Abstract
Objective
Chronic subdural hematoma (CSDH) is known to have a significant recurrence rate. There are different criteria defining the recurrence of CSDH. We evaluated the postoperative course of CSDH and tried to propose the reasonable criteria of recurrence.
Methods
We retrospectively examined the medical records and pre- and postoperative CT scans of 149 consecutive patients who underwent surgery from January 2005 to December 2009. Diagnosis was confirmed by CT scanning or MRI. The postoperative courses were either resolved or recurrent. The resolved CSDH was one of the three types; early resolution, delayed resolution, or late resolution. The recurrent CSDH was one of the four types; recurrence without resolution, early recurrence after resolution, late recurrence after resolution, or recurrent-and-resolved type.
Results
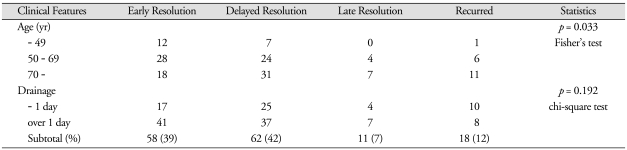
The CSDH was resolved within 30 days after surgery in 58 (39%) patients, between 1 to 3 months in 62 (42%), and after 3 months in 11 (7%) patients. The CSDH was recurred in 18 (12%) patients. Late resolution or recurrence was more common in the aged. The recurrent hematoma was seen on the same side in 11 patients, on the different side in 7 patients. Recurrence was significantly more common in the thick hematomas.
Conclusion
For a working criteria of the recurrence of CSDH, we propose the early recurrence as return of symptoms or reaccumulation of the hematoma after a surgery within 3 months regardless of the location, amount or repeated operations. The late recurrence can be defined as reappearance or enlargement of a liquefied hematoma within the cranial cavity surrounded by the membranes or persistent CSDH beyond 3 months after surgery.
Keywords: Chronic subdural hematoma, Craniocerebral trauma, Diagnosis, Recurrence, Risk Factors
INTRODUCTION
Chronic subdural hematoma (CSDH) is known to have a significant recurrence rate. The rate of recurrence of chronic subdural hematoma after surgery ranges from roughly 5% to 30%1,7,16,18-20,22). Many authors1,7,16,18-20,22) reported the recurrence rates of the CSDH, often without any criteria of the recurrence. There are some criteria defining the recurrence of CSDH, however, they are different from each other (Table 1)1,6,13,14,16-19,21,23). The simplest criteria are re-accumulation of the hematoma within the postoperative hematoma cavity and the reappearance of neurological symptoms14,16). However, the reappearance of neurologic symptoms is not always helpful to diagnose the recurrence. Because the symptoms of CSDH are often equivocal5), the diagnosis of a CSDH is not usually suspected at the time of initial presentation in majority of cases2,4). Residual fluid can be detected on computed tomography (CT) in as many as 80% of the patients, a majority of them is asymptomatic and clinically insignificant2). It is difficult to differentiate the reaccumulation from the incomplete removal. There are no established criteria of recurrence, yet. We evaluated the postoperative course of CSDH and tried to propose the reasonable criteria of recurrence.
Table 1.
Reported criteria of recurrence in chronic subdural hematomas
MATERIALS AND METHODS
We retrospectively examined the medical records and pre- and postoperative CT scans of 149 consecutive patients who underwent surgery from January 2005 to December 2009. Diagnosis was confirmed by CT scanning or MRI in all patients. The preferred surgical technique was single burr hole drainage (81%) under the local anesthesia. For the bilateral CSDHs, bilateral single burr hole drainage (15%) without irrigation was performed. Double burr holes with saline irrigation were used in only 3 patients (2%). Another 3 patients underwent a craniotomy. We placed a soft silicon drain in all cases, which was usually removed within 2 days. We obtained postoperative CT scans within 3 days after surgery. Routine follow-up CT scans were done until regression of the subdural collection and their recovery to premorbid functional status.
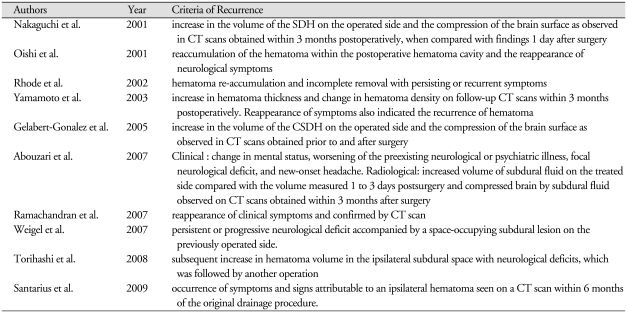
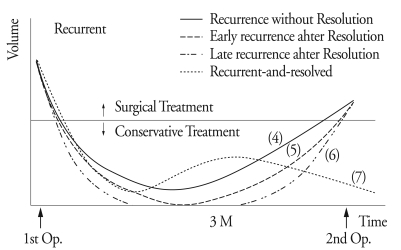
The postoperative courses were either resolved or recurrent. The resolved CSDH was one of the three types : early resolution, delayed resolution and late resolution (Fig. 1). Early resolution (Type 1) was defined as resolution of the hematoma within 30 days after surgery. Delayed resolution (Type 2) was defined as resolution of the hematoma between 1 to 3 months (Fig. 2). Late resolution (Type 3) was defined as resolution of the hematoma after 3 months or more (Fig. 3). The recurrent CSDH was one of the four types; recurrence without resolution, early recurrence after resolution, late recurrence after resolution, and recurrent-and-resolved type (Fig. 4). Recurrence without resolution (Type 4) was defined as increased volume of the hematoma without resolution. Early recurrence after resolution (Type 5) was defined as recurrence of the resolved hematoma within 3 months after surgery. Late recurrence after resolution (Type 6) was defined as recurrence of the resolved hematoma after 3 months or more. There is one additional type of recurrence, the recurrent-and-resolved type (Type 7), which was the spontaneously resolved recurrent CSDH.
Fig. 1.
Postoperative course of the resolved chronic subdural hematoma. Early resolution is defined as resolution of the hematoma within 30 days after surgery (1). Delayed resolution is defined as resolution of the hematoma between 1 to 3 months (2). Late resolution is defined as resolution of the hematoma after 3 months or more (3).
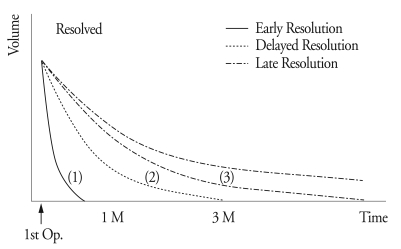
Fig. 2.
An example of a delayed resolution. The hematoma is resolved within 3 months.
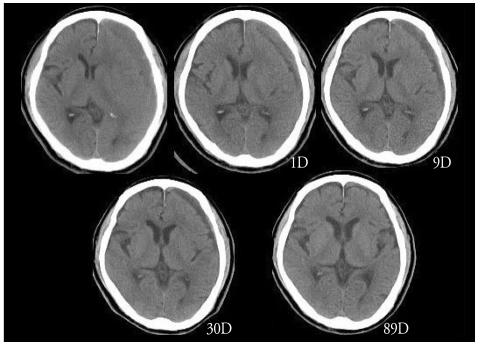
Fig. 3.
An example of a late resolution. The hematoma is remained till 292 days after surgery.
Fig. 4.
Postoperative courses of the recurrent chronic subdural hematoma. Recurrence without resolution is defined as increased volume of the hematoma without resolution (4). Early recurrence after resolution is defined as recurrence of the resolved hematoma within 3 months after surgery (5). Late recurrence after resolution is defined as recurrence of the resolved hematoma after 3 months or more (6). Finally, the recurrent-and-resolved type, which is the spontaneously resolved recurrent CSDH (7).
The thickness of the hematoma was measured on CT scan, which revealed the maximal thickness of the hematoma15). The degree of midline shift was measured near the level of the third ventricle or septum pellucidum on CT scan.
Statistical significance was tested using the chi-square test. It was considered significant, when p < 0.05.
RESULTS
Postoperative courses
The CSDH was resolved within 30 days after surgery in 58 (39%) patients, between 1 to 3 months in 62 (42%), and after 3 months in 11 (7%) patients. The CSDH was recurred in 18 (12%) patients. Late resolution or recurrence was more common in the aged (Table 2). This difference was statistically significant (p = 0.033).
Table 2.
Clinical features and postoperative courses of the chronic subdural hematoma
For the statistical comparison, we used the late resolution and the recurred as one group
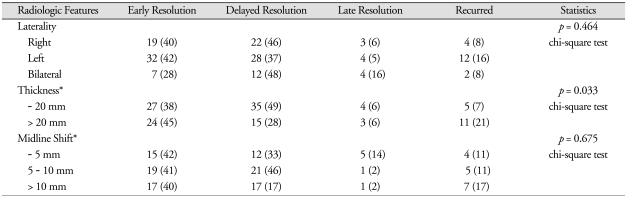
According to the laterality of the CSDH, delayed resolution was more common in the bilateral or right CSDHs (Table 3). Late resolution was also more common in the bilateral CSDHs. The recurrence rate was 16% in the left CSDH, while it was 8% in either the right or bilateral CSDHs. However, this difference is not statistically significant (p = 0.464).
Table 3.
Radiologic features and postoperative courses of the chronic subdural hematoma
For the statistical comparison, we used the late resolution and the recurred as one group. *except bilateral hematomas
Recurrence
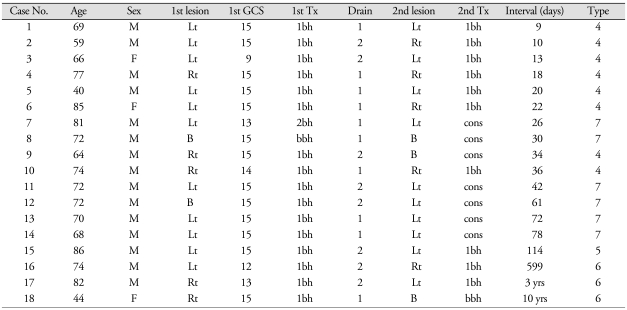
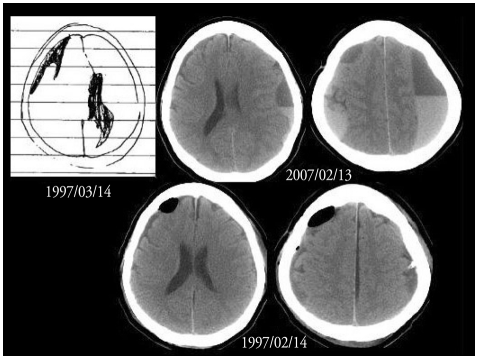
The CSDH was recurred in 18 patients (Table 4). The recurrent hematoma was on the same side in 11 patients, on the different side in 7 patients. The hematoma was recurred within 3 months in 14 patients, usually without complete resolution (Type 4). Recurrence without resolution (Type 4) was observed in 8 patients. Early recurrence after resolution (Type 5) was observed in only 1 patient. Late recurrence after resolution (Type 6) was found in 3 patients. In 3 patients, the hematoma recurred on more than a year after the surgery. The longest interval from the first operation to the diagnosis of the recurrence was about 10 years in case 18. In this case, we could confirm the episode of CSDH in her medical record (Fig. 5). We operated again for the recurrent hematomas in 11 patients. In 7 patients, the recurrent hematomas were resolved spontaneously, i.e. the recurrent-and-resolved type (Type 7). Hepatoma was found in the case 3 and case 16.
Table 4.
Clinical features of the recurrent chronic subdural hematomas
GCS : Glasgow Coma Score, Tx : treatment, Interval : interval from the 1st operation to the diagnosis of the recurrence, Type : type of recurrence, see Fig. 4. Lt : left, Rt : right, bh : burr hole, bbh : bilateral burr holes, cons : conservative
Fig. 5.
The case 18 had a history of chronic subdural hematoma on the right frontal region in March 1997. She was suffered from the schizophrenia for more than 10 years. Chronic subdural hematomas were found on both sides in February 2007. She recovered form the chronic subdural hematoma by bilateral burr holes.
Recurrence was significantly (p = 0.033) more common in the thick (more than 20 mm) hematomas (Table 3). Although the recurrence rate was relatively high in the hematomas with severe (more than 10 mm) midline shift (Table 3), this difference is not statistically significant (p = 0.675).
DISCUSSION
The postoperative courses of CSDH were either resolved or recurrent. We proposed seven different postoperative courses of the CSDH. Early or delayed resolution (Type 1 & 2) was the most common postoperative courses of the CSDH. As criteria of the recurrence, some proposed the time interval, such as within 3 months1,13,23) or 6 months of the initial surgery18). Pathologically, CSDH is defined as liquefied hematomas accumulated within the so-called subdural space surrounded by the outer and inner membranes12). If the CSDH remained till one month after the drainage, the lesion is a liquefied hematoma encapsulated by membranes. Although the hematoma is clinically corresponding to the incomplete removal, it is a CSDH in pathologic term. Strict differentiation between reaccumulation and incomplete removal is very difficult to deal together17). More than a half of the recurrent CSDHs recurred within 30 days, and 70% of them recurred within 3 months. However, follow up for at least 2 months after surgery11) is not enough to check recurrence, since more than one third of recurrences were diagnosed after 2 months in this study.
The recurrent hematoma was on the same side in 11 patients, on the different side in 7 patients. Recurrence, based on medical dictionary, is defined as the return of symptoms after a remission. If the same pathologic lesion reappears after surgical removal, we can use the term recurrence regardless of the interval. Although someone may insist the term reaccumulation of the hematoma on the previously operated side, the reappearance of cancer cells in another location is also a recurrence. In the case 18, the second lesion was pathologically CSDH, the same pathology of the initial lesion, although the interval was about 10 years. To diagnose the recurrence, Torihashi et al.19) required another operation after the initial surgery. However, repeated operations can be done for the incompletely removed hematomas. Spontaneous resolution of CSDH occurs by maturation of the neomembrane and stabilization of the neovasculature8). Steroid therapy is a feasible and safe option in the management of CSDH, which was able to cure or improve two thirds of the patients with CSDH4). In such cases, another operation is not necessary. After removal of a large unilateral CSDH with marked midline-shift, a small subdural hygromas may result on both sides, which became a recurrent bilateral CSDH. Even if we don't remove the hematoma surgically, the lesion is pathologically CSDH, which would be composed of liquefied hematoma within the inner and outer membranes. A repeated operation is not necessary to diagnose the recurrent CSDH.
The resolution was faster in relatively younger patients in this study. The resolution of the CSDH after burr hole drainage depends on the rate of cerebral re-expansion. The reexpansion rate was relatively low in patients who were 70 years old or more than that of patients who were less than 70 years old11). This is the reason why late resolution or recurrence was more common in the aged. Not only the age, pre-existing cerebral infarction, and air in the subdural space after surgery were thought to be the factors affecting re-expansion of the brain after surgery. Although there was a report that bilateral CSDH was an independent risk factor for the recurrence of CSDH19), the recurrence rate of the bilateral CSDHs was not higher than the unilateral hematomas in this study. Theoretically, patients with bilateral CSDH tend to have previous brain atrophy, which may lead to poor brain re-expansion. However, expansion of the CSDH from the bilateral subdural hygroma is not always equal in both sides. This hemispheric pressure difference may result from premorbid brain status or head tilting. Expansion of the CSDH in one side abolishes the opposite lesion, which changes bilateral lesions into unilateral lesions9). At this time, thickness of the hematoma becomes more important to the bilaterality itself. The recurrence rate was significantly high in the thick CSDHs in this study. Some10) reported that the thickness was a risk factor for the recurrence, while others19) reported that the bilaterality was a risk factor for the recurrence. Many risk factors for recurrence of CSDH have been reported, including old age, cerebral atrophy, large hematoma, bilateral hematomas, hematoma density, inflammation markers, alcohol ingestion, bleeding tendency, and some technical aspects of surgery3,5,6,11,13,14,23). Recently, an upright posture after surgery was reported as a risk factor of CSDH recurrence1). Majority of these risk factors are related to the poor brain re-expansion.
Although grouping such as early, delayed and late according to the time interval is arbitrary, we have to differentiate the recurrence from the incomplete removal at least clinically. Because 70% of the recurrence occurred within 3 months, it seems reasonable to use the term early recurrence when there is a return of symptoms or reaccumulation of the hematoma after a surgery within 3 months, regardless of the second operation. Persistent CSDH beyond 3 months after surgery can be regarded as a late recurrence regardless of the location, amount or repeated operations.
CONCLUSION
For working criteria of the recurrence of CSDH, we propose the early recurrence as return of symptoms or reaccumulation of the hematoma after a surgery within 3 months regardless of the location, amount or repeated operations. The late recurrence can be defined as reappearance or enlargement of a liquefied hematoma within the cranial cavity surrounded by the membranes or persistent CSDH beyond 3 months after surgery.
References
- 1.Abouzari M, Rashidi A, Rezaii J, Esfandiari K, Asadollahi M, Aleali H, et al. The role of postoperative patient posture in the recurrence of traumatic chronic subdural hematoma after burr-hole surgery. Neurosurgery. 2007;61:794–797. doi: 10.1227/01.NEU.0000298908.94129.67. [DOI] [PubMed] [Google Scholar]
- 2.Adhiyaman V, Asghar M, Ganeshram KN, Bhowmick BK. Chronic subdural hematoma in the elderly. Postgrad Med J. 2002;78:71–75. doi: 10.1136/pmj.78.916.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amirjamshidi A, Abouzari M, Eftekhar B, Rashidi A, Rezaii J, Esfandiari K, et al. Outcomes and recurrence rates in chronic subdural hematoma. Br J Neurosurg. 2007;21:272–275. doi: 10.1080/02688690701272232. [DOI] [PubMed] [Google Scholar]
- 4.Delgado-López PD, Martín-Velasco V, Castilla-Díez JM, Rodríguez-Salazar A, Galacho-Harriero AM, Fernández-Arconada O. Dexamethasone treatment in chronic subdural hematoma. Neurocirugia (Astur) 2009;20:346–359. doi: 10.1016/s1130-1473(09)70154-x. [DOI] [PubMed] [Google Scholar]
- 5.Fogelholm R, Heiskanen O, Waltimo O. Chronic subdural hematoma in adults. Influence of patient's age on symptoms, signs, and thickness of hematoma. J Neurosurg. 1975;42:43–46. doi: 10.3171/jns.1975.42.1.0043. [DOI] [PubMed] [Google Scholar]
- 6.Gelabert-González M, Iglesias-Pais M, García-Allut A, Martínez-Rumbo R. Chronic subdural hematoma : surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg. 2005;107:223–229. doi: 10.1016/j.clineuro.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Ko BS, Lee JK, Seo BR, Moon SJ, Kim JH, Kim SH. Clinical analysis of risk factors related to recurrent chronic subdural hematoma. J Korean Neurosurg Soc. 2008;43:11–15. doi: 10.3340/jkns.2008.43.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee KS. Natural history of chronic subdural hematoma. Brain Inj. 2004;18:351–358. doi: 10.1080/02699050310001645801. [DOI] [PubMed] [Google Scholar]
- 9.Lee KS, Bae WK, Yoon SM, Doh JW, Bae HG, Yun IG. Location of the chronic subdural hematoma : role of the gravity and cranial morphology. Brain Inj. 2001;15:47–52. doi: 10.1080/02699050150209129. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto K, Akagi K, Abekura M, Ryujin H, Ohkawa M, Iwasa N, et al. Recurrence factors for chronic subdural hematomas after burr-hole craniostomy and closed system drainage. Neurol Res. 1999;21:277–280. doi: 10.1080/01616412.1999.11740931. [DOI] [PubMed] [Google Scholar]
- 11.Mori K, Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases : clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo) 2001;41:371–381. doi: 10.2176/nmc.41.371. [DOI] [PubMed] [Google Scholar]
- 12.Murakami H, Hirose Y, Sagoh M, Shimizu K, Kojima M, Gotoh K, et al. Why do chronic subdural hematomas continue to grow slowly and not coagulate? Role of thrombomodulin in the mechanism. J Neurosurg. 2002;96:877–884. doi: 10.3171/jns.2002.96.5.0877. [DOI] [PubMed] [Google Scholar]
- 13.Nakaguchi H, Tanishima T, Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg. 2001;95:256–262. doi: 10.3171/jns.2001.95.2.0256. [DOI] [PubMed] [Google Scholar]
- 14.Oishi M, Toyama M, Tamatani S, Kitazawa T, Saito M. Clinical factors of recurrent chronic subdural hematoma. Neurol Med Chir (Tokyo) 2001;41:382–386. doi: 10.2176/nmc.41.382. [DOI] [PubMed] [Google Scholar]
- 15.Okada Y, Akai T, Okamoto K, Iida T, Takata H, Iizuka H. A comparative study of the treatment of chronic subdural hematoma-burr hole drainage versus burr hole irrigation. Surg Neurol. 2002;57:405–409. doi: 10.1016/s0090-3019(02)00720-6. [DOI] [PubMed] [Google Scholar]
- 16.Ramachandran R, Hegde T. Chronic subdural hematomas-causes of morbidity and mortality. Surg Neurol. 2007;67:367–372. doi: 10.1016/j.surneu.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Rohde V, Graf G, Hassler W. Complications of burr-hole craniostomy and closed-system drainage for chronic subdural hematomas : a retrospective analysis of 376 patients. Neurosurg Rev. 2002;25:89–94. doi: 10.1007/s101430100182. [DOI] [PubMed] [Google Scholar]
- 18.Santarius T, Kirkpatrick PJ, Ganesan D, Chia HL, Jalloh I, Smielewski P, et al. Use of drains versus no drains after burr-hole evacuation of chronic subdural hematoma : a randomised controlled trial. Lancet. 2009;374:1067–1073. doi: 10.1016/S0140-6736(09)61115-6. [DOI] [PubMed] [Google Scholar]
- 19.Torihashi K, Sadamasa N, Yoshida K, Narumi O, Chin M, Yamagata S. Independent predictors for recurrence of chronic subdural hematoma : a review of 343 consecutive surgical cases. Neurosurgery. 2008;63:1125–1129. doi: 10.1227/01.NEU.0000335782.60059.17. [DOI] [PubMed] [Google Scholar]
- 20.Tsai TH, Lieu AS, Hwang SL, Huang TY, Hwang YF. A comparative study of the patients with bilateral or unilateral chronic subdural hematoma : precipitating factors and postoperative outcomes. J Trauma. 2010;68:571–575. doi: 10.1097/TA.0b013e3181a5f31c. [DOI] [PubMed] [Google Scholar]
- 21.Weigel R, Hohenstein A, Schlickum L, Weiss C, Schilling L. Angiotensin converting enzyme inhibition for arterial hypertension reduces the risk of recurrence in patients with chronic subdural hematoma possibly by an antiangiogenic mechanism. Neurosurgery. 2007;61:788–792. doi: 10.1227/01.NEU.0000298907.56012.E8. [DOI] [PubMed] [Google Scholar]
- 22.Wilberger JE. Pathophysiology of evolution and recurrence of chronic subdural hematoma. Neurosurg Clin N Am. 2000;11:435–438. [PubMed] [Google Scholar]
- 23.Yamamoto H, Hirashima Y, Hamada H, Hayashi N, Origasa H, Endo S. Independent predictors of recurrence of chronic subdural hematoma : results of multivariate analysis performed using a logistic regression model. J Neurosurg. 2003;98:1217–1221. doi: 10.3171/jns.2003.98.6.1217. [DOI] [PubMed] [Google Scholar]