Abstract
Background
This study was performed to determine the utility of dobutamine stress test results for predicting myocardial infarction (MI) and cardiac death in patients with chest pain and left ventricular hypertrophy (LVH).
Methods and Results
Three hundred fifty-three (353) participants, aged 64±12 years (54% men) underwent dobutamine cardiovascular magnetic resonance (DCMR) stress testing and then were followed for 6±2 (range 0.5 to 11.5) years to assess the post-DCMR occurrence of MI or cardiac death. Left ventricular mass and the presence or absence of ischemia were determined; LVH was defined as > 96 g/m2 in men and > 77 g/m2 in women. LVH was present in 62 participants (18 % of the men and 17% of the women, p=0.90). Seventy-one (20%) participants experienced a MI or cardiac death during follow-up. The MI and cardiac death rate was more frequent in those with versus without LVH (32% vs. 17%, p=0.009). In multivariable analysis that accounted for the presence of pre-existing coronary artery disease, hypertension, diabetes, stress induced ischemia, and reduced LVEF, LVH was an independent predictor of MI and cardiac death (hazard ratio = 1.99, 95% confidence interval = 1.13-3.50; p=0.02).
Conclusions
Left ventricular hypertrophy is predictive of future MI and cardiac death in patients with or without inducible ischemia during dobutamine cardiac stress testing. As a result, LVH should be reported in those referred for dobutamine cardiac stress tests, particularly those without inducible ischemia, in whom otherwise one would assume a favorable cardiac prognosis.
Keywords: hypertrophy, magnetic resonance imaging, myocardial infarction
Introduction
In those with chest pain referred for dobutamine stress testing, the absence of inducible ischemia has been shown to confer a low risk of future adverse cardiac events.1 More recently, left ventricular hypertrophy (LVH), a strong, independent predictor of cardiovascular events and all-cause mortality,2,3 has become a relatively frequent finding in patients with chest pain referred for noninvasive dobutamine cardiac stress testing. At present, it is unknown whether the absence of inducible LV wall motion abnormalities indicative of ischemia (that normally carries a favorable prognosis in the absence of LVH) confers a negative cardiac prognosis in patients with LVH. The purpose of this study was to determine the importance of LVH in patients with chest pain referred for dobutamine cardiovascular stress testing procedures.
Methods
Our study was approved by the Institutional Review Board at the Wake Forest University School of Medicine, and all participants provided informed consent. Between 1997 and 2004, 362 dobutamine cardiovascular magnetic resonance (DCMR) studies were performed to diagnose inducible ischemia in which left ventricular wall motion (LVWM) was visualized throughout testing and left ventricular (LV) mass could be measured from the acquired images. Patients’ routine use of medications, including beta-receptor antagonists, was not altered before testing. Comprehensive demographic data regarding health status and medication use were collected at the time of testing. For the purposes of our study, prior myocardial infarction (MI), hypertension (HTN), diabetes (DM), and hypercholesterolemia were defined according to previously published criteria.1 The study was designed as a prospective cohort analysis in which cardiac outcomes were assessed after participants received stress DCMR.
DCMR procedure
DCMR was performed according to previously published techniques1 using single-slice, multi-phase gradient-echo images of the left ventricle acquired on a Horizon 1.5T whole-body imaging system (General Electric Medical Systems) at rest and then after dobutamine was infused to achieve 80% of the maximum predicted heart rate response for age. Image parameters included a 256 × 128 matrix, a 35 to 48 cm field of view, a 10-ms repetition time, a 4-ms echo time, a 20-degree flip angle, an 8 mm thick slice, and a 40-ms temporal resolution. Images were analyzed during and directly after the examination with the use of a software program designed for display of DCMR in multi-window, synchronized format. Inducible LVWM abnormalities were defined as deterioration of LVWM (with the exception of akinesis to dyskinesis) in any myocardial segment observed in 2 orthogonal planes of the left ventricle during infusion of dobutamine.1,4 The resting left ventricular ejection fraction (LVEF) was measured using a bi-plane area-length technique.5 Left ventricular mass was calculated from wall thickness measurements using a truncated ellipsoid technique6,7 that was indexed to body surface area (BSA).8 Papillary muscle mass was included in the LV cavity and excluded from the LV mass determination. According to previously published techniques, threshold values of LV mass (96 g/m2, and 77 g/m2, in men and women, respectively) were used to define LVH.8 Alternative analyses were also performed that defined LVH according to values indexed by height.8
Follow-up
Personnel blinded to DCMR stress testing results contacted each subject (or if deceased, an immediate family member) to determine whether cardiac events had occurred since their DCMR test. The date of the contact was used to calculate follow-up times. All events were confirmed by review of the participants’ medical record or death certificate. Hard events were defined as MI (angina of ≥ 30 min duration, and either ≥ 2 mm, or 1mm ST-segment elevation in men and women, respectively, in 2 consecutive electrocardiographic leads, or detection of a rise in cardiac biomarkers (troponin-I) with at least one value above the 99th percentile of the upper reference limit),9 or cardiac death (death in the presence of acute coronary syndrome, significant cardiac arrhythmia, or refractory congestive heart failure (CHF)). Electrocardiographic (ECG), enzymatic, or autopsy data were used to substantiate cardiac events including (when available) cardiac mortality.
Statistical analysis
Differences in baseline characteristics stratified by LVH were compared using Chi-square and Student’s t-tests for categorical and continuous characteristics, respectively. Kaplan-Meier methods were used to estimate the probability of hard cardiac events as a function of five years of follow-up and unadjusted differences were compared using log-rank test with weight given during these five years. Multivariable Cox proportional hazards regression models were performed to identify independent predictors of the time to cardiac events. Factors known to predict of cardiovascular events that were recorded were included in the model. The risk of a given variable was expressed by a hazard ratio (HR) with corresponding 95% confidence intervals. Variables were considered significant if the null hypothesis of no contribution could be rejected at the 5% two-sided level of significance. In addition to the primary analysis of comparing time to hard events between those with and without hypertrophy, secondary analyses estimated the increase risk associated with hypertrophy in subgroups defined by prior MI, ischemia, and LVEF<40; although, preliminarily there was felt somewhat insufficient power to detect clinically important hazard ratios with the number of events per subgroup.
Results
Of the 362 patients who underwent DCMR, and had follow-up, 9 underwent immediate (within 4 weeks) coronary artery revascularization and were excluded from further analysis in order to avoid the possibility of a hard event resulting from a revascularization procedure. This exclusion resulted in a study population of 353 participants; LVH was present in 62 participants or 17.6% of the study population including 18% of the men and 17% of the women (p=0.90). The demographic and clinical characteristics of the participants are shown in Table 1. The age (63 versus 64 years, p=0.80), gender (men 55% versus 54%, p=0.90) and body mass index (31 g/m2 versus 31 g/m2, p=0.91) were similar between those with or without LVH.
Table 1.
Baseline Characteristics of the participants*
| All | LV Hypertrophy | p value† | ||
|---|---|---|---|---|
| (n=353) | No (n=291) | Yes (n=62) | ||
| Demographics | ||||
| Age (yrs) | 64±12 | 64±12 | 63±12 | 0.80 |
| Men | 191(54) | 157(54) | 34(55) | 0.90 |
| BMI (g/m2) | 31±7 | 31±7 | 31±8 | 0.91 |
| Historical Data (%) | ||||
| Smoking | 150(42) | 123(42) | 27(44) | 0.89 |
| Hypertension | 243(69) | 197(68) | 46(74) | 0.56 |
| Diabetes mellitus | 126(36) | 93(32) | 33(53) | 0.006 |
| Hypercholesterolemia | 194(55) | 158(54) | 36(58) | 0.79 |
| CHF | 55(16) | 41(14) | 14(23) | 0.23 |
| Prior MI | 126(36) | 98(34) | 28(45) | 0.09 |
| Medication (%) | ||||
| ACE inhibitor/ARB | 107(30) | 77(26) | 30(48) | 0.003 |
| Statin | 106(30) | 90(31) | 16(26) | 0.62 |
| β-blocker | 123(35) | 97(33) | 26(42) | 0.33 |
| Hemodynamic | ||||
| Rest HR | 73±14 | 73±14 | 73±14 | 0.86 |
| Rest SBP | 142±23 | 141±22 | 144±26 | 0.42 |
| Rest DBP | 79±13 | 79±12 | 78±18 | 0.83 |
| Peak HR | 130±16 | 131±15 | 125±18 | 0.03 |
| Peak SBP | 148±27 | 148±26 | 149±28 | 0.87 |
| Peak DBP | 77±16 | 77±16 | 79±16 | 0.37 |
| Cardiac MRI | ||||
| LVEF (%) | 55±13 | 57±12 | 48±14 | <0.001 |
| LV mass(g) | 139±47 | 124±32 | 207±47 | <0.001 |
| LV mass index(g/m2) | 69±22 | 62±13 | 105±19 | <0.001 |
| Inducible ischemia | 110(31) | 80(27) | 30(48) | 0.001 |
| Event (%) | ||||
| Hard event | 71(20) | 51(17) | 20(32) | 0.009 |
| Cardiac death | 43(12) | 27(9) | 15(24) | <0.001 |
| MI | 38(11) | 30(10) | 7(11) | 0.79 |
For continuous variables, data are presented as mean ± standard deviation. For dichotomous variables, the number and percentage of participants with the condition are shown.
LV hypertrophy defined as LV mass index >96 g/m2 and 77 g/m2 in men and women, respectively.
p value for comparison of patients with or without LVH
Abbreviations: ACE = Angiotensin-converting enzyme; ARB= Angiotensin receptor blocker; BMI= Body mass index; CHF= Congestive heart failure; DBP = diastolic blood pressure; LVEF= Left ventricular ejection fraction; HR= heart rate; MI= Myocardial infarction; SBP= systolic blood pressure
Fifty-four participants (37 with inducible ischemia, 12 upon receipt of maximal stress, and 5 with angina) did not achieve 80% of the maximum predicted heart rate response for age. Participants with LVH exhibited more DM; prior history of MI, CHF, and smoking were similar between the groups. Hypertension was prevalent in all study participants and trended higher in those with LVH. Overall, renin-angiotensin converting enzyme inhibitors were prescribed more frequently in participants with LVH (26% versus 48%, p=0.007). Both participant groups did not differ according to resting heart rate and blood pressure values.
For the study participants, the LV mass index (LVMI) averaged 69±22g/m2. LV mass was greater in men versus women even after accounting for BSA (75±21 g/m2 versus 63±21 g/m2, p<0.001), respectively. Mean LVMI was 62±13 g/m2 in patients without LVH versus 105±19 g/m2 in patients with LVH (p<0.001). Compared to those without LVH, patients with LVH had a lower LVEF (48±14% vs. 57±12%, p<0.001) and a higher prevalence of inducible ischemia during DCMR (48% vs. 27% with ischemia, p=0.001). In 27% of patients (17 of 62) with LVH, the resting wall motion was normal, whereas in patients without LVH, 49% (136 of 279) had no segmental wall motion abnormalities (p=0.02).
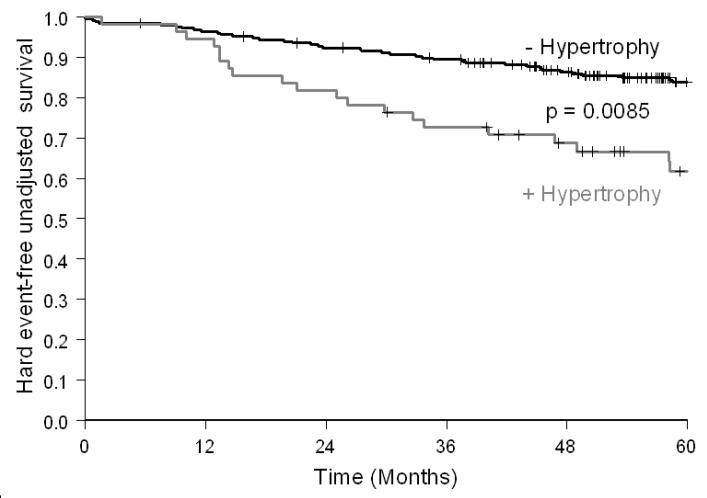
During a mean follow up of 6±2 (range 0.5 to 11.5) years, 138 participants or 39% of the participants experienced at least 1 cardiac event. Of these 138 participants, 71 (51%) experienced a hard event (including 32 subjects with non-fatal MIs and 39 subjects with cardiac deaths). The hard cardiac event rate at 5 years was greater in patients with LVH than in those without LVH (32% vs. 14%, p=0.003). Men and women with LVH had similar 5-year hard cardiac event rates (20% vs.16%, p=0.31). As shown in Figure 1, LVH was associated with a reduced event-free survival for hard events (p<0.001).
Figure 1.
Kaplan-Meier survival curves for MI and cardiac death in participants with chest pain referred for dobutamine stress stratified by LVH. Compared to patients without LVH, event free survival was reduced in participants with LVH.
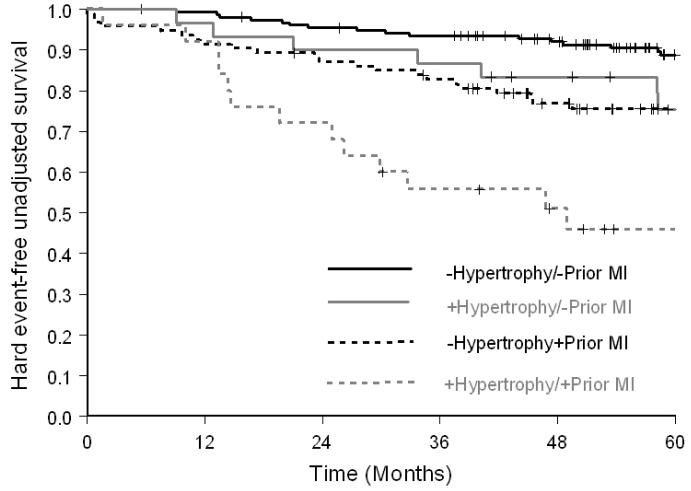
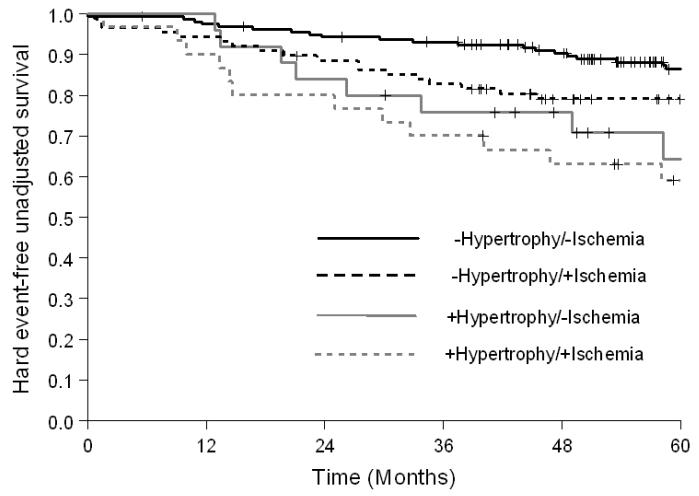
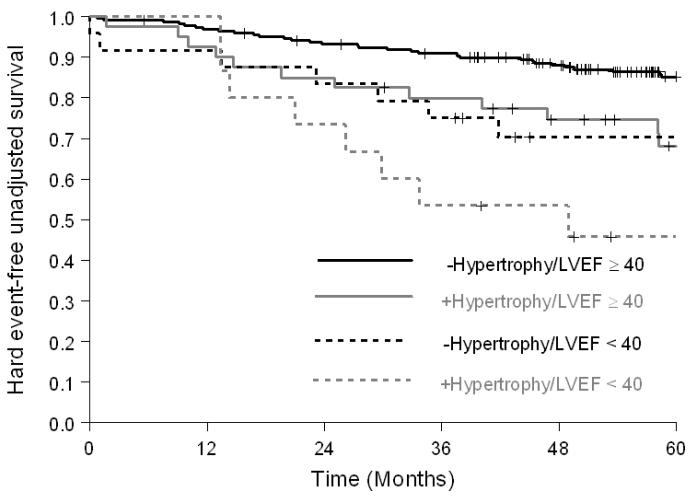
As shown in Figure 2, among participants with a history of prior MI, the risk of hard cardiac events was greater in participants with LVH (HR=2.34; p=0.01; 5-year hard event value of 48% versus 23% without LVH); for those without prior MI, the risk of hard events was also higher in those with LVH (HR=2.31; p=0.05; 5-year event rate of 22% versus 10% in those without LVH). Among participants with or without inducible ischemia, participants with LVH experienced more hard cardiac events (HR=1.88 [5-year event rate of 41% versus 23% in those without LVH] and 2.67 [5-year event rate of 28% in those without ischemia versus 11% in those without LVH]); however, it was only statistically significant in the non-ischemic group (p=0.08 and 0.01, respectively, Figure 3). When analysis was performed to evaluate participants with a LVEF ≥ 40%, or < 40%, the incidence of hard cardiac events was higher in participants with LVH for both groups (HR=2.21 [5-year event rate of 27% versus 13% in those without LVH] and 1.94 [5-year event rate of 54% versus 30% in those without LVH]); but was only statistically significant in those with a LVEF ≥40% (p=0.02 and 0.19, respectively, Figure 4).
Figure 2.
Kaplan-Meier survival curves for MI and cardiac death in participants receiving dobutamine stress with or without prior history of prior MI stratified by LVH. Compared to patients without history of prior MI and LVH, event free survival was significantly reduced in participants with LVH.
Figure 3.
Kaplan-Meier survival curves for MI and cardiac death in participants receiving dobutamine stress with or without inducible ischemia, stratified by LVH. Compared to participants without inducible ischemia and LVH, event free survival was significantly reduced in participants with LVH. Note the reduced event-free survival in participants with LVH and no ischemia relative to those without LVH, but with inducible ischemia.
Figure 4.
Kaplan-Meier survival curves for MI and cardiac death in participants receiving dobutamine stress with a LVEF < or ≥40%, stratified by LVH. Compared to patients with LVEF≥40% without LVH, event free survival was reduced in participants with EF< 40% with LVH. Participants with LVH and a LVEF ≥40% exhibited a similar adverse prognosis as compared to those with a LVEF <40%.
The univariable and multivariable HRs for cardiac events are displayed in Table 2. After adjustment for age, gender, body mass index, and other risk factors for cardiac events, LVH, inducible ischemia, and prior history of MI were independent predictors of an increased risk of hard cardiac events. Eight subjects exhibited a biphasic response during dobutamine stress. The incidence of hard events at 5 years was 40% in these 8 subjects compared with a 26% (p=0.61 for difference) incidence of hard events in individuals with ischemia that did not exhibit a biphasic response.
Table 2.
The relationship of LV mass to MI and cardiac death.
| Model | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR(95%CI) | p Value | HR(95%CI) | p-value | |
| LVH (g/m2)* | 2.59(1.51-4.43) | <0.001 | 1.99(1.13-3.50) | 0.02 |
| LVEF < 40% | 3.20(1.78-5.76) | <0.001 | 1.90(0.98-3.69) | 0.06 |
| Inducible ischemia | 2.44(1.47-4.05) | <0.001 | 1.87(1.06-3.31) | 0.03 |
| Age ≥ 65 years | 1.53(0.91-2.57) | 0.10 | 1.56(0.90-2.71) | 0.12 |
| Male gender | 1.36(0.81-2.28) | 0.24 | 0.85(0.48-1.50) | 0.57 |
| BMI | 0.97(0.94-1.01) | 0.16 | 0.98(0.93-1.02) | 0.28 |
| Prior Q-wave MI | 2.95(1.76-4.93) | <0.001 | 2.72(1.59-4.66) | <0.001 |
| Hypercholesterolemia | 1.01(0.60-1.67) | 0.98 | 1.04(0.61-1.76) | 0.89 |
| Hypertension | 1.36(0.76-2.43) | 0.30 | 1.28(0.68-2.40) | 0.45 |
| Diabetes mellitus | 1.30(0.78-2.18) | 0.31 | 0.86(0.49-1.51) | 0.60 |
| Smoking | 1.49(0.90-2.47) | 0.12 | 1.35(0.79-2.33) | 0.27 |
Abbreviations: BMI= Body mass index; LVH = Left ventricular hypertrophy; LVEF= Left ventricular ejection fraction; MI= Myocardial infarction; and MRI=Magnetic resonance imaging.
LVH defined as LV mass index >96 g/m2 and 77 g/m2 in men and women, respectively.
If LV mass indexed to body surface area was treated as a continuous rather than a dichotomous variable, there remained a significant association between LV mass and cardiac events (p<0.03). Analyses were performed to determine whether different indices of LV mass were predictive of cardiac events after accounting for the results of dobutamine stress. As shown in Table 3, all measures of LVH were predictive of MI or cardiac death.
Table 3.
The relationship of different indices of LV mass to MI and cardiac death
| Model | Unadjusted | |
|---|---|---|
| HR (95% CI) | p-value | |
| LV mass (g) | 1.005 (1.00-1.01) | 0.03 |
| LV mass /BSA (g/m2) | 1.019 (1.019-1.03) | <0.001 |
| LV mass/height (g/m) | 3.17 (1.40-7.19) | 0.005 |
| LV mass /height ≥ or < 1 (g/m) | 2.23 (1.30-3.82) | 0.003 |
Abbreviations: BSA = Body surface area; g = grams; m = meters
Discussion
The major finding of this study relates to the importance of LVH in individuals with chest pain that are referred for dobutamine stress tests. The results of this study indicate that LVH is an independent predictor of cardiovascular events in patients with chest pain referred for dobutamine stress testing. This is true regardless of whether LV mass is indexed to BSA or height. Importantly, in those without inducible LV wall motion abnormalities indicative of ischemia during dobutamine stress, the presence of LVH portends a poor cardiac prognosis. This finding is important given that in the absence of inducible LV wall motion abnormalities indicative of ischemia, often termed a “negative” stress test result, these negative dobutamine stress results are frequently used to ascribe a low risk of future MI or cardiac death. The results of this study indicate clearly that individuals with chest pain and LVH that have no inducible LV wall motion abnormalities during dobutamine, continue to be at risk of MI and cardiac death.
The presence of LVH has been shown to reduce the sensitivity of LV wall motion analyses assessed during dobutamine stress for identifying inducible ischemia due to >50% coronary arterial luminal narrowings.10 Recently, our group identified that increased LV end-diastolic wall thickness was associated with MI and cardiac death in individuals with a resting LVEF >55% and no inducible LV wall motion abnormalities indicative of ischemia after intravenous dobutamine.11 Participants in the current study included those with chest pain referred for dobutamine stress to identify LV wall motion abnormalities indicative of inducible ischemia. All variables known to be associated with a risk of cardiovascular events recorded in our dataset were included in our models to predict MI or cardiac death. Inclusion of all variables was performed in order to avoid bias by simply selecting variables that may have reached statistical significance. In this study, we recognize there is insufficient power to detect hazard ratios of 1.0 – 2.0 within subgroups of individuals with LVH that exhibited a significant hazard ratio for cardiac events within our multivariate model. Importantly, however, we were able to determine that LVH is an independent predictor of adverse cardiovascular events within our multivariable model that includes known predictors for CV events.
Results of this study indicate that LVH should be reported in individuals referred for dobutamine wall motion stress testing. As shown in Figure 3, the adverse prognosis associated with LVH in individuals without inducible ischemia was similar to those without LVH and inducible ischemia. Of note, the poor prognosis of those with LVH, but without ischemia, is also similar to populations with ischemia reported in other MRI stress studies.12 The results of this study raise important concerns for individuals that develop hyperdynamic wall motion responses during dobutamine echocardiography or CMR, but concomitantly exhibit LVH. Data from this study indicate that these individuals, while exhibiting no apparent inducible wall motion abnormalities to suggest an adverse prognosis, do indeed continue to have an adverse prognosis related to the presence of LVH.
Since image data for participants in this study were acquired primarily before the year 2000, newer, more rapid acquisitions of myocardial perfusion or delayed enhancement were not employed during the stress CMR protocol. For this reason, we were unable to determine if myocardial fibrosis, or dobutamine induced perfusion defects were present in our study participants. As demonstrated by Kwong et al,13 the presence of delayed enhancement in patients with chest pain referred for dobutamine stress may serve as a marker of adverse cardiac prognosis. Perhaps other imaging or bio-markers could be used to identify those at risk for future cardiac events in patients with chest pain and LVH that are currently referred for dobutamine wall motion stress tests.
In this study, we performed analyses stratifying our measures of LV mass based on gender. Several studies have performed similar analyses due to discrepancies in both heart and body size in men versus women.2,14-15 In those studies, women with LVH were found to have a higher risk of adverse outcomes compared with men.14 In our study, those with either gender and LVH exhibited an increase in adverse cardiac outcomes. Differences between our results and prior studies may be due to the fact we enrolled few black women, and many of the men had known CAD. Both of these conditions elevate the risk of cardiac events. The results of this study and others15 indicate that women with LVH and chest pain referred for dobutamine stress exhibit an elevated risk of cardiac events in the presence or absence of dobutamine induced LV wall motion abnormalities indicative of ischemia.
Several criteria have been used previously to define LVH. We found that higher LV mass indexed to body surface area predicted a 2.1 fold risk of subsequent CV events. In addition to expressing LVH as a dichotomous variable, we performed analyses using LVH as a continuous variable. The relationship between LV mass and increased cardiovascular risk was continuous, even down to values below the conventional cut-off for echocardiographically detected LVH (nominally at least 125 g/m2 in men and at least 110 g/m2 in women).16 Previously, Schillaci, et al., have shown a powerful relationship between LV mass and risk of cardiovascular events over a wide range of LV mass values, even below the current “upper normal” limits.16
We found that LVMI exhibited similar prognostic importance, whether indexed to BSA or height. We also assessed LV mass to height ratio of ≥ or < 1 g/cm and found a positive association for those with a LVMI ≥ 1 and future cardiovascular events (Table 3). This relatively easily remembered metric could be useful in further clinical studies.
Study limitations
We recognize the following limitations to our study: first, the majority of our patients were Caucasian. Reliable evaluation of the relationship of ethnicity in relationship to LV mass and cardiovascular events during dobutamine stress will require additional study. Second, research has demonstrated reversal of LVH in patients receiving various antihypertensive medications. Unfortunately, the role of medications during the time between DCMR and participants’ events could not be addressed effectively in our study because information about medication use was limited to that at the time of the DCMR exam. Third, spoiled gradient-echo sequences were used at the time of data collection with DCMR. We are uncertain how results obtained with steady state free precession (SSFP) techniques would compare to those in this study. Fourth, studies were not performed using multislice determinations of LV volume or mass, or with perfusion or late gadolinium enhancement techniques. Further studies using these methodologies may offer additional prognostic information in subjects with suspected LVH. Fifth, the studies main goal was to estimate the association between hypertrophy and MI or cardiac death. It was not designed with optimal power to detect potentially important HRs when participants were further divided into subgroups.
Clinical implications and Conclusion
Patients with chest pain syndromes and LVH that may or may not exhibit inducible wall motion abnormalities indicative of ischemia during dobutamine stress are at high risk for future myocardial infarction or cardiac death. LVH should be reported in patients referred for dobutamine stress testing, and one should not assume a low future risk of MI and cardiac death in patients with LVH that do not exhibit inducible LV wall motion abnormalities indicative of ischemia during intravenous dobutamine stress.
Inducible left ventricular wall motion abnormalities (due to flow-limiting epicardial coronary artery disease [CAD]) observed during dobutamine stress testing are used to identify individuals with myocardial ischemia and those at risk for future cardiovascular events. Previously, it has been shown that patients with concentric left ventricular remodeling or hypertrophy that may have flow-limiting CAD do not exhibit inducible left ventricular wall motion abnormalities during dobutamine stress. In this study, we examined the prognostic utility of the absence of dobutamine-induced wall motion abnormalities for predicting future myocardial infarction or cardiac death. The results indicate that those individuals with left ventricular hypertrophy (LVH) and no inducible wall motion abnormalities during dobutamine exhibit the same adverse cardiovascular prognosis as individuals with inducible wall motion abnormalities indicative of ischemia that do not have LVH. These findings suggest that one should identify LVH during dobutamine stress studies and not assume a favorable cardiovascular risk profile in the absence of inducible wall motion abnormalities if LVH is present. In this situation, one should consider other forms of testing to ascertain cardiovascular risk.
Acknowledgments
Sources of Funding Research supported in part by: National Institutes of Health grants R01HL076438, MOI-RR07122S, and P30AG21332.
Footnotes
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hundley WG, Morgan TM, Neagle CM, Hamilton CA, Rerkpattanapipat P, Link KM. Magnetic resonance imaging determination of cardiac prognosis. Circulation. 2002;106:2328–33. doi: 10.1161/01.cir.0000036017.46437.02. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Gordon T, Castelli WP, Margolis JR. Electrocardiographic left ventricular hypertrophy and risk of coronary heart disease. The Framingham study. Ann Intern Med. 1970;72:813–22. doi: 10.7326/0003-4819-72-6-813. [DOI] [PubMed] [Google Scholar]
- 3.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 4.Chen C, Li L, Chen LL, Prada JV, Chen MH, Fallon JT, Weyman AE, Waters D, Gillam L. Incremental doses of dobutamine induce a biphasic response in dysfunctional left ventricular regions subtending coronary stenoses. Circulation. 1995;92:756–66. doi: 10.1161/01.cir.92.4.756. [DOI] [PubMed] [Google Scholar]
- 5.Lawson MA, Blackwell GG, Davis ND, Roney M, Dell’Italia LJ, Pohost GM. Accuracy of biplane long-axis left ventricular volume determined by cine magnetic resonance imaging in patients with regional and global dysfunction. Am J Cardiol. 1996;77:1098–104. doi: 10.1016/s0002-9149(96)00140-3. [DOI] [PubMed] [Google Scholar]
- 6.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiog. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 7.Papavassiliu T, Kuhl HP, van Dockum W, Hofman MB, Bondarenko O, Beek IA, van Rossum AC. Accuracy of one- and two-dimensional algorithms with optimal image plane position for the estimation of left ventricular mass: A comparative study using magnetic resonance imaging. J Cardiovasc Magn Reson. 2004;6:845–54. doi: 10.1081/jcmr-200036157. [DOI] [PubMed] [Google Scholar]
- 8.Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging. 2003;17:323–9. doi: 10.1002/jmri.10262. [DOI] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–95. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Smart SC, Knickelbine T, Malik F, Sagar KB. Dobutamine-atropine stress echocardiography for the detection of coronary artery disease in patients with left ventricular hypertrophy. Importance of chamber size and systolic wall stress. Circulation. 2000;101:258–63. doi: 10.1161/01.cir.101.3.258. [DOI] [PubMed] [Google Scholar]
- 11.Walsh TF, Dall’Armellina E, Chughtai H, Morgan TM, Ntim W, Link KM, Hamilton CA, Kitzman DW, Hundley WG. Adverse effect of increased left ventricular wall thickness on five year outcomes of patients with negative dobutamine stress. J Cardiovasc Magn Reson. 2009;11:25–32. doi: 10.1186/1532-429X-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jahnke C, Nagel E, Gebker R, Kokocinski T, Kelle S, Manka R, Fleck E, Paetsch I. Prognostic value of cardiac magnetic resonance stress tests: Adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation. 2007;115:1769–76. doi: 10.1161/CIRCULATIONAHA.106.652016. [DOI] [PubMed] [Google Scholar]
- 13.Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, Davis RB. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–43. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 14.Liao Y, Cooper RS, Mensah GA, McGee DL. Left ventricular hypertrophy has a greater impact on survival in women than in men. Circulation. 1995;92:805–10. doi: 10.1161/01.cir.92.4.805. [DOI] [PubMed] [Google Scholar]
- 15.East MA, Jollis JG, Nelson CL, Marks D, Peterson ED. The influence of left ventricular hypertrophy on survival in patients with coronary artery disease: Do race and gender matter? J Am Coll Cardiol. 2003;41:949–54. doi: 10.1016/s0735-1097(02)03006-1. [DOI] [PubMed] [Google Scholar]
- 16.Schillaci G, Verdecchia P, Porcellati C, Cuccurullo O, Cosco C, Perticone F. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension. 2000;35:580–6. doi: 10.1161/01.hyp.35.2.580. [DOI] [PubMed] [Google Scholar]