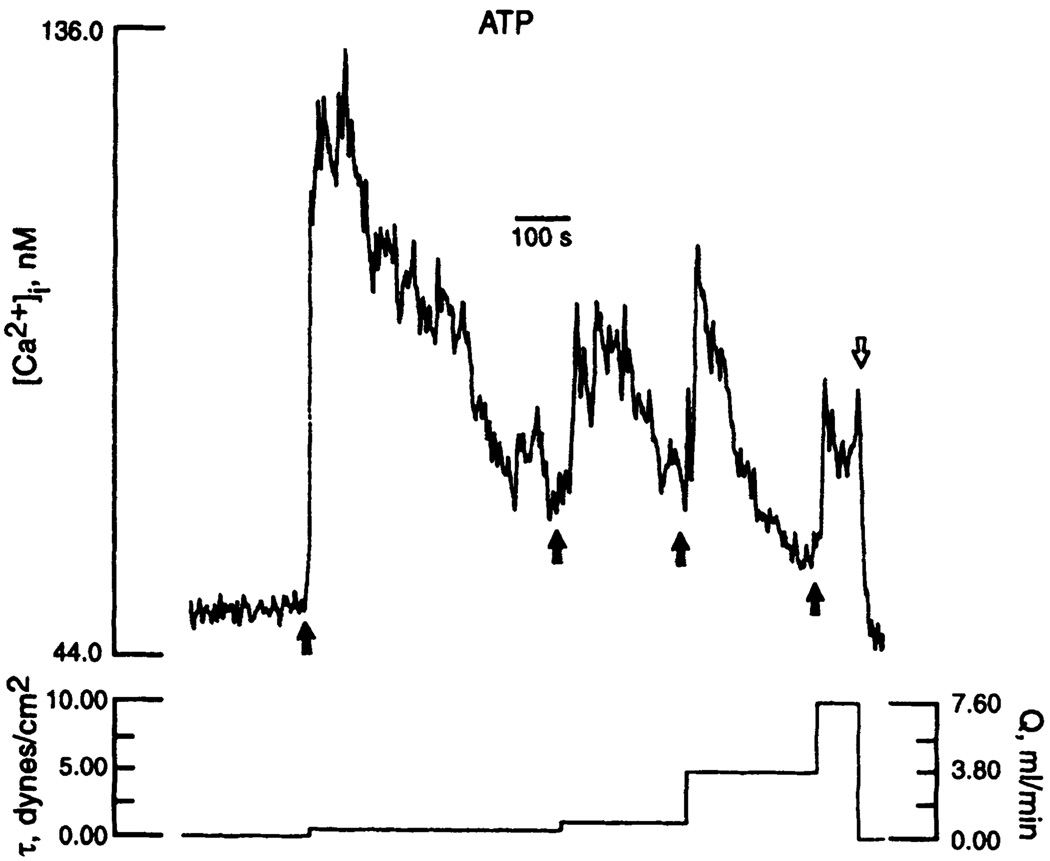
When direct mechanical forces stimulate mechanotransduction, the stimulus is coupled to structural components that may act as selective filters responsive only to certain mechanical stimuli. The classic example is the Pacinian corpuscle, which transmits only high-frequency stimuli throughout the structure of the organ (200); manipulation of the structure modifies the frequency response. In a similar fashion, the cytoskeleton may filter hemodynamic stimuli in endothelial cells. The frequency of flow-mediated [Ca2+]i oscillations that have been reported in the presence (340) or absence (133) of agonists may also reflect selective filtration of the force.
Endothelial cells subjected to pulsatile flow fail to respond at certain frequencies. This may be because the positive stimulus for response needs to be sustained. For example, pinocytosis rates that increase with steady shear stress (8 dyn/cm2) were unaffected by 1-Hz oscillations of shear stress around the same mean value (8 ± 5 dyn/cm2) (66). Endothelin-1 mRNA that normally downregulates at 15 dyn/cm2 failed to change when cells were subjected to back-and-forth reversing flow of root-mean-square shear stress magnitude 15 dyn/cm2 but average magnitude of zero (214). Further evidence of differential behavior to oscillatory versus steady flow, and to different types of oscillatory patterns, was reported by Helmlinger et al. (133), who showed that endothelial cells can discriminate between different flow environments. Recently, the same group has reported that the amplitude, delay time, and rise time of [Ca2+]i levels in endothelial monolayers were strongly dependent on the steady and pulsatile characteristics of the flow (133; R. M. Nerem, personal communication). A purely oscillatory flow failed to induce changes of average [Ca2+]i levels, although it resulted in a larger number of cells exhibiting oscillating [Ca2+]i levels.
V. WHERE TRANSMISSION BECOMES TRANSDUCTION: CANDIDATE MECHANOTRANSDUCERS
A. Structural Considerations
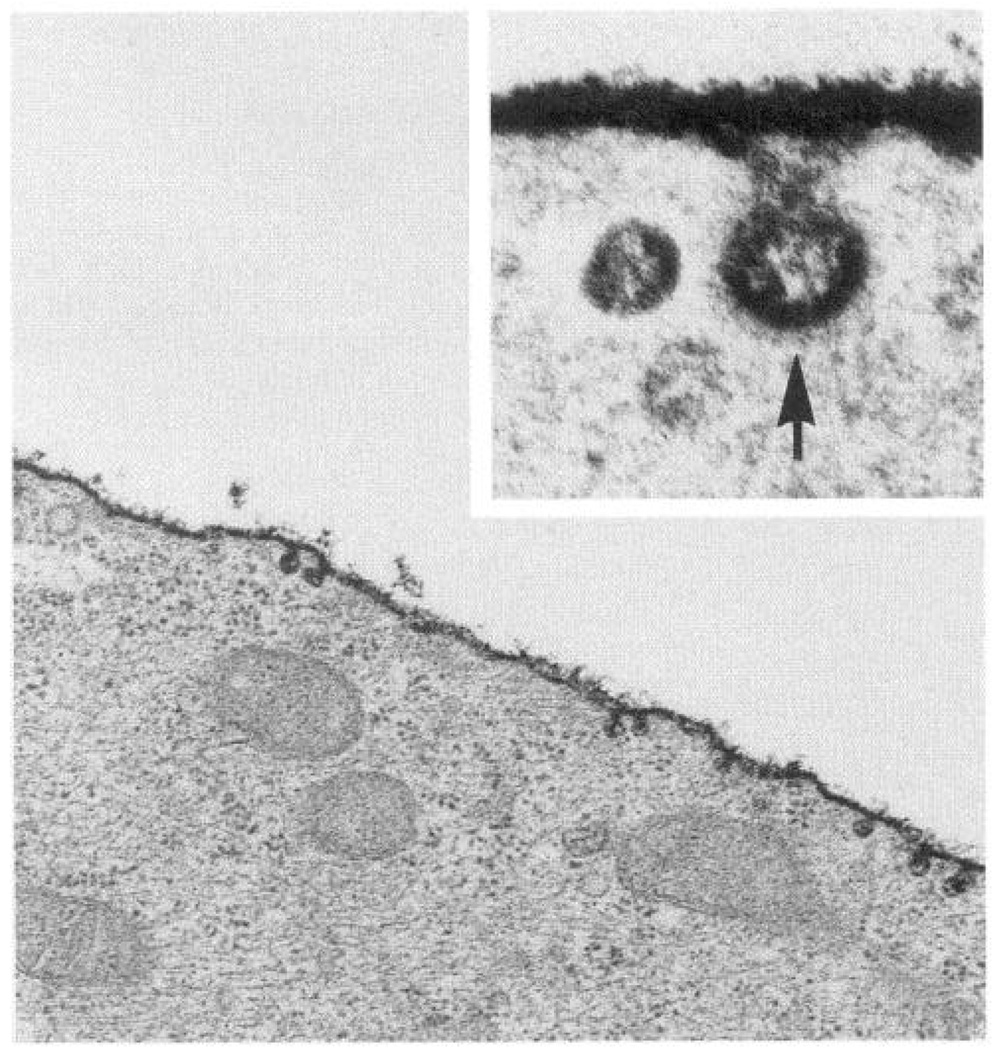
Transfer of fluid shear stress forces to the cell occurs first at the luminal cell surface. Plasma membrane molecules are therefore candidate mechanotransducers that generate intracellular biochemical signals. The cell membrane is heterogeneous in composition and, as demonstrated above, the complex surface geometry results in significant variations of stresses on a subcellular scale. Staining of the cell surface with ruthenium red and similar markers reveals a prominent glycocalyx (Fig. 6), a fringe of carbohydrate-rich glycoproteins, many of which are themselves receptors or are linked to receptors anchored in the plasma membrane (205). The glycocalyx may be a significant structure in mechanotransduction in several ways. First, transmembrane glycoproteins extending into the extracellular space occupied by the glycocalyx may be physically displaced by the frictional hemodynamic shear stress to provoke an intracellular response. Second, the fluid layer associated with the glycocalyx represents a microenvironment for agonist-receptor interactions. It acts as a discontinuous medium between bulk fluid flow and the solid body characteristics of the cell surface. Agonists and chemokines present in the plasma or secreted from the cell are likely to be present in this relatively unstirred layer in quite different concentrations than in the bulk fluid and interact with receptors as a function of flow-related convective and diffusive transport rates (82, 83, 260, 339). This indirect effect of flow on cell signaling, however, is unlikely to occur completely independently of direct mechanical effects and is considered more fully in section VII.
FIG. 6.
Endothelial cell luminal plasma membrane glycocalyx stained with ruthenium red. Inset: invagination of surface at a clathrin-coated pit. Transmission electron microscope magnification: ×40,000; inset, ×175,000.
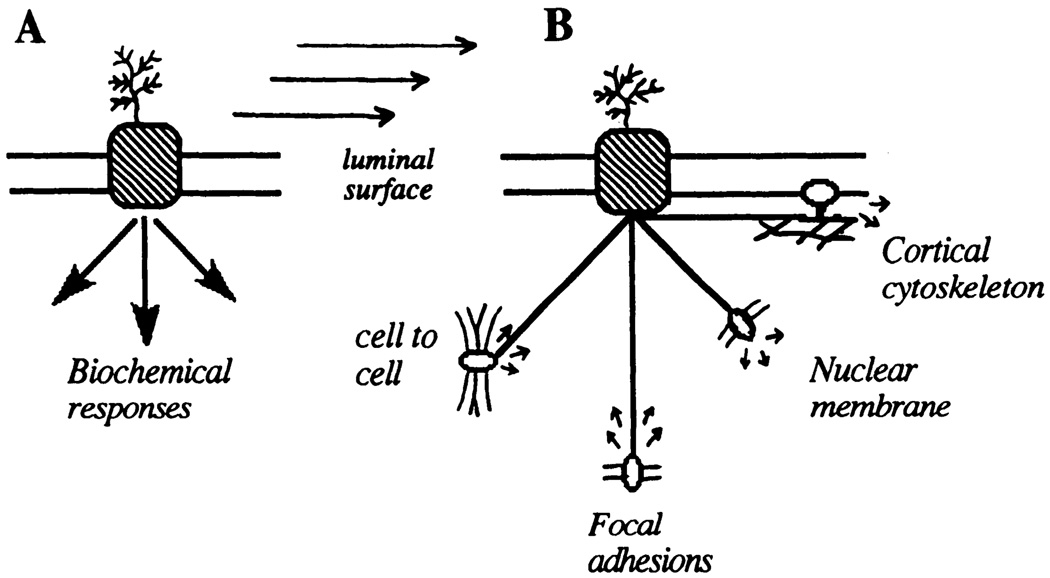
A prototypic mechanical sensor at the luminal surface may be a molecule or molecular complex whose function is related to its stressed state. Intramolecular forces, defined by structural configuration, set the stress state. The specialized function of a mechanosensor might be expected to be reflected in a specialized structure. This could manifest itself by a particular conformation (e.g., unusually long glycoprotein extensions into the extracellular space; intracellular conformational changes favoring association with soluble second messengers) or by sensitivity to physical displacement of intracellular linkages to cytoskeletal elements. It is suggested therefore that one view of mechanotransduction mechanisms restricts all of the mechanics to the luminal membrane proteins that, once displaced (activated), generate a biochemical cascade(s) at the cytoplasmic face of the membrane. Here the data fit a model of mechanoreceptor activation by stress, the generation of second messengers, and the activation of cytosolic transcription factors that subsequently regulate gene transcription in the nucleus (Fig. 7A).
FIG. 7.
Centralized and decentralized mechanotransduction models in endothelial cells. A: shear stress transduction occurs at a primary site on luminal cell surface after which biochemical/electrophysiological pathways control responses through serial and parallel pathways. B: shear stress transduction occurs at multiple sites that are mechanically coupled by cytoskeleton. Each site, with different sensitivities, response times, and local environment, can generate bioresponses.
The next level of structural complexity in mechanotransduction involves transmission of the stress throughout the cell via the cytoskeleton (refer to Fig. 3). Here the membrane molecules participate in two ways: 1) to passively transfer the stress to the cytoskeleton in one part of the cell and 2) to respond to cytoskeletal deformations at sites remote from the stimulus. The latter may occur at any site of connection between membrane proteins and cytoskeleton including the luminal cell surface (383), intercellular junctions (361), abluminal focal attachment sites (35), and the nuclear membrane (286). Because a reasonable case can be made that these sites are mechanically connected, [for example, nuclear shape depends on cell adhesion events that are mediated through integrin-adhesion protein interactions (155, 154)], mechanotransduction mechanisms may be decentralized. This scenario is consistent with multiple pathways of biochemical responses that lead to quite different end results in the cell. Rather than a serial hierarchy of responses emanating from a primary mechanotransducer, decentralization will allow several mechanisms to respond to a variety of stress configurations (Fig. 7B). For example, the ionic conductance of Ca2+ and Na+ in muscle cell membrane changes as cytoskeletal strain is changed. The resulting depolarization opens voltage-dependent potassium channels that initiate repolarization (150), thereby contributing to reversal of the response and the setting of a new mechanical threshold (121); similar mechanisms are applicable to stretch and shear stress-activated ion channels in endothelial cells (see below). The “decentralized mechanism” (Fig. 7B) more readily accommodates temporal differences in responses and a greater diversity of end responses.
Wherever mechanotransduction occurs, the process is likely to be modulated by changes in the stiffness of a receptor(s), the stiffness of the mechanical coupling between the receptor and other structures such as the cytoskeleton, the stiffness of the cytoskeleton itself, and the integrated structure and geometry of the tissue including its association with the extracellular matrix.
Although from structural studies it is obvious that the cytoskeleton interacts mechanically with the plasma membrane, it is only recently that stress transmission to the cytoskeleton via transmembrane proteins has been directly demonstrated. Using ferromagnetic microbeads coated with adhesion peptides or antibodies to integral membrane proteins (integrins), Wang et al. (383) were able to measure the mechanical resistance to deformation of the endothelial cytoskeleton when the beads were twisted in a magnetic field. They noted that the resistance to twisting was a function of the bead/membrane association and the integrity of the cytoskeleton. Only when beads were coated with integrin ligands was a significant mechanical connection maintained. Disruption of the actin microfilaments by cytochalasin D greatly suppressed the stiffening response; disruption of other cytoskeletal components added to the suppression. The studies were directed primarily at β1-integrin, a common member of the β-subunit integrin family (151). They concluded that β1-integrin can act as a cell-surface mechanoresponsive element that can transfer mechanical forces across the cell surface via a specific molecular pathway to the actin stress fibers. This conclusion is likely to be applicable to all integrin-rich regions of the cell where cytoskeletal structures connect with the membrane proteins. Tension and compression in the cytoskeleton of other cells have been directly measured (75). Stress distribution to the cell junctions, focal adhesion sites, and nuclear membrane of endothelial cells subjected to flow is consistent with these models.
The distribution of stress throughout the cell via an integrated structural network might be explained in part by the tensegrity model of tension as developed for cellular structures by Ingber and co-workers (156–158). A notable finding of Wang et al. (383) was that cytoskeletal stiffness increased linearly with the applied stress, a property often noted in living cells (228, 287) and less commonly found in synthetic inert materials. The tensegrity model requires continuous tension to maintain an array of interconnected rigid structures. When tension is changed, the components rearrange to new positions, a response that depends less on the individual components than on the integrated structure. Linear cellular responses to altered stress fit this model well, as does the redistribution of tension to a variety of putative mechanotransduction sites in the cell. While tensegrity is unlikely to provide a complete explanation of flow-related stress transmission in the endothelium (e.g., compression elements may play some role in cytoskeletal mechanics; metabolic regulation undoubtedly is involved), it is a useful model for considerations of cellular mechanotransmission and transduction mechanisms.
B. Plasma Membrane Proteins as Mechanoreceptors
If there are proteins at the endothelial surface that function as mechanotransducers, what characteristics might be required to fulfill such a function? The receptors would be located at a site where either the stress acts directly or to which the stress can be efficiently transmitted. The efficiency of signaling across the membrane to the interior of the cell would be improved if the receptor is a transmembrane protein with significant cytoplasmic sequence perhaps arranged in loops and/or a long tail. Its activity would ideally be associated with a well-defined intracellular signaling pathway(s).
Receptor families that meet these criteria include ion channels, integrins, G protein-linked receptors, and mitogen-activated receptors. The last two groups include several receptors for agonists whose secretion from endothelial cells is stimulated by flow (purinoceptors, neurotransmitter receptors, and growth factor receptors).
C. Mechanosensitive Ion Channels
Endothelial cells and their cell membranes exhibit certain ion channel responses to mechanical forces that appear to be identical to those identified in simpler life forms (222, 318). Other ionic responses, however, are endothelial specific (271). Change of intracellular ion concentrations, particularly calcium and potassium, are implicated in many of the second messenger pathways and in gene regulatory changes discussed in this review. Cationpermeable channels of varying degrees of selectivity have been demonstrated in cultured endothelial cells (2, 61, 136, 190, 269, 270).
Mechanically activated potassium-specific, calcium-specific, and nonspecific cation channel activities are present in cultured endothelial cells. Some interactions between them are mediated by the consequences of their opening (2, 136). For example, potassium channel activation usually leads to a shift in the membrane potential (Vm) toward the reversal potential for potassium, resulting in cellular hyperpolarization that may influence the activity of other channels in the cell. Although voltage-gated calcium channels appear not to be present in cultured endothelial cells (2, 271, 360), hyperpolarization can lead to increased calcium influx via a calcium/phosphatidylinositol/hyperpolarization-activated calcium-permeable channel (202, 204, 270), and hyperpolarization increases the electrochemical gradient for calcium, resulting in calcium influx (136). The opposite effect, depolarization, attenuates cellular functions that rely on calcium influx, e.g., nitric oxide (NO) release and vasodilation (43, 203). The different vectors inherent in hemodynamic forces, pressure, and shear stress also appear to selectively activate ion channels. Thus there are common stretch-activated nonspecific cation channels in endothelial cells (190) that contrast with shear stress-activated potassium channel activity (271). Such cell activities can have opposite consequences for the Vm of the cell, which in turn will influence downstream signaling pathways (61). Presumably, in vivo there will be a differential exposure to the components of blood flow forces such that one or more of these pathways will dominate, depending on the local hemodynamic conditions. An important consideration regarding shear stress-activated channel activity is whether the ion channels are activated downstream from some other kind of mechanical receptor or whether there may be direct alteration of the conformation of the proteins comprising the ion channels to allow increased ion permeability.
Levitan (195) proposed ion channel phosphorylation some years ago. Recently, Olesen and Bundgaard (270) have demonstrated that an inward rectifying potassium channel in bovine aortic endothelial cells requires phosphorylation to remain open. Inside-out patches required administration of ATP to the cytosolic side to maintain ion channel activity. These findings suggest that a prominent inward rectifying potassium channel in endothelial cells has some similar characteristics to the rat kidney channel cloned by Ho et al. (137) and for which a putative ATP binding site impli ated in phosphorylation was identified. However, attempts to clone endothelial potassium channels on the basis of structural homology have been unsuccessful to date.
1. Shear stress-activated potassium channels in endothelial cells
These were first identified by Olesen et al. (271) using whole cell recordings of bovine aortic endothelial cells grown in microcapillary flow tubes. When flow was imposed, a membrane current developed as a function of shear stress with half-maximal activation of 0.7 dyn/cm2. Channel activity reached a plateau near 20 dyn/cm2. On the basis of ion selectivity and reversal potential, the current was identified as an inward rectifying potassium current and was designated IKS. The current was rapidly activated in response to flow, did not rapidly desensitize, and was inactivated when flow was stopped. Recently, single-channel recordings in the cell-attached mode have demonstrated activation of an inward rectifying potassium channel that often demonstrated a delayed opening and closing response to shear stress (163). Because the single channels were not directly exposed to the shear stress (being protected by the micropipette), these preliminary findings suggest that the channel is activated secondarily to other signaling events initiated by shear stress elsewhere on the same cell and transmitted to the channels in the micropipette.
The IKS activity, which was blocked by Ba2+ and Cs+, resulted in hyperpolarization of the endothelial cell. Neither atrial myocytes nor vascular smooth muscle cells expressed IKS, suggesting some degree of endothelial specificity. Endothelial hyperpolarization as a function of shear stress was also reported using membrane potential-sensitive fluorescent dyes (250). Furthermore, studies of unidirectional Rb+ efflux by Alevriadou et al. (6) have confirmed a shear stress-dependent plasma membrane permeability to K+. Cooke and co-workers (51, 265) using pharmacological inhibitors have demonstrated the association of flow-sensitive endothelial potassium channel activity with the release of an endogenous nitrovasodilator in arterial rings. They have also provided circumstantial evidence that activation of a potassium channel is associated with G protein coupling and the elevation of endothelial guanosine 3′,5′-cyclic monophosphate (cGMP). Again, however, it is unclear whether the potassium channel activity is secondary to upstream mechanoreceptors. The potassium channel blockers tetraethylammonium and Ba2+ inhibit IKS and shear-induced downregulation of endothelin-1 mRNA expression (A. Malek, personal communication). Because chelation of [Ca2+]i also inhibits endothelin downregulation, the potassium channel blockers, by preventing hyperpolarization, may inhibit calcium influx.
A link between cyclic nucleotides and potassium channels that may be of general relevance was recently reported (330). Adenylyl cyclase isolated from Paramecium flagellum was reconstituted in a lipid bilayer. The enzyme acted as a potassium channel in which the hyper-polarized state of the enzyme regulated adenosine 3′,5′-cyclic monophosphate (cAMP) production. Thus ion flux and generation of an important biochemical second messenger were regulated by an enzyme that exhibited some of the characteristics of an ion channel.
2. Stretch-activated ion channels
This kind of mechanotransducing ion channel has been demonstrated in a wide variety of cells ranging from primitive organisms to mammalian cells (see review by Sachs, Ref. 318). For example, stretch-activated ion channels have been found in snail neurons (244), skeletal muscle (122), ventricular cells (55), renal tubular epithelium (319), choroid plexus epithelium (45), and vascular endothelial cells (190).
When a micropipette is applied to the cell surface and attached to the cell membrane by suction to form a tight seal, distension of the membrane patch captured in the pipette can be controlled by negative pressure. With the use of such an approach, the degree of stretch has been found to be related to the electrical activity of the membrane and, more specifically, to the opening of transmembrane cation channels. In vascular endothelial cells, Lansman et al. (190) first described mechanosensitive ion channels in membrane patches. During suction pulses of 1–20 mmHg pressure in a cell-attached patch-clamp configuration, single-channel data were collected. The stretch-sensitive channels were cation specific, with a conductance of ~40 pS with both fast and slow components to their opening. A principal consequence of the activation of these channels is the influx of calcium, resulting in depolarization of the cell. The resulting influx of calcium would also be expected to have profound effects on many of the bioresponses to hemodynamic forces as outlined in Table 1.
TABLE 1.
Shear stress and related mechanical stress responses in endothelial cells (arranged in order of response time)
| Effect | Force | Cell Type and Response Time |
Significance | Reference Number |
|---|---|---|---|---|
| K+ channel activation, hyperpolarization (Whole cell recording) |
LSS; 0.2–16.5 dyn/cm2 | BAEC; s | Related to vasorelaxation | 51, 163, 271 |
| Rb+ efflux stimulated | LSS, 1–10 dyn/cm2 | PAEC | Graded transient increase of K+ permeability |
6 |
| Hyperpolarization (Vm-sensitive dyes) | LSS; 10–120 dyn/cm2 | BPAEC; steady state at 60 s |
As above | 250 |
| Activation of nonselective cation channels (membrane patch) |
Suction (pressure, stretch); 10–20 mmHg |
PAEC; ms | Endothelial stretch-activated channels |
190 |
| Intracellular Ca2+ rise (fluo 3) | Mechanical poking and dimpling |
HUVEC; s | Stretch-activated Ca2+ channels; depolarization. |
115 |
| Large increase in release of NO | LSS; 8 dyn/cm2 | BAEC; s | Flow-mediated vasorelaxation |
51, 96, 258, 262, 364, 375, 376 |
| Release of ATP, acetylcholine, endothelin, and substance P |
Flow through microcarrier bed | HUVEC; s | Neurotransmitter release | 26, 27, 237, 294 |
| Stimulation of PA and PE hydrolysis | LSS; 1.4 and 22 dyn/cm2 | HUVEC; 10–30 s | Additional sources of AA; implies activation of PLA2 |
22 |
| Decrease of intracellular pH | LSS; 0.5–13.4 dyn/cm2 | BAEC | Modulation of ionic balance | 406 |
| Transient elevation of IP3; biphasic (BAEC) |
LSS; 30 and 60 dyn/cm2; cyclic stretch |
BAEC, HUVEC; >15– 30 s; major peak at 5 min (BAEC) |
Phosphoinositides as second messenger for shear stress transduction |
20, 22, 260 |
| Intracellular Ca2+ rise; Ca2+ oscillations |
LSS; 0.2–4.0 dyn/cm2 | BAEC; 15–40 s | Ca2+ as second messenger | 7, 106, 332, 339 |
| Flow modulation of effects of vasoactive agonists ATP and bradykinin |
LSS; 0–30 dyn/cm2 | BAEC; s | Indirect stimulation via agonist receptor mechanisms |
82, 83, 239, 261, 341 |
| cGMP increased 3-fold via a NO- dependent mechanism; endothelial K+ channel implicated |
LSS; 0–40 dyn/cm2 | BAEC; 60 s | Vasoregulation mechanisms | 264 |
| Transient elevation of IP3 | Cyclic strain; 24% deformation; 1 Hz |
HSVEC | Phosphoinositides as second messengers for strain deformation |
293, 310 |
| Sustained PGI2 release; G proteins implicated |
LSS; 0–9 and 14.0 dyn/cm2 | HUVEC; 2 min | PGI2 regulation of vascular tone | 19, 95 |
| Pulsed PGI2 release at higher level than steady flow |
LSS (pulsatile); mean 10 dyn/ cm2 |
HUVEC; <1 min | Antithrombotic properties | 118 |
| Augmented factor Xa production (indicative of enhanced tissue factor activity) |
LSS; 0.7 and 2.7 dyn/cm2 | Activated HUVEC; min |
Enhanced procoagulant activity | 119 |
| Vascular free radical generation | Perfusion rates 2–12 ml/min | Ex vivo artery; 10 min |
Unknown | 191 |
| Stimulation of mitogen-activated protein kinases (MAPK); 35 dyn/ cm2 peak |
LSS; 3.5–117 dyn/cm2 | BAEC; 5 min, peak 20–30 min |
Involvement of membrane mitogen receptor-like pathway in shear transduction |
B. Berk, personal communication |
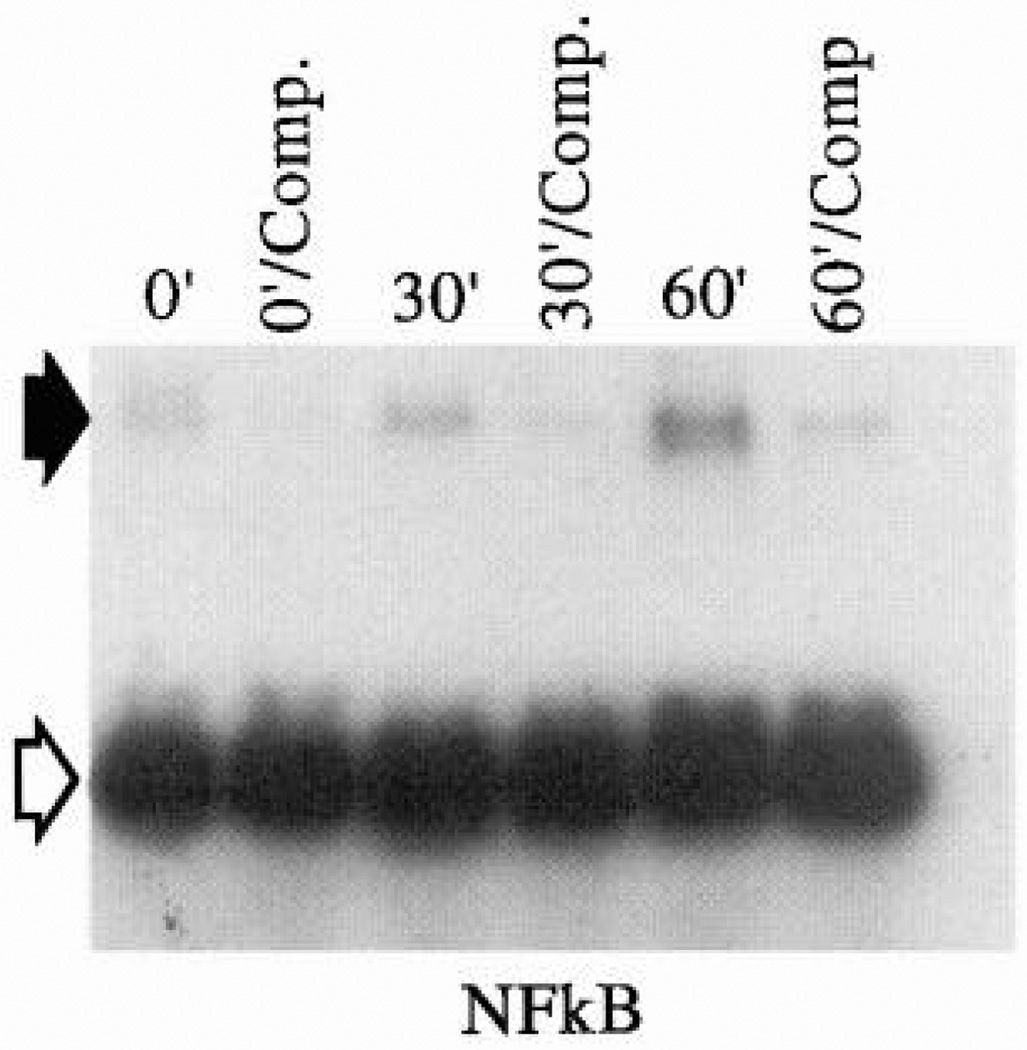
| Activation of NFκB | LSS; 10 dyn/cm2 | BAEC; 20 min | Transcription factor activation | 186, 301 |
| Induction of c-myc, jun | LSS; 10 dyn/cm2 | BAEC; 30 min | Immediate early growth response genes |
146, 186 |
| Activation of adenylyl cyclase | Cyclic stretching; osmotic swelling |
BAEC, HUVEC; min | cAMP as second messenger for stretch |
192, 385 |
| Directional remodeling of focal adhesion sites; Realignment with flow (>8 h) |
LSS; 10 dyn/cm2 | BAEC; min, h | Cell attachment site involvement in transmission and/or transduction of stress |
68, 304 |
| Tension controls cell shape, pH, and growth via extracellular matrix- integrin binding |
Modulation of inherent cell tension |
Capillary EC; <1 h | Integrins regulate cell growth via cell tension |
156 |
| Downregulation of VCAM-1 expression |
LSS; 0–7.2 dyn/cm2 | Mouse lymph node endothelial cells; >1 h |
Preferential leukocyte adhesion at low shear stress |
266, 321 |
| 10-Fold enhancement of PDGF-A mRNA; PDGF-A peak at 1.5–2 h |
LSS; 0–51 dyn/cm2 | HUVEC, BAEC; >1 h | Enhanced mitogen secretion; regulation of SMC growth |
147, 216, 238 |
| 2- to 3-fold increase of PDGF-B mRNA followed by 4-fold decrease by 9 h; PKC dependence controversial |
LSS; 10–36 dyn/cm2 steady, pulsatile, turbulent |
HUVEC, BAEC; 1–9 h | Identification of shear stress response element of PDGF-B gene |
147, 238, 301 |
| bFGF mRNA stimulated 1.5- to 5-fold | LSS; 15 and 36 dyn/cm2 | BAEC; 0.5–9 h, peak at 6 h |
Peptide growth factor regulation |
216 |
| Pinocytosis stimulated; adaptation by 6 h |
LSS; >5 dyn/cm2 | BAEC; <2 h | Plasma membrane vesicle formation rate transiently elevated |
61 |
| Induction of c-fos; 50% block by PKC inhibitor |
LSS; 4–25 dyn/cm2 | BAEC; 1–2 h | Early growth response gene | 146, 148, 295 |
| Increased TGF-β1 mRNA and biologically active TGF protein |
LSS; 20 dyn/cm2 | BAEC; 2 h | Inhibition of smooth muscle growth |
265 |
| Upregulation of ICAM-1 mRNA and protein and enhanced lymphocyte adhesion; downregulated by 6 h |
LSS; 2.5–46 dyn/cm2 | HUVEC; 2 h | Enhanced binding of LFA-1- positive cells |
249, 321 |
| Stimulation of IL-6 secretion and gene expression |
LSS; >10 dyn/cm2 | HUVEC; 2 h | Cytokine secretion | 223 |
| Redistribution of Golgi apparatus and MTOC to upstream location in cell; normalized by 24 h |
LSS; 22 and 88 dyn/cm2 | BCAEC; >2 h | Temporary displacement of organelles |
47 |
| Induction of protein kinase C | LSS; >10 dyn/cm2 | BAEC; <3 h | Regulation of protein phosphorylation |
181 |
| Endothelin mRNA and protein secretion reported to be both stimulated and downregulated; mechanism appears to involve filamentous actin and microtubules |
LSS; 5–20 dyn/cm2 | PAEC and BAEC; peak at 2–6 h |
Regulation of vasoconstriction |
214, 215, 243, 338, 398 |
| NO synthase mRNA and protein stimulated |
LSS; 15 dyn/cm2; flow through microcarrier bed; 0.5–2.0 ml/min |
BAEC; 3 h | Vasorelaxation |
182, 258, 285, 375, 376 |
| HSP 70 mRNA increased 2- to 4-fold | LSS; bidirectional | BAEC; 4 h | HSP 70 shock response to flow | 139 |
| tPA mRNA expression and secretion stimulated |
LSS; 15 and 25 dyn/cm2 | HUVEC; 5 h | Enhancement of fibrinolytic activity |
79–81 |
| Cell proliferation in quiescent monolayer |
Turbulent flow; average shear stress 1.5–15.0 dyn/cm2 |
BAEC; >3 h | Loss of contact inhibition of growth by disturbed flow |
63 |
| Cell alignment in direction of flow; function of time and magnitude of shear stress |
LSS; >5 dyn/cm2 | All types; >6 h | Minimizes drag on cell |
78, 85, 187, 193, 194 |
| F-actin cytoskeletal and fibronectin rearrangement |
LSS; >5 dyn/cm2 and in vivo | All types; >6 h | Associated with cell realignment |
78, 97, 169, 189, 274, 388, 389 |
| Differential cell shape and alignment responses; corresponding F-actin changes |
LSS; pulsatile 1 Hz; sinusoidal flows of various patterns up to 60 dyn/cm2 |
BAEC; >6 h | Discrimination between different types of pulsatile flows |
133, 134, 254, 255 |
| Cell realignment perpendicular to strain; protein synthesis increased; F-actin redistribution perpendicular to strain |
Cyclic biaxial deformation; 0.78–12%; 1-Hz frequency 20–24% strain; 0.9–1.0 Hz |
BPAEC; >7 h HUVEC, HSVEC; 15 min |
Stretching of artery by blood pulsation; separation of strain and shear stress effects |
152, 343, 358 |
| Histamine release and histamine decarboxylase activity stimulated |
Oscillatory LSS; range, 1.6–8.2 dyn/cm2 |
BAEC; >6 h | Modulation of endothelial permeability barrier |
348 |
| Decreased thrombomodulin mRNA and protein at 15 and 36 dyn/cm2 |
LSS; 4, 15, and 36 dyn/cm2 | BAEC; 9 h | Protective role against thrombosis in regions of low shear stress |
217 |
| Increased thrombomodulin activity (synthesis of activated protein C) |
LSS; 25 dyn/cm2 | HUVEC; 24 h | Protective in regions of higher shear stress |
321 |
| Downregulation of fibronectin synthesis |
LSS; 24 dyn/cm2 + 20 mmHg hydrostatic pressure |
HUVEC; 12 and 48 h | Altered cell adhesion; platelet- endothelial interactions |
124 |
| Regional cell cycle stimulation in confluent monolayer |
Disturbed laminar flow (flow separation, vortex, reattachment); 0–10 dyn/cm2 |
BAEC; 12 h | Steep shear gradients stimulate cell turnover; focal hemodynamic effects |
76 |
| Reorganized topography of luminal surface at subcellular resolution |
LSS; 12 dyn/cm2 | BAEC; 24 h | Reduced gradients of shear stress in aligned cells; force transmission altered |
15, 16, 395 |
| Mechanical stiffness of cell surface proportional to extent of realignment to flow |
LSS; 10–85 dyn/cm2 | BAEC; 24 h | Decreased deformability of subplasma membrane cortical complex |
323 |
| LDL metabolism stimulated | LSS; 30 and 60 dyn/cm2 | BAEC; 24 h | Endothelial cholesterol balance | 351 |
| Increase in class I and induction of class II MHC antigen expression |
LSS; 3–36 dyn/cm2 | Fat, omentum, and brain microvessels; 24–36 h |
Role of flow in immune response |
221 |
| Inhibition of endothelial cell division | LSS | BAEC; 24–48 h | Regulation of endothelial regeneration |
407 |
| Inhibition of collagen synthesis and stimulation of cell growth |
Cyclic biaxial stretch; 3 cycles/ min; 24% deformation |
BAEC, myocytes; 5 days |
Inverse relationship related to endothelial repair mechanisms |
359 |
LSS, laminar shear stress; BAEC, bovine aortic endothelial cells; Vm, membrane potential; BPAEC, bovine pulmonary artery endothelial cells; PAEC, porcine aortic endothelial cells; BCAEC, bovine carotid artery endothelial cells; IP3, inositol trisphosphate; HUVEC, human umbilical vein endothelial cells; HAEC, human aortic endothelial cells; PGI2, prostaglandin I2 (prostacyclin); tPA, tissue plasminogen activator; NO, nitric oxide; AA, arachidonic acid; PLA2, phospholipase A2; PDGF-A, PDGF-B, platelet-derived growth factor A and B chains, respectively; SMC, vascular smooth muscle cells; HSVEC, human saphenous vein endothelial cells; MTOC, microtubule organizing center; LDL, low-density lipoproteins; MHC, major histocompatibility complex; HSP 70, 70-kDa heat-shock protein.
The mechanisms by which mechanical forces may control stretch-activated ion channel gating are largely unknown; the little information available has been conducted on nonvascular cells (318). Open probability increases exponentially with the square of differential pressure across the membrane (122). It appears unlikely that the lipid bilayer significantly transmits force to the channel to open it because of limitations in the area of bilayer required to do so (318). Consequently, it seems more likely that the underlying cytoskeleton in some way is connected to the ion channels such that when membrane is captured in a pipette and distended, the cortical cytoskeleton is also stretched; this in turn activates the channel. This may be a very local event because both cell-attached patches and isolated inside-out patches demonstrate similar stretch sensitivity, suggesting that an intact cytoskeleton throughout the cell is not required (122). Sachs (318) has calculated the distribution of these channels to be ~3/µm2 of membrane surface area and has proposed a model in which the channels are located at the nodes of a network of submembranous cytoskeletal elements. A short delay after the application of stretch before the channel opens also suggests an elastic component involved in directing tension to the channel, and treatment with drugs that disrupt the cytoskeleton clearly shift the open probability curve toward lower pressures. Together, these observations suggest that intrinsic cell tension and its alteration by externally loaded forces play a key role in the regulation of these channels.
Variations of stretch-sensitive channels include mechanosensitive potassium ions in the renal medulla that are calcium activated (364). They appear to be involved in osmotic swelling and volume regulation. Other variations found in nonvascular cells include stretch inactivated potassium-selective channels side by side with potassium-selective stretch-activated channels in snail neurons (244). Their activation will antagonize the effects of stretch-activated channels in the same cell and presumably impart a finer regulation of potassium permeability at intermediate membrane tensions. They have not yet been reported in higher animals.
The physiological significance of diverse channel sensitivity to stretch and shear stress in endothelial cells may be related to the high variability of hemodynamic forces in the arterial circulation. In regions of complex disturbed laminar flow, where shear stresses and pressures vary over short distances and throughout the cardiac cycle (60), shear stretch and stress may activate synergistic or antagonistic effects mediated through ion channels. Corresponding regional hyperpolarization/depolarization responses may regulate local tone at such sites (61).
Recently, inward rectifying potassium channels have been cloned from heart and kidney tissues to reveal structures that are quite different from voltage-gated potassium channels (137, 179). Attempts to clone the endothelial counterparts of such channels by sequence homology, however, have yet to prove fruitful (M. Volin, L. Joseph, and P. F. Davies, unpublished data; G. Droogmans, personal communication). Because prominent inward rectifying potassium channel activity is clearly present in endothelial cells as observed by electrophysiological recordings, it seems likely that these channels express unique characteristics.
The mechanism of potassium channel activation by shear stress could be 1) direct displacement of the channel proteins or 2) secondary to a mechanoreceptor and second messengers specific for the channels. The latter explanation would be consistent with similar hyperpolarizing responses to a variety of vasodilatory agonists originating from platelets or erythrocytes in the blood (ATP, ADP, 5-hydroxytryptamine) (145) and from the endothelium itself (acetylcholine, ATP, substance P, histamine, endothelin, arginine vasopressin, bradykinin, angiotensin II) (26, 27, 37, 168, 197, 199, 237). It should be noted, however, that unlike these agonist-receptor interactions, shear stress does not always lead to increased [Ca2+]i, as discussed in section VIII.
D. Focal Adhesions and Integrins as Mechanotransducers
Focal adhesions are regions of the abluminal cell surface where membrane components contact the extracellular matrix (35, 161, 278, 369). They are the sites primarily responsible for cell adhesion and are involved in the regulation of endothelial cell signaling, morphology, proliferation, migration, and differentiation (117, 130, 132, 155, 159, 154, 180, 210, 213, 292, 309, 331, 399, 400). They provide anchorage essential for the maintenance of tension within the cell (hence cell shape) by the association of actin stress fibers on the cytoplasmic face of the plasma membrane with an array of cytoplasmic proteins that link the cytoskeleton to transmembrane integrins (5, 32, 35, 131, 379). This provides a continuum between the intracellular cytoskeleton and extracellular adhesion proteins to which the integrins are bound (73, 87, 144, 247, 276). Focal adhesions are the most intensively studied of the possible cytoskeletal-linked mechanotransduction sites to which cell tension is transmitted by flow. Until several years ago, the sites were regarded as relatively inert, (105, 354, 370). However, work over the past 7 years has identified focal adhesions as sites of concentrated activity of protein kinase homologues of viral oncogenes (142, 143, 371–374) and protein kinase C (372) and implicated them in cell signaling (87). In endothelial cells, the link between focal adhesions and mechanotransduction became apparent when it was demonstrated that shear stress applied to the luminal surface of cultured bovine endothelial cells or human umbilical vein endothelial cells (HUVECs) resulted in directional remodeling of the abluminal focal adhesion sites (69, 70, 304). This was observed directly in living cells using tandem scanning confocal microscopy (Fig. 8), a technique that when combined with digitized image analysis provides quantitative information about the dynamics of membrane-substratum interactions (69). Endothelial focal adhesion sites undergo rapid remodeling even in quiescent cells within the confluent monolayer, but the direction of extension and retraction of membrane contact is random. During flow (10-dyn/cm2 unidirectional shear stress), however, periods of directional remodeling occurred that were sometimes immediate, often delayed, and led to the alignment of focal adhesions in the direction of flow together with fusion of smaller contact regions to form a reduced number of larger adhesion sites (Fig. 9). Girard and Nerem (111) also observed directional redistribution of proteins associated with focal adhesion sites including vinculin and integrins. The cytosolic proteins focal adhesion kinase (p125FAK), tensin, and paxillin, all enriched at focal adhesions, show similar alignment (unpublished data). The area of membrane/substratum contact does not vary greatly through out the remodeling process, and calculations of cell adhesion from measurements of contact area and separation distance between membrane and substratum showed adhesion to be constant despite the dynamic characteristics of the remodeling process (69, 71). These studies demonstrate little change in adhesion as defined by the physical proximity of membrane and substratum; however, they did not directly address the attachment strength of the focal adhesions (although there is good correlation between focal adhesion area and centrifugal force required to detach cells).
FIG. 8.
Endothelial abluminal cell surface geometry observed in real time by tandem scanning confocal microscopy after image processing (Quantex QX-7) and computer enhancement (Silicon Graphics Indigo II, IDL software). Membrane is organized into focal adhesion sites that extend downward to (invisible) substratum. Because this is a perspective view, scale marker refers to center of image only. (Courtesy of A. Robotewskyj and Dr. M. L. Griem, The University of Chicago, Chicago, IL.)
FIG. 9.
Tandem scanning confocal microscopy of endothelial cell focal adhesion site rearrangement during flow. Detailed real-time images 1 min apart of reorganizing focal contacts in a cell within a confluent monolayer during flow. Width of field is 2.5 µm. Progressive fusion and alignment of regions occurred over a short interval. Note that small area to left of newly forming aligned site did not significantly change, whereas other sites, above and below, migrated in direction of flow (shown by arrow). Cell boundaries did not significantly change during this period. [From Davies et al. (70). Reproduced from The Journal of Clinical Investigation, 1993, vol. 91, p. 2640–2652 by copyright permission of The Society for Clinical Investigation.]
The cytoskeletal events that may alter the structure of attachment sites and hence change their strength have been recently investigated by Ward and Hammer (384). They modeled focal adhesions as integrin clusters, linked to the cytoskeleton, that bind extracellular adhesion proteins. The extent of adhesive strengthening when focal adhesions assemble depends on the elastic rigidity of the cytoskeletal connections. They concluded from theoretical and experimental calculations that rigid cytoskeletal connections favor much greater attachment strengths. This conclusion is also consistent with decreased membrane deformability (323) and increased resistance to detachment when endothelial cells have aligned with the flow, although streamlining of the luminal surface (15) will decrease the effective shear stress and also favor attachment.
Another quantitative aspect accessible from image analysis is the rate of remodeling, defined as the two-dimensional focal adhesion area gained and lost per unit time. Equivalent rates were measured whether flow was present or not (70). Thus, during flow, only the direction of the remodeling event was different, not the quantity of remodeling. In contrast, however, the rate of remodeling was markedly changed when the composition of the extracellular adhesion proteins was modified; this presumably is mediated through different integrins. These studies demonstrated that focal adhesions are dynamic structures, an observation consistent with their signaling function, and that frictional shear stress at the luminal endothelial surface is transmitted to the abluminal focal adhesion sites via the cytoskeleton.
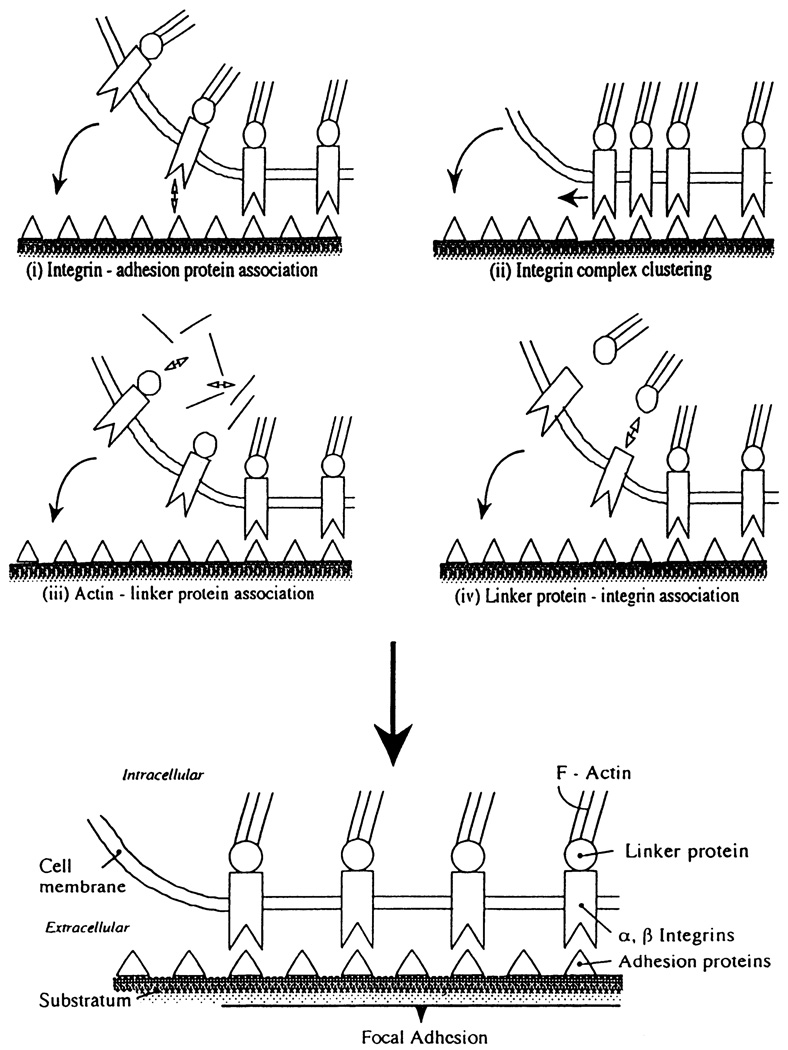
The molecular mechanisms responsible for focal adhesion remodeling are unclear. Transmembrane integrins bind adhesion molecules of the extracellular matrix on the outside and connect to the cytoskeleton via linker proteins on the inside. During remodeling, cell membrane must approach close enough (10–15 nm) to the substratum to form new focal adhesion area, a process that may involve changes of integrin-adhesion protein association, lateral migration of existing integrin-adhesion protein complexes, and/or association of cytoskeletal elements and integrins with linker proteins to establish structural continuity from inside to outside the cell (Fig. 10). The most relevant integrins in endothelial cells appear to be the fibronectin receptors α5β1 and αvβ1, laminin receptor α6β1, vitronectin receptors αvβ3 and αvβ5, and the basement membrane receptor α6β4 (44, 171). As a consequence of integrin specificity for different adhesion proteins, the composition of the extracellular matrix influences focal adhesion remodeling rates (70) and endothelial adhesion (328) during flow.
FIG. 10.
Mechanisms of focal adhesion formation/remodeling. Changes in affinities of principal proteins include those between integrins and adhesion proteins (i); between cytoskeleton and linker proteins such as talin, vinculin, tensin, paxillin, p125FAK, and α-actinin (iii); and between linker proteins and integrins (iv). Clustering of integrin complexes (ii) may also be involved.
Recently, several cytoplasmic proteins have been identified that are tyrosine phosphorylated during cell adhesion to extracellular matrix proteins but not when the cells are attached to uncoated plastic or to poly-l-lysine-coated surfaces (25, 213). The proteins include p125FAK, a tyrosine kinase localized to focal adhesions (325), paxillin (373, 371), tensin, and actin binding protein (25). Inhibition of tyrosine kinase activity led to diminished phosphorylation and inhibited the formation of focal adhesions and stress fibers (36). Phosphorylation of linker proteins on the cytoplasmic face of the plasma membrane is intimately involved in cell adhesion and focal adhesion dynamics in fibroblasts. We have recently examined the phosphorylation state of endothelial cells. Antiphosphotyrosine Western blots of lysates of adherent endothelial cell monolayers demonstrated two prominent groups of proteins at 116–125 kDa and 65–75 kDa that were identified on immunoblots as p125FAK and paxillin, respectively. Both were shown to be associated with endothelial focal adhesion sites by immunofluorescence. Within 2 h of exposure to unidirectional laminar flow at 12-dyn/cm2 shear stress, paxillin tyrosine phosphorylation increased greater than twofold when compared with no-flow monolayers (A. Banega, H. Tipping, and P. F. Davies, unpublished data). In contrast, p125FAK phosphorylation remained near control levels. Paxillin phosphorylation may be involved in the intracellular mechanotransduction of blood flow forces in endothelial cells. Romer et al. (309) have demonstrated increased tyrosine phosphorylation of p125FAK in HUVECs during migration into a wound in the monolayer in the absence of flow; however, no changes in paxillin were identified, although Burridge et al. (36) have shown a role for tyrosine phosphorylation of paxillin as well as p125FAK in cytoskeletal assembly. The cytoskeletal reorganization during endothelial exposure to flow would be consistent with changes of paxillin activity. Absence of changes in p125FAK phosphorylation is consistent with a constant level of cell adhesion as measured by digital image analysis (70). Signaling mechanisms that involve different members of the integrin family are poorly understood but appear to involve specific phosphorylation events. p125FAK and proteins that contain homologous domains of src binding domains (SH-2 and SH-3) appear to be primarily involved (87, 325, 402).
Recent work suggests convergence of signaling pathways at the integrin-rich focal adhesion sites. Hansen et al. (128) reported the localization of a heterotrimeric G protein γ-subunit (γ5) to these regions and to adjacent stress fibers in a variety of cells. The distribution of γ5 was most similar to that of zyxin, a protein that binds α-actinin (56) and is considered to be involved in focal adhesion signal transduction (87). When the G protein-coupled receptors for endothelin and bombesin were stimulated by agonist binding, there was immediate phosphorylation of p125FAK (402), suggesting the localization of these receptors to focal adhesion sites. The colocalization of the γ5-subunit of G proteins is consistent with convergence of signaling pathways involving two families of transmembrane proteins: G protein-linked receptors and integrins. Heterotrimeric G proteins may therefore also be involved in integrin receptor signaling. Arcangeli et al. (11) have shown that pertussis toxin inhibition of G protein activation interfered with a cell adhesion-activated potassium channel that is linked to integrin-extra-cellular matrix binding.
Considering the dynamic state of focal adhesion sites and their involvement in mechanical responses of endothelial cells to shear stress (63, 64, 70), it seems reasonable that both phosphorylation and G protein-linked pathways may be involved in mechanical signaling at these sites, as well as at the luminal plasma membrane.
E. G Protein-Linked Receptors
In a review of sensory transduction, Shepherd (342) drew attention to common aspects between vision, hearing, photoreception, olfactory transduction, and mechanoreception at the molecular level. The conversion of stimulus energy to a sensory response that eventually involves ion channels resides primarily in two types of mechanisms: 1) direct displacement, as for example in hearing (149) or mechanical poking (115), and 2) the interactions of molecules (or photons) with transmembrane receptors, as occurs for vision, olfaction, and taste (342). This distinction is essentially the same as that discussed for alternative (displacement vs. agonist-mediated) mechanisms of shear stress responses alluded to earlier in this review and discussed more fully in sections VII and VIII. In sensory transduction systems, seven transmembrane domain G protein-linked receptors (serpentine receptors) are commonly involved (342). Considering that G proteins play a prominent role in the regulation of the cardiovascular system (174, 303), what is the evidence that the serpentine superfamily of transmembrane receptors may participate in mechanotransduction of hemodynamic forces in the endothelial cell?
One aspect has already been discussed in the context of G protein γ5-subunit localization to focal adhesions and the role of focal adhesions in the integration of force transmission in the endothelial cell. Serpentine receptors, however, are distributed over the entire surface of the cell. They are integral membrane proteins whose sequences include seven hydrophobic domains corresponding to transmembrane regions that span the membrane (387). They represent a widespread system that upon stimulation by the appropriate ligand transmits signals into the cell by interaction with G proteins at the cytoplasmic COOH-terminal of the receptor. The G proteins are heterotrimeric, being composed of α-, β-, and γ-subunits, each encoded by separate gene families; the family that encodes α-subunits is especially diverse (46). There are, therefore, many possible heterotrimeric combinations of subunits. In the resting state, GDP is bound to the α-subunit of the intact heterotrimer. Upon binding of agonist to the serpentine receptor, the G protein interacts with the cytoplasmic tail of the receptor, and GDP dissociates from the G protein complex. In a Mg2+-dependent activation step, GTP binds to the unoccupied guanine nucleotide-binding site causing a conformational change and the dissociation of the heterotrimer to a GTP α-subunit and a βγ-dimer (48, 387). The subunits then regulate metabolic pathways resulting in the activation of various second messengers, enzymes, and ion channels (363). The mechanism is switched off by hydrolysis of bound GTP and the reassociation of GDP α-subunit with βγ-dimer. Activation of G protein can be manipulated by fluoride (that mimics bound GTP; Ref. 120), nonhydrolyzable analogues of GTP [e.g., guanosine 5′-O-(3-thiotriphosphate) and 5′-guanylyl-imidodiphosphate that resist guanosinetriphosphatase (GTPase)-mediated switch off], and cholera toxin (that also suppresses GTPase activity). In contrast, pertussis toxin inhibits the activated state by ADP-ribosylation of the α-subunit, resulting in uncoupling from the receptor. A number of endothelial receptors, including those activated by ATP, thrombin, and bradykinin, are G protein linked. They appear, however, to elicit intracellular effects through the activity of diverse G proteins, some of which are pertussis toxin sensitive (5-hydroxytryptamine and histamine receptors), whereas the majority either are insensitive (thrombin and purinergic receptors) or the data are ambiguous (bradykinin receptor) (136).
1. Second messengers associated with activated G proteins
Both GTP α- and βγ-subunits of G proteins regulate downstream signaling (46, 251). There is a wide diversity of G proteins involved in signal transduction (346). Types of activity include Gi proteins that inhibit adenylyl cyclase and activation of potassium channels, Gs proteins that stimulate adenylyl cyclase and activate calcium channels, and Gq proteins that activate phospholipase C (PLC) with the release of 1,2-diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). Two isoenzymes of PLC are particularly sensitive to activation by βγ-dimers while other isoenzymes are less responsive. Inositol 1,4,5-trisphosphate stimulates the mobilization of [Ca2+]i, and DAG mediates the activation of the protein kinase C (PKC) family of enzymes (18, 235). This is a pertussis toxin-sensitive mechanism of phosphoinositide hydrolysis in which both the α- and βγ-subunits are involved (24). Other PLC isoenzymes are not activated through this mechanism but via tyrosine phosphorylation of mitogen-stimulated receptors (see sect. vF). Both Gi and Gs act on adenylyl cyclase to inhibit or stimulate, respectively, the generation of cAMP; Gi is also directly implicated in the activation of potassium channels (46).
2. Evidence for G protein-linked receptors in mechanotransduction
By inference to G proteins, G protein-linked receptors are likely to be involved in mechanotransduction. Activation may be secondary to flow-mediated binding of a known receptor, or the receptor may itself be uniquely mechanosensitive. The data supporting a role for G proteins and G protein-linked mechanoreceptors can be summarized for a number of shear stress-related responses in endothelial cells.
The consequences of G protein stimulation of PLC activity are common to several flow-initiated endothelial responses. These include the generation of IP3 and DAG (21, 261, 293) that may lead to elevated [Ca2+]i and activation of PKC (259). Cleavage of arachidonic acid from DAG by diacylglycerol hydrolase or from phospholipids by phospholipase A2 leads to the subsequent release of prostaglandin (PG) I2 (prostacyclin; Refs. 95, 118). Endothelin-1 release is also mediated by a PLC-PKC pathway (84). A similar series of events associated with PGI2 release is stimulated when endothelial cells are treated with aluminum fluoride (211), a G protein activator that binds to the nucleotide site adjacent to the β-phosphate of GDP and mimics the phosphate of bound GTP (23). Some PLC isoenzymes are pertussis toxin sensitive while others are not, and a mixture of these isoforms when combined with a variety of pertussis toxin-sensitive and -insensitive α and βγ-subunits (387) appears to be the likeliest explanation of variable sensitivity of shear stress responses to this toxin. For example, with respect to flow-stimulated NO production, there are conflicting reports of pertussis toxin-sensitive (265) and -insensitive (181) G proteins associated with PLC activation. Furthermore, prostacyclin release was shown to be pertussis toxin-sensitive in HUVECs in which inhibition of both early and late phases of the response occurred (19, 20); in contrast, PGI2 release in no-flow cells was pertussis toxin insensitive.
There is some evidence of G protein-mediated regulation of PDGF gene expression by shear stress via a PKC-dependent mechanism (147).
Stimulation of phospholipase A2 activity with the release of arachidonic acid has been reported for several G protein-linked receptors (196, 252). This would provide another pathway for prostacyclin release by shear stress.
The activation of shear stress-sensitive potassium channels (271) may be directly mediated by G proteins. Clapham (46) has recently examined the evidence for direct G protein interaction with ion channels.
There are specific associations between the cytoskeletal protein tubulin and G proteins, suggesting a role in signaling when cell tension changes (296).
Both Gi and Gs regulate adenylyl cyclase and the synthesis of cAMP in endothelial cells. Shear stress at 4 dyn/cm2 was reported to increase cAMP at least fourfold in HUVECs (298). However, it should be noted that Malek et al. (216) reported no changes in cAMP in bovine arterial endothelial cells subjected to 20 dyn/cm2.
Taken together, these findings suggest a role for G proteins in mechanotransduction. If an endothelial mechanosensitive G protein-linked receptor is identified, it will be important to determine whether it is a primary mechanoreceptor, a flow-sensitive ligand-coupled receptor (secondary mechanoreceptor), or a flow-regulated hormone receptor that is only indirectly involved in mechanotransduction.
F. Mitogen-Activated Protein Kinase Signaling
It has recently been demonstrated that in mammalian cells, G protein-linked receptors activate a mitogen-activated protein (MAP) kinase cascade that transmits growth and differentiation signals from the cell surface to the nucleus (49, 50). The MAP kinase pathway was first discovered in relation to mitogen receptors that activate the cascade by tyrosine phosphorylation of the receptor tail. A key molecular switch between the surface signal and the MAP kinase pathway is Ras, a GTPase (126). Mitogens that use G protein-linked receptors, e.g., thrombin, can acutely activate the MAP kinase cascade by Ras-dependent and -independent pathways, but sustained activation of MAP kinase and DNA synthesis requires Ras (50). Recently, it has been shown that the MAP kinase cascade is activated by GTP α- and βγ-subunits of G proteins (57). The requirement for Ras resides in its ability to interact with a MAP kinase, Raf, that phosphorylates MAP kinases Erkl and Erk2, which then translocate to the nucleus where they phosphorylate key transcription factors (368). Ras interacts with Raf to localize it to the plasma membrane (126), an important requirement for subsequent Raf phosphorylation of Erkl and Erk2. Is this signal transduction cascade, which is common to both mitogen receptors and G protein-linked receptors, involved in mechanotransduction?
Berk (B. C. Berk, personal communication) has demonstrated phosphorylation of 42- and 44-kDa MAP kinases in endothelial cells subjected to shear stresses in the range of 3.5–117 dyn/cm2. The maximal effect was at 35 dyn/cm2 and was preventable by the nonhydrolyzable GDP analogue guanosine 5′-O-(2-thiodiphosphate) and by PKC inhibition or downregulation. Mitogen-activated protein kinase activation by shear stress was independent of [Ca2+]i and was unaffected by modulators and inhibitors of potassium channels and stretch-activated channels but was blocked by inhibition of tyrosine kinase activity. These studies are consistent with a phosphorylation-dependent mechanoreceptor somehow linked to the MAP kinase cascade and possibly involving G proteins. With the ubiquitous control of MAP kinase systems by Ras, it will be of interest to see if this molecular switch plays any role in mechanotransduction.
G. Other Receptors
A number of other endothelial plasma membrane receptors of diverse functions have been reported to be regulated by shear stress.
1. Thrombomodulin
Thrombomodulin (TM) is an integral membrane glycoprotein. Protein C is activated when thrombin binds to TM. Activated protein C forms a complex with protein S that inactivates factors Va and VIIIa, resulting in the inhibition of thrombin formation (86). Thrombomodulin also indirectly influences thrombin-thrombin receptor interactions, and consequently receptor signaling (281). Malek et al. (217) have reported a small transient increase followed by a significant decrease in TM mRNA over a period of 9-h exposure of bovine arterial endothelial cells to shear stresses of 15 and 36 dyn/cm2. Thrombomodulin protein declined 33% after 36 h of shear stress at 15 dyn/cm2. The decreases, which were independent of the characteristics of flow (steady or unsteady flow), were reversible within 6 h. In contrast, bovine smooth muscle cells showed no response. In HUVECs, however, McIntire (L. V. McIntire, personal communication) has reported a fourfold increase in TM activity as measured by synthesis of activated protein C after 24-h exposure to 25 dyn/cm2. These inconsistencies may be due to species differences or to some independence between TM activity and protein expression.
Changes in TM are of relevance to hemodynamic regulation of coagulation. When considered in conjunction with flow-dependent stimulation of tissue plasminogen activator (79–81), TM activity is predicted to vary with the characteristics of the hemodynamic field, an effect that may have some bearing on hemodynamic regulation of pro- and anticoagulant regions of arteries in vivo.
2. Adhesion molecules of the immunoglobulin superfamily
These transmembrane glycoproteins are important in inflammation, during which they specifically bind circulating blood cells to the endothelial surface (see Ref. 151 for a review of adhesion receptors). Intracellular adhesion molecule 1 (ICAM-1), constitutively expressed in endothelial cells, binds β2-integrin receptors such as lymphocyte function associated antigen-1 on circulating lymphocytes, monocytes, and polymorphonuclear leukocytes. Vascular cell adhesion molecule 1 (VCAM-1), upregulated in endothelium overlying atherosclerotic lesions (58), binds the integrin very late activation antigen-4 present on monocytes and lymphocytes but not on neutrophils. Nagel et al. (249) have reported specific upregulation of ICAM-1 expression by HUVECs exposed to shear stresses in the range of 2.5–46 dyn/cm2, with a correlative increase of leukocyte cell adhesion. The effect was time dependent but not force dependent and was not attributable to flow-secreted cytokines (which independently induce ICAM-1). Expression of ICAM-1 mRNA increased up to 8 h and remained above control levels at 24 h. In contrast, VCAM-1 levels were not stimulated. Ohtsuka et al. (266) have reported decreased expression of VCAM-1 in mouse endothelial cells exposed to a lower range of shear stress levels (0–7 dyn/cm2) for up to 24 h. This downregulation appeared to be force dependent. A progressive decrease of VCAM-1 expression was noted within 6 h in HUVECs by McIntire (personal communication), who also observed a different pattern of ICAM-1 responses than that reported above (249). Instead of a sustained elevation of ICAM-1, expression was slightly elevated at 1 h but declined below basal levels within 6 h. Interestingly, the ICAM-1 gene but not VCAM-1 contains a shear stress response element (SSRE; see sect. IX) in its promoter (72), suggesting that the regulation of this molecule is secondary to hemodynamic signaling rather than ICAM-1 itself being a primary mechanoreceptor.
VI. TEMPORAL CONSIDERATIONS OF HEMODYNAMIC RESPONSES
Endothelial responses to flow tend to fit into related groups that can be sorted on the basis of response times. In 1988, a table listing a half-dozen hemodynamic responses of endothelial cells represented the then-current knowledge in this area (61). The huge expansion of effort in endothelial mechanotransduction research during the last 6 years is reflected in Table 1. The entries, arranged in approximate order of response time to mechanical stress, follow a similar format as that used previously (71). It is useful to consider the interrelationships of these events, which are condensed into temporal groupings in Table 2.
TABLE 2.
Endothelial responses to unidirectional shear stress: temporal groupings
| Within 1 min | |
 | |
| 1 min to 1 h | |
| G protein activation | Signaling cascades Transcription factor activation and initiation of gene regulation |
| MAP kinase signaling | |
| NFκB activation | |
| SSRE-dependent gene regulation: PDGF-B, c-jun | |
| bFGF upregulation | |
| Pinocytosis stimulated | |
| 1–6 h | |
| SSRE-dependent gene regulation: eNOS, tPA, TGF-β, ICAM-1, c-fos, MCP-1 |
Gene regulation and protein synthesis Beginning of cell-wide adaptive responses |
| Stimulation of HSP 70 | |
| Downregulation of ET-1 | |
| Cytoskeletal rearrangement | |
| Focal adhesion rearrangement | |
| Transient rearrangement of Golgi and MTOC | |
| >6 h | |
| Reorganization of luminal surface | Adaptive responses to new hemodynamic conditions |
| Cell alignment | |
| Completion of cytoskeletal rearrangement | |
| Increased mechanical stiffness | |
| Decreased fibronectin synthesis | |
| Changes of TM expression | |
| Stimulation of histidine decarboxylase | |
| Enhanced LDL metabolism | |
| Induced MHC antigen expression | |
DAG, 1,2-diacylglycerol; bFGF, basic fibroblast growth factor; TGF-β, transforming growth factor-β; ICAM-1, intracellular adhesion molecule 1; ET-1, endothelin-1; MAP; mitogen-activated protein; SSRE, shear stress response element; NFκB, nuclear factor kappa B; eNOS, endothelial nitric oxide synthase; TM, thrombomodulin; MCP-1, monocyte chemoattractant protein 1. Other definitions are as in Table 1.
The fastest detectable changes after mechanical stimulation occur within seconds and include a variety of electrophysiological and biochemical responses. They may quickly lead to end responses such as vasotonic adjustment within a few seconds (Table 2). Presumably, the response cascades also terminate quickly. In some instances, desensitization is associated with sustained stimulation.
Delayed responses (minutes to several hours) are associated with cytoplasmic and nuclear changes linked to transcription factor activation and the regulation of gene transcription. The group includes variably timed responses ranging from the generation of third messenger transcription factors within a few minutes (186) to the induction of PDGF mRNA expression over several hours (147). This time interval overlaps with protein translation that has far-reaching consequences for endothelial structure and function as reflected in major morphological and metabolic adaptations.
Several possibilities for interactive stress mechanisms are implicit in Table 2. First, it is likely that there are separate pathways connecting elements within each group of responses. For example, rapid calcium elevation leading to calcium/calmodulin-dependent stimulation of endothelial NO synthase activity may be distinguished from transcription factor-dependent induction of the PDGF B chain (PDGF-B) gene. Second, the fastest responses are likely to be closer to a primary mechanotransduction mechanism than later responses, and this may provide the means to identify a mechanosensor. For example, the quest to clone flow-sensitive potassium channels is driven not only by the central role that they appear to have in both agonist and flow-mediated vasodilation, but also by the possibility of identifying accessory proteins that function as cofactors or membrane mechanoreceptors for channel activation. A second example is the involvement of G protein-like activities in flow signaling (51, 265) that when considered in relation to flow-induced autocrine secretion of powerful endothelial agonists, might implicate seven transmembrane G protein-linked mechanoreceptors. Therefore, with the arrangement of mechanical responses in a time sequence, useful clues regarding different patterns of interactions may be revealed.
A striking feature of many of the stress-related responses in Table 1, especially the early responses, is that they are similar to those that often occur following ligand-receptor binding. Membrane proteins displaced by mechanical force may therefore be members of existing receptor families. Alternatively, ligand-receptor binding of soluble effectors (both known and unknown) may represent an indirect mechanism of flow signaling that plays an important role in mechanotransduction as discussed in section VII.
VII. INDIRECT MECHANISMS OF HEMODYNAMIC RESPONSES: FLOW MODULATION OF AGONIST MASS TRANSPORT AND RECEPTOR-LIGAND INTERACTIONS
Endothelial mechanotransduction mechanisms can be considered within two conceptually distinct mechanisms: 1) physical displacement of a structure at the cell surface or displacement of an integral part of the cell tension system, a direct force mechanism, as has been discussed so far in this review, and 2) an indirect effect of flow that changes the concentration of solutes at the endothelial surface. This latter mechanism is undoubtedly of importance when high local concentrations of labile agonists are released close to the endothelial surface, e.g., ADP during platelet aggregation and neurotransmitters released from the vessel wall.
The local concentration of a plasma solute in the unstirred boundary layer at the cell surface is similar to its concentration in the bulk fluid if the rate of removal from the cell surface is slow. For most ligands, removal is typically by receptor-mediated endocytosis, a relatively slow process (minutes) (31). However, when the solutes are labile ligands and/or substrates for endothelial surface enzymes, the convective mass transport characteristics of the bulk fluid at a given flow rate (or at changing rates as in pulsatile flow) can influence the local agonist concentration, modify agonist-receptor interactions, and consequently modulate the cellular response. Some agonists are removed very rapidly by degradation at the cell surface, e.g., adenine nucleotides, bradykinin, and angiotensin (98, 123, 273). When the removal rate exceeds convective and diffusive delivery from the bulk fluid, a steep concentration gradient will exist between the bulk fluid and the cell surface, and this in turn will be influenced by the flow characteristics. Chemical gradients in the opposite direction will also be influenced. If an agonist or chemokine secreted by the endothelial cells or other cellular components of the vessel wall can interact with endothelial receptors, the concentration gradient near the surface will also be affected by the flow and thereby influence the autocrine interactions. Thus by interfering with mass transport, flow influences the availability of agonists for their receptors at the cell surface (Fig. 11). There is experimental evidence to support the mass transport hypothesis in vitro and to suggest that the direct and indirect effects of flow on endothelial responses are not mutually exclusive.
FIG. 11.
Mass transport at endothelial surface, Availability of an agonist in boundary layer adjacent to cell surface is determined by diffusion and convective delivery, balanced by removal via degradation and binding/internalization. If degradation is rapid, mass transport becomes rate limiting. Flow influences rate constants for mass transport and therefore indirectly regulates agonist availability to the cell.
The mass transport hypothesis has been studied most intensively with respect, to mobilization of endothelial [Ca2+]i (8, 9, 82, 83, 239, 260, 272). Conditions for depletion of the boundary-layer concentration of agonists exist when a potent degradation system for a particular agonist is present at the endothelial cell surface. Using adenine nucleotides as the agonist and [Ca2+]i mobilization as the response, several groups (82, 239) have demonstrated flow regulation of ATP- and ADP-calcium coupling via an imbalance between the mass transport delivery of ATP/ ADP to the cell surface (which is increased by flow) and the degradation by ectonucleotidases. It follows that small changes in the rate constants for convection of ATP from the bulk fluid to the cell surface or for the degradation of ATP at or near its receptor (the P2y purinoceptor) significantly influence the boundary-layer ATP concentration. Calculations of the mass transport rates indicate a change in concentration within 10 s when flow rate is changed, consistent with rapid on-off calcium responses (83). Similar conclusions that the rate of delivery of ATP from the bulk fluid exceeds the degradation of ATP as flow (and shear rate) increases were reached by Nollert et al. (260), who estimated the concentrations of ATP at the endothelial cell surface to be 60 and 80% of that in the bulk fluid at a shear stress of 10 and 100 dyn/cm2, respectively, while at 10−2 dyn/cm2, the concentration is predicted to be effectively zero. However, Shen et al. (339) have demonstrated that when the intermediate product of ATP degradation, ADP, is included in the calculations, the concentration of adenine nucleotides (ATP + ADP) at the cell surface is unlikely to drop below critical values for activation of the P2y purinoceptor. Adenosine 5′-diphosphate is equipotent with ATP in eliciting [Ca2+]i responses via purinoceptors. The latter study suggests that at least a part of the mechanism of flow-regulated calcium signaling in endothelial cells is a direct effect on the sensitivity of agonist/purinoceptor signaling or cell-surface ectonucleotidase activity as proposed earlier (82, 83).
Intracellular calcium responses were highly sensitive to the fluid motion at the cell surface (Fig. 12); consecutive small increases of flow stimulated large [Ca2+]i transients, the levels returning to baseline at the new flow rate within 4 min (82, 83, 239). The characteristics of [Ca2+]i transients were also influenced by decreasing flow (83). When a nonhydrolyzable ATP analogue, adenosine 5′-O-(2-thiodiphosphate) (ADPβS), that resists degradation by surface ATPases was used instead of ATP, only an initial calcium transient was observed, and subsequent changes of flow rate did not influence [Ca2+]i. This occurred because the boundary-layer concentration of nonhydrolyzable ADPβS was the same as in the bulk flow, resulting in saturation of the receptors. When steady-state mass transport coefficients for ATP under various flow conditions were compared with the estimated rate constant for surface degradation of ATP to adenosine, ratios close to unity were obtained (83), suggesting that both boundary layer mass transport and ATP clearance rates can be rate limiting for flow-mediated activation of the P2y purinoceptor.
FIG. 12.
Flow modulates intracellular calcium ([Ca2+]i) response to purinergic agonist (extracellular ATP) in endothelial cells. A small change in flow rate (Q) [and shear stress (τ), dyn/cm2] disturbs mass transport of ATP in boundary layer, thereby favoring ATP-P2y purinoceptor interaction, which induces a calcium response. Concentration of ATP in bulk flow was constant at 1 mM. Up arrows indicate step increase of flow rate; down arrows indicate cessation of flow. [From Dull et al. (83).]
There could be a stimulation of adenosinetriphosphatase (ATPase) activity when ATP associates with the purinoceptor under flow conditions. Alternatively, shear stress itself may influence ATPase activity; several enzyme systems have been reported to be shear sensitive (107, 350, 367). If ATPase activity increased several seconds after a step increase in flow, the boundary-layer ATP concentration would be restored below a critical threshold for activation of the purinoceptor, and [Ca2+]i would decline. However, such a mechanism does not explain the absence of repeated responses in the presence of ADPβS. This nonhydrolyzable analogue would be expected to continuously stimulate [Ca2+]i because ATPase activity would now be irrelevant. However, as shown by Dull et al. (83), [Ca2+]i usually returned to 120% of basal levels in the presence of ADPβS; therefore, this mechanism of ATPase activation can only account for part of the experimental observations. Nevertheless, in the absence of specific inhibitors, activation of ATPase enzyme activity cannot be discounted.
The sensitivity of the purinoceptor may be altered during flow by intracellular feedback following signal transduction. In this case, the ability of the P2y purinoceptor to bind ATP would be altered such that higher concentrations of ATP are required for restimulation of [Ca2+]i. Because ATP is substrate for both the receptor and the ectonucleotidase (ATPase), an interaction between them whereby activation of the enzyme influences ATP/receptor binding also cannot be ruled out. Although it has been proposed that the P2y purinoceptor and ecto-ATPase are the same molecule (127, 212), Pearson and Cusack (282) have demonstrated that each has distinct characteristics regarding stereospecific requirements for ATP; therefore, they are likely to be two distinctly different molecules. On the cytoplasmic side of the plasma membrane, receptor phosphorylation by calcium-dependent kinases inactivates some receptors until calcium is removed, reducing kinase activity and allowing phosphatases to dephosphorylate the receptor (74, 208, 344, 380). Thus receptor activation, modulated by flow-induced changes of mass transport, may be responsible for an autofeedback mechanism that alters the sensitivity of signal transduction to match the prevailing hemodynamic conditions. These mechanisms may involve a complex playoff between direct effects of shear stress on the receptor system and mass transport of the ATP in the boundary layer.
Differential changes in the shear rate and shear stress at the surface of endothelial cells can be achieved by varying the viscosity of the fluid. Using such techniques, Ando et al. (9) have recently provided evidence for a direct mechanism of force transduction in evoking [Ca2+]i responses that is additional to the effects of flow on ATP mass transport. Furthermore, in the presence of ATP and ADP, Shen et al. (340) noted [Ca2+]i oscillations whose frequency was dependent on the magnitude of shear stress in laminar flow. When the adenine nucleotides were replaced by nonhydrolyzable analogues, [Ca2+]i oscillations showed a similar dependence on shear stress, suggesting that the flow-mediated [Ca2+]i response was not simply accounted for by hydrolysis of nucleotides at the cell surface. While the present discussion concerns ATP, the flow-dependent responsiveness of bradykinin has also been demonstrated (239), and the general features of the ATP mechanism apply to other labile agonists such as angiotensin and substance P.
A series of papers from Burnstock and co-workers (26, 27, 237, 294) have reported the release of ATP, acetylcholine, endothelin, and substance P from endothelial cells subjected to flow in vitro. Because there are receptors for these agonists on the endothelial surface, an autocrine mechanism of endothelial stimulation is implicated. There is precedent for neurotransmitter release from endothelial cells. By the action of thrombin, ATP can be released from aortic endothelial cells (283), an effect that was not attributable to cell damage. Although the concentrations of secreted agonists measured in the bulk fluid are low, they may reflect high local concentrations at the cell surface and, consequently, the creation of a steep concentration gradient in the boundary layer, the profile of which will be influenced by changes of flow. This would represent a flow effect on autocrine stimulation of the endothelium. The secretion of arginine vasopressin, another neurotransmitter, was unaffected by increased shear stress (27). These observations may account for the results of in vivo studies that suggested the involvement of endothelial cells in the vasodilatory response to hypoxia and the suggestion that a number of vasodilatory and vasoconstrictive agents can be released from the endothelium by increased flow (37, 141, 316). Busse et al. (38) have reported evidence of a similar mechanism for bradykinin release in intact arteries. The release of neurotransmitters directly from endothelial cells provides an important mechanism for flow-induced vasodilation, one that uses existing receptor systems to generate relaxing factor(s). However, while the consequences of shear stress-induced neurotransmitter released from the endothelial cells are reasonably well understood, they represent a secondary event, the mechanisms initiating the release being the primary flow-induced signal (and which remain elusive).
VIII. INTRACELLULAR CALCIUM
Intracellular calcium is an important signaling molecule that mediates other critical intracellular pathways after stimulation of endothelial cells by a variety of agonists (127). Release of the vasodilatory factors NO and prostacyclin by endothelium in response to agonists (adenine nucleotides, bradykinin, and thrombin) is stimulated by PLC-mediated cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) to generate IP3 and DAG (18). Then, IP3 mediates release of [Ca2+]i from the endoplasmic reticulum storage pool and may also stimulate the influx of extracellular calcium. The translocation of PKC to the plasma membrane and PKC activation by DAG are calcium dependent, and in turn, PKC activation changes the sensitivity of agonist/Ca2+-mediated prostacyclin release (42). Consequently, a wide range of downstream targets for protein phosphorylation are likely to be influenced by [Ca2+]i. A role for calcium in endothelial mechanotransduction is therefore to be expected, considering the variety of second messengers that appear to be common to both flow-stimulated and agonist-stimulated responses. However, although several groups have reported clear-cut endothelial [Ca2+]i responses to well-defined shear stress forces (106, 340; L. Thibault, personal communication), most investigators have been unable to obtain such responses (7, 82, 83, 239, 260, 326, 327, 406), and the current situation is confusing.
In 1988, Ando et al. (8) first reported stimulation of [Ca2+]i in cultured endothelial cells. About the same time, investigators in two other labs were unable to obtain similar results and realized that the major experimental difference was the use of medium 199 in the flow experiments by Ando et al. (8). Medium 199 contains 1.8 µM ATP, a potent stimulator of [Ca2+]i via the endothelial P2y purinoceptor. Intracellular calcium was not stimulated by flow when Dulbecco’s modified Eagle’s medium or minimal media that lack known endothelial agonists were used (82, 239). Intracellular calcium responses were restored by the addition of exogenous ATP or ADP. Subsequently, Ando et al. (8) obtained similar results, confirming the maintenance of baseline [Ca2+]i in the presence of shear stress (Refs. 7, 9; J. Ando, personal communication). An important outcome of these experiments was the discovery of mass transport-mediated mechanisms of endothelial stimulation by agonists at the cell surface (82, 239), as discussed in section VII. However, in 1992, Shen et al. (340) reported transient increases in calcium using flow medium that appears to lack known endothelial agonists. Peak values were reached within 40 s before return to baseline levels over the ensuing 40–80 s, a pattern very similar to that observed during agonist stimulation (127). Intracellular calcium oscillations with diminishing frequency were also noted. Intracellular calcium oscillations have also been reported during histamine (162) and ATP (82) stimulation of endothelial cells when the agonist concentrations were low. Shen et al. (340) noted that peak [Ca2+]i response increased with shear stress in the range of 0.2–4.0 dyn/cm2 then reached a plateau. The responses occurred as readily in flow-aligned monolayers as in unaligned cells. Chelation of extracellular calcium or the blocking of calcium channels by lanthanum had little effect, suggesting that intracellular storage pools of calcium were stimulated (although specific manipulation of [Ca2+]i mobilization with agents such as thapsigargin were not tested). The magnitude of [Ca2+]i responses diminished with repetitive shear stimulation, an effect possibly explained by desensitization or the washout of a secreted endogenous agonist; however, the addition of apyrase (an adenine nucleotidase) had no effect, suggesting that endogenous ATP secretion was not involved. Secretion of other endogenous chemokines was not tested in these experiments. Intracellular calcium responses to shear stress were heterogeneous within the monolayer. When individual cells were monitored, it was noted that about one-third did not respond to the mechanical force. A similar heterogeneity of calcium mobilization has been reported in endothelial cells stimulated by agonists.
Concurrent studies by Geiger et al. (106) supported a flow-dependent stimulation of [Ca2+]i. These authors used image digitization to analyze the spatial and temporal patterns of [Ca2+]i stimulation by shear stress in the range of 0.5–30 dyn/cm2. This study was in close agreement with that of Shen et al. (340); the temporal profiles were similar, extracellular calcium appeared not to be required, [Ca2+]i oscillations were noted, and desensitization to the shear stress stimulus occurred. In addition, Geiger et al. (106) noted that the number of responding cells within the monolayer increased with the magnitude of the shear stress to ~70% respondents, and the rate of increase of [Ca2+]i varied with the shear stress. Spatial analysis revealed that the largest and the most consistent increases of [Ca2+]i were in the nuclear region of the cell.
In other studies, Schwarz et al. (332) blew a stream of physiological solution onto a single endothelial cell to induce shear stress in the range of 0–50 dyn/cm2. They noted calcium transients that depended on the presence of 10 mM extracellular calcium (in contrast to the 2 studies just discussed) and that were abolished in depolarized cells, where the driving force for calcium entry was reduced. However, this study was not conducted under carefully controlled fluid dynamic conditions. The responses were similar to those observed when stretch-activated nonspecific cationic channels are stimulated (115, 190) rather than true shear stress activation of the responses.
It is unclear why other investigators have been unable to detect significant flow-dependent [Ca2+]i activity. Exchange of cells between “respondent” and “nonrespondent” laboratories has failed to resolve the differences. Another explanation may lie in the use of different batches of serum to grow the cells, with the possibility of residual serum components retained in the system even when measurements were subsequently conducted in serum-free medium. Recently, Helmlinger (133) and Nerem (personal communication) have observed differential flow-induced [Ca2+]i signaling in serum-free medium vs. serum-containing medium, observations that imply the presence of calcium-stimulating agonists in the serum. Preliminary data by Thibault (personal communication) suggest that the rate of onset of the flow-force may also influence the ability of the cell to respond; the probability of calcium response diminished if the onset interval was >200 ms. Both these latter observations warrant further investigation.
Cytoskeletal organization may play some regulatory role in [Ca2+]i independently of mechanical coupling to stretch-activated Ca2+ channels. Phospholipase C-mediated hydrolysis of PIP2 (to yield IP3 and DAG) is inhibited by profilin, a protein that binds both actin and PIP2 (114).
The major uncertainty associated with endothelial [Ca2+]i response to flow is whether it is an agonist-mediated effect or the direct result of displacement of a mechanosensor, or both. These are difficult mechanisms to separate and are complicated by effects of shear stress on agonist-receptor signaling that occur at the same time as mass transport effects on agonist-receptor signaling. The absence of flow stimulation of [Ca2+]i as measured by fluorescence techniques does not necessarily rule out an important role for this ion in shear stress-regulated mechanotransduction; as Nollert et al. (260) have suggested, there may be regulation of calcium-sensitive enzymes without any direct corresponding increase in bulk cytosolic calcium concentration.
IX. TRANSCRIPTION FACTORS AND STRESS RESPONSE ELEMENTS
The molecular mechanisms responsible for transducing shear stress and stretch into gene transcriptional changes are poorly understood. Second messengers located at the plasma membrane and in the cytoplasm are coupled to “third messenger” transcription factors activated posttranslationally and that serve as readily available elements for information transfer to the nucleus where they induce the expression of immediate early response genes (IERG). These, in turn, can further regulate gene expression (392). Endothelial IERGs that are known to be responsive to changes of shear stress include c-myc, c-fos, and c-jun (146, 148). Two transcription factor families that are present in the cytoplasm of most cells including endothelium are Rel-related nuclear factor kappa B (NFκB) and nuclear factor activator protein 1 (AP-1). They are typically activated by hormone stimulation (198, 257). The promoter regions of several inducible endothelial genes, including c-myc, interleukins (IL-1, IL-6, and IL-8), E-selectin, VCAM-1, granulocyte colony-stimulating factor-1, inducible NO synthase, and tissue factor contain recognition elements for NFκB (297). In the cytoplasm, NFκB consists of subunits p50 and p65 complexed with inhibitory protein IκB. Upon activation, IκB dissociates from the complex allowing p50/p65 translocation to the nucleus, where the subunits bind to DNA recognitionsites. Fos/Jun heterodimers (assembled by leucine zipper formation) bind primarily at the AP-1 site of DNA where they activate transcription (108). Recently, both shear stress (186) and cyclic stretch (B. Sumpio, personal communication) have been shown to stimulate the formation of NFκB and AP-1 complexes with DNA of cultured bovine aortic endothelial cells. By gel mobility shift assays, there was increased formation of complexes between 32P-labeled immunoglobulin-κB sequence and endothelial proteins extracted from cells exposed to unidirectional shear stress in laminar flow (Fig. 13). The NFκB-DNA complex formation increased steadily during a 1-h exposure to flow, after which there was no further increase. Gel supershifts demonstrated the inhibition of NFκB-DNA binding following preincubation of reaction mixtures with polyclonal antibody directed against the p65 subunit of NFκB. Whereas under no-flow conditions endothelial cells express very little binding of nuclear factor AP-1 to DNA, significant binding is induced by both shear stress and cell stretch. Exposure to flow resulted in a biphasic induction of AP-1 binding, reaching an initial peak at 10–20 min, a decline, then a second rise by 2 h. The c-fos and c-jun mRNA levels have been shown to increase modestly in human endothelial cells subjected to steady and pulsatile flow (146, 148) and to cyclic strain (Sumpio, personal communication), findings consistent with increased AP-1-DNA complex formation. Fos/Jun dimers have a higher affinity for the AP-1 site than Jun/Jun homodimers; Fos/ Fos homodimers are unable to efficiently bind. Protein kinase C has been reported to influence protein binding at the AP-1 site via phosphorylation of c-jun (29). There is also evidence that PKC regulates endothelin-1 release (181) and PDGF gene expression (147) in endothelial cells exposed to flow. Thus the AP-1 binding site may be linked to flow via PKC.
FIG. 13.
Nuclear factor κ B (NFκB)-DNA complex formation is stimulated by shear stress. Electrophoretic mobility shift assay of nuclear extracts isolated from confluent bovine aortic endothelial cells immediately after exposure to unidirectional laminar flow is shown. Control cells (lane 0') were placed in apparatus without flow. Other monolayers were exposed to steady shear stress of 12 dyn/cm2 for 30 and 60 min. Cell extracts were incubated with radiolabeled oligonucleotide containing consensus sequence for κB in the presence of nonspecific DNA-binding inhibitor poly(dI)-(dC), Extracts were incubated in absence (t') and presence (t'/comp) of a 100-fold molar excess of unlabeled oligonucleotide. DNA-protein complexes were resolved in 4% polyacrylamide gels, and gel shifts were documented by autoradiography. Closed arrow, NFκB-DNA complexes; open arrow, free κB oliognucleotide. [From Lan et al. (186).]
Both NFκB and AP-1 are widely distributed transcription factors that respond to a number of defined stimuli as well as flow. As has been noted with other elements of the signal transduction response to shear stress, it appears likely that these pathways are shared with receptor-mediated responses.
Are there unique shear stress or stretch-regulated transcription factors and corresponding DNA binding sites? An important step to investigate this possibility was the discovery of a SSRE first noted in the promoter of the PDGF-B gene and now found to be a consensus sequence in a number of other flow-responsive genes (301). Transcript levels of PDGF-B in bovine aortic endothelial cells are elevated after several hours of exposure to laminar shear stress (147, 301). A 1.3-kb fragment of the human PDGF-B promoter coupled to chloramphenicol acetyltransferase (CAT) construct acted as a reporter gene. Transfection of this gene to endothelial cells enhanced the PDGF transcript response to flow as reported by enhanced CAT activity. The PDGF-B-CAT construct was then systematically deleted to identify a core cis-acting sequence within the PDGF-B promoter necessary for the shear stress responsiveness. Using this approach, Resnick et al. (301) identified a 12-bp component that was interactive with nuclear proteins derived from shear stressed endothelial cells. A 6-bp core component, GAGACC, was further defined by deletion mutation in gel shift assays and has been designated a SSRE. This sequence has now been identified in a number of other flow-responsive endothelial genes including tissue plasminogen activator, ICAM-1, c-fos, c-jun, monocyte chemoattractant protein 1 (MCP-1), endothelial NO synthase, transforming growth factor-β1, and endothelin-1.
In recent studies, N. Resnick (personal communication) has demonstrated activation of the SSRE by 10-min exposure to shear stress in laminar flow. Furthermore, its specificity for endothelial cells was supported by the inability of smooth muscle cell extract to bind oligonucleotides containing the shear stress responsive consensus sequence. A most important recent experiment is the transfection of the reporter gene containing SSRE (GA-GACC/CAT, and the complementary simian virus 40/ CTCTGG/CAT) into nonresponsive cells which resulted in inducible CAT activity upon exposure to flow (M. A. Gimbrone, Jr., personal communication). It will be of interest to determine whether deletion mutation of the consensus sequence will inactivate those genes that contain it and which have been shown to be flow responsive.
Is the sequence GAGACC the only SSRE or a member of a family of SSREs? The rapid activation of the endothelial SSRE suggests that it plays an early role in transcriptional regulation. Some progress has been made concerning the identity of the transcription factors interacting with SSREs in endothelial cells. Khachigian et al. (168a) report functional interactions of NFκB with the SSRE of the PDGF-B promoter. The finding is consistent with NFκB activation by flow as reported earlier (186). Shyy and Chien (343a) have reported a new endothelial SSRE in the promoter of the MCP-1 gene. A phorbol ester response element, TGACTCC, was identified as both a hemodynamic regulatory element as well as a phorbol ester regulatory element. There may be a superfamily of SSREs, some of which also respond to influences other than mechanical signaling. At the level of gene expression, this would allow effects to modulate endothelial responses to other signals, and vice versa, and may account for occasional contradictory results in the literature. It is possible that upstream regulation may reside in other transcriptional events analogous to the immediate early growth response genes. Identification of the SSRE binding proteins is of high priority, and the unique nature of this consensus sequence will be confirmed or reassessed based on transfection experiments using a variety of related constructs and selective deletions.
Medford and co-workers (R. Medford, personal communication) have proposed a redox-sensitive mechanism that links VCAM-1 expression (of relevance in atherogenesis) and biomechanical signaling. Cytokine activation of VCAM-1 gene expression was inhibited in endothelial cells subjected to laminar shear stress (5–20 dyn/cm2) for 24 h. The effects are similar to antioxidant sensitivity of VCAM-1 expression observed in no-flow endothelial monolayers (222a). Medford and co-workers (4a) suggest that biomechanical-redox interactions may influence transcription factor binding to SSRE, perhaps via enhancer motifs. Although correlative at present, the mechanism is consistent with flow activation of NFκB (186), NFκB binding to the SSRE of the PDGF-B promoter (168a), and antioxidant inhibition of NFκB activation in other cells (328a).
A concise review of hemodynamic regulation of endothelial gene expression is available in Reference 301a.
X. SHEAR STRESS-MEDIATED VASOREGULATION
An important rapid physiological consequence of hemodynamic force transduction is the acute regulation of arterial diameter. When flow increases, arteries dilate by endothelial-dependent nervous system-independent relaxation of smooth muscle cells.
Although Thoma (366) first suggested that blood flow may regulate vessel tone in 1921, it was Schretzenmayr (329) who first experimentally demonstrated vasomotion in response to altered blood flow. The role of the endothelium in this process was proposed by Rodbard (305, 306), who suggested that the endothelium may sense the shear stress generated by flowing blood. In 1980, Furchgott and Zawadski (104) discovered that agonist-mediated vasodilation requires participation by the endothelium. The dependence of flow-mediated vasodilation on an intact endothelium was quickly confirmed in large-conduit arteries as well as in resistance-sized vessels (138, 166, 232, 233, 236, 288, 315, 314, 349). Intra-arterial pressure appears to have little effect on endothelial control of vasoactivity; the contractile response of smooth muscle cells to increasing pressure (the myogenic response) is smooth muscle dependent and largely endothelial independent (164, 313). In contrast, the principal endothelial regulator of arterial diameter is shear stress. This distinction was demonstrated by changing the fluid viscosity flowing through segments of arteries while maintaining constant physiological pressure and constant flow rate (172, 233). Shear stress is directly proportional to viscosity. Viscosity was changed either by hemoconcentration/dilution (233) or by addition of dextrans (172). Vasodilation developed as a function of the increasing shear stress incurred by increasing the viscosity of the fluid without changing the flow rate, thereby confirming that shear stress is the most relevant endothelial-regulated hemodynamic force in vasoregulation. There is now good evidence that the mechanism involves the enhanced release of endothelial-derived relaxing factors (EDRF), the principal component of which is NO and closely related nitroderivatives (241, 279, 280). Additional flow-mediated vasotonic control is provided by prostacyclin (relaxation) and endothelin-1 (contraction).
A. Nitric Oxide
Nitric oxide is the principal EDRF in the vasculature as determined by an overwhelming number of in vivo, ex vivo, and in vitro experiments (reviewed in Refs. 153 and 241). Nitric oxide is an endogenous activator of the soluble form of guanylate cyclase. Much of the work that led to the identification of NO and other relaxing and contracting factors (377) was conducted using bioassays of arterial rings perfused with run-off from intact vessels or endothelial-coated beads in chromatography columns or stirred cell systems. Nitric oxide is a short-lived molecule (half-life in physiological buffer of a few seconds) readily scavenged by hemoglobin (33), and its effect is potentiated by superoxide dismutase, an enzyme that destroys free radicals that sequester NO (279, 280, 317). Nitric oxide activates cGMP in endothelium, in smooth muscle cells, and in platelets, where it results in inhibition of shape change and aggregation (12, 246, 353). There are membrane-bound cGMP-dependent protein kinases in bovine endothelial cells (209). The enzyme NO synthase (NOS) converts l-arginine to l-citrulline with release of NO (279, 280). Endothelial NOS (eNOS), which has been mapped to chromosome 7 (218), is constitutively expressed at a basal level, and its activity is calcium/calmodulin dependent (94, 201). Consideration of the mechanism(s) by which flow induces NO-mediated vasodilation must focus on the regulation of eNOS activity. Most information about NOS activation, however, has been obtained for other forms of NOS, particularly inducible NOS of macrophages (iNOS) and neuronal NOS (nNOS) from rat cerebellum (reviewed in Ref. 334). The enzyme is a cytochrome P-450 protein containing a heme prosthetic group (391). In the presence of molecular oxygen, l-arginine is converted to an intermediate form N-hydroxy-l-arginine by electron donation from NADPH; the intermediate is further oxidized to NO and citrulline (357). Both steps appear to involve the same active site of NOS. Several cofactors are required for NOS activation including protoporphyrin IX, flavin nucleotides, and tetrahydrobiopterin (334). Nitric oxide synthase is unique in being the only mammalian enzyme that simultaneously catalyzes cytochrome P-450-related hydroxylation and NADPH reduction. It is also the only known example of a soluble cytochrome P-450 enzyme (334). Endothelial NOS and nNOS, both calcium/calmodulin-dependent enzymes, are 60% homologous, but a unique feature of eNOS is a myristoylation site of unknown function at the NH2-terminal (218, 258, 335, 334). This site may anchor eNOS to the plasma membrane (334) and in some way may be involved in shear activation of eNOS. There are heme, calmodulin, and l-arginine binding sites in all NOS, and the enzyme is subject to feedback regulation by NO (307). The calmodulin consensus sequence is composed of arginine and lysine residues arranged in a α-helix (263), but calmodulin binding is also believed to be influenced by adjacent amino acids. The activation of eNOS by flow involves increase of [Ca2+]i and calmodulin binding. However, the mechanisms of shear stress transduction are unclear because of the large number of regulatory possibilities associated with the cofactors for activation of this enzyme, the presence of common transcription factor binding sites (including AP-1, AP-2, NF-1, SSRE) in the 5′-flanking region, and the availability of recently identified phosphorylation sites (334). Nevertheless, a number of important observations with respect to flow and NO production have been reported.
Endothelial NOS from bovine aortic endothelial cells has been cloned and characterized (258, 289, 336). Nishida et al. (258) reported significantly increased eNOS mRNA and protein expression after exposure to 15-dyn/cm2 shear stress in laminar flow for 24 h. Further studies (375, 376) reported a twofold increase of eNOS message as early as 3 h. The increase was prevented by inhibition of protein synthesis and was independent of PKC activation or inhibition. A potassium channel antagonist, tetraethylammonium chloride (TEA), blocked the response, suggesting some relationship with potassium channel opening; however, this antagonist does not discriminate between a range of potassium channels and is likely to promote many other unrelated changes in the cells that could indirectly influence eNOS activity. The inhibitor of microtubule polymerization nocadozole also abolished the flow-related eNOS effect without altering basal expression in unsheared cells. Endothelial NOS expression has been compared in cells exposed to laminar, periodic laminar, and turbulent flows (262). Steady laminar flow induced synthesis of NO that was dependent on shear stress magnitude in the range of 2–12 dyn/cm2 and upregulated the level of eNOS mRNA. A further increase of NO synthesis was observed when HUVECs were subjected to step change increases of laminar shear stress of the same magnitude. In contrast, turbulent flow failed to upregulate eNOS mRNA, and NO release remained at basal levels (A. Remuzzi, personal communication).
In vivo, both acute and chronic increases of blood flow enhance endothelium-dependent vasodilation. Here, the mechanism involves the release of nitrogen oxides linked to specific increases of eNOS gene expression in endothelial cells obtained directly from the aorta. Thus eNOS expression is increased in vivo in agreement with in vitro studies. Under conditions of chronic cardiac pacing or exercise training (337, 382), this mechanism is likely to have an overall beneficial effect. In contrast, when blood flow is reduced, as in congestive heart failure, flow-mediated vasodilation in vivo is depressed (165).
Endothelial NOS, in common with other NOS, contains consensus sequences for phosphorylation by protein kinases A and C and calmodulin kinase II (334). A threefold increase in the phosphorylation of eNOS on serine or threonine residues within 1 min of exposure to 10-dyn/cm2 shear stress in laminar flow has recently been reported in preliminary form (54). Further indirect support for a role of phosphorylation in eNOS activation by phosphorylation has been reported in abstract form (285). Arterial endothelial cells on collagen-coated dextran beads were perfused in a column at different flow rates while [Ca2+]i was simultaneously monitored. Nitric oxide production was stimulated to fivefold higher levels than when the cells were exposed to calcium ionophore. Furthermore, when [Ca2+]i increase was blocked with the chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid, agonist-induced increases of calcium were blocked, but flow-stimulated NO production remained highly elevated, suggesting a calcium/calmodulin-independent mechanism of activation of eNOS (285). Further supporting these observations is a demonstration that NO production is biphasic following the onset of laminar flow with an initial rapid rise at the onset of flow followed by slow development of a sustained increased level of production (20, 182). The characteristics of each phase were found to be quite different. The rapid initial production of NO was independent of shear stress magnitude (range 6–25 dyn/cm2), whereas the sustained release phase was shear dependent. With the use of selective inhibitors, the initial phase was shown to be calcium/calmodulin dependent in contrast to the sustained phase, which appeared to be independent of these messengers. Preincubation with dexamethasone, a chemical that blocks induction of iNOS, had no effect on the later phase, confirming that iNOS is not present in primary HUVECs. Together, these reports suggest two mechanisms of activation of eNOS: by calcium/calmodulin and by a mechanism perhaps involving phosphorylation of the protein. Endothelial NO synthesis has also been demonstrated to be both calcium dependent and calcium independent in response to agonists (245). In a preliminary report, Awolesi et al. (14) have described eNOS gene upregulation by cyclic strain. It is unclear how the shear stress/strain stimuli relate to the effects of hemodynamics in vivo in the regulation of eNOS activity.
Endothelial cells in vitro respond to shear stress by stimulation of cGMP, the elevation of which is proportional to the intensity of the shear stress up to ~40 dyn/cm2. The increase appears to be regulated by a flow-induced activation of soluble guanylate cyclase which, in turn, is mediated by autocrine production of NO. Ohno et al. (265) have reported circumstantial evidence linking such activation to a Gi or Go protein inhibitor and concomitant potassium channel activation. The nonselective potassium channel antagonist TEA completely abolished flow-induced elevation of intracellular cGMP, and the effect was also pertussis toxin sensitive, thereby supporting the role of G proteins. Although the selectivity of inhibitors used in these experiments is debatable, the data are suggestive of a G protein-linked mechanism for eNOS activation and, as discussed in section V, are consistent with the idea of a G protein-linked receptor(s) involved in mechanotransduction. Insights into separate mechanisms of eNOS activation by agonist in comparison with shear stress were provided in experiments in which the intracellular calcium stores of endothelial cells were emptied using the chemical thapsigargin. This agent selectively blocked agonist-dependent release of NO without effect on the shear stress-mediated NO production (207). These data suggest that mobilization of [Ca2+]i from inositol phosphate-sensitive calcium stores is not required for the eNOS response. Thus endothelial cells may contain both a calcium-independent and a calcium/calmodulin-activatable NOS, each of which may be under multiple controls for activation by shear stress or by agonists.
Unlike iNOS, constitutive eNOS can act as a substrate for endothelial kinases, resulting in a severalfold increase in eNOS phosphorylation after stimulation of endothelial cells with bradykinin, ionomycin, and nitroprusside (234). Phosphorylation has also been reported in response to shear stress activation of eNOS. Bradykinin-induced eNOS phosphorylation was inhibited by a calmodulin antagonist but not by inhibitors of protein kinase A, PKC, calmodulin kinase II, and myosin light-chain kinase, suggesting that phosphorylation of eNOS is a calmodulin-mediated event acting perhaps via a calmodulin-activated kinase distinct from kinase II. Bradykinin-induced eNOS phosphorylation also involves translocation of membrane-bound eNOS to the cytosol, a phenomenon blocked by calmodulin antagonist (335). Sessa (334) has drawn a parallel between eNOS phosphorylation and translocation and the regulation of a similarly structured kinase substrate, the myristoylated alanine-rich C kinase substrate (MARCKS) protein in leukocyte membranes (3). For this molecule, subsequent dephosphorylation allows MARCKS to rebind to the plasma membrane (365); Sessa (334) suggests that a similar mechanism (regulation by phosphatases) may occur with eNOS.
Certain combinations of the cytokines tumor necrosis factor-α and interferon-γ stimulate an inducible form of calcium/calmodulin-independent NOS (iNOS type) in murine and porcine endothelial cells but not in their bovine and human counterparts (185, 334). It is unclear at present whether cytokine-induced iNOS expression or activity is influenced by flow.
B. Prostacyclin
Prostaglandin I2, also known as prostacyclin, is the most potent natural inhibitor of platelet aggregation (240). Its synthesis and release from endothelial cells occur not only in response to a number of agonists, but also in the presence of increased shear stress (95, 118); flow-induced prostacyclin changes were one of the earliest documented responses of endothelial cells to shear stress. Prostacyclin is also a vasodilator, although of inferior potency than NO. Its half-life is longer than NO (~3 min at physiological pH in vitro vs. a few seconds for NO). In HUVECs, prostacyclin synthesis and release are stimulated after exposure to step increases in laminar shear stress. The response is biphasic; after an initial rapid release, production declines for several hours before recovering to maintain a steady release rate (20). The second phase depends on an exogenous source of arachidonic acid and is directly related to the magnitude of the shear stress. If the force is applied in a pulsatile fashion, the steady-state level of prostacyclin is significantly increased when compared with the same average shear stress applied in steady laminar flow (118). The early rapid phase of flow-mediated prostacyclin production may be related to the availability of arachidonic acid pools in the cell which when exhausted become rate limiting for prostaglandin synthesis (160). Arachidonic acid is released from phosphatidylethanolamine and phosphatidylinositol by the actions of phospholipase A2 (direct release of arachidonate from the C-2 position), PLC (releasing DAG, which is hydrolyzed by a diglycerol lipase to release arachidonic acid), or phospholipase D (to generate phosphatidic acid which is subsequently cleaved to DAG) (18, 140, 219, 220). In the presence of inhibitors of diglycerol lipase, flow-mediated prostacyclin production is inhibited (22), pointing perhaps to a preferential role for PLC and possibly phospholipase D in the mediation of this response.
Rapid increases of IP3 and DAG in HUVECs are also consistent with activation of PLC cleavage of phosphatidylinositol (21, 261). These transient increases, however, are consistent only with the early part of the biphasic PGI2 response to shear stress and may be linked to IP3-driven mobilization of [Ca2+]i. Mechanisms responsible for the second (longer) phase of PGI2 synthesis are unclear, may be calcium independent, and related to an upregulation of PGI2 synthase (20).
What are the relative contributions of PGI2 and NO to flow-mediated vasodilation? In arterioles of rat muscle, it appears that both NO and PGI2 are involved in endothelial mediation of dilation after increases in blood flow. In contrast, however, in hypertensive rats, the NO-mediated arteriolar dilation was impaired, whereas the PGI2 component was largely unaffected (173). In other vascular beds such as the basilar artery (102) and coronary microcirculation (183), the role of PGI2 is less clear, and NO-mediated effects appear to be dominant.
C. Endothelin-1
Endothelin-1 (ET-1) is a potent and long-lasting vasoconstrictor synthesized and released by endothelial cells (396). It is a 21-amino acid peptide derived from a 39-amino acid precursor (“big endothelin”) that in turn is the cleavage product of the 202-amino acid peptide preproendothelin. Several isoforms of endothelin exist of which only ET-1 is synthesized by the endothelium. Endothelin-1 is also a growth factor for smooth muscle cells and therefore may modify arterial diameter by reorganization of the smooth muscle cellular and extracellular composition (175). Release of ET-1 from aorta is inhibited by NO (28). Following reports of vasoconstrictive activity in endothelial cell-conditioned medium, Yanigisawa et al. (396) identified ET-1 as the active component and isolated, purified, cloned, and sequenced it from porcine aortic endothelial cells. The influence of flow on ET-1 was first reported anecdotally in the same paper as a decrease in mRNA expression and peptide levels. Some confusion was engendered, however, when the same group reported transient stimulation of ET-1 mRNA (1–4 h) and peptide release by porcine endothelial cells exposed to shear stress estimated to be in the range of 5 dyn/cm2 when compared with no-flow control (398). However, mRNA levels were unchanged compared with controls when the cells were exposed to 8 dyn/cm2 for 48 h during which time they became aligned with the flow.
Sharefkin et al. (338) used reverse transcription linked to polymerase chain reaction amplification of preproendothelin transcript to demonstrate that HUVEC exposure to shear stress (25 dyn/cm2) suppressed both mRNA transcript levels after 24 h as well as the rate of ET-1 peptide release. The latter inhibition began 2–4 h after the onset of flow. The downregulation was confirmed and extended to bovine aortic endothelial cells by Izumo and co-workers (214, 216). Endothelin-1 mRNA decreased fivefold during exposure to steady shear stress at 15 dyn/cm2. The decline, which was evident by 1 h after the onset of flow and complete by 2 h, was force dependent and saturated at 15 dyn/cm2. Peptide levels of ET-1 also declined by greater than one-half. Similar decreases in mRNA were obtained when shear stress was applied in turbulent or pulsatile laminar flow (1-Hz frequency) but not during sinusoidal reversing flow (frequency, 0.25 Hz; average shear stress, 0 ± 21 dyn/cm2). In further studies, Malek et al. (216) reported a transcriptional mediation of the downregulation and noted that it appeared to be independent of PKC and cAMP. Experiments with DNA transfection suggested that a SSRE of the ET-1 gene is located between −2.5 and −2.9 kb of the 5′-upstream region. The basal transcription level of the ET-1 gene is critically controlled by a GATA motif at bp −135 to −132 upstream of the transcription start site and an AP-1 consensus sequence. However, neither of these sites nor their DNA binding factors appear to be required for ET-1 down-regulation by shear stress. The failure of PKC inhibitors to influence the downregulation is consistent with AP-1 as a target site for PKC activation, i.e., neither seems to be involved in controlling ET-1 downregulation. In contrast to flow, agonist-mediated stimulation of ET-1 release occurs via the PKC pathway, and cGMP is implicated as an inhibitor of thrombin-mediated ET-1 production.
Recently, interpretation of flow-mediated ET-1 gene regulation has again become more complicated. Kuchan and Frangos (181) have reported that HUVEC ET-1 peptide release is stimulated by low levels of shear stress (1.8 dyn/cm2), whereas 6- to 25-dyn/cm2 shear stress for periods of >6 h inhibited ET-1 release. Endothelin-1 peptide stimulation was prevented by an inhibitor of PKC, whereas the suppression could be rescued by N-nitro-l-arginine, a competitive inhibitor of l-arginine that prevents NO and cGMP synthesis, suggesting that NO and/or cGMP is involved in ET-1 downregulation. Direct treatment with 8-bromo-cGMP also inhibited ET-1 release, consistent with inhibition of ET-1 by endogenous cGMP. An inhibitor of cGMP-dependent protein kinase failed to influence the flow responses, suggesting that regulation was not via such a kinase. It should be noted that mRNA levels were not measured in these experiments, the bioassay being radioimmunoassay of ET-1 peptide. Taken together, all of these studies suggest that there can be a transient elevation of ET-1, both mRNA and peptide release, followed by a highly significant suppression. The role of PKC appears to be confined to the stimulatory segment of the biphasic response, since there is no evidence for its involvement in downregulation. Although it is curious that Malek et al. (214) failed to report a stimulatory phase of ET-1, it should be pointed out that in Figure 3 of their paper there is indeed a 20% stimulation within 30 min of exposure to 15 dyn/cm2 (although not commented on). It seems likely that in different laboratories with different cells and under different conditions the stimulatory phase is expressed to varying extents.
Morita et al. (243) have proposed an alternative mechanism for some of the disparities noted in ET-1 responses to flow. They demonstrated a conversion of F-actin to G-actin within 5 min of exposure to 5-dyn/cm2 shear stress in porcine endothelial cells, the response returning to basal levels by 24 h. Endothelin-1 mRNA levels followed a similar pattern. Because stabilization of F-actin abolished the shear stress-induced, but not chemical-induced, increases in ET-1 mRNA, they suggested that actin fibers must play an important role in shear stress-induced ET-1 expression. In addition, inhibition of tubulin dimerization abolished shear stress-induced increases in ET-1 expression. Morita et al. (243) suggested that the discrepancies between the effects of low and high shear stress on ET-1 gene expression reported from various laboratories may reflect different cytoskeletal configuration or equilibrium conditions. In support of this interpretation, A. Malek and S. Izumo (personal communication) have observed that depolymerization of F-actin with cytochalasin D results in a time-and dose-dependent decrease in ET-1 mRNA levels, as well as cell retraction and rounding. Depolymerization of microtubules with nocodazole or colchicine prevented not only the shear-induced ET-1 downregulation, but also the morphological changes and alignment in the direction of flow. Conversely, treatment with taxol to stabilize microtubules did not interfere with ET-1 downregulation, although it slowed and attenuated the morphological adaptation to shear. Furthermore, by plating bovine aortic endothelial cells on increasing concentrations of recombinant fibronectin residues (pronectin F), cell shape was regulated and a correlation was demonstrated between cell spreading and ET-1 mRNA levels. This phenomenon was specific to ET-1, since another flow-regulated gene product, TM, was not affected. In recent unpublished studies, Malek and Izumo (personal communication) have demonstrated that shear stress at 20 dyn/cm2 induces increased phosphotyrosine content of a number of proteins (the most prominent having molecular mass of 55 kDa) in a rapid fashion. This increase in phosphotyrosine was blocked by pretreatment with quin 2-AM (10 µM, 30 min), the membrane-permeable chelator of [Ca2+]i, and herbimycin A (875 nM, 24 h), a tyrosine kinase inhibitor. Furthermore, both of these inhibitors prevented the downregulation of ET-1 mRNA as well as the endothelial morphological and cytoskeletal adaptations in response to shear stress. As noted above, however, because the cytoskeleton plays a key role in transmitting the forces throughout the cell via integrin receptors, it is not surprising that its disruption or modification parallels the regulation of force-related gene expression.
D. Other Vasoactive Responses
Other responses relevant to vasoregulation include stimulation of histamine decarboxylase activity and histamine levels by oscillating shear stress (348). Histamine is a potent vasodilator. The effects were independent of the average magnitude of the force, although flow reversal was not included in these experiments.
In a recent paper, Laurindo et al. (191) have demonstrated flow-triggered free radical production in ex vivo-perfused rabbit arteries and extracorporeal arterial loops. A spin trap was injected that formed radical adducts that were detected by paramagnetic resonance spectroscopy. Free radical accumulation increased with flow rate, was endothelial dependent, and was blocked by superoxide dismutase. Confirmation of increased free radical production was also obtained by measurement of ascorbyl radical, a defense against oxygen-derived radicals and an index of free radical release. Laurindo et al. (191) argue that in addition to pathological events, free radical generation is part of a normal regulatory mechanism for autocrine control of vascular diameter, and hence, shear stress.
XI. FLOW-DEPENDENT ARTERIAL REMODELING
In flow-mediated vasodilation, the shear stress is usually returned to its previous level (15–20 dyn/cm2) by the change in arterial geometry (112, 113). In other words, a change in the mechanical environment alters cellular responses that reequilibrate after a few seconds, and the flow environment of the sensing system is then returned to its previous state. Essentially, a similar sequence of events occurs during remodeling of the arterial wall in response to a chronic change of mechanical stimulus except that the adaptation occurs over a much longer interval (weeks to months) and involves changes in gene expression to effect the remodeling. This is an example of close integration of artery wall mechanics and metabolic functions.
Chronically decreased flow, with attendant reduction in shear stress, induces intimal thickening and the reduction of lumen diameter (189). As the vessel narrows, the flow velocity increases to restore the shear stress to 15–20 dyn/cm2. Increased flow, on the other hand, results in chronically enlarged lumen diameter (166). Both of these responses (shown in Fig. 14) involve fundamental changes in artery wall thickness, matrix composition, and the organization of the artery wall (224, 405, 403) and, as in acute vasotonic responses, the endothelium plays a key role (189); removal of the endothelium before coarctation (decreased flow) or before creation of an arteriovenous fistula (increased flow) resulted in a failure of the artery wall to adapt. The endothelial cell is therefore a signaling interface for the mechanically stimulated remodeling process. It is presently unclear how the medial and intimal reorganization involving regulation of extracellular matrix synthesis by smooth muscle cells is mediated by the endothelium. Changes of stretch and tensile stress in endothelial and smooth muscle cells that lead to altered synthesis and secretion of collagen, elastin, and connective tissue proteases (358, 359) appear to be under the overall control of the endothelium. Perhaps changes in the secretion of endothelial-derived growth factors or chemokines (215, 264) are responsible.
FIG. 14.
Arterial wall remodeling as a consequence of chronic alterations in flow. a: substantial decrease of rabbit carotid artery diameter 6 wk after flow had been reduced by coarctation. Top panel: vascular casts. Bottom panel: histological sections. Top and bottom panels show artery from treated and contralateral sides, respectively. Effects were dependent on presence of endothelium. [From Langille and O’Donnel (189).] b: increased diameter of rabbit carotid artery caused by an abnormally high flow rate by an arteriovenous fistula. Blood flow was increased 20-fold, resulting in a 4-fold increase in arterial diameter after 2 wk. Arrowheads indicate artery diameter. (Photograph courtesy of Dr. James McKinsey, Department of Surgery, University of Chicago, Chicago, IL.)
Ohno et al. (264) have recently demonstrated that increased laminar shear stress induces the expression of transforming growth factor-β1 (TGF-β1) and the increased secretion of biologically active TGF-β1. Transforming growth factor-β1 is a pleiotropic factor that inhibits the growth of vascular smooth muscle cells (277) and thus could act in a paracrine fashion to modify the underlying vascular wall. Regulation of endothelial TGF-β1 was at the transcriptional level and appeared to be closely linked to hyperpolarization of the cells; inhibition of endothelial potassium channels inhibited activation of gene transcription. The conversion of inactive TGF-β1 to a biologically active form can be promoted by plasmin, which is generated from plasminogen by tissue plasminogen activator (tPA; Ref. 324). Because tPA is increased by shear stress (Table 1 and Refs. 79–81), cosecretion of inactive TGF-β1 and tPA may mediate the activation of TGF-β1. These studies may also be relevant to the inhibition of intimal hyperplasia associated with neointimal thickening in porous grafts (176).
XII. HEMODYNAMICS AND THE FOCAL ORIGIN OF ATHEROSCLEROSIS
In the late 15th century, Leonardo Da Vinci documented the potential importance of hemodynamics for cardiovascular tissue. Having drawn a dissected heart valve, he postulated that blood flow through the valve and beyond would be influenced by the geometry of the surrounding tissue. This included recirculation of the flow immediately downstream of the valve. Da Vinci wrote that, having observed the flow of water around obstacles in nature, he expected blood flow to be subjected to similar principles in the heart. In the 19th century, the great pathologists Rokitansky (308) and Virchow (378) recognized that the distribution of atherosclerotic lesions was nonuniform and postulated that mechanical forces operating in different regions of the arterial tree may be responsible. In the early part of this century, Aschoff (13) restated this hypothesis during a period of renewed interest in the study of atherosclerosis following the demonstration of its induction by hypercholesterolemia in animal models (10).
As indicated in Figure 15, atherosclerotic lesions in humans tend to develop in regions where there is separation from unidirectional laminar blood flow, typically near branches, bifurcations, regions of arterial narrowing, and curvatures in the arteries (52, 53, 355, 397). Certain vessels are more susceptible than others. The carotid bifurcation, coronary arteries, abdominal aorta, and the iliofemoral arteries are lesion prone while other arteries are spared, despite the presence of complex flow profiles (for example, the internal mammary and renal arteries). While these differences can be explained in part by structural differences in the artery wall, hemodynamic forces also play a key role. Transendothelial permeability to macromolecules, including low-density lipoprotein, in normal rabbits (333) and minipigs (101) is greatest in regions predisposed to atherosclerosis. The flow characteristics in susceptible regions of complex geometry are intricate (167, 177, 178), and it was not until estimates of the local fluid dynamics became available that some understanding of the mechanisms responsible for the pathology were possible. Fry (100) and Caro and co-workers (39, 40) investigated the fluid dynamics of lesion-susceptible sites of large arteries and established several important concepts with respect to the role of hemodynamics in atherogenesis. They were able to estimate stresses and strains in the artery wall and the shear stress forces acting at the endothelial surface. In addition, they established that the flow environment modified the mass transport of blood elements to the artery wall. Fry (100) was able to demonstrate endothelial damage at high levels of shear stress by directing a jet of fluid onto the surface of excised arteries. The levels of shear stress required to detach the endothelium, however, were extremely high, on the order of 400 dyn/cm2, and further developments by Caro et al. (39) established that atherosclerosis usually developed in regions of the artery that correlated with an average low level of shear stress rather than an injuriously high level, a finding confirmed by others (00, 113, 178, 404). Some reconciliation of what appeared to be opposing views has occurred, most recently with the demonstration that the gradients of shear stress may be of importance to the endothelium (68, 76). Such gradients exist in regions of both average high and low shear stress. Classic investigations of the hemodynamic role in lesion distribution have been carried out by Glagov, Zarins, Giddens, and co-workers (177, 178, 404). Their studies have convincingly demonstrated that atherosclerosis develops in regions of average low shear stress that are usually associated with flow separation and flow reversal. However, the molecular mechanisms linking endothelial flow responses to the pathogenesis of atherosclerosis are complex and difficult to dissect (135). Significant progress in this direction occurred following the proposal of Glagov and co-workers (112, 113) that arterial adaptation to the development of an atherosclerotic lesion and arterial remodeling and adaptation to changing flow were similar in some respects. During atherogenesis, an intact endothelium is retained over the developing lesion (67, 88, 116), but normal endothelial function may be compromised (67, 65, 110); for example, agonist-mediated vasodilation is inhibited in atherosclerotic arteries (227). It is not known whether hypercholesterolemia and atherosclerosis selectively interfere with those hemodynamic aspects of endothelial function that regulate normal arterial structure and function (206), nor is it clear how this may relate to the pathological changes occurring in the intima as the lesion develops. Clarification is likely to come from the integration of atherogenic and remodeling mechanisms (113), from better real-time imaging of endothelial cells in arteries, and from studies of the effects of hypercholesterolemia on hemodynamically related endothelial function; for example, Flavahan and co-workers (91–93) have proposed that EDRF dysfunction in hypercholesterolemia may result from uncoupling of endothelial G protein mechanisms. Such compromise of vasodilation in atherosclerosis extends to the microcirculation (183).
FIG. 15.
Localization of human atherosclerosis in abdominal aorta. a: digitization of sudanophilia intensity demonstrates lesions to be associated with regions of predicted complex hemodynamic profiles near branch arteries. b: detail of a showing distribution near right and left renal arteries (RR, LR) as well as superior mesenteric (SM) and celiac branches (C). Arrow indicates overall direction of flow, although detailed flow is complex. [From Cornhill et al. (52).]
The “reaction to injury” hypothesis of atherogenesis (311, 312) considers the endothelium as a prime target for “insult” or altered function resulting in perturbations of the normal interactions between blood and artery wall. The localization of atherosclerosis to regions of disturbed flow suggests that hemodynamic forces may induce greater wear and tear on endothelial cells at these locations, particularly since low and high shear stress and shear stress gradients have been shown to modify endothelial function in vitro. McNeil (229) has shown that the cells of many tissues subjected to mechanical stress in vivo suffer transient disruptions of the plasma membrane, thereby allowing the influx of molecules as large as immunoglobulins into the cytoplasm (230). In the rat aorta, 6.5% of endothelial cells took up a circulating marker (rat serum albumin) that is normally excluded from the cell cytoplasm (248, 401), and the distribution of uptake throughout the aorta was similar to that of dead endothelial cells identified by immunoglobulin uptake (129). However, there was a 10-fold greater number of rat serum albumin-positive cells than dead cells. The transiently injured cells were noted in clusters around branch arteries and as streaks in the long axis of the aorta. McNeil (229) has suggested that transient disruption of the plasma membrane by hemodynamic forces is an alternative to secretion as a release mechanism for cytosolic endothelial products. In particular, basic fibroblast growth factor, a potent growth factor (302) that lacks a signal peptide required for secretion (381), could be released in this way. Approximately 40% of total basic fibroblast growth factor was released from cultured endothelial cells by scraping them from the dish, a process that created transient disruption of the plasma membrane (231). The role of shear stress in nonfatal mechanical injury of endothelial cells therefore deserves further investigation.
XIII. FUTURE DIRECTIONS
Which are the most prominent questions in cellular mechanotransduction that are likely to be addressed during the next five years? Advances in several “hot” areas of signaling research should find relevance in this field. They include MAP kinases, for which exciting preliminary studies in endothelial flow responses have already been conducted. Although shear stress does not per se induce endothelial proliferation, MAP kinase signaling pathways associated with stress responses appear to exist. Strong evidence for G protein responses to shear stress implicates endothelial serpentine receptors as putative flow sensors. Both of these pathways could provide a key missing element for flow sensing, a transmembrane receptor sensitive to displacement by the frictional shear stress. The consequences of intervention in receptor function can then be more precisely addressed. However, a confounding and difficult problem is the separation of chemical mass transport changes at the cell surface from structural displacement of cellular components, intertwining relationships that will complicate the identification of flow receptors. Related to the existence of flow sensors is their distribution on and/or throughout the cell. It is clear that the cytoskeleton and other structural components can transmit and modulate changes of tension into (and perhaps through) the cell to focal adhesions, extracellular matrix, and cell junctions. The involvement of bioengineers, fluid dynamicists, and other physical scientists, all of whom have been well represented in this field since its inception, is essential to elucidate the structural aspects of stress distribution. The spatial information that will be required to complete such studies is already being provided from three-dimensional reconstructions of intracellular structures created using laser confocal microscopy or fluorescence image deconvolution techniques coupled to computer-assisted imaging using high-powered image analysis software available in the public domain (e.g., Khorus from the University of New Mexico). Downstream of flow-sensing structures are the complex biochemical cascades that elicit acute changes of ionic composition of the cell, mediated by ion channels and gap junctions. Progress in the cloning of endothelial-specific channels is likely. Further along the signaling cascades, a major initial step has been the identification of some transcription factors and response elements that are activated by shear stress. A number of SSREs and cofactor sequences in flow responsive genes are likely to soon emerge in this fast-moving area. The creation of transgenic animals and gene knockouts will quickly follow, with fascinating results. Finally, in vitro investigations of flow responsiveness have at last begun to mimic more closely the environment of endothelial cells in arteries. These cells are highly adapted to the prevailing flow forces, and their putative sensors are likely to be set at different threshold levels and have different characteristics than the typical culture studies in which the cells are not adapted to the flow. Investigations of adapted cells are likely to provide some surprises.
Acknowledgments
I thank many colleagues working on mechanotransduction who not only graciously sent me preprints and reprints and permission to quote their unpublished work but also engaged in stimulating discussions of our common research interests. I gratefully acknowledge the constructive criticism of my colleagues, postdocs, and students at the University of Chicago and the invaluable assistance of Gloria Johnson during preparation of the manuscript.
This work was supported by National Heart, Lung, and Blood Institute Grants HL-15062 and HL-36049 and American Heart Association Grant 91–1557.
REFERENCES
- 1.Achard C, Aynaud M. Recherches sur l’impregnation histologique de l’endothelium. Arch. Med. Exp. 1907;19:437–450. [Google Scholar]
- 2.Adams DJ, Barakeh J, Laskey R, Van Breemen C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB J. 1989;3:2389–2400. doi: 10.1096/fasebj.3.12.2477294. [DOI] [PubMed] [Google Scholar]
- 3.Aderem A. The MARCKS brothers: a family of protein kinase C substrates. Cell. 1992;71:713–716. doi: 10.1016/0092-8674(92)90546-o. [DOI] [PubMed] [Google Scholar]
- 4.Agard DA, Sedat JW. Three-dimensional architecture of a polytene nucleus. Nature Lond. 1983;302:676–681. doi: 10.1038/302676a0. [DOI] [PubMed] [Google Scholar]
- 4a.Ahmad M, Marui N, Alexander RW, Medford RM. Cell type specific transactivation of the VCAM-1 promoter through an NFκB enhancer motif. J. Biol. Chem. doi: 10.1074/jbc.270.15.8976. In press. [DOI] [PubMed] [Google Scholar]
- 5.Albelda SM, Buck CA. Integrins and other cell adhesion molecules. FASEB J. 1990;4 2868–2800. [PubMed] [Google Scholar]
- 6.Alevriadou BR, Eskin SG, McIntire SV, Schilling WP. Effect of shear stress on 86Rb+ efflux from calf pulmonary artery endothelial cells. Ann. Biomed. Eng. 1993;21:1–7. doi: 10.1007/BF02368159. [DOI] [PubMed] [Google Scholar]
- 7.Ando J, Kamiya A. Blood flow and vascular endothelial cell function. Front. Med. Biol. Eng. 1993;5:245–264. [PubMed] [Google Scholar]
- 8.Ando J, Komatsuda T, Kamiya A. Cytoplasmic calcium responses to fluid shear stress in cultured vascular endothelial cells. In Vitro Cell Dev. Biol. 1988;24:871–877. doi: 10.1007/BF02623896. [DOI] [PubMed] [Google Scholar]
- 9.Ando J, Ohtsuka A, Korenaga R, Kawamura T, Kamiya A. Wall shear stress rather than shear rate regulates cytoplasmic Ca2+ responses to flow in vascular endothelial cells. Biochem. Biophys. Res. Commun. 1993;190:716–723. doi: 10.1006/bbrc.1993.1108. [DOI] [PubMed] [Google Scholar]
- 10.Anitschkof N. Experimentelle atherosklerose der aorta bein near schweinchen. Ziegler’s Beitrage. 1922;70:104–145. [Google Scholar]
- 11.Arcangeli A, Becchetti A, Mannini A, Mugnai G, Philippi P, Tarone G, Del Bene M, Barletta E, Wanke E, Olivotto M. Integrin-mediated neurite outgrowth in neuroblastoma cells depends on activation of potassium channels. J. Cell Biol. 1993;122:1131–1143. doi: 10.1083/jcb.122.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′,5′-cyclic monophosphate levels in various tissue preparations. Proc. Natl. Acad. Sci. USA. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aschoff L. Lectures in Pathology. Berlin: Hueber; 1924. [Google Scholar]
- 14.Awolesi N, Sessa WC, Sumpio B. Cyclic strain up-regulates the endothelial cell nitric oxide synthase gene (Abstract) FASEB J. 1993;7:4360. [Google Scholar]
- 15.Barbee KA, Davies PF, Lal R. Shear stress-induced reorganization of the surface topography of living endothelial cells imaged by atomic force microscopy. Circ. Res. 1994;74:163–171. doi: 10.1161/01.res.74.1.163. [DOI] [PubMed] [Google Scholar]
- 16.Barbee KA, Mundel T, Lal R, Davies PF. Subcellular distribution of shear stress at the surface of flow aligned and non-aligned endothelial monolayers. Am. J. Physiol. doi: 10.1152/ajpheart.1995.268.4.H1765. In press. [DOI] [PubMed] [Google Scholar]
- 17.Barrett TB, Gajdusek CM, Schwartz SM, McDougall JK, Benditt EP. Expression of the sis gene by endothelial cells in culture and in vivo. Proc. Natl. Acad. Sci. USA. 1984;81:6772–6777. doi: 10.1073/pnas.81.21.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berridge MJ. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu. Rev. Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- 19.Berthiaume F, Frangos JA. Flow-induced prostacyclin production is mediated by a pertussis toxin-sensitive G protein. FEES Lett. 1992;308:277–279. doi: 10.1016/0014-5793(92)81292-t. [DOI] [PubMed] [Google Scholar]
- 20.Berthiaume F, Frangos JA. Flow effects on endothelial cell signal transduction, function, and mediator release. In: Bevan J, Kaley G, Rubanyi G, editors. Flow-Dependent Regulation of Vascular Function. New York: Oxford Univ. Press; 1994. [Google Scholar]
- 21.Bhagyalakshmi A, Berthiaume F, Reich KM, Frangos JA. Fluid shear stress stimulates membrane phospholipid metabolism in cultured human endothelial cells. J. Vasc. Res. 1992;29:443–449. doi: 10.1159/000158963. [DOI] [PubMed] [Google Scholar]
- 22.Bhagyalakshmi A, Frangos JA. Mechanisms of shear induced prostacyclin production in endothelial cells. Biochem. Biophys. Res. Commun. 1989;158:33–37. doi: 10.1016/s0006-291x(89)80172-x. [DOI] [PubMed] [Google Scholar]
- 23.Bijay J, Deterre P, Pfister C, Chabre M. Fluoroaluminates activate transducin-GDP by mimicking the γ phosphate of GTP in its binding site. FEBS Lett. 1985;191:181–185. doi: 10.1016/0014-5793(85)80004-1. [DOI] [PubMed] [Google Scholar]
- 24.Blank JL, Brattain KA, Exton JH. Activation of cytosolic phosphoinositide phospholipase C by G protein βγ subunits. J. Biol. Chem. 1992;267:23069–23075. [PubMed] [Google Scholar]
- 25.Bockholt SM, Burridge K. Cell spreading on extracellular matrix proteins induces tyrosine phosphorylation of tensin. J. Biol. Chem. 1993;268:14565–14567. [PubMed] [Google Scholar]
- 26.Bodin P, Bailey D, Burnstock G. Increased flow-ATP release from isolated vascular endothelial cells but not smooth muscle cells. Br. J. Pharmacol. 1991;103:1203–1205. doi: 10.1111/j.1476-5381.1991.tb12324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bodin P, Milner P, Winter R, Burnstock G. Chronic hypoxia changes the ratio of endothelin to ATP release from rat aortic endothelial cells exposed to high flow. Proc. R. Soc. Lond. B Biol. Sci. 1992;247:131–135. doi: 10.1098/rspb.1992.0019. [DOI] [PubMed] [Google Scholar]
- 28.Boulanger C, Luscher TF. Release of endothelin from the porcine aorta: inhibition by endothelium-derived nitric oxide. J. Clin. Invest. 1990;85:587–590. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyle WJ, Smeal T, Defize LH, Angel P, Woodgett JR, Karin M, Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991;64:573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- 30.Braam J, Davis RW. Rain, wind, and touch-induced expression of calmodulin and calmodulin-related genes in. Arabidopsis. Cell. 1990;60:357–364. doi: 10.1016/0092-8674(90)90587-5. [DOI] [PubMed] [Google Scholar]
- 31.Brown MS, Anderson RG, Goldstein JL. Recycling receptors: the round trip itinerary of migrant membrane proteins. Cell. 1983;32:663–667. doi: 10.1016/0092-8674(83)90052-1. [DOI] [PubMed] [Google Scholar]
- 32.Buck C, Horwitz A. Integrin, a transmembrane glycoprotein complex mediating cell-substratum adhesion. J. Cell Sci. Suppl. 1987;8:231–250. doi: 10.1242/jcs.1987.supplement_8.13. [DOI] [PubMed] [Google Scholar]
- 33.Buga GM, Gold ME, Fukuto JM, Ignarro LJ. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension Dallas. 1991;17:187–193. doi: 10.1161/01.hyp.17.2.187. [DOI] [PubMed] [Google Scholar]
- 34.Burridge K. Substrate adhesions in normal and transformed fibroblasts: organization and regulation of cytoskeletal, membrane, and extracellular matrix components at focal contacts. Cancer Res. 1986;4:18–78. [Google Scholar]
- 35.Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu. Rev. Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- 36.Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J. Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Busse R, Lamontagne D. Endothelium-derived bradykinin is responsible in the increase in calcium produced by angiotensin converting enzyme inhibitors in human endothelial cells. Naunyn-Schmiedebergs Arch. Pharmacol. 1991;344:126–129. doi: 10.1007/BF00167392. [DOI] [PubMed] [Google Scholar]
- 38.Busse R, Pohl U, Kellner C, Klemm U. Endothelial cells are involved in the vasodilatory response to hypoxia. Pfluegers Arch. 1983;397:78–80. doi: 10.1007/BF00585175. [DOI] [PubMed] [Google Scholar]
- 39.Caro CG, Fitzgerald JM, Schroter RC. Arterial wall shear and distribution of early atheroma in man. Nature Lond. 1969;223:1159–1161. doi: 10.1038/2231159a0. [DOI] [PubMed] [Google Scholar]
- 40.Caro CG, Nerem RM. Transport of 14C-cholesterol between serum and wall in the perfused dog common carotid artery. Circ. Res. 1973;32:187–194. doi: 10.1161/01.res.32.2.187. [DOI] [PubMed] [Google Scholar]
- 41.Carrington WA, Fogarty KE, Fay FS. Noninvasive Techniques In Cell Biology. New York: Wiley-Liss; 1990. Three-D fluorescence imaging of single cells using image restoration; pp. 53–72. [Google Scholar]
- 42.Carter TD, Hallam TJ, Pearson JD. Protein kinase C activation alters the sensitivity of agonist-stimulated endothelial cell prostacyclin production to intracellular Ca2+ Biochem. J. 1989;262:431–437. doi: 10.1042/bj2620431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chand N, Altura MB. Acetylcholine and bradykinin relax intrapulmonary arteries by acting on endothelial cells: role in lung vascular disease. Science Wash. DC. 1981;213:1376–1379. doi: 10.1126/science.7268440. [DOI] [PubMed] [Google Scholar]
- 44.Charo IF, Nannizzi L, Smith JW, Cheresh DA. The vitronectin receptor alpha v beta 3 binds fibronectin and acts in concert with alpha 5 beta 1 in promoting cellular attachment and spreading on fibronectin. J. Cell Biol. 1990;111:2795–2800. doi: 10.1083/jcb.111.6.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christensen O. Mediation of cell volume regulation by calcium influx through stretch-activated channels. Nature Lond. 1987;330:66–68. doi: 10.1038/330066a0. [DOI] [PubMed] [Google Scholar]
- 46.Clapham DE. Direct G protein activation of ion channels? Annu. Rev. Neurosci. 1994;17:441–464. doi: 10.1146/annurev.ne.17.030194.002301. [DOI] [PubMed] [Google Scholar]
- 47.Coan DE, Wechezak AR, Viggers RF, Sauvage LR. Effect of shear stress upon localization of the Golgi apparatus and microtubule organizing center in isolated cultured endothelial cells. J. Cell Sci. 1993;104:1145–1153. doi: 10.1242/jcs.104.4.1145. [DOI] [PubMed] [Google Scholar]
- 48.Codina J, Hildebrandt JD, Birirnbaumer L, Sekura RD. Effects of guanine nucleotides and Ng on human erythrocyte Ni and Ns, the regulatory components of adenylyl cyclase. J. Biol Chem. 1984;259:11408–11418. [PubMed] [Google Scholar]
- 49.Cook SJ, McCormick F. Ras blooms on sterile ground. Nature Lond. 1994;369:361–362. doi: 10.1038/369361a0. [DOI] [PubMed] [Google Scholar]
- 50.Cook SJ, Rubinfeld B, Albert I, McCormick F. RapV12 antagonizers Ras-dependent activation of Erk-1 and Erk-2 by LPA and EGF in rat-1 fibroblasts. EMBO J. 1993;12:3475–3485. doi: 10.1002/j.1460-2075.1993.tb06022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cooke JP, Rossitch E, Andon NA, Loscalzo J, Dzau V. Flow activates an endothelial potassium channel to release an endogenous nitrovasodilator. J. Clin. Invest. 1991;88:1663–1671. doi: 10.1172/JCI115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cornhill JF, Henderick EE, Stary HC. Topography of human aortic sudanophilic lesions. Monogr. Atherosclerosis. 1990;15:13–19. [PubMed] [Google Scholar]
- 53.Cornhill JF, Roach MR. A quantitative study of the localization of atherosclerotic lesions in the rabbit aorta. Atherosclerosis. 1976;23:489–499. doi: 10.1016/0021-9150(76)90009-5. [DOI] [PubMed] [Google Scholar]
- 54.Corson MA, Berk BC, Navas JP, Harrison DG. Phosphorylation of endothelial nitric oxide synthase in response to shear stress (Abstract) Circulation. 1993;88:1–183. doi: 10.1161/01.res.79.5.984. [DOI] [PubMed] [Google Scholar]
- 55.Craelius W, Chen V, El-Sharif N. Stretch-activated ion channels in ventricular myocytes. Biol. Sci. Rep. 1988;8:407–414. doi: 10.1007/BF01121637. [DOI] [PubMed] [Google Scholar]
- 56.Crawford AW, Michelsen JW, Beckerle MC. An interaction between zyxin and α-actinin. J. Cell Biol. 1992;116:1381–1393. doi: 10.1083/jcb.116.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crespo P, Xu N, Simons WF, Gutkind JS. Ras-dependent activation of MAP kinase pathway mediated by G protein βγ subunits. Nature Lond. 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- 58.Cybulsky MI, Gimbrone MA., Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science Wash. DC. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 59.Darnell JE, Lodish H, Baltimore D. Molecular Cell Biology. New York: Scientific American; 1986. [Google Scholar]
- 60.Davies PF. Endothelial cells, hemodynamic forces, and the localization of atherosclerosis. In: Ryan U, editor. Endothelial Cells. vol. II. Boca Raton, FL: CRC; 1986. pp. 123–139. [Google Scholar]
- 61.Davies PF. How do vascular endothelial cells respond to flow? News Physiol. Sci. 1989;4:22–26. [Google Scholar]
- 62.Davies PF. Endothelium as a signal transduction interface for flow forces: cell surface dynamics. Thromb. Haemostasis. 1993;70:124–128. [PubMed] [Google Scholar]
- 63.Davies PF. Flow-mediated signal transduction in endothelial cells. In: Bevan J, Kaley G, Rubanyi G, editors. Flow Dependent Regulation of Vascular Function in Health and Disease. New York: Academic; 1984. pp. 46–61. [Google Scholar]
- 64.Davies PF, Barbee KA. Endothelial cell surface imaging: insights into hemodynamic force transmission and transduction. News Physiol. Sci. 1994;9:153–158. [Google Scholar]
- 65.Davies PF, Barbee KA, Lal R. Hemodynamics and atherosclerosis: endothelial surface dynamics in flow signal transduction. Ann. NY Acad. Sci. 1995;748:86–104. [PubMed] [Google Scholar]
- 66.Davies PF, Dewey CF, Jr., Bussolari SR, Gordon EJ, Gimbrone MA., Jr. Influence of hemodynamic forces on vascular endothelial function. J. Clin. Invest. 1983;73:1121–1129. doi: 10.1172/JCI111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davies PF, Reidy MA, Goode TB, Bowyer DE. Scanning electron microscopy in the evaluation of endothelial integrity of the fatty lesion in atherosclerosis. Atherosclerosis. 1976;25:125–130. doi: 10.1016/0021-9150(76)90054-x. [DOI] [PubMed] [Google Scholar]
- 68.Davies PF, Remuzzi A, Gordon ES, Dewey CF, Jr., Gimbrone MA., Jr. Turbulent shear stress induces vascular endothelial turnover in vitro. Proc. Natl. Acad. Sci. USA. 1986;83:2114–2118. doi: 10.1073/pnas.83.7.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davies PF, Robotewskyj A, Griem ML. Endothelial cell adhesion in real-time. Measurements in vitro by tandem scanning confocal image analysis. J. Clin. Invest. 1993;91:2640–2652. doi: 10.1172/JCI116503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davies PF, Robotewskyj A, Griem ML. Quantitative studies of endothelial cell adhesion: directional remodeling of focal adhesion sites in response to flow forces. J. Clin. Invest. 1994;93:2031–2038. doi: 10.1172/JCI117197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davies PF, Tripathi SC. Mechanical stress mechanisms and cell. An endothelial paradigm. Circ. Res. 1993;72:239–245. doi: 10.1161/01.res.72.2.239. [DOI] [PubMed] [Google Scholar]
- 72.Degitz K, Jie L, Caughman SW. Cloning and characterization of the 5′-transcriptional regulatory region of the human intercellular adhesion molecule-1 gene. J. Biol. Chem. 1991;266:14024–14030. [PubMed] [Google Scholar]
- 73.Dejana E, Colella S, Conforti G, Abbadini M, Gaboli M, Marchisio PC. Fibronectin and vitronectin regulate the organization of their respective Arg-Gly-Asp adhesion receptors in cultured human endothelial cells. J. Cell Biol. 1988;107:1215–1222. doi: 10.1083/jcb.107.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Demolle D, Lecomte M, Boeynaems JM. Pattern of protein phosphorylation in aortic endothelial cell. J. Biol. Chem. 1988;263:18459–18465. [PubMed] [Google Scholar]
- 75.Dennerll TJ, Joshi HC. Tension and compression in the cytoskeleton of PC-12 neurites. II. Quantitative measurements. J. CeU Biol. 1988;107:665–674. doi: 10.1083/jcb.107.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Depaola N, Gimbrone MA, Jr., Davies PF, Dewey CF., Jr. Vascular endothelium responds to fluid shear stress gradients. Arteriosclerosis Thromb. 1992;12:1254–1257. doi: 10.1161/01.atv.12.11.1254. [DOI] [PubMed] [Google Scholar]
- 77.Dewey CF. Dynamics of arterial flow. Adv. Exp. Med. Biol. 1979;115:55–89. [PubMed] [Google Scholar]
- 78.Dewey CF, Jr., Bussolari SR, Gimbrone MA, Jr., Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J. Biomech. Eng. 1981;103:177–188. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- 79.Diamond SL, Eskin SG, McIntire LV. Fluid flow stimulates tissue plasminogen activator secretion by cultured human endothelial cells. Science Wash. DC. 1989;243:1483–1485. doi: 10.1126/science.2467379. [DOI] [PubMed] [Google Scholar]
- 80.Diamond SL, Sharefkin JB, Dieffenbach CW, Eskin SG, McIntire LV. Regulation of endothelial cell gene expression by hemodynamic forces: implications for intimal hyperplasia and graft patency. In: Yao J, Pearce WH, editors. Technologies in Vascular Surgery. Philadelphia, PA: Saunders; 1992. pp. 12–24. [Google Scholar]
- 81.Diamond SL, Sharefkin JB, Dieffenbach C, Frazier-Scott K, McIntire LV, Eskin SG. Tissue plasminogen activator mRNA levels increase in cultured human endothelial cells exposed to laminar shear stress. J. Cell. Physiol. 1990;143:364–371. doi: 10.1002/jcp.1041430222. [DOI] [PubMed] [Google Scholar]
- 82.Dull RO, Davies PF. Flow modulation of agonist (ATP)-response (Ca2+) coupling in vascular endothelial cells. Am. J. Physiol. 1991;261:H149–H156. doi: 10.1152/ajpheart.1991.261.1.H149. (Heart Circ. Physiol. 30) [DOI] [PubMed] [Google Scholar]
- 83.Dull RO, Tarbell JM, Davies PF. Mechanisms of flow-mediated signal transduction in endothelial cells: kinetics of ATP surface concentrations. J. Vasc. Res. 1992;28:410–419. doi: 10.1159/000158959. [DOI] [PubMed] [Google Scholar]
- 84.Emori T, Hirata Y, Ohta K, Kanno KK, Eguchi S, Imai T, Shichiri M, Marumo F. Cellular mechanism of endothelin-1 release by angiotensin and vasopressin. Hypertension Dallas. 1991;18:165–170. doi: 10.1161/01.hyp.18.2.165. [DOI] [PubMed] [Google Scholar]
- 85.Eskin SG, Ives CL, McIntire LV, Navarro LT. Response of cultured endothelial cells to steady flow. Microvasc. Res. 1984;28:87–93. doi: 10.1016/0026-2862(84)90031-1. [DOI] [PubMed] [Google Scholar]
- 86.Esmon CT. The roles of protein C and thrombomodulin in the regulation of blood coagulation. J. Biol. Chem. 1989;264:4743–4746. [PubMed] [Google Scholar]
- 87.Ezzell C. Just the FAK(s), Ma’am: researchers investigate a new signalling enzyme. J. NIH Res. 1993;5:49–54. [Google Scholar]
- 88.Faggiotto A, Ross R, Harker L. Studies of hypercholesterolemia in the non human primate. Changes that lead to fatty streak formation. Arteriolosclerosis. 1984;4:323–341. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- 89.Falcone JC, Kuo L, Meininger GA. Endothelial cell calcium increases during flow-induced dilation in isolated arterioles. Am. J. Physiol. 1993;264:H653–H659. doi: 10.1152/ajpheart.1993.264.2.H653. (Heart Circ. Physiol. 33) [DOI] [PubMed] [Google Scholar]
- 90.Flaherty JT, Pierce JE, Ferrans VJ, Patel DJ, Tucker WK, Fry DL. Endothelial nuclear patterns in the canine arterial tree with particular reference to hemodynamic events. Circ. Res. 1972;30:23–33. doi: 10.1161/01.res.30.1.23. [DOI] [PubMed] [Google Scholar]
- 91.Flavahan NA. Atherosclerosis or lipoprotein-induced endothelial dysfunction. Potential mechanisms underlying reduction in EDRF/nitric oxide activity. Circulation. 1992;85:1927–1938. doi: 10.1161/01.cir.85.5.1927. [DOI] [PubMed] [Google Scholar]
- 92.Flavahan NA, Shimokawa T, Vanhoutte PM. Pertussis toxin inhibits endothelium-dependent relaxations to certain agonists in porcine coronary arteries. J. Physiol. Lond. 1989;408:549–560. doi: 10.1113/jphysiol.1989.sp017475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Flavahan NA, Vanhoutte PM. G proteins and endothelial responses. Blood Vessels. 1990;27:218–229. doi: 10.1159/000158813. [DOI] [PubMed] [Google Scholar]
- 94.Forstermann U, Pollock JS, Schmidt HH, Heller M, Murad F. Calmodulin-dependent endothelium-derived relaxing factor synthase activity is present in the particulate and cytosolic fractions of bovine aortic endothelial cells. Proc. Natl. Acad. Sci. USA. 1991;88:1788–1792. doi: 10.1073/pnas.88.5.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science Wash. DC. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 96.Frangos JA, Kuchan MJ. Fluid flow activates G proteins that are coupled to Ca-dependent and -independent EDRF production in cultured endothelial cells (Abstract) FASEB J. 1991;5:A1820. [Google Scholar]
- 97.Franke RP, Grafe M, Schnittler H, Seiffge D, Mit-Termayer C, Drenckhahn D. Induction of human vascular endothelial stress fibers by fluid shear stress. Nature Lond. 1984;307:648–650. doi: 10.1038/307648a0. [DOI] [PubMed] [Google Scholar]
- 98.Friedli B, Kent G, Olley PM. Inactivation of bradykinin in the pulmonary vascular bed of newborn fetal lambs. Circ. Res. 1973;33:421–427. doi: 10.1161/01.res.33.4.421. [DOI] [PubMed] [Google Scholar]
- 99.Friedman MH, Hutchins GM, Bargeron CB, Deters OJ, Mark FF. Correlation between intimal thickness and fluid shear in human arteries. Atherosclerosis. 1981;39:425–436. doi: 10.1016/0021-9150(81)90027-7. [DOI] [PubMed] [Google Scholar]
- 100.Fry DL. Atherogenesis: initiating factors. Ciba Found. Symp. 1973;12:96–118. [Google Scholar]
- 101.Fry DL, Herderick EE, Johnson DK. Local intimal-medial uptakes of 125I-albumin, 125I-LDL, and parenteral Evans Blue dye protein complex along the aortas of normocholesterolemic minipigs as predictors of subsequent hypercholesterolemic atherogenesis. Arteriosclerosis Thromb. 1993;13:1193–1204. doi: 10.1161/01.atv.13.8.1193. [DOI] [PubMed] [Google Scholar]
- 102.Fujii K, Heistad DD, Faraci FM. Flow-mediated dilatation of the basilar artery in vivo. Circ. Res. 1991;69:697–705. doi: 10.1161/01.res.69.3.697. [DOI] [PubMed] [Google Scholar]
- 103.Fung YC, Liu SQ. Elementary mechanics of the endothelium of blood vessels. J. Biomech. Eng. 1993;115:1–12. doi: 10.1115/1.2895465. [DOI] [PubMed] [Google Scholar]
- 104.Furchgott RF, Zawadski JV. The obligatory role of the endothelial cells in the relaxation of arterial smooth muscle cells by acetylcholine. Nature Lond. 1981;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 105.Geiger B, Avnur Z, Kreis TE, Schlessinger J. The dynamics of cytoskeletal organization in areas of cell contact. Cell Muscle Motil. 1984;5:195–234. doi: 10.1007/978-1-4684-4592-3_5. [DOI] [PubMed] [Google Scholar]
- 106.Geiger RV, Berk BC, Alexander RW, Nerem RM. Flow-induced calcium transients in single endothelial cells: spatial and temporal analysis. Am. J. Physiol. 1992;262:C1411–C1417. doi: 10.1152/ajpcell.1992.262.6.C1411. (Cell Physiol. 31) [DOI] [PubMed] [Google Scholar]
- 107.Gemmell C, Nemerson Y, Turitto V. The effects of shear rate on the enzymatic activity of the tissue-factor VIIa complex. Microvasc. Res. 1990;40:327–340. doi: 10.1016/0026-2862(90)90031-l. [DOI] [PubMed] [Google Scholar]
- 108.Gentz R, Rauscher FJ, Abate C, Curran T. Parallel association of Fos and Jun leucine zippers juxtaposes DNA binding domains. Science Wash. DC. 1989;243:1695–1699. doi: 10.1126/science.2494702. [DOI] [PubMed] [Google Scholar]
- 109.Giddens DN, Zarins CK, Glagov S. Response of arteries to near-wall fluid dynamic behavior. Appl. Mech. Rev. 1990;43 Suppl.:S98–S102. [Google Scholar]
- 110.Gimbrone MA., Jr. Endothelial dysfunction and the pathogenesis of atherosclerosis. In: Gotto AM, Smith LC, Allen B, editors. Atherosclerosis V. New York: Springer-Verlag; 1980. pp. 415–425. [Google Scholar]
- 111.Girard PR, Nerem RM. Endothelial cell signaling and cytoskeletal changes in response to shear stress. Front. Med. Biol. Eng. 1993;5:31–36. [PubMed] [Google Scholar]
- 112.Glagov S, Weisenberg E, Zarins CK, Stankuna-Vicius R, Kolettis G. Compensatory enlargement of human atherosclerotic coronary arteries. N. Engl. J. Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 113.Glagov S, Zarins CK, Giddens DP, Ku DN. Hemodynamics in atherosclerosis: insights and perspectives gained from studies of human arteries. Arch. Pathol. Lab. Med. 1988;112:1018–1031. [PubMed] [Google Scholar]
- 114.Goldschmidt-Clermont PJ, Machesky LM, Baldassare JJ, Pollard TD. The actin-binding protein profilin binds to PIP2 and inhibits its hydrolysis by phospholipase C. Science Wash. DC. 1990;247:1575–1578. doi: 10.1126/science.2157283. [DOI] [PubMed] [Google Scholar]
- 115.Goligorsky MS. Mechanical stimulation induces transients and membrane depolarization in cultured endothelial cells. Effects on in co-perfused smooth muscle cells. FEBS Lett. 1988;240:59–64. doi: 10.1016/0014-5793(88)80340-5. [DOI] [PubMed] [Google Scholar]
- 116.Goode T, Davies PF, Reidy MA, Bowyer DE. Aortic endothelial cell morphology observed in situ by scanning electron-microscopy during atherogenesis in the rabbit. Atherosclerosis. 1977;27:235–251. doi: 10.1016/0021-9150(77)90061-2. [DOI] [PubMed] [Google Scholar]
- 117.Gospodarowicz D, Greenburg G, Birdwell CR. Determination of cellular shape by the extracellular matrix and its correlation with the control of cellular growth. Cancer Res. 1978;38:4155–4171. [PubMed] [Google Scholar]
- 118.Grabowski EF, Weksler BB, Jaffe EA. Prostacyclin production by cultured endothelial cell monolayers exposed to step increases in shear stress. J. Lab. Clin. Med. 1985;105:36–43. [PubMed] [Google Scholar]
- 119.Grabowski EF, Zuckerman DB, Nemerson Y. The functional expression of tissue factor by fibroblasts and endothelial cells under flow conditions. Blood. 1993;81:3265–3270. [PubMed] [Google Scholar]
- 120.Graier WF, Schmidt K, Kukowetz WR. Effect of sodium fluoride on eytosolic-free calcium concentrations and cGMP levels in endothelial cells. Cell Signal. 1990;2:369–375. doi: 10.1016/0898-6568(90)90067-k. [DOI] [PubMed] [Google Scholar]
- 121.Griggs P. Biophysical studies of mechanoreceptors. J. Appl. Physiol. 1986;60:1107–1115. doi: 10.1152/jappl.1986.60.4.1107. [DOI] [PubMed] [Google Scholar]
- 122.Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue cultured embryonic chick skeletal muscle cells. J. Physiol. Lond. 1984;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gunther S, Alexander RW, Atkinson W, Gimbrone MA., Jr. Functional angiotensin II receptors in cultured vascular smooth muscle cells. J. Cell Biol. 1982;92:195–204. doi: 10.1083/jcb.92.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gupte A, Frangos JA. Effects of flow on the synthesis and release of fibronectin by endothelial cells. In Vitro Cell Dev. Biol. 1990;26:57–60. doi: 10.1007/BF02624155. [DOI] [PubMed] [Google Scholar]
- 125.Guyton JR. Mechanical control of smooth muscle growth. In: Seidel CL, Weisbrodt NW, editors. Hypertrophic Response in Smooth Muscle. vol. II. Boca Raton, FL: CRC; 1987. pp. 121–152. [Google Scholar]
- 126.Hall A. A biochemical function for Ras at last. Science Wash. DC. 1994;264:1413–1414. doi: 10.1126/science.8197454. [DOI] [PubMed] [Google Scholar]
- 127.Hallam TJ, Pearson JD. Exogenous ATP raises cytoplasmic free calcium in piglet aortic endothelial cells. FEBS Lett. 1986;207:95–99. doi: 10.1016/0014-5793(86)80019-9. [DOI] [PubMed] [Google Scholar]
- 128.Hansen CA, Schroering AG, Carey DJ, Robishaw JD. Localization of a heterotrimeric G protein γ subunit to focal adhesions and associated stress fibers. J. Cell Biol. 1994;126:811–829. doi: 10.1083/jcb.126.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hansson GK, Chao S, Schwartz SM, Reidy MA. Aortic endothelial cell death and replication in normal and lipopolysaccharide-treated rats. Am. J. Pathol. 1985;121:123–127. [PMC free article] [PubMed] [Google Scholar]
- 130.Hay ED, Meier S. Stimulation of corneal differentiation by interaction between cell surface and extracellular matrix. II. Further studies on the nature and site of transfilter “induction.”. Dev. Biol. 1976;52:141–157. doi: 10.1016/0012-1606(76)90014-2. [DOI] [PubMed] [Google Scholar]
- 131.Heath JP, Dunn GA. Cell to substratum contacts of chick fibroblasts and their relation to the microfilament system. A correlated interference reflection and high voltage electonmicroscope study. J. Cell Sci. 1978;29:197–212. doi: 10.1242/jcs.29.1.197. [DOI] [PubMed] [Google Scholar]
- 132.Heidemann SR, Buxbaum RE. Tension as a regulator and integrator of axonal growth. Cell. Motil. Cytoskeleton. 1990;17:6–10. doi: 10.1002/cm.970170103. [DOI] [PubMed] [Google Scholar]
- 133.Helmlinger G. Effect of Pulsatile Flow on the Intracellular Free Calcium Concentration of Cultured Vascular Endothelial Cells (PhD thesis) Atlanta: Georgia Institute of Technology; 1994. [Google Scholar]
- 134.Helmlinger G, Geiger RV, Schreck S, Nerem RM. Effects of pulsatile flow on cultured vascular endothelial cell morphology. J. Biomech. Eng. 1991;113:123–134. doi: 10.1115/1.2891226. [DOI] [PubMed] [Google Scholar]
- 135.Henry PD, Chen CH. Inflammatory mechanisms of atheroma formation. Influence of fluid mechanics and lipid-derived inflammatory mediators. Am. J. Hypertens. 1993;6 Suppl.:328S–334S. doi: 10.1093/ajh/6.11.328s. [DOI] [PubMed] [Google Scholar]
- 136.Himmel HM, Whorton AR, Strauss HC. Intracellular calcium, current, and stimulus-response coupling in endothelial cells. Hypertension Dallas. 1993;21:112–127. doi: 10.1161/01.hyp.21.1.112. [DOI] [PubMed] [Google Scholar]
- 137.Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature Lond. 1993;362:31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- 138.Holtz J, Forstermann U, Pohl U, Giesler M, Bassenge E. Flow-dependent, endothelium-mediated dilatation of epicardial arteries in concious dogs: effects of cyclo-oxygenase inhibition. J. Cardiovasc. Pharmacol. 1984;6:1161–1169. [PubMed] [Google Scholar]
- 139.Honda HM, Wortham C, Navab M, Demer LL. Disturbed flow induces heat shock protein-70 mRNA in bovine and human aortic endothelial cells (Abstract) Circulation. 1992;86 Suppl. I:I-224. [Google Scholar]
- 140.Hong SL, Deykin D. Activation of phospholipases A2 and C in pig aortic endothelial cells synthesizing prostacyclin. J. Biol. Chem. 1982;257:7151–7154. [PubMed] [Google Scholar]
- 141.Hopwood AM, Lincoln J, Kirkpatrick KA, Burnstock G. Adenosine-5′-trisphophate, adenosine and endothelium-derived relaxing factor in hypoxic vasodilatation of the heart. Eur. J. Pharmacol. 1989;165:323–326. doi: 10.1016/0014-2999(89)90730-9. [DOI] [PubMed] [Google Scholar]
- 142.Horvath AR, Elmore MA, Kellie S. Differential tyrosine-specific phosphorylation of integrin in Rous sarcoma virus transformed cells with differing transformed phenotypes. Oncogene. 1990;5:1349–1357. [PubMed] [Google Scholar]
- 143.Horvath AR, Kellie S. Regulation of integrin mobility and cytoskeletal association in normal and RSV-transformed chick embryo fibroblasts. J. Cell Sci. 1990;97:307–315. doi: 10.1242/jcs.97.2.307. [DOI] [PubMed] [Google Scholar]
- 144.Horwitz A, Duggan K, Buck C, Beckerle MC, Burridge K. Interaction of plasma membrane fibronectin receptor with talin: a transmembrane linkage. Nature Lond. 1986;320:531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- 145.Houston DS, Schepherd JT, Vanhoutte PM. Adenine nucleotides, serotonin, and endothelium-dependent relaxations to platelets. Am. J. Physiol. 1985;248:H389–H395. doi: 10.1152/ajpheart.1985.248.3.H389. (Heart Circ. Physiol. 17) [DOI] [PubMed] [Google Scholar]
- 146.Hsieh HJ, Li NQ, Frangos JA. Pulsatile and steady flows increase proto-oncogenes c-fos and c-myc mRNA levels in human endothelial cells (Abstract) FASEB J. 1991;5:A1820. [Google Scholar]
- 147.Hsieh HJ, Li NQ, Frangos JA. Shear-induced platelet derived growth factor gene expression in human endothelial cells is mediated by protein kinase C. J. Cell. Physiol. 1992;150:552–558. doi: 10.1002/jcp.1041500316. [DOI] [PubMed] [Google Scholar]
- 148.Hsieh HJ, Li NQ, Frangos JA. Pulsatile and steady flow induces c-fos expression in human endothelial cells. J. Cell. Physiol. 1993;154:143–151. doi: 10.1002/jcp.1041540118. [DOI] [PubMed] [Google Scholar]
- 149.Hudspeth AJ. How the ear’s works work. Nature Lond. 1989;341:397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- 150.Hunt CC, Wilkinson RS, Fukami Y. Ionic basis of the receptor potential in primary endings of mammalian muscle spindles. J. Gen. Physiol. 1978;71:683–698. doi: 10.1085/jgp.71.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hynes RO. Integrins: versatility, modulation, and signalling and cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 152.Iba T, Sumpio BE. Morphological response of human endothelial cells subjected to cyclic strain in vitro. Microvasc. Res. 1991;42:245–254. doi: 10.1016/0026-2862(91)90059-k. [DOI] [PubMed] [Google Scholar]
- 153.Ignarro LJ. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu. Rev. Pharmacol. Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- 154.Ingber D. Integrins as mechanochemical transducers. Curr. Opin. Cell Biol. 1991;3:841–848. doi: 10.1016/0955-0674(91)90058-7. [DOI] [PubMed] [Google Scholar]
- 155.Ingber D, Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J. Cell Biol. 1989;199:317–330. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ingber DE. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J. Cell Sci. 1993;104:613–627. doi: 10.1242/jcs.104.3.613. [DOI] [PubMed] [Google Scholar]
- 157.Ingber DE, Dike L, Hansen L, Karp S, Liley H, Maniotis A, McNamee H, Mooney D, Plopper G, Sims J, Wang N. Cellular tensegrity: exploring how mechanical changes in the cytoskeleton regulate cell growth, migration, and tissue pattern during morphogenesis. Int. Rev. Cytol. 1994;150:173–224. doi: 10.1016/s0074-7696(08)61542-9. [DOI] [PubMed] [Google Scholar]
- 158.Ingber DE, Jamieson JD. Cells as tensegrity structures: architectural regulation of histodifferentiation by physical forces transduced over basement membrane. In: Andersson LC, Gahmberg CG, Ekblom P, editors. Gene Expression During Normal and Malignant Differentiation. Orlando, FL: Academic; 1985. pp. 13–32. [Google Scholar]
- 159.Ingber DE, Prusty D, Frangioni JV, Cragoe EG, Lechene C, Schwartz MA. Control of intracellular pH and growth by fibronectin in capillary endothelial cells. J. Cell Biol. 1990;110:1803–1811. doi: 10.1083/jcb.110.5.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Irvine RF. How is the level of free arachidonic acid controlled in mammalian cells? Biochem. J. 1982;204:3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Izzard CS, Lochner LR. Formation of cell-to-substrate contacts during fibroblast motility: an interference-reflexion study. J. Cell Sci. 1980;42:81–116. doi: 10.1242/jcs.42.1.81. [DOI] [PubMed] [Google Scholar]
- 162.Jacob R, Merritt JE, Hallam TJ, Rink TJ. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature Lond. 1988;335:40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- 163.Jacobs E, Cheliakine C, Gebremedhin D, Davies PF, Harder DR. Shear-activated channels in cell-attached patches of aortic endothelial cells (Abstract) FASEB J. 1993;7:71. doi: 10.1007/BF00374386. [DOI] [PubMed] [Google Scholar]
- 164.Johnson PC. Handbook of Physiology. The Cardiovascular System. Vascular Smooth Muscle. Bethesda, MD: Am. Physiol. Soc.; 1981. The myogenic response; pp. 409–442. sect. 2, vol. II, chapt. 15. [Google Scholar]
- 165.Kaiser L, Spickard RC, Oliver NB. Heart failure depresses endothelium dependent responses in canine femoral artery. Am. J. Physiol. 1989;259:H962–H967. doi: 10.1152/ajpheart.1989.256.4.H962. (Heart Circ. Physiol. 28) [DOI] [PubMed] [Google Scholar]
- 166.Kamiya A, Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am. J. Physiol. 1980;239:H14–H21. doi: 10.1152/ajpheart.1980.239.1.H14. (Heart Circ. Physiol. 8) [DOI] [PubMed] [Google Scholar]
- 167.Karino T, Motomiya M. Flow visualization in isolated transparent natural blood vessels. Biorheology. 1983;20:119–127. doi: 10.3233/bir-1983-20202. [DOI] [PubMed] [Google Scholar]
- 168.Kawashima K, Watanabe N, Oohata H, Fujimoto K, Suzuki T, Ishizaki Y, Morita I, Murota S. Synthesis and release of acetylcholine by cultured bovine arterial endothelial cells. Neurosci. Lett. 1990;119:156–158. doi: 10.1016/0304-3940(90)90822-q. [DOI] [PubMed] [Google Scholar]
- 168a.Khachigian LM, Resnick N, Gimbrone MA, Jr., Collins T. Nuclear factor κB interacts functionally with the shear stress response element in the platelet derived growth factor κB chain promoter. FASEB J. doi: 10.1172/JCI118106. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kim DW, Gotlieb AI, Langille BL. In vivo modulation of endothelial F-actin microfilaments by experimental alterations in shear stress. Arteriosclerosis. 1989;9:439–445. doi: 10.1161/01.atv.9.4.439. [DOI] [PubMed] [Google Scholar]
- 170.Kim DW, Langille BL, Wong MK, Gotlieb AI. Patterns of endothelial microfilament distribution in the rabbit aorta in situ. Circ. Res. 1989;64:21–31. doi: 10.1161/01.res.64.1.21. [DOI] [PubMed] [Google Scholar]
- 171.Klein S, Giancotti FG, Presta M, Arbelda SM, Buck CA, Rifkin DB. Basic FGF modulates integrin expression in microvascular endothelial cells. Mol. Biol. Cell. 1993;4 doi: 10.1091/mbc.4.10.973. 973–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Koller A, Sun D, Kaley G. Role of shear stress and endothelial prostaglandins in flow- and viscosity-induced dilation of arterioles in vitro. Circ. Res. 1993;72:1276–1284. doi: 10.1161/01.res.72.6.1276. [DOI] [PubMed] [Google Scholar]
- 173.Koller AS, Huang A. Impaired nitric oxide-mediated flow-induced dilation in arterioles of spontaneously hypertensive rats. Circ. Res. 1994;74:416–421. doi: 10.1161/01.res.74.3.416. [DOI] [PubMed] [Google Scholar]
- 174.Komaru T, Wang Y, Akai K, Sato K, Sekiguchi N, Sugimura A, Kumagai T, Kanatsuke H, Shirato K. Pertussis toxin-sensitive G protein mediates coronary microvascular control during autoregulation and ischemia in canine heart. Circ. Res. 1994;75:556–566. doi: 10.1161/01.res.75.3.556. [DOI] [PubMed] [Google Scholar]
- 175.Komuro I, Kurihara H, Sugiyama HT, Yoshizumi M, Takaku F, Yazaki Y. Endothelin stimulates c-fos and c-myc expression and proliferation of vascular smooth muscle cells. FEBS Lett. 1988;238:249–252. doi: 10.1016/0014-5793(88)80489-7. [DOI] [PubMed] [Google Scholar]
- 176.Kraiss LW, Kirkman TR, Kohler TR, Zierler B, Clowes AW. Shear stress regulates smooth muscle proliferation and neo-intimal thickening in porous polytetrafluoroethylene grafts. Arteriosclerosis Thromb. 1991;11:1844–1852. doi: 10.1161/01.atv.11.6.1844. [DOI] [PubMed] [Google Scholar]
- 177.Ku DN, Giddens DP. Pulsatile flow in a model carotid bifurcation. Arteriosclerosis. 1983;3:31–39. doi: 10.1161/01.atv.3.1.31. [DOI] [PubMed] [Google Scholar]
- 178.Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low and oscillating shear stress. Arteriosclerosis. 1985;5:293–301. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- 179.Kubo Y, Baldwin TJ, Jan YN, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature Lond. 1993;362:127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- 180.Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminar and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J. Cell Biol. 1988;107:1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kuchan MJ, Frangos JA. Shear stress regulates endothelin-1 release via protein kinase C and cGMP in cultured endothelial cells. Am. J. Physiol. 1993;264:H150–H156. doi: 10.1152/ajpheart.1993.264.1.H150. (Heart Circ. Physiol. 33) [DOI] [PubMed] [Google Scholar]
- 182.Kuchan MJ, Frangos JA. Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. Am. J. Physiol. 1994;266:C628–C636. doi: 10.1152/ajpcell.1994.266.3.C628. (Cell Physiol. 35) [DOI] [PubMed] [Google Scholar]
- 183.Kuo L, Davis MJ, Cannon MS, Chilian WM. Pathophysiological consequences of atherosclerosis extends into the microcirculation. Restoration of endothelium-dependent responses by L-arginine. Circ. Res. 1992;70:465–476. doi: 10.1161/01.res.70.3.465. [DOI] [PubMed] [Google Scholar]
- 184.Lal R, John SA. Biological applications of atomic force microscopy. Am. J. Physiol. 1994;266:C1–C21. doi: 10.1152/ajpcell.1994.266.1.C1. (Cell Physiol. 35) [DOI] [PubMed] [Google Scholar]
- 185.Lamas S, Michel T, Brenner BM, Marsden PA. Nitric oxide synthesis in endothelial cells: evidence for a pathway inducible by TNF-α. Am. J. Physiol. 1991;261:C634–C641. doi: 10.1152/ajpcell.1991.261.4.C634. (Cell Physiol. 30) [DOI] [PubMed] [Google Scholar]
- 186.Lan Q, Mercurius KO, Davies PF. Stimulation of transcription factors NFκB and AP1 in endothelial cells subjected to shear stress. Biochem. Biophys. Res. Commun. 1994;201:950–956. doi: 10.1006/bbrc.1994.1794. [DOI] [PubMed] [Google Scholar]
- 187.Langille BL, Adamson SL. Relationship between blood flow direction and endothelial cell orientation at arterial branch sites in rabbits and mice. Circ. Res. 1981;48:481–488. doi: 10.1161/01.res.48.4.481. [DOI] [PubMed] [Google Scholar]
- 188.Langille BL, Graham JJ, Kim D, Gotlieb AI. Dynamics of shear-induced redistribution of F-actin in endothelial cells in vivo. Arteriosclerosis Thromb. 1991;11:1814–1820. doi: 10.1161/01.atv.11.6.1814. [DOI] [PubMed] [Google Scholar]
- 189.Langille BL, O’Donnel F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science Wash. DC. 1986;231:405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- 190.Lansman JB, Hallam TJ, Rink TJ. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers? Nature Lond. 1987;325:811–812. doi: 10.1038/325811a0. [DOI] [PubMed] [Google Scholar]
- 191.Laurindo FRM, De Pedro MA, Barbeiro HV, Pileggi F, Carvalho MHC, Augusto O, Da Luz PL. Vascular free radical release ex vivo and in vivo evidence for a flow-dependent endothelial mechanism. Circ. Res. 1994;74:700–709. doi: 10.1161/01.res.74.4.700. [DOI] [PubMed] [Google Scholar]
- 192.Letsou GV, Rosales O, Maitz S, Vogt A, Sumpio BE. Stimulation of adenylate cyclase activity in cultured endothelial cells subjected to cyclic stretch. J. Cardiovasc. Surg. 1990;31:634–639. [PubMed] [Google Scholar]
- 193.Levesque MJ, Liepsch D, Moravec S, Nerem RM. Correlation of endothelial cell shape and wall shear stress in a stenosed dog aorta. Arteriosclerosis. 1986;6:220–229. doi: 10.1161/01.atv.6.2.220. [DOI] [PubMed] [Google Scholar]
- 194.Levesque MJ, Nerem RM. The study of rheological effects on vascular endothelial cells in culture. Biorheology. 1989;26:345–357. doi: 10.3233/bir-1989-26218. [DOI] [PubMed] [Google Scholar]
- 195.Levitan IB. Phosphorylation of ion channels. J. Membr. Biol. 1985;87:177–190. doi: 10.1007/BF01871217. [DOI] [PubMed] [Google Scholar]
- 196.Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 197.Linnik MD, Moskowitz MA. Identification of immunoreactive substance P in human and other mammalian endothelial cells. Peptides. 1989;10:957–962. doi: 10.1016/0196-9781(89)90175-7. [DOI] [PubMed] [Google Scholar]
- 198.Liou HC, Baltimore D. Regulation of the NF-κB/rel transcription factor and IκB inhibitor system. Curr. Opin. Cell Biol. 1993;5:477–487. doi: 10.1016/0955-0674(93)90014-h. [DOI] [PubMed] [Google Scholar]
- 199.Loesch A, Bodin P, Burnstock G. Co-localization of endothelin, vasopressin and serotonin in cultured endothelial cells of rabbit aorta. Peptides. 1991;12:1095–1103. doi: 10.1016/0196-9781(91)90065-w. [DOI] [PubMed] [Google Scholar]
- 200.Loewenstein WR, Skalak R. Mechanical transmission in a Pacinian corpuscle. An analysis and a theory. J. Physiol. Lond. 1981;182:346–378. doi: 10.1113/jphysiol.1966.sp007827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lopez-Jaramillo P, Gonzalez MC, Palmer RM, Moncada S. The crucial role of physiological calcium concentrations in the production of endothelial nitric oxide and the control of vascular tone. Br. J. Pharmacol. 1990;101:489–493. doi: 10.1111/j.1476-5381.1990.tb12735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Luckhoff A, Busse R. Activators of potassium channels enhance calcium influx into endothelial cells as a consequence of potassium currents. Naunyn-Schmiedebergs Arch. Pharmacol. 1990;342:94–99. doi: 10.1007/BF00178979. [DOI] [PubMed] [Google Scholar]
- 203.Luckoff A, Busse R. Calcium influx into endothelial cells and formation of endothelium derived relaxing factor is controlled by the membrane potential. Pfluegers Arch. 1990;416:305–311. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- 204.Luckhoff A, Clapham DE. Inositol 1,3,4,5-tetrakisphos-phate activates an endothelial Ca2+-permeable channel. Nature Lond. 1992;355:356–358. doi: 10.1038/355356a0. [DOI] [PubMed] [Google Scholar]
- 205.Luft JH. The structure and properties of the cell surface coat. Int. Rev. Cytol. 1976;45:291–382. doi: 10.1016/s0074-7696(08)60081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Luscher TF, Tanner FC. Endothelial regulation of vascular tone and growth. Am. J. Hypertens. 1993;6 Suppl.:283S–293S. doi: 10.1093/ajh/6.7.283s. [DOI] [PubMed] [Google Scholar]
- 207.Macarthur H, Hecker M, Busse R, Vane JR. Selective inhibition of agonist-induced but not shear stress-dependent release of endothelial autacoids by thapsigargin. Br. J. Pharmacol. 1993;108:100–105. doi: 10.1111/j.1476-5381.1993.tb13446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mackie K, Lai Y, Nairn AC, Greengard P, Pitt BR, Lazo JS. Protein phosphorylation in cultured endothelial cells. J. Cell. Physiol. 1986;128:367–374. doi: 10.1002/jcp.1041280304. [DOI] [PubMed] [Google Scholar]
- 209.Macmillan-Crow LA, Murphy-Ullrich JE, Lincoln TM. Identification and possible localization of cGMP-dependent protein kinase in bovine aortic endothelial cells. Biochem. Biophys. Res. Commun. 1994;201:531–537. doi: 10.1006/bbrc.1994.1734. [DOI] [PubMed] [Google Scholar]
- 210.Madri JA, Pratt BM, Yannariello-Brown J. Matrix driven cell size change modulates aortic endothelial cell proliferation and sheet migration. Am. J. Pathol. 1988;132:18–27. [PMC free article] [PubMed] [Google Scholar]
- 211.Magnusson MK, Halldorsson H, Kjeld M, Thorgeirsson G. Endothelial inositol phosphate generation and prostacyclin production in response to G protein activation by AlF4. Biochem. J. 1989;264:703–711. doi: 10.1042/bj2640703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Maguire MH, Satchell DG. Purinergic receptors in visceral smooth muscle. In: Burnstock G, editor. Purinergic Receptors. London: Clapham and Hall; 1981. [Google Scholar]
- 213.Maher PA, Pasquale EB, Yang JY, Singer SJ. Phosphotyrosine-containing proteins are concentrated in focal adhesions and intercellular junctions in normal cells. Proc. Natl. Acad. Sci. USA. 1985;82:6576–6580. doi: 10.1073/pnas.82.19.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Malek A, Izumo S. Physiological fluid shear stress causes down-regulation of endothelin-1 mRNA in bovine aortic endothelium. Am. J. Physiol. 1992;263:C389–C396. doi: 10.1152/ajpcell.1992.263.2.C389. (Cell Physiol. 32) [DOI] [PubMed] [Google Scholar]
- 215.Malek AM, Gibbons GH, Dzau VJ, Izumo S. Fluid shear stress differentially modulates expression of genes encoding basic fibroblast growth factor and platelet-derived growth factor B chain in vascular endothelium. J. Clin. Invest. 1993;92:2013–2021. doi: 10.1172/JCI116796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Malek AM, Greene AL, Izumo S. Regulation of endothelin 1 gene by fluid shear stress in transcriptionally mediated and independent of protein kinase C and cAMP. Proc. Natl. Acad. Sci. USA. 1993;90:5999–6003. doi: 10.1073/pnas.90.13.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Malek AM, Jackman R, Rosenberg RD, Izumo S. Endothelial expression of thrombomodulin is reversibly regulated by fluid shear stress. Circ. Res. 1994;74:852–860. doi: 10.1161/01.res.74.5.852. [DOI] [PubMed] [Google Scholar]
- 218.Marsden PA, Heng HH, Scherer SW, Stewart RJ, Hall AV, Shi XM, Tsui L, Schappert KT. Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase. J. Biol. Chem. 1993;268:17478–17488. [PubMed] [Google Scholar]
- 219.Martin TW. Formation of diacylglycerol by a phospholipase D-phosphatase pathway specific for phosphatidylcholine in endothelial cells. Biochim. Biophys. Acta. 1988;962:282–296. doi: 10.1016/0005-2760(88)90258-5. [DOI] [PubMed] [Google Scholar]
- 220.Martin TW, Wysolmerski RB. Ca2+-dependent pathways for release of arachidonic acid from phosphatidylinositol in endothelial cells. J. Biol. Chem. 1987;262:13086–13092. [PubMed] [Google Scholar]
- 221.Martin-Mondiere CF, Caprani A, Desgranges PC, Loisance DY, Charron DJ. Shear stress affects expression of major histocompatibility complex antigens on human endothelial cells. ASAIO Trans. 1989;35:288–290. doi: 10.1097/00002480-198907000-00036. [DOI] [PubMed] [Google Scholar]
- 222.Martinac B, Buechner M, Delcour AH, Adler J, Kung C. Pressure sensitive ion channels in E. coli. Proc. Natl. Acad. Sci. USA. 1987;84:2279–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222a.Marui N, Offermann MK, Swerlick R, Kunsch C, Rosen CA, Ahmad M, Alexander RW, Medford RM. VCAM-1 gene transcription and expression are regulated through an antioxidant sensitive mechanism in human vascular endothelial cells. J. Clin. Invest. 1993;92:1866–1874. doi: 10.1172/JCI116778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Massop DW, Wright JG, Smead WL, Sadoun ET, Cornhill JF. Interleukin 6 secretion and gene expression by human aortic endothelial cells in response to laminar shear stress (Abstract) Circulation. 1990;82:206. [Google Scholar]
- 224.Masuda H, Bassiouny HS, Glagov S, Zarins CK. Artery wall restructuring in response to increased flow. Surg. Forum. 1989;40:285–286. [Google Scholar]
- 225.Masuda H, Shozawa T, Hosoda S, Kanda M, Kamiya A. Cytoplasmic microfilaments in endothelial cells are flow loaded and canine carotid arteries. Heart Vessels. 1985;1:65–69. doi: 10.1007/BF02066350. [DOI] [PubMed] [Google Scholar]
- 226.Masuda M, Fujiwara K. The biased lamellipodium development and microtubule organizing center position in vascular endothelial cells migrating under the influence of fluid flow. Biol. Cell. 1993;77:237–245. doi: 10.1016/s0248-4900(05)80193-5. [DOI] [PubMed] [Google Scholar]
- 227.McLenachan JM, Williams JK, Fish RD, Ganz P, Selwyn AP. Loss of flow-mediated endothelium-dependent dilation occurs early in the development of atherosclerosis. Circulation. 1991;84:1273–1278. doi: 10.1161/01.cir.84.3.1273. [DOI] [PubMed] [Google Scholar]
- 228.McMahon TA. Muscles, Reflexes, and Locomotion. Princeton, NJ: Princeton Univ. Press; 1984. [Google Scholar]
- 229.McNeil PL. Cellular and molecular adaptations to injurious mechanical stress. Trends Cell Biol. 1993;3:302–307. doi: 10.1016/0962-8924(93)90012-p. [DOI] [PubMed] [Google Scholar]
- 230.McNeil PL, Murphy RF, Lanni F, Taylor DL. A method for incorporating macromolecules into adherent cells. J. Cell Biol. 1984;98:1556–1564. doi: 10.1083/jcb.98.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.McNeil PL, Muthukrishman L, Warder E, D’Amore PA. Growth factors are released by mechanically wounded endothelial cells. J. Cell Biol. 1989;109:811–822. doi: 10.1083/jcb.109.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Melkumyants AM, Balashov SA. Blood flow velocity: a constantly acting factor in dilatation of large arteries. Bull. Exp. Biol. Med. 1985;99:135–138. [PubMed] [Google Scholar]
- 233.Melkumyants AM, Balashov SA, Khayutin VM. Endothelium dependent control of arterial diameter by blood viscosity. Cardiovasc. Res. 1989;23:741–747. doi: 10.1093/cvr/23.9.741. [DOI] [PubMed] [Google Scholar]
- 234.Michel T, Li GK, Busconi L. Phosphorylation and subcellular translocation of endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA. 1993;90:6252–6256. doi: 10.1073/pnas.90.13.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Michell RH. Inositol lipids in cellular signalling mechanisms. Trans. Biochem. Sci. 1992;17:274–276. doi: 10.1016/0968-0004(92)90433-a. [DOI] [PubMed] [Google Scholar]
- 236.Miller VM, Vanhoutte PM. Enhanced release of endothelium-derived relaxing factor by chronic increases in blood flow. Am. J. Physiol. 1988;255:H446–H451. doi: 10.1152/ajpheart.1988.255.3.H446. (Heart Circ. Physiol. 24) [DOI] [PubMed] [Google Scholar]
- 237.Milner P, Kirkpatrick KA, Ralevic V, Toothill V, Pearson JS, Burnstock G. Endothelial cells cultured from human umbilical vein release ATP, substance P and acetylcholine in response to increased flow. Proc. R. Soc. Lond. B Biol. Sci. 1990;241:245–248. doi: 10.1098/rspb.1990.0092. [DOI] [PubMed] [Google Scholar]
- 238.Mitsumata M, Fishel RS, Nerem RM, Alexander RW, Berk BC. Fluid shear stress stimulates platelet-derived growth factor expression in endothelial cells. Am. J. Physiol. 1993;265:H3–H8. doi: 10.1152/ajpheart.1993.265.1.H3. (Heart Circ. Physiol. 34) [DOI] [PubMed] [Google Scholar]
- 239.Mo M, Eskin SG, Schilling WP. Flow-induced changes in calcium signalling of vascular endothelial cells: effects of shear stress and ATP. Am. J. Physiol. 1991;260:H1698–H1707. doi: 10.1152/ajpheart.1991.260.5.H1698. (Heart Circ. Physiol. 29) [DOI] [PubMed] [Google Scholar]
- 240.Moncada S, Gryglewsky R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature Lond. 1976;263:663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- 241.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology and pharmacology of nitric oxide. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 242.Monck JR, Oberhauser AF, Keating TJ, Fernandez JM. Thin-section ratiometric Ca2+ images obtained by optical sectioning of fura-2 loaded mast cells. J. Cell Biol. 1992;116:745–759. doi: 10.1083/jcb.116.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Morita T, Kurihara H, Maemura K, Yoshizumi M, Yazaki Y. Disruption of cytoskeletal structures mediates shear stress-induced endothelin-1 gene expression in cultured porcine aortic endothelial cells. J. Clin. Invest. 1993;92:1706–1712. doi: 10.1172/JCI116757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Morris C, Sigurdson WS. Stretch inactivated ion channels coexist with stretch activated ion channels. Science Wash. DC. 1989;243:807–809. doi: 10.1126/science.2536958. [DOI] [PubMed] [Google Scholar]
- 245.Mulsch A, Bassenge E, Busse R. Nitric oxide synthesis in endothelial cytosol: evidence for a calcium dependent and a calcium independent mechanism. Naunyn-Schmiedebergs Arch. Pharmacol. 1989;340:767–770. doi: 10.1007/BF00169688. [DOI] [PubMed] [Google Scholar]
- 246.Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J. Clin. Invest. 1986;78:1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Murphy-Ullrich JE, Hook M. Thrombospondin modulates focal adhesions in endothelial cells. J. Cell Biol. 1989;109:1309–1319. doi: 10.1083/jcb.109.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Muthukrishman L, Warder E, McNeil PL. Basic fibroblast growth factor is efficiently released from a cytosolic storage site through plasma membrane disruptions of endothelial cells. J. Cell. Physiol. 1991;148:1–16. doi: 10.1002/jcp.1041480102. [DOI] [PubMed] [Google Scholar]
- 249.Nagel T, Resnick N, Atkinson WJ, Dewey CF, Jr., Gimbrone MA., Jr. Shear stress selectively upregulates ICAM-1 expression in cultured human vascular endothelial cells. J. Clin. Invest. 1994;94:885–891. doi: 10.1172/JCI117410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Nakache M, Gaub HE. Hydrodynamic hyperpolarization of endothelial cells. Proc. Natl. Acad. USA. 1988;85:1841–1843. doi: 10.1073/pnas.85.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Neer EJ, Clapham DE. Roles of G protein subunits in transmembrane signalling. Nature Lond. 1988;333:129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- 252.Nemenoff RA, Winitz S, Qian NX, Van Putten V, Johnson GL, Heasley LE. Phosphorylation and activation of a high molecular weight form of phospholipase A2 by p42 microtubule-associated protein 2 kinase and protein kinase C. J. Biol. Chem. 1993;268:1960–1964. [PubMed] [Google Scholar]
- 253.Nerem RM, Levesque MJ, Cornhill JF. Vascular endothelial morphology as an indicator of the pattern of blood flow. J. Biomech. Eng. 1981;103:172–177. doi: 10.1115/1.3138275. [DOI] [PubMed] [Google Scholar]
- 254.Nerem RM. Vascular fluid mechanics, the arterial wall and atherosclerosis. J. Biomech. Eng. 1992;114:274–282. doi: 10.1115/1.2891384. [DOI] [PubMed] [Google Scholar]
- 255.Nerem RM, Harrison DG, Taylor WR, Alexander RW. Hemodynamics and vascular endothelial biology. J. Cardiovasc. Pharmacol. 1993;21 Suppl.:S6–S10. doi: 10.1097/00005344-199321001-00002. [DOI] [PubMed] [Google Scholar]
- 256.Newby AC, Henderson AH. Stimulus-secretion coupling in vascular endothelial cells. Annu. Rev. Physiol. 1990;52:661–674. doi: 10.1146/annurev.ph.52.030190.003305. [DOI] [PubMed] [Google Scholar]
- 257.Nicolaides NC, Correa I, Casadevall C, Travali S, Soprano KJ, Calabretta B. The Jun family members, c-Jun and JunD, transactivate the human c-myb promoter via an Ap1-like element. J. Biol. Chem. 1992;267:19665–19672. [PubMed] [Google Scholar]
- 258.Nishida K, Harrison DG, Navas JP, Fisher AA, Dockery SP, Uematsu M, Nerem RM, Alexander RW, Murphy TJ. Molecular cloning and characterization of the constitutive bovine aortic endothelial cell nitric oxide synthase. J. Clin. Invest. 1992;90:2092–2096. doi: 10.1172/JCI116092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Nishizuka Y. Studies and prospectives of protein kinase C. Science Wash. DC. 1986;233:305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- 260.Nollert MU, Diamond SL, McIntire LV. Hydrodynamic shear stress and mass transport modulation of endothelial cell metabolism. Biotech. Bioeng. 1991;38:588–602. doi: 10.1002/bit.260380605. [DOI] [PubMed] [Google Scholar]
- 261.Nollert MU, Eskin SG, McIntire LV. Shear stress increases inositol trisphosphate levels in human endothelial cells. Biochem. Biophys. Res. Commun. 1990;170:281–289. doi: 10.1016/0006-291x(90)91271-s. [DOI] [PubMed] [Google Scholar]
- 262.Noris M, Morigi M, Donadelli R, Aiello S, Foppolo M, Todeschini M, Orisio S, Remuzzi G, Remuzi A. Nitric oxide synthesis by cultured endothelial cells is modulated by flow conditions. Circ. Res. doi: 10.1161/01.res.76.4.536. In press. [DOI] [PubMed] [Google Scholar]
- 263.O’Neill KT, Degrado WF. How calmodulin binds its targets: sequence independent recognition of amphiphilic α-helices. Trends Biochem. Sci. 1990;15:59–64. doi: 10.1016/0968-0004(90)90177-d. [DOI] [PubMed] [Google Scholar]
- 264.Ohno M, Cooke JP, Dzau VJ, Gibbons GH. Fluid shear stress induces endothelial TGF-β1 transcription and production: modulation by potassium channel blockade. J. Clin. Invest. doi: 10.1172/JCI117787. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ohno M, Gibbons GH, Dzau V, Cooke JP. Shear stress elevates endothelial cGMP. Role of a potassium channel and G-protein coupling. Circulation. 1993;88:193–197. doi: 10.1161/01.cir.88.1.193. [DOI] [PubMed] [Google Scholar]
- 266.Ohtsuka A, Ando J, Korenaga R, Kamiya A, Toyamasorimaci N, Miyasaka M. The effect of flow on the expression of vascular adhesion molecule-1 by cultured mouse endothelial cells. Biochem. Biophys. Res. Commun. 1993;193:303–310. doi: 10.1006/bbrc.1993.1624. [DOI] [PubMed] [Google Scholar]
- 267.Ojha M. Spatial and temporal variations of wall shear stress within an end-to-side arterial anastomosis model. J. Biomech. 1993;26:1377–1388. doi: 10.1016/0021-9290(93)90089-w. [DOI] [PubMed] [Google Scholar]
- 268.Okano M, Yoshida Y. Influence of shear stress on endothelial cell shapes and junction complexes at flow dividers of aortic bifurcations in cholesterol-fed rabbits. Front. Med. Biol. Eng. 1993;5:95–120. [PubMed] [Google Scholar]
- 269.Olesen SP, Bundgaard M. Chloride-selective channels of large conductance in bovine aortic endothelial cells. Acta Physiol. Scand. 1992;144:191–198. doi: 10.1111/j.1748-1716.1992.tb09285.x. [DOI] [PubMed] [Google Scholar]
- 270.Olesen SP, Bundgaard M. ATP-dependent closure and reactivation of inward rectifier K+ channels in endothelial cells. Circ. Res. 1993;73:492–495. doi: 10.1161/01.res.73.3.492. [DOI] [PubMed] [Google Scholar]
- 271.Olesen SP, Clapham DE, Davies PF. Hemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature Lond. 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- 272.Oliver JA, Chase HS., Jr. Changes in luminal flow rate modulate basal and bradykinin-stimulated cell [Ca2+] in aortic endothelium. J. Cell. Physiol. 1992;151:37–40. doi: 10.1002/jcp.1041510107. [DOI] [PubMed] [Google Scholar]
- 273.Olsson RA, Pearson JD. Cardiovascular purinoceptors. Physiol Rev. 1990;70:761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- 274.Ookawa K, Sato M, Ohshima N. Changes in the microstructure of cultured porcine aortic endothelial cells in the early stage after applying a fluid-imposed shear stress. J. Biomech. 1992;25:1321–1328. doi: 10.1016/0021-9290(92)90287-b. [DOI] [PubMed] [Google Scholar]
- 275.Ookawa K, Sato M, Ohshima N. Morphological changes of endothelial cells after exposure to fluid-imposed shear stress: differential responses induced by extracellular matrices. Biorheology. 1993;30:131–140. doi: 10.3233/bir-1993-30204. [DOI] [PubMed] [Google Scholar]
- 276.Otey CA, Pavalko FM, Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J. Cell Biol. 1990;111:721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Owens GK, Geisterfer AT, Yang WH, Komoriya A. Transforming growth factor β-induced growth inhibition and cellular hypertrophy in cultured vascular smooth muscle cells. J. Cell Biol. 1988;107:771–780. doi: 10.1083/jcb.107.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Paddock SW. Tandem scanning reflected light microscopy of cell-substratum adhesions and stress fibres in Swiss 3T3 cells. J. Cell Sci. 1989;93:143–146. doi: 10.1242/jcs.93.1.143. [DOI] [PubMed] [Google Scholar]
- 279.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature Lond. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 280.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature Lond. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 281.Parkinson JF, Bang NU, Garcia JG. Recombinant human thrombomodulin attenuates human endothelial cell activation by human thrombin. Arteriosclerosis Thromb. 1993;13:1119–1123. doi: 10.1161/01.atv.13.7.1119. [DOI] [PubMed] [Google Scholar]
- 282.Pearson JD, Cusack NJ. Investigation of the preferred Mg(II)-adenine nucleotide complex at the active site of ectonucleotidases in intact vascular cells using phosphorothioate analogs of ADP and ATP. Eur. J. Biochem. 1985;151:373–375. doi: 10.1111/j.1432-1033.1985.tb09111.x. [DOI] [PubMed] [Google Scholar]
- 283.Pearson JD, Gordon JL. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature Lond. 1979;281:384–386. doi: 10.1038/281384a0. [DOI] [PubMed] [Google Scholar]
- 284.Pennisi E. Thicker than water. Biochemistry blends with fluid dynamics to yield a vascular science. Sci. News. 1991;140:220–223. [Google Scholar]
- 285.Perry PB, O’Neill WC. Flow stimulates nitric oxide synthesis in endothelial cells through a calcium-independent mechanism (Abstract) Circulation. 1993;88:1–134. [Google Scholar]
- 286.Pienta KJ, Coffey DS. Nuclear-cytoskeletal interactions: evidence for physical connections between the nucleus and cell periphery and their alteration by transformation. J. Cell. Biochem. 1992;49:357–365. doi: 10.1002/jcb.240490406. [DOI] [PubMed] [Google Scholar]
- 287.Pinto JG, Fung YC. Mechanical properties of the heart muscle in the passive state. J. Biomech. 1973;6:597–606. doi: 10.1016/0021-9290(73)90017-1. [DOI] [PubMed] [Google Scholar]
- 288.Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension Dallas. 1986;8:37–47. doi: 10.1161/01.hyp.8.1.37. [DOI] [PubMed] [Google Scholar]
- 289.Pollock JP, Forstermann U, Mitchell JA, Warner TD, Schmidt HH, Nakane M, Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc. Natl Acad. Sci. USA. 1991;88:10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Poole JC, Florey HW. Changes in the endothelium of the aorta and the behavior of macrophages in experimental atheroma of the rabbit. J. Pathol. Bacteriol. 1958;75:245–271. doi: 10.1002/path.1700750202. [DOI] [PubMed] [Google Scholar]
- 291.Pourageaud F, Freslon JL. Effects of flow direction and endothelium on reactivity of rat coronary arteries in vitro (Abstract) Circulation. 1993;88 Suppl. I:I-36. [Google Scholar]
- 292.Pouysegur J, Seuwen K. Transmembrane receptors and intracellular pathways that control cell proliferation. Annu. Rev. Physiol. 1992;54:195–210. doi: 10.1146/annurev.ph.54.030192.001211. [DOI] [PubMed] [Google Scholar]
- 293.Prasad ARS, Logan SA, Nerem RM, Schwartz CJ, Sprague EA. Flow-related responses of intracellular inositol phosphate levels in cultured aortic endothelial cells. Circ. Res. 1993;72:827–836. doi: 10.1161/01.res.72.4.827. [DOI] [PubMed] [Google Scholar]
- 294.Ralevic V, Milner P, Hudlicka O, Kristek F, Burnstock G. Substance P is released from the endothelium of normal and capsaicin-treated hindlimb vasculature in vivo by increased flow. Circ. Res. 1990;66:1178–1183. doi: 10.1161/01.res.66.5.1178. [DOI] [PubMed] [Google Scholar]
- 295.Ranjan V, Diamond SL. Fluid shear stress induces synthesis and nuclear localization of c-fos in cultured human endothelial cells. Biochem. Biophys. Res. Commun. 1993;196:79–84. doi: 10.1006/bbrc.1993.2218. [DOI] [PubMed] [Google Scholar]
- 296.Rasenick MM, Wang N, Yan K. Specific associations between tubulin and G proteins: participation of cytoskeletal elements in cellular signal transduction. Adv. Second Messenger Phosphoprotein Res. 1990;24:381–386. [PubMed] [Google Scholar]
- 297.Read MA, Whitley MZ, Williams AJ, Collins T. NF-κB and I kBa: an inducible regulatory system in endothelial activation. J. Exp. Med. 1994;179:503–512. doi: 10.1084/jem.179.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Reich KM, Gay CV, Frangos JA. Fluid shear stress as a mediator of osteoblast cyclic adenosine monophosphate production. J. Cell Physiol. 1990;143:100–104. doi: 10.1002/jcp.1041430113. [DOI] [PubMed] [Google Scholar]
- 299.Reidy MA, Langille BL. The effect of local blood flow patterns on endothelial cell morphology. Exp. Mol. Pathol. 1980;32:276–289. doi: 10.1016/0014-4800(80)90061-1. [DOI] [PubMed] [Google Scholar]
- 300.Remuzzi A, Dewey CF, Davies PF, Gimbrone MA., Jr. Orientation of endothelial cells in shear fields in vitro. Biorheology. 1984;21:617–630. doi: 10.3233/bir-1984-21419. [DOI] [PubMed] [Google Scholar]
- 301.Resnick N, Colons T, Atkinson W, Bonthron DT, Dewey CF, Jr., Gimbrone MA., Jr. Platelet-derived growth factor B chain promoter contains a cis-acting fluid shear-stress-responsive element. Proc. Natl. Acad. Sci. USA. 1993;90:4591–4595. doi: 10.1073/pnas.90.10.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301a.Resnick N, Gimbrone MA., Jr. Hemodynamic forces are complex regulators of endothelial gene expression. FASEB J. doi: 10.1096/fasebj.9.10.7615157. In press. [DOI] [PubMed] [Google Scholar]
- 302.Rifkin DB, Moscatelli D. Recent developments in the cell biology of basic fibroblast growth factor. J. Cell Biol. 1989;109:1–6. doi: 10.1083/jcb.109.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Robishaw JD, Foster KA. Role of G proteins in the regulation of the cardiovascular system. Annu. Rev. Physiol. 1989;51:229–244. doi: 10.1146/annurev.ph.51.030189.001305. [DOI] [PubMed] [Google Scholar]
- 304.Robotewskyj A, McKibben S, Dull RO, Griem ML, Davies PF. Dynamics of focal adhesion site remodeling in living endothelial cells in response to shear stress forces using confocal image analysis (Abstract) FASEB J. 1991;5:A527. [Google Scholar]
- 305.Rodbard S. Negative feedback mechanisms in the architecture and function of the connective and cardiovascular tissues. Perspect. Biol. Med. 1970;13:507–527. doi: 10.1353/pbm.1970.0054. [DOI] [PubMed] [Google Scholar]
- 306.Rodbard S. Vascular caliber. Cardiology. 1975;60:4–49. doi: 10.1159/000169701. [DOI] [PubMed] [Google Scholar]
- 307.Rogers NE, Ignarro LJ. Constitutive nitric oxide synthase from cerebellum is reversibly inhibited by nitric oxide formed from l-arginine. Biochem. Biophys. Res. Commun. 1992;189:242–249. doi: 10.1016/0006-291x(92)91550-a. [DOI] [PubMed] [Google Scholar]
- 308.Rokitansky C. A Manual of Pathological Anatomy. vol. 4. London: Sydenham Soc.; 1952. The pathological anatomy of the organs of respiration and circulation. translated from the German by G. E. Day. [Google Scholar]
- 309.Romer LH, McLean N, Turner CE, Burridge K. Tyrosine kinase activity, cytoskeletal organization, and motility in human vascular endothelial cells. Mol. Biol. Cell. 1994;5:349–361. doi: 10.1091/mbc.5.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Rosales OR, Sumpio BE. Changes in cyclic strain increase inositol trisphophate and diacylglycerol in endothelial cells. Am. J. Physiol. 1992;262:C956–C962. doi: 10.1152/ajpcell.1992.262.4.C956. (Cell Physiol. 31) [DOI] [PubMed] [Google Scholar]
- 311.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature Lond. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 312.Ross R, Glomset JA. The pathogenesis of atherosclerosis. N. Engl J. Med. 1977;295:369–381. doi: 10.1056/NEJM197608122950707. [DOI] [PubMed] [Google Scholar]
- 313.Rubanyi GM. Endothelium-dependent pressure-induced contraction of isolated canine carotid arteries. Am. J. Physiol. 1988;255:H783–H788. doi: 10.1152/ajpheart.1988.255.4.H783. (Heart Circ. Physiol. 24) [DOI] [PubMed] [Google Scholar]
- 314.Rubanyi GM, Freay AD, Kauser K, Johns A, Harder DR. Mechanoreception by the endothelium: mediators and mechanisms of pressure-and flow-induced vascular responses. Blood Vessels. 1990;27:246–257. doi: 10.1159/000158816. [DOI] [PubMed] [Google Scholar]
- 315.Rubanyi GM, Ramiro JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am. J. Physiol. 1986;250:H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. (Heart Circ. Physiol. 19) [DOI] [PubMed] [Google Scholar]
- 316.Rubanyi GM, Vanhoutte PM. Hypoxia releases a vasoconstrictor substance from the canine vascular endothelium. J. Physiol. Lond. 1985;364:45–56. doi: 10.1113/jphysiol.1985.sp015728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium derived relaxing factor. Am. J. Physiol. 1986;250:H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. (Heart Circ. Physiol. 19) [DOI] [PubMed] [Google Scholar]
- 318.Sachs F. Mechanical transduction in biological systems. CRC Crit. Rev. Biomed. Eng. 1988;16:141–169. [PubMed] [Google Scholar]
- 319.Sackin H. Stretch-activated potassium channels in renal proximal tubule. Am. J. Physiol. 1987;253:F1253–F1262. doi: 10.1152/ajprenal.1987.253.6.F1253. (Renal Fluid Electrolyte Physiol. 22) [DOI] [PubMed] [Google Scholar]
- 320.Sakurai A, Nakano A, Yamaguchi T, Masuda M, Fujiwara K. A computational fluid mechanical study of flow over cultured endothelial cells. Adv. Bioeng. 1991;20:299–302. [Google Scholar]
- 321.Sampath R, Kukielka GL, Smith CW, Panaro NJ, Eskin SG, McIntire LV. Shear stress-mediated changes in the expression of surface receptors on human umbilical vein endothelial cells in vitro. Ann. Biomed. Eng. doi: 10.1007/BF02584426. In press. [DOI] [PubMed] [Google Scholar]
- 322.Satcher RL, Bussolari SR, Gimbrone MA, Jr., Dewey CF., Jr. The distribution of fluid forces on model arterial endothelium using computational fluid dynamics. J. Biomech. Eng. 1992;114:309–316. doi: 10.1115/1.2891388. [DOI] [PubMed] [Google Scholar]
- 323.Sato M, Levesque MJ, Nerem RM. Micropipette aspiration of cultured bovine aortic endothelial cells exposed to shear stress. Arteriosclerosis. 1987;7:276–286. doi: 10.1161/01.atv.7.3.276. [DOI] [PubMed] [Google Scholar]
- 324.Sato Y, Rifkin DB. Inhibition of endothelial movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor β1-like molecule by plasma during co-culture. J. Cell Biol. 1989;109:309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc. Natl. Acad. Sci. USA. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Schilling WP. Effect of membrane potential on cytosolic calcium of bovine aortic endothelial cells. Am. J. Physiol. 1989;257:H778–H784. doi: 10.1152/ajpheart.1989.257.3.H778. (Heart Circ. Physiol. 26) [DOI] [PubMed] [Google Scholar]
- 327.Schilling WP, Mo M, Eskin SG. Effect of shear stress on cytosolic Ca2+ of calf pulmonary artery endothelial cells. Exp. Cell Res. 1992;198:31–35. doi: 10.1016/0014-4827(92)90145-x. [DOI] [PubMed] [Google Scholar]
- 328.Schnittler HJ, Franke RP, Akbay U, Mrowietz C, Drenckhahn D. Improved in vitro Theological system for studying the effect of fluid shear stress on cultured cells. Am. J. Physiol. 1993;265:C289–C298. doi: 10.1152/ajpcell.1993.265.1.C289. (Cell Physiol. 34) [DOI] [PubMed] [Google Scholar]
- 328a.Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor κB activation in intact cells. J. Exp. Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Schretzenmayr A. Uber kreislaufregulatorische vorgange an den grossen arterien bei der muskelarbeit. Pfluegers Arch. 1933;232:743–748. [Google Scholar]
- 330.Schultz JE, Klumpp S, Benz R, Schurhoff-Goeters WJ, Schmid A. Regulation of adenyl cyclase from Paramecium by an intrinsic potassium conductance. Science Wash. DC. 1992;255:600–603. doi: 10.1126/science.1371017. [DOI] [PubMed] [Google Scholar]
- 331.Schwartz MA, Ingber DE, Lawrence M, Springer TA, Lechene C. Multiple integrins share the ability to induce elevation of intracellular pH. Exp. Cell Res. 1991;195:533–535. doi: 10.1016/0014-4827(91)90407-l. [DOI] [PubMed] [Google Scholar]
- 332.Schwarz G, Callewaert G, Droogmans G, Nilius B. Shear stress-induced calcium transients in endothelial cells from human umbilical cord veins. J. Physiol. Lond. 1992;458:527–538. doi: 10.1113/jphysiol.1992.sp019432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Schwenke DE, Carew TE. Quantification in vivo of increased LDL content and rate of LDL degradation in normal rabbit aorta occurring at sites susceptible to early atherosclerotic lesions. Circ. Res. 1988;62:699–710. doi: 10.1161/01.res.62.4.699. [DOI] [PubMed] [Google Scholar]
- 334.Sessa WC. The nitric oxide synthase family of proteins. J. Vasc. Res. 1994;31:131–143. doi: 10.1159/000159039. [DOI] [PubMed] [Google Scholar]
- 335.Sessa WC, Barber CM, Lynch KR. Mutation of N-myristoylation site converts endothelial cell nitric oxide synthase from a membrane to a cytosolic protein. Circ. Res. 1993;72:921–924. doi: 10.1161/01.res.72.4.921. [DOI] [PubMed] [Google Scholar]
- 336.Sessa WC, Harrison JK, Barber CM, Zeng D, Durieux ME, D’Angelo DD, Lynch KR, Peach MJ. Molecular cloning and expression of a cDNA encoding endothelial cell nitric oxide synthase. J. Biol. Chem. 1992;267:15274–15276. [PubMed] [Google Scholar]
- 337.Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ. Res. 1994;74:349–353. doi: 10.1161/01.res.74.2.349. [DOI] [PubMed] [Google Scholar]
- 338.Sharefkin JB, Diamond SL, Eskin SG, McIntire LV, Dieffenbach CW. Fluid flow decreases preproendothelin mRNA levels and suppresses endothelin-1 peptide release in cultured human endothelial cells. J. Vasc. Surg. 1991;14:1–9. [PubMed] [Google Scholar]
- 339.Shen J, Gimbrone MA, Jr., Luscinskas FW, Dewey CF., Jr. Regulation of adenine nucleotide concentration at endothelium-fluid interface by viscous shear flow. Biophys. J. 1993;64:1323–1330. doi: 10.1016/S0006-3495(93)81498-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Shen J, Luscinskas FW, Connolly A, Dewey CF, Jr., Gimbrone MA., Jr. Fluid shear stress modulates cytosolic free calcium in vascular endothelial cells. Am. J. Physiol. 1992;262:C384–C390. doi: 10.1152/ajpcell.1992.262.2.C384. (Cell Physiol. 31) [DOI] [PubMed] [Google Scholar]
- 341.Shen J, Luscinskas FW, Gimbrone MA, Jr., Dewey CF., Jr. Fluid flow modulates vascular endothelial cytosolic calcium responses to adenine nucleotides. Microcirculation. 1994;1:67–78. doi: 10.3109/10739689409148263. [DOI] [PubMed] [Google Scholar]
- 342.Shepherd GM. Sensory transduction: entering the mainstream of membrane signalling. Cell. 1991;67:845–851. doi: 10.1016/0092-8674(91)90358-6. [DOI] [PubMed] [Google Scholar]
- 343.Shirinsky VP, Antonov AS, Birukov KG, Sobolevsky AV, Romanov YA, Kabaeva NV, Antonova GN, Smirnov VN. Mechano-chemical control of human endothelium orientation and size. J. Cell Biol. 1989;109:331–339. doi: 10.1083/jcb.109.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343a.Shyy J-Y, Chien S. The molecular basis for shear stress regulated gene expression (Abstract); Abstracts of the AHA Scientific Conference on the Functional and Structural Aspects of Vascular Wall Salt Lake City UT 1995. [Google Scholar]
- 344.Sibley DR, Banovic JL, Caron MG, Lefkowitz RJ. Regulation of transmembrane signalling by receptor phosphorylation. Cell. 1987;48:913–922. doi: 10.1016/0092-8674(87)90700-8. [DOI] [PubMed] [Google Scholar]
- 345.Sigurdson WJ, Sachs F, Diamond SL. Mechanical perturbation of cultured human endothelial cells causes rapid increases of intracellular calcium. Am. J. Physiol. 1993;264:H1745–H1752. doi: 10.1152/ajpheart.1993.264.6.H1745. (Heart Circ. Physiol. 33) [DOI] [PubMed] [Google Scholar]
- 346.Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science Wash. DC. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- 347.Sims JR, Karp S, Ingber DE. Altering the cellular mechanical force balance results in integrated changes in cell, cytoskeletal and nuclear shape. J. Cell Sci. 1992;103:1215–1222. doi: 10.1242/jcs.103.4.1215. [DOI] [PubMed] [Google Scholar]
- 348.Skarlatos SI, Hollis TM. Cultured bovine aortic endothelial cells show increased histamine metabolism when exposed to oscillatory shear stress. Atherosclerosis. 1987;64:55–61. doi: 10.1016/0021-9150(87)90054-2. [DOI] [PubMed] [Google Scholar]
- 349.Smiesko V, Kozik J, Dolezel S. Role of endothelium in the control of arterial diameter by blood flow. Blood Vessels. 1985;22:247–251. [PubMed] [Google Scholar]
- 350.Spaeth EE, Roberts GW, Yadwadkar SR, Ng PK, Jackson CM. The influence of fluid shear on the kinetics of blood coagulation reactions. Trans. Am. Soc. Artif. Intern. Organs. 1973;19:179–187. doi: 10.1097/00002480-197301900-00033. [DOI] [PubMed] [Google Scholar]
- 351.Sprague EA, Steinbach BL, Nerem RM, Schwartz CJ. Influence of a laminar steady state fluid imposed wall shear stress on the binding, internalization and degradation of LDL by cultured arterial endothelium. Circulation. 1987;76:648–656. doi: 10.1161/01.cir.76.3.648. [DOI] [PubMed] [Google Scholar]
- 352.Springer TA. Adhesion receptors of the immune system. Nature Lond. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 353.Stamler J, Mendelsohn ME, Amarante P, Smick D, Andon N, Davies PF, Cooke JP, Loscalzo J. N-acetylcysteine potentiates platelet inhibition by endothelium-derived relaxing factor. Circ. Res. 1989;65:789–795. doi: 10.1161/01.res.65.3.789. [DOI] [PubMed] [Google Scholar]
- 354.Stehbens WE. The basal attachment of endothelial cells. J. Ultrastruct. Res. 1966;15:388–400. doi: 10.1016/s0022-5320(66)80115-6. [DOI] [PubMed] [Google Scholar]
- 355.Stehbens WE. Haemodynamics and atherosclerosis. Exp. Mol. Pathol. 1974;20:412–425. doi: 10.1016/0014-4800(74)90071-9. [DOI] [PubMed] [Google Scholar]
- 356.Stepp MA, Spurr-Michand S, Tisdale A, Elwell J, Gipson IK. α6β4 Integrin heterodimer is a component of hemidesmosomes. Proc. Natl. Acad. Sci. USA. 1990;87:8970–8974. doi: 10.1073/pnas.87.22.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Stuehr DJ, Kwon NS, Navan C, Griffith O, Feldman P, Wiseman J. Nω-hydroxy-l-arginine is an intermediate in the biosynthesis of nitric oxide from l-arginine. J. Biol. Chem. 1991;266:6259–6266. [PubMed] [Google Scholar]
- 358.Sumpio BE, Banes AJ, Buckley M, Johnson G. Alterations in aortic endothelial cell morphology and cytoskeletal protein synthesis during cyclic tensional deformation. J. Vasc. Surg. 1987;7:130–138. [PubMed] [Google Scholar]
- 359.Sumpio BE, Banes AJ, Link GW, Iba T. Modulation of endothelial phenotype by cyclic stretch: inhibition of collagen production. J. Surg. Res. 1990;48:415–420. doi: 10.1016/0022-4804(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 360.Takeda K, Schini V, Stoeckel H. Voltage-activated potassium, but not calcium currents in cultured bovine aortic endothelial cells. Pfluegers Arch. 1987;410:385–393. doi: 10.1007/BF00586515. [DOI] [PubMed] [Google Scholar]
- 361.Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988;102:639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- 362.Taniguchi J, Guggino WB. Membrane stretch: a physiological stimulator of Ca2+-activated K+ channels in thick ascending limb. Am. J. Physiol. 1989;257:F347–F352. doi: 10.1152/ajprenal.1989.257.3.F347. (Renal Fluid Electrolyte Physiol. 26) [DOI] [PubMed] [Google Scholar]
- 363.Taylor CW. The role of G proteins in transmembrane signalling. Biochem. J. 1990;272:1–13. doi: 10.1042/bj2720001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Taylor WR, Hanson DG, Nerem RM, Peterson TE, Alexander RW. Characterization of the release of endothelial-derived nitrogen oxides by shear stress (Abstract) FASEB J. 1991;5:A1727. [Google Scholar]
- 365.Thelen M, Rosen A, Nairn AC, Aderem AA. Regulation by phosphorylation of reversible association of myristoylated protein kinase C substrate with the plasma membrane. Nature Lond. 1991;351:320–322. doi: 10.1038/351320a0. [DOI] [PubMed] [Google Scholar]
- 366.Thoma R. Uber die intima der arterien. Virchows Arch. A. 1921;230:1–45. [Google Scholar]
- 367.Tirrell M, Middleman S. Shear modification of enzyme kinetics. Biotechnol. Bioeng. 1975;17:299–303. [Google Scholar]
- 368.Treisman R. Ternary complex factors: growth factor regulated transcriptional activators. Curr. Opin. Genet. Dev. 1994;4:96–101. doi: 10.1016/0959-437x(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 369.Truskey GA, Burmeister JS, Grapa E, Reichert WM. Total internal reflection fluorescence microscopy (TIRFM). II. Topographical mapping of relative cell/substratum separation distances. J. Cell Sci. 1992;103:491–499. doi: 10.1242/jcs.103.2.491. [DOI] [PubMed] [Google Scholar]
- 370.Ts’Ao CH, Glagov S. Basal endothelial attachment. Tenacity at cytoplasmic dense zones in the rabbit aorta. Lab. Invest. 1970;23:510–516. [PubMed] [Google Scholar]
- 371.Turner CE. Paxillin is a major phosphotyrosine-containing protein during embryonic development. J. Cell Biol. 1991;115:201–207. doi: 10.1083/jcb.115.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Turner CE, Burridge K. Transmembrane molecular assemblies in cell-extracellular matrix interactions. Curr. Opin. Cell Biol. 1991;3:849–853. doi: 10.1016/0955-0674(91)90059-8. [DOI] [PubMed] [Google Scholar]
- 373.Turner CE, Glenney JR, Jr., Burridge K. Paxillin: a new vinculin-binding protein present in focal adhesions. J. Cell Biol. 1990;111:1059–1068. doi: 10.1083/jcb.111.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Turner CE, Miller JT. Primary sequence of paxillin contains putative SH2 and SH3 domain binding motifs and multiple LIM domains: identification of a vinculin and pp125FAK binding region. J. Cell Sci. 1994;107:1583–1591. doi: 10.1242/jcs.107.6.1583. [DOI] [PubMed] [Google Scholar]
- 375.Uematsu M, Navas JP, Nishida K, Ohara Y, Murphy TJ, Alexander RW, Nerem RM, Harrison DG. Mechanisms of endothelial cell NO synthase induction by shear stress (Abstract) Circulation. 1993;88 Suppl. I:I-184. [Google Scholar]
- 376.Uematsu M, Navas JP, Nishida K, Ohara Y, Murphy TJ, Alexander RW, Nerem RM, Harrison DG. Regulation of endothelial cell nitric oxide synthase expression by shear stress. Circ. Res. doi: 10.1152/ajpcell.1995.269.6.C1371. In press. [DOI] [PubMed] [Google Scholar]
- 377.Vanhoutte PM, Eber B. Endothelium-derived relaxing and contracting factors. Wien. Klin. Wochenschr. 1991;103:405–411. [PubMed] [Google Scholar]
- 378.Virchow R. Cellular Pathology as Based Upon Physiological and Pathological Histology. London: Churchill; 1860. translated from the German by F. Clance. [DOI] [PubMed] [Google Scholar]
- 379.Volk T, Fessler LI, Fessler JH. A role for integrin in the formation of sarcomeric cytoarchitecture. Cell. 1990;63:525–536. doi: 10.1016/0092-8674(90)90449-o. [DOI] [PubMed] [Google Scholar]
- 380.Walsh DA, Newsholme P, Cawley KC, Van Patten SM, Angelos KL. Motifs of protein phosphorylation and mechanisms of reversible covalent regulation. Physiol. Rev. 1991;71:285–304. doi: 10.1152/physrev.1991.71.1.285. [DOI] [PubMed] [Google Scholar]
- 381.Walter P, Lingappa V. Mechanism of protein translocation across the endoplasmic reticulum membrane. Annu. Rev. Cell Biol. 1986;2:499–516. doi: 10.1146/annurev.cb.02.110186.002435. [DOI] [PubMed] [Google Scholar]
- 382.Wang J, Wolin MS, Hintze TH. Enhanced flow-dependent endothelium-derived dilation of epicardial coronary artery in conscious dogs. Circ. Res. 1993;73:829–838. doi: 10.1161/01.res.73.5.829. [DOI] [PubMed] [Google Scholar]
- 383.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science Wash. DC. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 384.Ward MD, Hammer DA. A theoretical analysis for the effect of focal contact formation on cell-substrate attachment strength. Biophys. J. 1993;64:936–959. doi: 10.1016/S0006-3495(93)81456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Watson PA. Direct stimulation of adenylate cyclase by mechanical forces in S49 mouse lymphoma cells during hyposmotic swelling. J. Biol. Chem. 1990;265:6569–6575. [PubMed] [Google Scholar]
- 386.Watson PA. Function follows form: generation of intracellular signals by cell deformation. FASEB J. 1991;5:2013–2019. doi: 10.1096/fasebj.5.7.1707019. [DOI] [PubMed] [Google Scholar]
- 387.Watson S, Arkinstall S. The G-Protein Linked Receptor Facts Book. London: Academic; 1994. [Google Scholar]
- 388.Wechezak AR, Viggers RF, Sauvage LR. Fibronectin and F-actin redistribution in cultured endothelial cells exposed to shear stress. Lab. Invest. 1985;53:639–647. [PubMed] [Google Scholar]
- 389.Wechezak AR, Wight TN, Viggers RF, Sauvage LR. Endothelial adherence under shear stress is dependent upon microfilament reorganization. J. Cell. Physiol. 1989;139:136–146. doi: 10.1002/jcp.1041390120. [DOI] [PubMed] [Google Scholar]
- 390.White GE, Fujiwara K. Expression and intracellular distribution of stress fibers in aortic endothelium. J. Cell Biol. 1986;103:63–70. doi: 10.1083/jcb.103.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.White KA, A M. MARLETTA Nitric oxide synthase is a cytochrome P-450 type hemoprotein. Biochemistry. 1992;31:6627–6631. doi: 10.1021/bi00144a001. [DOI] [PubMed] [Google Scholar]
- 392.Wilson RE, Taylor SL, Atherton GT, Johnston D, Waters M, Norton JD. Early response gene signalling cascades activated by ionizing radiation in primary human B cells. Oncogene. 1993;8:3229–3237. [PubMed] [Google Scholar]
- 393.Wilson T, Sheppard C. Theory and Practice of Scanning Optical Microscopy. Orlando, FL: Academic; 1984. 1984. [Google Scholar]
- 394.Wong AJ, Pollard TD, Hermann IM. Actin-filament stress fibers in vascular endothelial cells in vivo. Science Wash. DC. 1983;219:867–869. doi: 10.1126/science.6681677. [DOI] [PubMed] [Google Scholar]
- 395.Yamaguchi T, Hoshiai K, Okino H, Kakurai A, Hanai S, Masuda M, Fujiwara K. Shear stress distribution over confluently cultured endothelial cells studied by computational fluid mechanics. Adv. Bioeng. 1993;20:167–170. [Google Scholar]
- 396.Yanagisawa N, Inoue A, Takuwa Y, Mitsui Y, Kobayachi M, Masaki T. The human preproendothelin-1 gene: possible regulation by endothelial phosphoinositide turnover signalling. J. Cardiovasc. Pharmacol. 1989;13 Suppl.:S13–S17. [PubMed] [Google Scholar]
- 397.Yoshida Y, Sue W, Okano M, Oyama T, Yamane T, Mitsumata M. The effects of augmented hemodynamic forces on the progression and topography of atherosclerotic plaques. Ann. NY Acad. Sci. 1990;598:256–273. doi: 10.1111/j.1749-6632.1990.tb42298.x. [DOI] [PubMed] [Google Scholar]
- 398.Yoshizumi M, Kurihara H, Sugiyama T, Takaku F, Yanagisawa M, Masaki T, Yazaki Y. Hemodynamic shear stress stimulates endothelin production by cultured endothelial cells. Biochem. Biophys. Res. Commun. 1989;161:859–864. doi: 10.1016/0006-291x(89)92679-x. [DOI] [PubMed] [Google Scholar]
- 399.Yost JC, Hermann IM. Substratum-induced stress fiber assembly in vascular endothelial cells during spreading in vitro. J. Cell Sci. 1990;95:507–520. doi: 10.1242/jcs.95.3.507. [DOI] [PubMed] [Google Scholar]
- 400.Young WC, Hermann IM. Extracellular matrix modulation of endothelial cell shape and motility following injury in vitro. J. Cell Sci. 1985;73:19–28. doi: 10.1242/jcs.73.1.19. [DOI] [PubMed] [Google Scholar]
- 401.Yu QC, McNeil PL. Transient disruptions of aortic endothelial cell plasma membrane. Am. J. Pathol. 1992;141:1349–1360. [PMC free article] [PubMed] [Google Scholar]
- 402.Zachary I, Rozengurt E. Focal adhesion kinase (p125FAK): a point of convergence in the action of neuropeptides, integrins and oncogenes. Cell. 1992;71:891–894. doi: 10.1016/0092-8674(92)90385-p. [DOI] [PubMed] [Google Scholar]
- 403.Zarins CK. Adaptive responses of arteries. J. Vasc. Surg. 1989;9:382–389. [Google Scholar]
- 404.Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ. Res. 1983;53:502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]
- 405.Zarins CK, Zatina MA, Giddens DP, Ku DN, Glagov S. Shear stress regulation of artery lumen diameter in experimental atherogenesis. J. Vasc. Surg. 1987;5:413–420. [PubMed] [Google Scholar]
- 406.Ziegelstein RC, Cheng L, Capogrossi MC. Flow dependent cytosolic acidification of vascular endothelial cells. Science Wash. DC. 1992;258:656–659. doi: 10.1126/science.1329207. [DOI] [PubMed] [Google Scholar]
- 407.Ziegler T, Nerem RM. Effect of flow on the process of endothelial cell division. Arteriosclerosis Thromb. 1994;14:636–643. doi: 10.1161/01.atv.14.4.636. [DOI] [PubMed] [Google Scholar]