Abstract
Objective
To evaluate the role of lumbar drainage in the prevention of shunt-dependent hydrocephalus after treatment of ruptured intracranial aneurysms by coil embolization in good-grade patients.
Methods
One-hundred-thirty consecutive patients with aneurysmal subarachnoid hemorrhage in good-grade patients (Hunt & Hess grades I-III), who were treated by coil embolization between August 2004 and April 2010 were retrospectively evaluated. Poor-grade patients (Hunt & Hess grades IV and V), a history of head trauma preceding the development of headache, negative angiograms, primary subarachnoid hemorrhage (SAH), and loss to follow-up were excluded from the study. We assessed the effects on lumbar drainage on the risk of shunt-dependent hydrocephalus related to coil embolization in patients with ruptured intracranial aneurysms.
Results
One-hundred-twenty-six patients (96.9%) did not develop shunt-dependent hydrocephalus. The 2 patients (1.5%) who developed acute hydrocephalus treated with temporary external ventricular drainage did not require permanent shunt diversion. Overall, 4 patients (3.1%) required permanent shunt diversion; acute hydrocephalus developed in 2 patients (50%). There was no morbidity or mortality amongst the patients who underwent a permanent shunt procedure.
Conclusion
Coil embolization of ruptured intracranial aneurysms may be associated with a lower risk for developing shunt-dependent hydrocephalus, possibly by active management of lumbar drainage, which may reflect less damage for cisternal anatomy than surgical clipping. Coil embolization might have an effect the long-term outcome and decision-making for ruptured intracranial aneurysms.
Keywords: Coil embolization, Hydrocephalus, Lumbar drainage, Shunt
INTRODUCTION
The incidence of hydrocephalus, a known complication of aneurysmal subarachnoid hemorrhage (SAH), has been reported to have a variable range (6-67%) early in the course [acute (during the first 3 days), or subacute (days 4-13)] or after the first 2 weeks (chronic or shunt-dependent)2,3,6,9,11,32).
Generally, the mechanism underlying hydrocephalus involves obstruction of the cerebrospinal fluid (CSF) pathway with blood clot and products (acute hydrocephalus) or adhesion that blocks CSF circulation within the ventricles and cisterns (chronic hydrocephalus and shunt-dependent hydrocephalus). Many factors have been associated with the development of hydrocephalus requiring permanent CSF diversion. Poorer neurologic outcomes and cognitive deficits have been cited by many authors as some of the adverse factors for hydrocephalus. Factors, such as increasing age, female gender, poor admission Hunt & Hess grade, diffuse or thick subarachnoid blood on computed tomography (CT) scan reflecting Fisher's grade, rebleeding, intraventricular hemorrhage (IVH), location (such as anterior communicating artery and posterior circulation aneurysms), and symptomatic vasospasm have been associated with the development of hydrocephalus among patients with aneurysmal SAH3,6,7,11,21,24,25,28,30,33,34).
The introduction of Guglielmi detachable coils in the early 1990s allowed physicians to treat intracranial aneurysms directly. This treatment has been accepted by more physicians, especially after a large study that compared clipping to coiling showed favorable results4,8,23,26). However, only a small number of studies2,3,7,25,32) have examined the effect of aneurysmal SAH after coil embolization on hydrocephalus and permanent ventricular shunt requirements. Because only surgery is likely to open the cisterns and remove blood clot and blood products directly, the risk of developing hydrocephalus and shunt dependency is hypothetically less for patients treated by surgical clipping than those treated with coil embolization. Two available treatments for aneurysmal SAH, clipping or coiling, may lead to differences in the need for CSF diversion.
Lumbar CSF drainage after aneurysmal SAH is performed to prevent cerebral vasospasm in many institutions. The rationale for using lumbar CSF drainage in all patients with aneurysmal SAH is that lumbar CSF drainage promotes CSF circulation from the ventricles through the subarachnoid space, which is related to the development and severity of vasospasm. However, the effect of lumbar CSF drainage on shunt-dependent hydrocephalus after coil embolization for aneurysmal SAH is unclear. In this study we determined whether or not lumbar drainage influences shunt-dependent hydrocephalus after coil embolization for aneurysmal SAH in good-grade patients.
MATERIALS AND METHODS
One-hundred-thirty consecutive patients who underwent endovascular coil embolization treatment of ruptured, atraumatic, intracranial aneurysms between August 2004 and April 2010 at our hospital were retrospectively studied. The patient ages ranged from 22-82 years (mean age, 56.3 years). The study population consisted of 52 males and 78 females. The diagnosis of SAH was made by clinical presentation, CT of the head, or lumbar puncture. The diagnosis of a cerebral aneurysm as a cause of SAH was made by conventional cerebral vessel or cerebral CT angiography. Poor-grade patients (Hunt & Hess grades IV and V), a history of head trauma preceding the development of headaches, negative angiography, primary SAH, and loss to follow-up were excluded from this study. All patients were admitted to the neurosurgical intensive care unit (NICU) before and after obliteration of the aneurysm and the ruptured aneurysm was treated within 48 hours of admission. All aneurysms were selected and performed by one neurosurgeon interventionalist on the basis of radiologic features and the patient's condition. Standard SAH management practices were followed in the NICU, which included prevention of rebleeding in the pre-operative period, as well as treatment of vasospasm and hydrocephalus in the postoperative period. After coil embolization, all patients underwent lumbar drainage. Amount of CSF drainage was 8 cc/hour. Amount of CSF drainage was gradually reduced according to result of follow-up CT image. When SAH resolved in follow-up CT image, lumbar drainage was removed. Usually, period of lumbar drainage did not exceed 1 week.
For all permanent shunt placements, clinical deterioration should have been associated with dilatation of the ventricles in radiologic images because of exclusion of the vasospasm or other medical problems.
The patients treated with coil embolization for ruptured intracranial aneurysms were classified into the following two groups : permanent shunt and non-shunt group. Data on each group included age, gender, presence of IVH, presence of intracerebral hemorrhage (ICH), presence of acute hydrocephalus, and location of the aneurysm. Additional data included clinical grade on the day of treatment using Hunt & Hess grade and hemorrhage pattern on CT scan obtained at the time of admission using the Fischer grade.
RESULTS
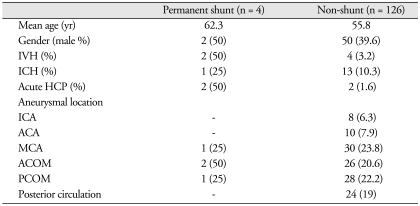
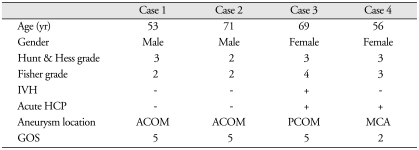
One-hundred-twenty-six patients (96.9%) did not develop shunt-dependent hydrocephalus (Table 1). Although two patients in the non-shunt group developed acute hydrocephlaus were treated with temporary external ventricular drainage, they did not require permanent shunt diversion. Overall, 4 patients (3.1%) required permanent shunt diversion (cases 2 & 3); acute hydrocephalus developed in 2 patients (50%) (Table 2). There was no morbidity or mortality in the patients who underwent permanent shunt procedures.
Table 1.
Characteristics of patients (n=130) treated with coil embolization for ruptured intracranial aneurysms
ICA : internal carotid artery, ACA : anterior cerebral artery, MCA : middle cerebral artery, ACOM : anterior communi cating artery, PCOM : posterior communicating artery, IVH : intraventricular hemorrhage, ICH : intracerebral hemorrhage, HCP : hydrocephalus
Table 2.
Patients treated with coil embolization for ruptured intracranial aneurysms and permanent shunt operation (n = 4)
ACOM : anterior communicating artery, PCOM : posterior communicating artery, MCA : middle cerebral artery, IVH : intraventricular hemorrhage, ICH : intracerebral hemorrhage, HCP : hydrocephalus, GOS : Glasgow outcome scale
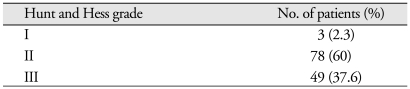
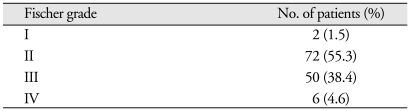
Six patients presented with IVH (2 patients in the permanent shunt group and 4 patients in the non-shunt group). Fourteen patients presented with ICH (1 patient in the permanent shunt group and 13 patients in the non-shunt group) in the following locations : middle cerebral artery aneurysms (30 patients); posterior communicating artery aneurysms (28 patients); and anterior communicating artery aneurysms (26 patients). The Hunt and Hess grades of these patients were as follows : grade I, 3 (2.3%); grade II, 78 (60%); and grade III, 49 (37.6%) (Table 3). The Fischer grades of these patients were as follows : grade I, 2 (1.5%); grade II, 72 (55.3%); grade III, 50 (38.4%); and grade IV, 50 (38.4%) (Table 4).
Table 3.
Hunt and Hess grades of patients (n = 130)
Table 4.
Fischer grades of patient (n = 130)
Complications that were related to lumbar drainage were none except infection of 1 patient. It was confirmed by CSF examination and, well treated with antibiotics.
DISCUSSION
Pathogenesis of post-SAH hydrocephalus
Shunt-dependent hydrocephalus in an unselected series of patients has an incidence between 18% and 45%1,2,9,10,21). Diverse theories have been advanced to explain the pathogenesis of post-SAH hydrocephalus. Acute hydrocephalus is thought to result from blockage of CSF flow. That is, blockade within the ventricular system at the outlet foramen or structural blockage of the arachnoid villi generates a pressure gradient and ultimately leads to enlargement of the ventricles. Acute hydrocephalus can, but does not necessarily, lead to chronic hydrocephalus. Chronic hydrocephalus occurs when CSF flow is permanently impeded or absorption is permanently reduced. The pathogenesis of chronic hydrocephalus involves arachnoid adhesions that are formed because of a meningeal reaction to blood products, impairing CSF absorption at the arachnoid villi and basal cisterns2,3,7,27,33,34).
The effect of IVH on the development of hydrocephalus has been well-established. In our series, 40% of patients who underwent definitive shunt placement also had IVH. Some authors assert that the presence of blood clots and high CSF viscosity can lead to an obstructive form of hydrocephalus and early CSF disturbances. Investigators noted no relationship between the development of hydrocephalus and the number of erythrocytes in the CSF, thus indicating that flow disturbances also play an important role in acute hydrocephalus2,7).
The patients with acute hydrocephalus and impaired consciousness after SAH, in contrast to patients with cerebral ischemia, usually have decreased cerebral blood flow predominantly in the basal parts of the brain. Apart from showing that impaired consciousness in acute hydrocephalus results from a flow disturbance predominantly in the basal parts of the brain, this study may also have practical implications. If a patient has deteriorated from acute hydrocephalus and does not respond to treatment, the explanation may be that insufficient CSF drainage or another complication, such as cerebral ischemia, has developed. The latter is most likely if single photon emission computed tomography (SPECT) scanning shows decreased regional cerebral blood flow in other than the basal parts of the brain, even if the CT shows no evidence of infarction10,18). One study has found a significant association of a history of hypertension, elevated blood pressure on admission, and hypertension post-operatively with both CT and clinical hydrocephalus. The mechanism involved in ventricular enlargement seemed to be a combination of at least two factors : possible failure of CSF absorption in the face of increased superior sagittal sinus venous pressure; and increased intraventricular pulse pressure from the choroid plexus6).
Coil embolization and post-SAH hydrocephalus
The risk of shunt-dependent hydrocephalus after treatment of ruptured intracranial aneurysms by coiling compared with clipping is unclear. Many reports on these issues report that the technical procedure used to treat aneurysms, whether clipping or embolization, does not significantly affect the development of hydrocephalus2,3,25,29,32).
Dorai et al.3) and Varelas et al.32) reported that a higher percentage of coiled patients required permanent shunting, compared with the clipped patients. They reasoned that irrigation and early evacuation of subarachnoid clots during microsurgery allow the blood to clear more rapidly, decreasing the probability of shunt-dependent hydrocephalus. However, the results of this study are that coiling is not associated with an increased risk of shunt procedure. In a prospective, non-randomized study, Dehdashti et al.2) reported that the percentage of shunt-dependent hydrocephalus in clipped and coiled patients was 14% and 19%, respectively (p value = 0.53). Another recent report by de Oliveira et al.25) showed similar results with the percentage of shunt-dependent hydrocephalus in clipped and coiled patients was 17.4% and 19.6%, respectively (p value = 0.59).
Comparing our results to the results of the previous studies, the percentage requiring a permanent shunt was much lower [3.1% (130 patients)]. The basis for the discrepancy between our results and the previous studies may be the exclusion of poor-grade SAH patients (Hunt & Hess grades IV and V) and active management of lumbar drainage to all patients with ruptured intracranial aneurysms during the peri-operative and vasospasm periods. In the previous studies the good-grade SAH patients (Hunt & Hess grades I-III) and performance of lumbar drainage in the peri-operative periods was not analyzed. Because lumbar drainage is able to remove blood clots in the subarachnoid space indirectly, the rate of shunt-dependent hydrocephalus in the coil-treated group was not higher. Lumbar drainage was highly related to less damage to cisternal anatomy in coiling than surgical clipping. Based on the data in this study, endovascular treatment did not raise the risk of shunt-dependent hydrocephalus. This finding is in agreement with data in the study conducted by Park et al.26), who showed a lower rate [5.9% (118 patients)] in shunt dependency in the endovascular treatment group compared with the surgical treatment group.
An important question is whether or not patients treated with endovascular coiling are more inclined than patients who are surgically treated with removal of clots within the basal cisterns to develop hydrocephalus. Recent preliminary studies have shown that patients with ruptured aneurysms who are treated exclusively with endovascular methods had no more vasospasm and/or hydrocephalus than those patients who are surgically treated, with the exception of patients with Fisher grade III or IV3,7,22,25,32). Because EVDs were placed intra-operatively, i.e., to facilitate dissection at the base of the brain rather than to treat extensive acute hydrocephalus, and because we were reluctant to place EVDs in patients selected for endovascular treatment with post-embolizaion heparinization, it is reasonable to assume that comparisons were not substantially confounded by the higher EVD placement rate in the surgical treatment group7).
Lumbar drainage and post-SAH hydrocephalus
The rationale we used for lumbar CSF drainage in all patients with ruptured intracranial aneurysms was that it promotes CSF circulation from the ventricles through the subarachnoid space, and that it also takes the bloody CSF from the spinal cistern. This means that CSF drainage from the ventricles may actually promote CSF flow stasis within the subarachnoid space. Thus, it may decrease the incidence of vasospasm and shunt-dependent hydrocephalus7,14,19,24,28). The beneficial effect of continuous lumbar drainage on reduction of incidence of hydrocephalus was well-demonstrated in some literature2,5,11,12,15-17,20,31). There are several possible different explanations on the effect of lumbar drainage of hydrocephalus. The most important mechanisms suggest removal of blood clot and avoidance of the disturbance of CSF circulation and/or absorption5). Another possible explanation is that draining a large amount of CSF during early stages after SAH induce lower intracranial pressure11,16). The phenomenon was confirmed by the statistically significant dose-response (drainage volume-hydrocephalus) relationship according to Hasan et al.13) and Kasuya et al.16,17). However, patients who survived a second bleeding episode and presented with IVH were highly correlated with a requirement of shunt placement31). That is a common presentation in poor-grade SAH patients.
Complications of lumbar drainage are well-recognized, but not common. Serial lumbar puncture provides CSF blockage in the subarachnoid space, but not in the ventricular system. The risk of infection with this procedure is small, and the fall in CSF pressure is probably more gradual15).
Limitations of our study
The main limitation in our study is that there was a small number of patients in the shunt-dependent hydrocephalus group with lumbar drainage. To evaluate the difference in the need for permanent CSF diversion according to treatment modality and variables, such as gender, age, Hunt & Hess grade, and Fisher grade, should be evaluated. More precise evaluation of shunt-dependent hydrocephalus according to treatment modality will be possible only when we exclude confounding factors.
CONCLUSION
In this study, coil embolization of ruptured intracranial aneurysms in good-grade patients may be associated with lower risk for developing shunt-dependent hydrocephalus, possibly by active management of lumbar drainage and lesser damage for cisternal anatomy during procedure. This might affect long-term outcome and decision-making of ruptured intracranial aneurysms. However, a randomized, prospective study of treatment modality with or without the use of lumbar drainage will be able to overcome the limitations of our study.
References
- 1.Auer LM, Mokry M. Disturbed cerebrospinal fluid circulation after subarachnoid hemorrhage and acute aneurysmal surgery. Neurosurgery. 1990;26:804–808. doi: 10.1097/00006123-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Dehdashti AR, Rilliet B, Rufenacht DA, de Tribolet N. Shunt-dependent hydrocephalus after rupture of intracranial aneurysms : a prospective study of the influence of treatment modality. J Neurosurg. 2004;101:402–407. doi: 10.3171/jns.2004.101.3.0402. [DOI] [PubMed] [Google Scholar]
- 3.Dorai Z, Hynan LS, Kopitnik TA, Samson D. Factors related to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2003;52:763–769. doi: 10.1227/01.neu.0000053222.74852.2d. discussion 769-771. [DOI] [PubMed] [Google Scholar]
- 4.Fanning NF, Willinsky RA, ter Brugge KG. Wall enhancement, edema, and hydrocephalus after endovascular coil occlusion of intradural cerebral aneurysms. J Neurosurg. 2008;108:1074–1086. doi: 10.3171/JNS/2008/108/6/1074. [DOI] [PubMed] [Google Scholar]
- 5.Fülöp B, Deak G, Mencser Z, Kuncz A, Barzó P. Factors affecting the development of chronic hydrocephalus following subarachnoid hemorrhage, with special emphasis on the role of ventricular and lumbar drainage. Ideggyogy Sz. 2009;62(7-8):255–261. [PubMed] [Google Scholar]
- 6.Graff-Radford NR, Torner J, Adams HP, Kassell NF. Factors asso-ciated with subarachnoid hemorrhage. Arch Neurol. 1989;46:744–752. doi: 10.1001/archneur.1989.00520430038014. [DOI] [PubMed] [Google Scholar]
- 7.Gruber A, Reinprecht A, Bavinzski G, Czech T, Richling B. Chronic shunt-depedent hydrocephalus after early surgical and early endovascular treatment of ruptured intracranial aneurysms. Neurosurgery. 1999;44:503–509. doi: 10.1097/00006123-199903000-00039. discussion 509-512. [DOI] [PubMed] [Google Scholar]
- 8.Guglielmi G, Vinuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2 : Preliminary clinical experience. J Neurosurg. 1991;75:8–14. doi: 10.3171/jns.1991.75.1.0008. [DOI] [PubMed] [Google Scholar]
- 9.Hasan D, Tanghe HL. Disturbance of cisternal blood in patients with acute hydrocephalus after subarachnoid hemorrhage. Ann Neurol. 1992;31:374–378. doi: 10.1002/ana.410310405. [DOI] [PubMed] [Google Scholar]
- 10.Hasan D, van Peski J, Loeve I, Krenning EP, Vermeulen M. Single photon emission computed tomography in patients with acute hydrocephalus or cerebral ischemia after subarachnoid hemorrhage. J Neurol Neurosurg Psychiatry. 1991;54:490–493. doi: 10.1136/jnnp.54.6.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasan D, Vermeulen M, Wijdicks EF, Hijdra A, van Gijn J. Management problems in acute hydrocephalus after subarachnoid hemorrhage. Stroke. 1989;20:747–753. doi: 10.1161/01.str.20.6.747. [DOI] [PubMed] [Google Scholar]
- 12.Hoekema D, Schmidt RH, Ross I. Lumbar drainage for subarachnoid hemorrhage : technical considerations and safety analysis. Neurocrit Care. 2007;7:3–9. doi: 10.1007/s12028-007-0047-3. [DOI] [PubMed] [Google Scholar]
- 13.Huttner HB, Schwab S, Bardutzky J. Lumbar drainage for communicating hydrocephalus after ICH with ventricular hemorrhage. Neurocrit Care. 2006;5:193–196. doi: 10.1385/NCC:5:3:193. [DOI] [PubMed] [Google Scholar]
- 14.Inagawa T, Kamiya K, Matsuda T. Effect of continuous cisternal drainage on cerebral vasospasm. Acta Neurochir (Wien) 1991;112:28–36. doi: 10.1007/BF01402451. [DOI] [PubMed] [Google Scholar]
- 15.Kasuya H, Shimuzu T, Kagawa M. The effect of continuous drainage of cerebrospinal fluid in patients with subarachnoid hemorrhage : a retrospective analysis of 108 patients. Neurosurgery. 1991;28:56–59. doi: 10.1097/00006123-199101000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Kasuya H, Shimizu T, Kagawa M. The effect of continuous drainage of cerebrospinal fluid in patients with subarachnoid hemorrhage : a retrospective analysis of 108 patients. Neurosurgery. 1991;28:56–59. doi: 10.1097/00006123-199101000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Kasuya H, Shimizu T, Okada T, Takahashi K, Summerville T, Kitamura K. A study of continuous cerebrospinal fluid drainage in patients with subarachnoid hemorrhage. No Shinkei Geka. 1988;16(5 Suppl):475–481. [PubMed] [Google Scholar]
- 18.Klinge PM, Berding G, Brinker T, Knapp WH, Samii M. A positron emission tomography study of cerebrovascular reserve before and after shunt surgery in patients with idiopathic chronic hydrocephalus. J Neurosurg. 1999;91:605–609. doi: 10.3171/jns.1999.91.4.0605. [DOI] [PubMed] [Google Scholar]
- 19.Kwon OY, Kim YJ, Kim YJ, Cho CS, Lee SK, Cho MK. The Utility and Benefits of External Lumbar CSF Drainage after Endovascular Coiling on Aneurysmal Subarachnoid Hemorrhage. J Korean Neurosurg Soc. 2008;43:281–287. doi: 10.3340/jkns.2008.43.6.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macdonald RL. Lumbar drainage after subarachnoid hemorrhage : does it reduce vasospasm and delayed hydrocephalus? Neurocrit Care. 2007;7:1–2. doi: 10.1007/s12028-007-0046-4. [DOI] [PubMed] [Google Scholar]
- 21.Milhorat TH. Acute hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1987;20:15–20. doi: 10.1227/00006123-198701000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Mitha AP, Wong JH, Lu JQ, Morrish WF, Hudon ME, Hu WY. Communicating hydrocephalus after endovascular coiling of unruptured aneurysms : report of 2 cases. J Neurosurg. 2008;108:1241–1244. doi: 10.3171/JNS/2008/108/6/1241. [DOI] [PubMed] [Google Scholar]
- 23.Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International Subarachnoid Aneurysm Trial(ISAT) of neurological clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms : a randomised trial. Lancet. 2002;360:1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 24.Nakagomi T, Takagi K, Narita K, Nagashima H, Tamura A. Cisternal washing therapy for the prevention of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl. 2001;77:161–165. doi: 10.1007/978-3-7091-6232-3_34. [DOI] [PubMed] [Google Scholar]
- 25.de Oliveira JG, Beck J, Setzer M, Gerlach R, Vatter H, Seifert V, et al. Risk of shunt-dependent hydrocephalus after occlusion of ruptured intracranial aneurysms by surgical clipping or endovascular coiling : a single-institution series and meta-analysis. Neurosurgery. 2007;61:924–933. doi: 10.1227/01.neu.0000303188.72425.24. [DOI] [PubMed] [Google Scholar]
- 26.Park HK, Horowitz M, Jungreis C, Genevro J, Koebbe C, Levy E, et al. Periprocedural morbidity and mortality associated with endovascular treatment of intracranial aneurysms. AJNR. 2005;26:506–514. [PMC free article] [PubMed] [Google Scholar]
- 27.Pietilä TA, Heimberger KC, Palleske H, Brook M. Influence of aneurysm location on the development of chronic hydrocephalus following SAH. Acta Neurochir (Wien) 1995;137:70–73. doi: 10.1007/BF02188784. [DOI] [PubMed] [Google Scholar]
- 28.Sakaki S, Ohta S, Kuwabara H, Shiraishi M. The role of ventricular and cisternal drainage in the early operation for ruptured intracranial aneurysms. Acta Neurochir (Wien) 1987;88:87–94. doi: 10.1007/BF01404143. [DOI] [PubMed] [Google Scholar]
- 29.Sethi H, Moore A, Dervin J, Clifton A, MacSweeney JE. Hydrocephalus: comparison of clipping and embolization in aneurysm treatment. J Neurosurg. 2000;92:991–994. doi: 10.3171/jns.2000.92.6.0991. [DOI] [PubMed] [Google Scholar]
- 30.Steinke D, Weir B, Disney L. Hydrocephalus following aneurysmal subarachnoid hemorrhage. Neurol Res. 1987;9:3–9. doi: 10.1080/01616412.1987.11739764. [DOI] [PubMed] [Google Scholar]
- 31.Vale FL, Bradley EL, Fisher WS., 3rd The relationship of subarachnoid hemorrhage and the need for postoperative shunting. J Neurosurg. 1997;86:462–466. doi: 10.3171/jns.1997.86.3.0462. [DOI] [PubMed] [Google Scholar]
- 32.Varelas P, Helms A, Sinson G, Spanaki M, Hacein-Bey L. Clipping or coiling of ruptured cerebral aneurysms and shunt-dependent hydrocephalus. Neurocrit Care. 2006;4:223–228. doi: 10.1385/NCC:4:3:223. [DOI] [PubMed] [Google Scholar]
- 33.Vermeij FH, Hasan D, Vermeulen M, Tanghe HL, van Gijn J. Predictive factors for deterioration from hydrocephalus after subarachnoid hemorrhage. Neurology. 1994;44:1851–1855. doi: 10.1212/wnl.44.10.1851. [DOI] [PubMed] [Google Scholar]
- 34.Yoshioka H, Inagawa T, Tokuda Y, Inokuchi F. Chronic hydrocephalus in elderly patients following subarachnoid hemorrhage. Surg Neurol. 2000;53:119–124. doi: 10.1016/s0090-3019(99)00185-8. discussion 124-125. [DOI] [PubMed] [Google Scholar]