Abstract
Sphingosine 1-phosphate (S1P)-metabolizing enzymes regulate the level of bioactive sphingolipids that have curative potential. Recently, S1P-metabolizing enzymes such as sphingosine kinase 1 and S1P lyase were shown to regulate influenza virus replication and the virus-induced cytopathogenicity. The mechanism appeared to employ a JAK/STAT type I interferon signaling pathway that induces anti-viral status. Further, sphingosine analogs altered cytokine responses upon influenza virus infection. This article focuses on recent discoveries about the sphingolipid system that influences on host protection from viral virulence and the involvement of cytokine signaling in its underlying mechanisms. Deciphering the steps of this pathway could help us envision how the modulation of sphingolipid metabolism can be applied as a therapeutic approach to overcome infectious diseases.
Keywords: sphingosine kinase, S1P lyase, influenza virus, type I IFN, TNF
1. Introduction
Sphingolipids are lipid mediators characterized by the presence of a serine headgroup with fatty acid tails (1, 2). The sphingolipids regulate multiple cellular functions including cell growth, survival, differentiation and migration. One of these sphingolipids, sphingosine 1-phosphate (S1P), is generated from sphingosine inside cells and triggers intracellular signaling and/or is secreted to an extracellular area, where it can bind to five G protein-coupled S1P receptors (S1P1 to S1P5) (2, 3). S1P1 is known to play important roles in angiogenesis and vascular maturation (4), in addition to the development and mobilization of lymphocytes (5). Also, S1P1 and S1P3 are up-regulated in maturing dendritic cells (DCs), which results in the increased migration of mature DCs to S1P (6). Recently, a synthetic sphingosine analog, FTY720, the phosphorylated form of which is a ligand for S1P1, S1P3, S1P4 and S1P5, has emerged as a candidate drug to treat patients with multiple sclerosis via oral administration and is currently being tested in phase III clinical trials (7).
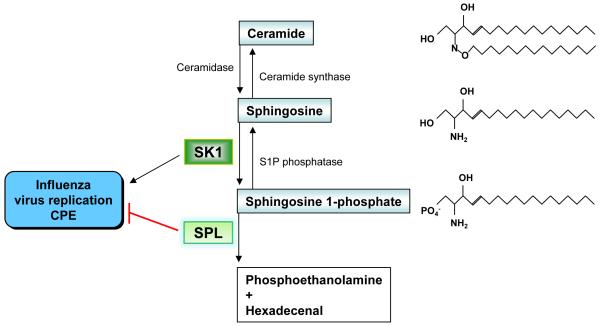
The physiologic level of S1P is tightly regulated by S1P-metabolizing enzymes. S1P is generated from sphingosine and ATP through the enzymatic activity of sphingosine kinase (SK) and is degraded by S1P lyase (SPL) (Fig. 1) (8). These enzymes are known to modulate responses to diverse cellular stresses induced by anti-cancer drugs, DNA damage or serum deprivation (9-14). For instance, cells overexpressing SK1 are resistant to anti-cancer drugs such as cisplatin, carboplatin, and doxorubicin (10), whereas SPL overexpression in cells greatly increases their sensitivity to drug-induced cell death (11). Therefore, S1P-metabolizing enzymes are potential therapeutic targets to improve the efficacy of conventional chemotherapeutic drugs.
Figure 1. Regulation of influenza virus propagation by S1P-metabolizing enzymes.
Sphingolipids with chemical structure and their metabolizing enzymes are shown. The impact of S1P-metabolizing enzymes on influenza virus replication and CPE is depicted.
Recently, these S1P-metabolizing enzymes were shown to modulate influenza virus replication and virus-induced cytopathic effects (CPE) (15). Pharmacological inhibition of SK1 or overexpression of SPL blocked influenza virus propagation and infection-induced cytopathogenicity (Fig. 1). Intriguingly, the rapid activation of Janus kinase (JAK)/signal transducer and activator of a transcription (STAT) type I interferon (IFN) signaling proved to be a mechanism of SPL-mediated cellular protection from influenza (15). Further, a sphingosine analog, AAL-R, reduced excessive cytokine responses and lessened immune pathologic tissue injury induced by influenza virus infection (16, 17). These findings indicate that the sphingolipid system modulates host defensive signaling and the immune response to virus infections. Here, we discuss these recent findings and, in particular, possible molecular mechanisms that connect S1P-metabolizing enzymes to cytokine signaling pathways of type I IFN and tumor necrosis factor (TNF).
2. Control of influenza virus replication
Overexpression of SPL, which degrades S1P, rendered HEK293 cells extremely resistant to influenza virus infection and virus-induced cellular apoptosis (Fig. 1) (15). Specific siRNA targeting SPL reversed that increased resistance to the infection confirming SPL’s protective function for the host. In contrast, SK1 overexpression increased cellular sensitivity to influenza virus infection (Fig. 1). The importance of this kinase activity of SK1 in the increased susceptibility to infection was demonstrated by using a specific inhibitor, N,N,-dimethylsphingosine (DMS), to block SK activity. Indeed, DMS potently blocked the expression of influenza viral nucleoprotein (NP) not only in SK1-overexpressing cells (15) but also in HEK293 and MDCK cells (data not shown). Therefore, the generation of S1P from sphingosine appears to stimulate influenza virus replication. However, exogenously supplied S1P failed to enhance virus replication. This suggests that intracellular S1P signaling (3), but not extracellular S1P receptor signaling (2), is crucial for the inhibitory pathway. Alternatively, S1P-meatbolizing enzyme regulating the balance of sphingolipids rather than the absolute levels of sphingolipids could be a better target for the control of virus replication. Modulation of S1P-metabolizing enzymes could also affect the quantities of diverse sphingolipids and their intermediate metabolites. For instance, the inhibition of SK activity by DMS treatment subsequently increased the level of ceramide (18), and a ceramide analog (d-threo-1-phenyl-2-decanoylamin-3-morpholino-propanol, D-PDMP) reduced glycosphingolipid content but enhanced DMS synthesis (19). These reports also raise the possibility that ceramide synthase or ceramide is involved in anti-viral activities against influenza virus replication, which remains to be explored. The consequence of modulation by S1P-metabolizing enzymes on influenza virus infection could be the concerted effect of an altered balance of sphingolipids in the cells.
In cancer biology studies, SPL was initially identified as a protein that mediates resistance to anti-cancer drugs such as cisplatin, and SPL overexpression was shown to increase cellular sensitivity to cisplatin-caused cellular death (10). A similarly increased susceptibility to cell death by SPL overexpression was detected when cells were stressed by serum deprivation or DNA damage (12-14). In contrast, SK1 enhanced cellular resistance to the anti-cancer drug-induced death signaling, showing that the two enzyme activities worked in opposition (10). These previous observations did not correlate well with the cells’ sensitivity to influenza virus-caused apoptosis. The disparity may be attributable to distinctly different intracellular mechanisms. Although signals from p38 mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) were critical for SPL-mediated anti-cancer drug-sensitivity (11), the JAK/STAT type I IFN signal and extracellular signal-regulated kinase (ERK) activation, but not p38 MAPK nor JNK signal pathways, were important for SPL’s defensive mechanism that protected infected cells from influenza virus (15). Additionally, influenza virus infection is thought to change cellular status swiftly and dramatically via dynamic interaction between viral components and cellular proteins. Perhaps, SPL overexpression or SK1 inhibition disturbs these virus-induced modifications of cellular molecules and thus affects virus replication. Since SPL expression/SK inhibition increased cell death caused by some anti-cancer drugs and also inhibited virus replication and viral CPE, molecules that inhibit SK activity or activate SPL could be developed into biomedical drugs that benefits patients suffering from cancers or influenza.
3. Linkage of type I IFN signaling to the sphingolipid system during virus infection
3.1. JAK/STAT signaling
Type I IFN is a member of the cytokine family that includes IFN-α, IFN-β, IFN -ε, IFN-κ and IFN-ω, and displays potent anti-viral and immunomodulatory activities (20-22). Following virus infection, type I IFN is produced by various cell types including epithelial cells, macrophages, and DCs. Plasmacytoid dendritic cells (PDCs) have been demonstrated as the most potent producers of type I IFN, since they synthesize up to 10 pg of type I IFN per cell (23, 24). However, the main cell type responsible for synthesizing this cytokine following infections seems to be dependent on the nature of the pathogen (25-28). Type I IFN receptor (IFNAR)-deficient mice were highly susceptible to infections with vesicular stomatitis virus (VSV), Semliki Forest virus (20), and the A/WSN/33 strain of influenza virus (29), indicating that a type I IFN system is required for effective host protection from numerous virus-induced diseases. The engagement of IFNAR mainly triggers the phosphorylation of STAT1/STAT2 through the activation of the JAK1 and Tyk2 (30-32). The phosphorylated STAT1 and STAT2 form an IFN-stimulated gene factor 3 (ISGF3) with IRF9, and ISGF3 is translocated into the nucleus to activate the transcription of IFN-stimulated genes (ISGs) and induce anti-viral status (33, 34). The activation of JAK/STAT is an important criterion of the type I IFN-mediated protective host action against pathogenic viral infections. Thus, the early activation of JAK/STAT observed in SPL-overexpressing cells infected with influenza virus could be one of the main mechanisms mediating these cells’ defense against virus infections.
Besides impacting JAK/STAT, SPL triggered the rapid activation of ERK upon influenza virus infection of the cells. Importantly, ERK2 has been associated with IFNAR or STAT1 after type I IFN treatment, thereby regulating ISG expression (35). Therefore, SPL-mediated, early ERK activation following virus infection also could contribute to potentiating type I IFN signaling and to reducing virus replication.
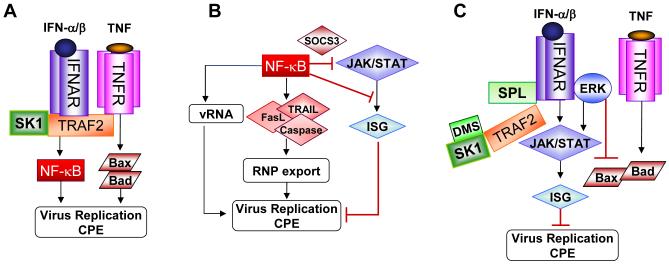
Interestingly, SK1 as well as intracellular S1P were demonstrated to interact with TNF receptor-associated factor 2 (TRAF2) to activate NF-κB signaling (Fig. 2A) (36, 37). Further, TRAF2 was reported to bind directly to IFNAR1 subunit of the receptor (38). Thus, a complex composed of SK1-TRAF2-IFNAR could form in SK1-overexpressing cells and alter anti-viral type I IFN signaling by activating the NF-κB signal pathway. Although NF-κB signaling is known to exhibit anti-viral function, influenza virus seems to utilize the signaling for its own replication and cytopathogenicity. This pro-viral mechanism of NF-κB signaling involves the synthesis of a suppressor of cytokine signaling 3 (SOCS3) to block STAT activation (39) and the direct inhibition of a promoter activity for the expression of ISGs (Fig. 2B) (40). The overexpression of SPL may hinder SK activity and decrease the level of intracellular S1P. This would unleash JAK/STAT type I IFN signaling by interfering with the physical complex formation of SK1-TRAF2-IFNAR or by disrupting the SK1 (or S1P)-TRAF2-mediated activation of its downstream NF-κB signal pathway (Fig. 2C). It is also conceivable that SPL activation increases ERK2’s association with IFNAR to further elevate STAT signaling (Fig. 2C). Along the same lines, the direct association of SPL with any of these signaling components, if proven, could provide a clue for a cause-effect relationship leading to the activation of type I IFN signaling.
Figure 2. Schematic models for S1P-metabolizing enzyme-mediated regulation of cytokine signaling to control virus replication.
(A) In SK1-overexpressing cells, SK1-TRAF2 may form a multi-complex with IFNAR to trigger NF-κB signaling and block type I IFN signaling, which results in the enhancement of virus replication. TNF could also increase apoptosis leading to enhancement of virus replication or CPE. (B) The action mode of possible pro-viral NF-κB signaling is shown. (C) In SPL-overexpressing cells or DMS-treated cells, the SK1-TRAF2 complex may be destabilized leading to the blockade of pro-viral NF-κB signaling; in addition, this action could allow the activation of type I IFN signaling, which would interfere with virus replication. ERK may increase type I IFN signal activation and/or impair TNF-induced Bax/Bad activation.
3.2. Type I IFN synthesis
Following virus infections, cellular recognition receptors such as toll-like receptors (TLRs) and retinoic acid-inducible gene I (RIG-I) like receptors detect viral components and intermediate replication products then rapidly induce type I IFN, one of the host’s most potent innate immune molecules (41, 42). Early activation of STAT proteins in SPL-overexpressing cells infected with influenza virus infection raises the possibility that SPL elevates the synthesis of type I IFN from influenza virus-infected cells. However, the mRNA level of IFN-α and IFN-β did not significantly increase in SPL-cells compared to that in control HEK cells (15). Despite the unchanged amount of type I IFN, the differential activation of JAK/STAT signaling, as shown, can result in different outcomes. Further, the ability of S1P-metabolizing enzymes to regulate type I IFN content could be dependent on cell type. Interestingly, locally administered sphingosine analog AAL-R suppressed the synthesis of type I IFN in the lungs of influenza virus-infected mice (data not shown). Therefore, another sphingolipid analog or a regulator of a sphingolipid-metabolizing enzyme might also enhance the synthesis of type I IFN following influenza virus infection. Viruses often block the production or signaling of type I IFN to evade host innate immune responses (43-47). If modulation of sphingolipid metabolism counteracts this viral evasive strategy, the result could protect infected hosts from the virulent aftermath. However, direct treatment with type I IFN or type I IFN inducers that act on most cells often causes harmful inflammatory responses (21, 48). This unwanted pathogenic type I IFN response could arise from signaling activation in uninfected cells. Therefore, the local induction of type I IFN or its signaling activation solely in influenza virus-infected cells mediated by sphingolipid-metabolizing enzymes could not only interfere with virus replication and spread but even avoid the detrimental inflammatory response.
3.3. Lymphocyte trafficking and activation
Although host innate immunity limits virus replication, pathogenic viruses frequently overcome this first line of defense requiring activation of the host’s adaptive immunity. Following virus infection, lymphocytes move to secondary lymphoid organs close to the infection site leading to transient lymphopenia in the bloodstream and the accumulation of lymphocytes in the secondary lymphoid organs, where virus-specific adaptive immunity develops. Accumulation of T cells, B cells, and PDCs in the lymph nodes and transient lymphopenia in the blood rely on type I IFN signaling (49, 50). Further, type I IFN signaling is important for S1P1-mediated lymphocyte egress from lymph nodes (51). Naïve T cell recirculation is well known to be dependent on S1P receptor signaling presumably via S1P1 signaling on T cells and/or the tightening of endothelial cell junctions (2, 5). Systemic administration of FTY720 via oral or intravenous injection into mice following lymphocytic choriomeningitis virus (LCMV) infection induced sequestration of virus-reactive T cells in secondary lymphoid organs (52, 53). However, this did not alter T cell activation, expansion, or memory. In contrast, sphingosine analog, AAL-R, locally instilled via intratracheal inoculation impaired DC capability of stimulating T cells following influenza virus infection (16, 17). This locally delivered AAL-R also induced transient lymphopenia in the bloodstream. Given that S1P-metabolizing enzymes were shown to regulate type I IFN signaling, it is likely that regulation of S1P metabolism and S1P signaling are involved in type I IFN-induced lymphocyte re-distribution and DC modulation upon virus infection.
Although type I IFN is well-established as a cytokine involved in host innate immunity, it could act directly on virus-specific T cells and B cells to regulate their activation and differentiation (21, 54-59). For instance, LCMV-specific CD8+ T cells lacking IFNAR that were adoptively transferred into wild type mice failed to expand and express granzyme B which is one of the effector molecules that destroys virus-infected cells (55, 60). Thus, if the sphingolipid system regulates type I IFN synthesis and/or signaling, its subsequent impact on host adaptive T cell and B cell immunity should be evaluated.
4. TNF signaling
TNF is a cytokine mediator of such cellular responses as apoptotic cell death, inflammation and inhibition of both tumorigenesis and virus replication (61). Its signaling is triggered through TNF receptor-associated proteins including TNFR1-associated death domain (TRADD), TRAF2 and receptor interacting protein (RIP) 1 (62, 63). TRADD in alliance with the intracellular domain of TNFR can trigger the modulation of several signal pathways including the activation of NF-κB and MAPK. As briefly described in section 3.1., SK1 and intracellular S1P, but not extracellular S1P, interact with TRAF2 to activate NF-κB signaling but not JNK signaling (36, 37). The result indicates that intracellular S1P signaling, but not S1P receptor signaling, is involved. The binding of TRAF2 to SK1 results in the enzymatic activation of SK1, which is thought to mediate activation of the NF-κB signal cascade (Fig. 2A). Previously several studies have suggested that the activation of the NF-κB pathway is involved in anti-viral activity by regulating the expression of numerous cytokines including type I IFN (64, 65). In contrast, other investigators reported that influenza virus utilizes the NF-κB signal to promote virus propagation (66, 67). Other than the inhibitory path via NF- κB blocking STAT signaling described here in section 3.1., several other possibilities were demonstrated (Fig. 2B): 1) NF-κB-dependent, TNF-related, apoptosis-inducing ligand (TRAIL) and Fas/FasL were cited as important proponents of viral CPE and efficient influenza virus propagation, because they enhance nuclear export of the viral ribonucleoprotein (RNP) complex (67). 2) NF-κB signal positively regulated viral RNA synthesis, but not complementary RNA generation, at an early post-entry step of virus infection (68). Thus, SK1 might enhance activation of the TNF signaling pathway via TRAF2-NF-κB regulation during influenza virus replication, elevating the latter process and inciting CPE.
TNF signaling increases apoptosis under certain conditions by activating the proapoptotic molecules Bax and Bad. Recently, Bax was identified as an important factor for virus-induced apoptosis and for efficient virus replication as well (69). SPL-overexpression greatly reduced Bax production following influenza virus infection, which raises the question whether the suppression of Bax was due to the inhibition of influenza virus replication that causes cellular apoptosis (SPL → virus inhibition → Bax inhibition) or SPL-mediated direct inhibition of Bax resulted in the inefficient virus replication (SPL → Bax inhibition → virus inhibition). Importantly, Bax translocation into mitochondria was inhibited by ERK (70), which was rapidly activated in virus-infected SPL-overexpressing cells. Thus, TNF-Bax activation could be inhibited by the SPL-ERK pathway (Fig. 2C). However, the involvement of ERK in regulation of influenza virus replication seems to be complicated, since ERK activation is also pivotal for virus replication. Possibly, a certain level of ERK activation is required for viral RNP export and continuing replication as reported (71), whereas the temporal regulation and further activation of ERK could be manipulated by S1P-metabolism to control influenza virus replication (15). In fact, influenza virus may well have evolved to use host cell signaling of ERK and TNF-induced NF-κB for its replication. However, the modulation of S1P-metabolizing enzymes could interfere with these viral strategies.
TNF is also produced by virus-specific T cells to eliminate cells infected with that virus (61). Although pathogen-specific T cell activation and infiltration into the target site are crucial for the resolution of most virus infections, excessive T cell activation frequently induces tissue destruction. TNF is often categorized as a cytokine that contributes to lymphocyte-mediated viral immunopathology (72, 73). However, local delivery of the sphingosine analog AAL-R, but not AAL-S (a non-phosphorylatable form of AAL-R), suppressed cytokine responses including TNF-α production following influenza virus infection in vivo (17). This suggests that modulation of S1P receptor signaling could regulate the cytokine synthesis and function upon virus infection. Still unknown is whether sphingolipid signaling acts directly on T cells to regulate TNF synthesis affecting effector function of activated T cells following virus infections.
5. Regulation of other cytokines
Complementing its effect on type I IFN and TNF, sphingolipid metabolism is known to regulate other cytokine responses. Inhibition of SK1 expression or activity blocked the production of several pro-inflammatory cytokines (74, 75), whereas inhibition of SK2 expression up-regulated the level of those cytokines in a mouse arthritis model (76). Also, an inhibitor of SK suppressed the production of IL-12 from DCs and IFN-γ from T cells, and down-regulated the expression of co-stimulatory molecules on DCs upon LPS stimulation (77). Thus, drugs targeting SK activity could be used for the treatment of inflammatory diseases. However, the inhibition of SK expression also enhanced the synthesis of Th1 type cytokines by CD4+ T cells (78), suggesting that the effect of modulating sphingolipid metabolism is influenced by the cell types and cellular conditions involved. Yet, the role of sphingolipid-metabolizing enzymes in the activities of these cytokines in terms of overall immunity to virus infections has not been studied.
S1P receptor signaling has been shown to regulate the level of cytokines released from cells of the immune system such as T cells and DCs. The engagement of S1P1 up-regulated c-Maf, Jun B, and Gata3 in T cells, resulting in the enhancement of IL-4 production and subsequent development of Th2 cells in transgenic mice overexpressing S1P1 on T cells (79). The activation of S1P4 inhibited the production of IL-4, IL-2 and IFN-γ from CD4+ T cells but increased secretion of the immunosuppressive cytokine IL-10 (80). Moreover, S1P reduced the production of IL-12 and TNF-α yet increased the level of IL-10 from DCs (81). These studies demonstrate an immunosuppressive action of S1P. Therefore, the therapeutic potential of sphingosine analogs that activate S1P receptors has been tested for ameliorating diverse autoimmune disorders and offsetting the adverse effects of transplantation (82). The production of IL-10 from effector T cells also played a key role in the control of influenza virus infection-induced inflammation (83). Thus, S1P signal-mediated IL-10 production from T cells or DCs could be applicable to alleviating virus-induced tissue injury involving a harmful immune response.
6. Perspectives
Our understanding of sphingolipid signaling pathways has rapidly progressed including the use of metabolite analogs and regulators of its metabolic enzymatic reactions. The finding that inhibition of SK1 or overexpression of SPL triggers host defensive cytokine signaling against influenza could provide new approaches to therapy for pathogenic infections. However, the consequences of regulating the sphingolipid system may depend on diverse factors including the nature of each infecting virus and the cell type. For translational purposes, each of these factors must be considered. For instance, the inhibitor DMS blocked SK1 activity and strongly inhibited influenza virus replication (15)(data not shown), but in other circumstances, the inhibition of SK1 activity enhanced the replication of bovine viral diarrhea virus (84). Defining the underlying molecular mechanisms should clarify the diverse cause-effect relationships under specific pathogenic conditions. The recognition of a linkage between sphingolipid biology and host defensive signaling against virus infection has raised numerous exciting questions with respect to molecular signaling pathways, the effect on host innate and adaptive immunity, and its translational potential. Extensive research activities on mechanistic details, in conjunction with the development of novel small molecules targeting sphingolipid metabolism, could provide a basis for designing new therapeutic approaches, and help us uncover novel cellular signaling pathways to better understand virus-host interactions. Additionally, the research should be of importance to medical scientists/clinicians who intend to use sphingolipid system-based therapy for patients with multiple sclerosis or cancer, since these patients are so vulnerable to a range of pathogens during the treatment.
Acknowledgement
We thank the editor for invitation of this review. This work was supported by NIH/NIAID grant AI088363 (to B.H.), a Fellow/Mentor Research grant from the Department of Surgery (to Y.-J.S. and B.H.), and the MU Research Board (to B.H.).
Vitae

Young-Jin Seo is a postdoctoral fellow at the School of Medicine, University of Missouri-Columbia. Dr. Seo completed a Ph. D. program at the Seoul National University, South Korea. During his doctoral work, he focused on the identifying the functions of CD4+ T cell-derived cytokines in activating B cell maturation and the role of regulatory T cells and cytokines in autoimmune arthritis based on the mouse model. Further, he conducted research on cellular signaling pathways in survival/apoptosis and migration of human leukemia cells under hypoxia. Since he joined Dr. Bumsuk Hahm’s laboratory in May 2008, he has specialized in the field of virology and immunology by investigating the role of sphingolipids and their metabolizing enzymes in host immunity and the defense against virus infections.

Stephen Alexander, Ph.D. is professor of Biological Sciences at the University of Missouri. He is interested in the cell biological roles of sphingolipids in normal physiology and development, and pathology. In particular his lab has investigated the function of sphingolipids in regulating the sensitivity of cells to chemotherapeutic drugs with the aim of making these drugs more effective in the treatment of cancer.

Bumsuk Hahm, Ph.D. performed research in the field of molecular virology by studying hepatitis C virus replication and the IRES-dependent translation mechanism during his training in master and doctoral degree programs at the Pohang University of Science and Technology in Korea. He then expanded his research scope to the field of viral immunobiology as a postdoctoral fellow of Dr. Michael Oldstone and a staff scientist at The Scripps Research Institute. Since Dr. Hahm’s move to the University of Missouri-Columbia as an assistant professor, his research has centered on the interplay between RNA viruses and host defensive signals of the immune system for the purpose of developing immunotherapeutic approaches.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors do not have any conflict of interest.
References
- 1.Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–59. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- 2.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–70. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 3.Taha TA, Hannun YA, Obeid LM. Sphingosine kinase: biochemical and cellular regulation and role in disease. J Biochem Mol Biol. 2006;39:113–31. doi: 10.5483/bmbrep.2006.39.2.113. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–61. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–60. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 6.Czeloth N, Bernhardt G, Hofmann F, Genth H, Forster R. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J Immunol. 2005;175:2960–7. doi: 10.4049/jimmunol.175.5.2960. [DOI] [PubMed] [Google Scholar]
- 7.Horga A, Montalban X. FTY720 (fingolimod) for relapsing multiple sclerosis. Expert Rev Neurother. 2008;8:699–714. doi: 10.1586/14737175.8.5.699. [DOI] [PubMed] [Google Scholar]
- 8.Spiegel S, Kolesnick R. Sphingosine 1-phosphate as a therapeutic agent. Leukemia. 2002;16:1596–602. doi: 10.1038/sj.leu.2402611. [DOI] [PubMed] [Google Scholar]
- 9.Alexander S, Min J, Alexander H. Dictyostelium discoideum to human cells: pharmacogenetic studies demonstrate a role for sphingolipids in chemoresistance. Biochim Biophys Acta. 2006;1760:301–9. doi: 10.1016/j.bbagen.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Min J, Mesika A, Sivaguru M, Van Veldhoven PP, Alexander H, Futerman AH, et al. (Dihydro)ceramide synthase 1 regulated sensitivity to cisplatin is associated with the activation of p38 mitogen-activated protein kinase and is abrogated by sphingosine kinase 1. Mol Cancer Res. 2007;5:801–12. doi: 10.1158/1541-7786.MCR-07-0100. [DOI] [PubMed] [Google Scholar]
- 11.Min J, Van Veldhoven PP, Zhang L, Hanigan MH, Alexander H, Alexander S. Sphingosine-1-phosphate lyase regulates sensitivity of human cells to select chemotherapy drugs in a p38-dependent manner. Mol Cancer Res. 2005;3:287–96. doi: 10.1158/1541-7786.MCR-04-0197. [DOI] [PubMed] [Google Scholar]
- 12.Oskouian B, Sooriyakumaran P, Borowsky AD, Crans A, Dillard-Telm L, Tam YY, et al. Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is down-regulated in colon cancer. Proc Natl Acad Sci U S A. 2006;103:17384–9. doi: 10.1073/pnas.0600050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reiss U, Oskouian B, Zhou J, Gupta V, Sooriyakumaran P, Kelly S, et al. Sphingosine-phosphate lyase enhances stress-induced ceramide generation and apoptosis. J Biol Chem. 2004;279:1281–90. doi: 10.1074/jbc.M309646200. [DOI] [PubMed] [Google Scholar]
- 14.Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, Poulton S, et al. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999;147:545–58. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seo YJ, Blake C, Alexander S, Hahm B. Sphingosine 1-phosphate-metabolizing enzymes control influenza virus propagation and viral cytopathogenicity. J Virol. 2010;84:8124–31. doi: 10.1128/JVI.00510-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsolais D, Hahm B, Edelmann KH, Walsh KB, Guerrero M, Hatta Y, et al. Local not systemic modulation of dendritic cell S1P receptors in lung blunts virus-specific immune responses to influenza. Mol Pharmacol. 2008;74:896–903. doi: 10.1124/mol.108.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsolais D, Hahm B, Walsh KB, Edelmann KH, McGavern D, Hatta Y, et al. A critical role for the sphingosine analog AAL-R in dampening the cytokine response during influenza virus infection. Proc Natl Acad Sci U S A. 2009;106:1560–5. doi: 10.1073/pnas.0812689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edsall LC, Van Brocklyn JR, Cuvillier O, Kleuser B, Spiegel S. N,N-Dimethylsphingosine is a potent competitive inhibitor of sphingosine kinase but not of protein kinase C: modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochemistry. 1998;37:12892–8. doi: 10.1021/bi980744d. [DOI] [PubMed] [Google Scholar]
- 19.Felding-Habermann B, Igarashi Y, Fenderson BA, Park LS, Radin NS, Inokuchi J, et al. A ceramide analogue inhibits T cell proliferative response through inhibition of glycosphingolipid synthesis and enhancement of N,N-dimethylsphingosine synthesis. Biochemistry. 1990;29:6314–22. doi: 10.1021/bi00478a028. [DOI] [PubMed] [Google Scholar]
- 20.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–21. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 21.Seo YJ, Hahm B. Type I Interferon Modulates the Battle of Host Immune System Against Viruses. Adv Appl Microbiol. 2010;73C:83–101. doi: 10.1016/S0065-2164(10)73004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiscott J. Triggering the innate antiviral response through IRF-3 activation. J Biol Chem. 2007;282:15325–9. doi: 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- 23.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–26. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 24.Barchet W, Cella M, Colonna M. Plasmacytoid dendritic cells--virus experts of innate immunity. Semin Immunol. 2005;17:253–61. doi: 10.1016/j.smim.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Briere F, et al. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med. 2002;195:517–28. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlender J, Hornung V, Finke S, Gunthner-Biller M, Marozin S, Brzozka K, et al. Inhibition of toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J Virol. 2005;79:5507–15. doi: 10.1128/JVI.79.9.5507-5515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diebold SS, Montoya M, Unger H, Alexopoulou L, Roy P, Haswell LE, et al. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature. 2003;424:324–8. doi: 10.1038/nature01783. [DOI] [PubMed] [Google Scholar]
- 28.Hahm B. Hostile communication of measles virus with host innate immunity and dendritic cells. Curr Top Microbiol Immunol. 2009;330:271–87. doi: 10.1007/978-3-540-70617-5_13. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Sastre A, Durbin RK, Zheng H, Palese P, Gertner R, Levy DE, et al. The role of interferon in influenza virus tissue tropism. J Virol. 1998;72:8550–8. doi: 10.1128/jvi.72.11.8550-8558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darnell JE, Jr., Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–21. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 31.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–86. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 32.Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science. 2002;296:1653–5. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 33.Darnell JE., Jr. STATs and gene regulation. Science. 1997;277:1630–5. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 34.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282:20059–63. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 35.David M, Petricoin E, 3rd, Benjamin C, Pine R, Weber MJ, Larner AC. Requirement for MAP kinase (ERK2) activity in interferon alpha- and interferon beta-stimulated gene expression through STAT proteins. Science. 1995;269:1721–3. doi: 10.1126/science.7569900. [DOI] [PubMed] [Google Scholar]
- 36.Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–8. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia P, Wang L, Moretti PA, Albanese N, Chai F, Pitson SM, et al. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-alpha signaling. J Biol Chem. 2002;277:7996–8003. doi: 10.1074/jbc.M111423200. [DOI] [PubMed] [Google Scholar]
- 38.Yang CH, Murti A, Pfeffer SR, Fan M, Du Z, Pfeffer LM. The role of TRAF2 binding to the type I interferon receptor in alternative NF kappaB activation and antiviral response. J Biol Chem. 2008;283:14309–16. doi: 10.1074/jbc.M708895200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pauli EK, Schmolke M, Wolff T, Viemann D, Roth J, Bode JG, et al. Influenza A virus inhibits type I IFN signaling via NF-kappaB-dependent induction of SOCS-3 expression. PLoS Pathog. 2008;4:e1000196. doi: 10.1371/journal.ppat.1000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei L, Sandbulte MR, Thomas PG, Webby RJ, Homayouni R, Pfeffer LM. NFkappaB negatively regulates interferon-induced gene expression and anti-influenza activity. J Biol Chem. 2006;281:11678–84. doi: 10.1074/jbc.M513286200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baum A, Garcia-Sastre A. Induction of type I interferon by RNA viruses: cellular receptors and their substrates. Amino Acids. 2010;38:1283–99. doi: 10.1007/s00726-009-0374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hiscott J. Convergence of the NF-kappaB and IRF pathways in the regulation of the innate antiviral response. Cytokine Growth Factor Rev. 2007;18:483–90. doi: 10.1016/j.cytogfr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 2006;312:879–82. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- 44.Katze MG, He Y, Gale M., Jr. Viruses and interferon: a fight for supremacy. Nat Rev Immunol. 2002;2:675–87. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- 45.Mibayashi M, Martinez-Sobrido L, Loo YM, Cardenas WB, Gale M, Jr., Garcia-Sastre A. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J Virol. 2007;81:514–24. doi: 10.1128/JVI.01265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Sastre A, Egorov A, Matassov D, Brandt S, Levy DE, Durbin JE, et al. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology. 1998;252:324–30. doi: 10.1006/viro.1998.9508. [DOI] [PubMed] [Google Scholar]
- 47.Fensterl V, Sen GC. Interferons and viral infections. Biofactors. 2009;35:14–20. doi: 10.1002/biof.6. [DOI] [PubMed] [Google Scholar]
- 48.Vilcek J. Fifty years of interferon research: aiming at a moving target. Immunity. 2006;25:343–8. doi: 10.1016/j.immuni.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 49.Gao Y, Majchrzak-Kita B, Fish EN, Gommerman JL. Dynamic accumulation of plasmacytoid dendritic cells in lymph nodes is regulated by interferon-beta. Blood. 2009;114:2623–31. doi: 10.1182/blood-2008-10-183301. [DOI] [PubMed] [Google Scholar]
- 50.Kamphuis E, Junt T, Waibler Z, Forster R, Kalinke U. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood. 2006;108:3253–61. doi: 10.1182/blood-2006-06-027599. [DOI] [PubMed] [Google Scholar]
- 51.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–4. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 52.Pinschewer DD, Ochsenbein AF, Odermatt B, Brinkmann V, Hengartner H, Zinkernagel RM. FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. J Immunol. 2000;164:5761–70. doi: 10.4049/jimmunol.164.11.5761. [DOI] [PubMed] [Google Scholar]
- 53.Walsh KB, Marsolais D, Welch MJ, Rosen H, Oldstone MB. Treatment with a sphingosine analog does not alter the outcome of a persistent virus infection. Virology. 2010;397:260–9. doi: 10.1016/j.virol.2009.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brinkmann V, Geiger T, Alkan S, Heusser CH. Interferon alpha increases the frequency of interferon gamma-producing human CD4+ T cells. J Exp Med. 1993;178:1655–63. doi: 10.1084/jem.178.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aichele P, Unsoeld H, Koschella M, Schweier O, Kalinke U, Vucikuja S. CD8 T cells specific for lymphocytic choriomeningitis virus require type I IFN receptor for clonal expansion. J Immunol. 2006;176:4525–9. doi: 10.4049/jimmunol.176.8.4525. [DOI] [PubMed] [Google Scholar]
- 56.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–30. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J, Lin Q, Langston H, Cooper MD. Resident bone marrow macrophages produce type 1 interferons that can selectively inhibit interleukin-7-driven growth of B lineage cells. Immunity. 1995;3:475–84. doi: 10.1016/1074-7613(95)90176-0. [DOI] [PubMed] [Google Scholar]
- 58.Chang WL, Coro ES, Rau FC, Xiao Y, Erle DJ, Baumgarth N. Influenza virus infection causes global respiratory tract B cell response modulation via innate immune signals. J Immunol. 2007;178:1457–67. doi: 10.4049/jimmunol.178.3.1457. [DOI] [PubMed] [Google Scholar]
- 59.Rau FC, Dieter J, Luo Z, Priest SO, Baumgarth N. B7-1/2 (CD80/CD86) direct signaling to B cells enhances IgG secretion. J Immunol. 2009;183:7661–71. doi: 10.4049/jimmunol.0803783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–50. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 62.Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–5. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 63.Liu ZG. Molecular mechanism of TNF signaling and beyond. Cell Res. 2005;15:24–7. doi: 10.1038/sj.cr.7290259. [DOI] [PubMed] [Google Scholar]
- 64.Panne D. The enhanceosome. Curr Opin Struct Biol. 2008;18:236–42. doi: 10.1016/j.sbi.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 65.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–66. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 66.Ludwig S, Planz O. Influenza viruses and the NF-kappaB signaling pathway - towards a novel concept of antiviral therapy. Biol Chem. 2008;389:1307–12. doi: 10.1515/BC.2008.148. [DOI] [PubMed] [Google Scholar]
- 67.Wurzer WJ, Ehrhardt C, Pleschka S, Berberich-Siebelt F, Wolff T, Walczak H, et al. NF-kappaB-dependent induction of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas/FasL is crucial for efficient influenza virus propagation. J Biol Chem. 2004;279:30931–7. doi: 10.1074/jbc.M403258200. [DOI] [PubMed] [Google Scholar]
- 68.Kumar N, Xin ZT, Liang Y, Ly H, Liang Y. NF-kappaB signaling differentially regulates influenza virus RNA synthesis. J Virol. 2008;82:9880–9. doi: 10.1128/JVI.00909-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McLean JE, Datan E, Matassov D, Zakeri ZF. Lack of Bax prevents influenza A virus-induced apoptosis and causes diminished viral replication. J Virol. 2009;83:8233–46. doi: 10.1128/JVI.02672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pucci B, Indelicato M, Paradisi V, Reali V, Pellegrini L, Aventaggiato M, et al. ERK-1 MAP kinase prevents TNF-induced apoptosis through bad phosphorylation and inhibition of Bax translocation in HeLa Cells. J Cell Biochem. 2009;108:1166–74. doi: 10.1002/jcb.22345. [DOI] [PubMed] [Google Scholar]
- 71.Pleschka S, Wolff T, Ehrhardt C, Hobom G, Planz O, Rapp UR, et al. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat Cell Biol. 2001;3:301–5. doi: 10.1038/35060098. [DOI] [PubMed] [Google Scholar]
- 72.Xu L, Yoon H, Zhao MQ, Liu J, Ramana CV, Enelow RI. Cutting edge: pulmonary immunopathology mediated by antigen-specific expression of TNF-alpha by antiviral CD8+ T cells. J Immunol. 2004;173:721–5. doi: 10.4049/jimmunol.173.2.721. [DOI] [PubMed] [Google Scholar]
- 73.Bruder D, Srikiatkhachorn A, Enelow RI. Cellular immunity and lung injury in respiratory virus infection. Viral Immunol. 2006;19:147–55. doi: 10.1089/vim.2006.19.147. [DOI] [PubMed] [Google Scholar]
- 74.Zhi L, Leung BP, Melendez AJ. Sphingosine kinase 1 regulates pro-inflammatory responses triggered by TNFalpha in primary human monocytes. J Cell Physiol. 2006;208:109–15. doi: 10.1002/jcp.20646. [DOI] [PubMed] [Google Scholar]
- 75.Lai WQ, Irwan AW, Goh HH, Howe HS, Yu DT, Valle-Onate R, et al. Anti-inflammatory effects of sphingosine kinase modulation in inflammatory arthritis. J Immunol. 2008;181:8010–7. doi: 10.4049/jimmunol.181.11.8010. [DOI] [PubMed] [Google Scholar]
- 76.Lai WQ, Irwan AW, Goh HH, Melendez AJ, McInnes IB, Leung BP. Distinct roles of sphingosine kinase 1 and 2 in murine collagen-induced arthritis. J Immunol. 2009;183:2097–103. doi: 10.4049/jimmunol.0804376. [DOI] [PubMed] [Google Scholar]
- 77.Jung ID, Lee JS, Kim YJ, Jeong YI, Lee CM, Baumruker T, et al. Sphingosine kinase inhibitor suppresses a Th1 polarization via the inhibition of immunostimulatory activity in murine bone marrow-derived dendritic cells. Int Immunol. 2007;19:411–26. doi: 10.1093/intimm/dxm006. [DOI] [PubMed] [Google Scholar]
- 78.Yang J, Castle BE, Hanidu A, Stevens L, Yu Y, Li X, et al. Sphingosine kinase 1 is a negative regulator of CD4+ Th1 cells. J Immunol. 2005;175:6580–8. doi: 10.4049/jimmunol.175.10.6580. [DOI] [PubMed] [Google Scholar]
- 79.Wang W, Huang MC, Goetzl EJ. Type 1 sphingosine 1-phosphate G protein-coupled receptor (S1P1) mediation of enhanced IL-4 generation by CD4 T cells from S1P1 transgenic mice. J Immunol. 2007;178:4885–90. doi: 10.4049/jimmunol.178.8.4885. [DOI] [PubMed] [Google Scholar]
- 80.Wang W, Graeler MH, Goetzl EJ. Type 4 sphingosine 1-phosphate G protein-coupled receptor (S1P4) transduces S1P effects on T cell proliferation and cytokine secretion without signaling migration. Faseb J. 2005;19:1731–3. doi: 10.1096/fj.05-3730fje. [DOI] [PubMed] [Google Scholar]
- 81.Idzko M, Panther E, Corinti S, Morelli A, Ferrari D, Herouy Y, et al. Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. Faseb J. 2002;16:625–7. doi: 10.1096/fj.01-0625fje. [DOI] [PubMed] [Google Scholar]
- 82.Japtok L, Kleuser B. The role of sphingosine-1-phosphate receptor modulators in the prevention of transplant rejection and autoimmune diseases. Curr Opin Investig Drugs. 2009;10:1183–94. [PubMed] [Google Scholar]
- 83.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–84. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yamane D, Zahoor MA, Mohamed YM, Azab W, Kato K, Tohya Y, et al. Inhibition of sphingosine kinase by bovine viral diarrhea virus NS3 is crucial for efficient viral replication and cytopathogenesis. J Biol Chem. 2009;284:13648–59. doi: 10.1074/jbc.M807498200. [DOI] [PMC free article] [PubMed] [Google Scholar]