Abstract
Previous studies suggested that FGF signaling is important for lens formation. However, the times at which FGFs act to promote lens formation, the FGFs that are involved, the cells that secrete them and the mechanisms by which FGF signaling may promote lens formation are not known. We found that transcripts encoding several FGF ligands and the four classical FGF receptors are detectable in the lens-forming ectoderm at the time of lens induction. Conditional deletion of Fgfr1 and Fgfr2 from this tissue resulted in the formation of small lens rudiments that soon degenerated. Lens placodes lacking Fgfr1 and 2 were thinner than in wild type embryos. Deletion of Fgfr2 increased cell death from the initiation of placode formation and concurrent deletion of Fgfr1 enhanced this phenotype. Fgfr1/2 conditional knockout placode cells expressed lower levels of proteins known to be regulated by FGF receptor signaling, but proteins known to be important for lens formation were present at normal levels in the remaining placode cells, including the transcription factors, Pax6, Sox2 and FoxE3 and the lens-preferred protein, αA-crystallin. Previous studies identified a genetic interaction between BMP and FGF signaling in lens formation and conditional deletion of Bmpr1a caused increased cell death in the lens placode, resulting in the formation of smaller lenses. In the present study, conditional deletion of both Bmpr1a and Fgfr2 increased cell death beyond that seen in Fgfr2CKO placodes and prevented lens formation. These results suggest that the primary role of autocrine or paracrine FGF signaling is to provide essential survival signals to lens placode cells. Because apoptosis was already increased at the onset of placode formation in Fgfr1/2 conditional knockout placode cells, FGF signaling was functionally absent during the period of lens induction by the optic vesicle. Since the expression of proteins required for lens formation was not altered in the knockout placode cells, we can conclude that FGF signaling from the optic vesicle is not required for lens induction.
Keywords: Lens induction, Fibroblast growth factors, FGF receptors, apoptosis, Pax6
INTRODUCTION
The tissue interactions that lead to lens formation begin at gastrulation. These create a “lens-forming bias” in the prospective lens-forming ectoderm, leading to specification of ectoderm cells to form a lens (Donner et al., 2006; Lang, 2004; Saha et al., 1989; Sullivan et al., 2004). In the final stage of lens formation, adhesion of the optic vesicle to the lens-forming ectoderm triggers “lens induction,” leading to the formation of the lens placode and its subsequent invagination to form the lens vesicle. Contact between the optic vesicle and the ectoderm may also shield the lens from inhibitory signals from neural crest mesenchyme (Bailey et al., 2006; Sullivan et al., 2004). Previous studies implicated several factors in lens induction, including Bmp4 and 7 (Furuta and Hogan, 1998; Jena et al., 1997; Rajagopal et al., 2009; Wawersik et al., 1999), FGFs (Faber et al., 2001; Gotoh et al., 2004; Nakayama et al., 2008; Pan et al., 2006; Vogel-Hopker et al., 2000) and the Notch ligand, Delta2 (Ogino et al., 2008). Of these, only Bmp4 (mouse), Fgf19 (zebrafish) and Delta2 (frog) are known to be expressed in the optic vesicle and required for normal lens formation (Furuta and Hogan, 1998; Nakayama et al., 2008; Ogino et al., 2008) and only BMP receptors are known to be required in the responding ectoderm (Rajagopal et al., 2009). It is not yet clear whether FGFs from the optic vesicle fulfill the criteria to be considered classical “lens inducers” in mammals (one or more ligands produced by the optic vesicle, with receptors required in the lens-forming ectoderm; see Discussion).
The transcription factor, Pax6, is required in the surface ectoderm cells for lens formation (Ashery-Padan et al., 2000). Pax6 heterozygous mice have smaller lenses that later develop cataracts (Grindley et al., 1997). Pax6 is expressed at low levels in the prospective lens ectoderm before placode formation (Pax6pre-placode) and at higher levels during placode formation (Pax6placode) (Lang, 2004). For these reasons, the amount of Pax6 protein in the nuclei of placode cells has been used as a measure of the extent of lens induction. If the inhibition of a signaling pathway decreases the accumulation Pax6, that pathway has been implicated in lens induction.
Several types of experiments have shown that FGF signaling participates in the establishment of lens competence, lens bias and lens specification [reviewed in (Donner et al., 2006; Lang, 2004)]. Additional experiments suggest that FGFs may also be involved in lens induction. Expression in the lens placode of a kinase-deleted form of FGF receptor-1 was reported to reduce levels of Pax6 in the nucleus of placode cells and resulted in the formation of small lenses (Faber et al., 2001). Treatment of eye rudiments with an inhibitor of FGF receptor tyrosine kinase activity reduced lens cell proliferation and lens size (Faber et al., 2001). Germline deletion of Ndst1, which encodes an enzyme required for the synthesis of heparan sulfate, a co-factor for FGF receptor activation, disrupted the formation of the lens and optic vesicle and, in more severely affected eyes, decreased Pax6 levels in the lens placode (Pan et al., 2006). Mutation of critical amino acids in Frs2α, an adapter protein that participates in FGF receptor signaling, also disrupted optic vesicle and lens formation and reduced Pax6 levels in the placode (Gotoh et al., 2004). However, the identity and source of the FGF ligands involved in lens formation and the requirement for FGF receptors in the ectoderm have not been established (Smith et al., 2010). We determined the cell-autonomous function of FGF signaling during lens induction by conditionally deleting the two FGF receptors that are most abundantly expressed in the lens placode.
MATERIALS AND METHODS
Mice
Mice carrying floxed alleles of Fgfr1 (Trokovic et al., 2003), Fgfr2 (Yu et al., 2003) and Bmpr1a (Mishina et al., 2002) were mated to mice carrying the Le-Cre transgene, which is expressed in lens-forming ectoderm cells at E9 (Ashery-Padan et al., 2000). Animals were genotyped by PCR using primers described previously (Huang et al., 2009; Rajagopal et al., 2009). Matings were set up such that all animals were homozygous for the floxed allele(s), with the females also carrying a single copy of the Le-Cre transgene. This resulted in pregnancies in which approximately half of the embryos were Cre-positive. For timed matings, noon on the day on which a vaginal plug was detected was considered E0.5. The Le-Cre transgene has an internal ribosome entry site that drives the expression of green fluorescent protein (Ashery-Padan et al., 2000). Cre-positive embryos were identified using an Olympus SZX7 dissecting microscope with fluorescence detection.
Microarray analysis
Wild type E9.5 or E10.0 embryos were frozen in OCT embedding compound and stored at −80° C. Frozen sections were stained with hematoxylin and lens placode cells were isolated using a Leica LMD6000 laser microdissection system (North Central Instruments, Maryland Heights, MO). Tissue collected from both eyes of one embryo was lysed and RNA was isolated using a Qiagen RNeasy Micro kit (Qiagen, Valencia, CA). Total RNA (~50 ng) was amplified using the NuGEN WT-Ovation™ Pico RNA Amplification System (NuGEN, San Carlos, CA). The amplified DNA products were quantified and their size determined using an Agilent 2100 Bioanalyzer and labeled using the NuGEN Encore™ Biotin Module. Labeled products were hybridized to Illumina Mouse6 v1.2 BeadArrays (Illumina, Inc., San Diego, CA), scanned on an Illumina® Beadstation 500, the images decoded with Illumina Beadscan software and the results analyzed using the Illumina BeadStation software, which reports the probability that transcripts were detected above background. Probe sets with detection p-values <0.05 were considered to represent transcripts that were expressed in the original samples. The results of 18 microarrays of wild type tissues were used to identify the FGF and FGF receptor transcripts present in the E9.5 lens placode.
PCR amplification
E9.5 lens placode cells were isolated by laser microdissection and total RNA was isolated as described above. Total RNA was also isolated from manually-dissected adult mouse lens epithelia. Approximately 50 ng of total RNA was used to synthesize and amplify cDNA using the NuGEN WT-Ovation™ RNA Amplification System. PCR primers were selected using Primer 3 software. Transcripts were routinely amplified for 40 PCR cycles using standard procedures. To provide a semi-quantitative estimate of the abundance of transcripts encoding the four FGF receptors, cDNA was amplified for 33 or 35 PCR cycles.
Immunostaining
Embryos were fixed overnight in 10% neutral buffered formalin, washed, dehydrated, embedded in paraffin and sectioned at 5 μm using standard procedures or embedded in 4% agarose and sectioned at 150 μm in an oscillating tissue slicer (Electron Microscopy Sciences, Hatfield, PA). Antibody staining was performed on tissue sections using standard methods and detected with fluorescent-labeled secondary antibodies or with the Vectastain Elite Mouse IgG ABC kit. Antibodies used were mouse anti-chicken Pax6 (Developmental Studies Hybridoma Bank, Iowa City, IA), rabbit anti-Pax6 (sc-7750; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-Pax6 (ab5790; ABCAM, Cambridge, MA), rabbit anti-phosphorylated Frs2α (AF5126; R and D systems, Minneapolis, MN), rabbit anti-Erm (Etv5; sc-22807; Santa Cruz Biotechnology, Santa Cruz, CA), anti-Er81 (Etv1; ab36788, Abcam, Cambridge, MA), rabbit anti-FoxE3 (gift of Dr. Peter Carlsson) and mouse monoclonal anti-αA-crystallin (gift from Dr. Usha Andley). Fluorescent images for Pax6, Sox2, FoxE3 and αA-crystallin were acquired on a Zeiss LSM-510 Zeiss confocal microscope or an Olympus wide-field fluorescence microscope (Washington University). Images for Pax6, pFrs2α, ER81 and Erm were collected on a Zeiss LSM-710 confocal microscope (Miami University).
Quantifying immunofluorescence
Immunofluorescent images from sections stained for Pax6 or Er81 were analyzed using ImageJ (http://rsbweb.nih.gov/ij/). Fluorescent staining intensity in the optic vesicle cells was used as an internal standard to compare the immunofluorescence of wild type and Fgfr1/2CKO lens placode cells. A box was drawn around the distal optic vesicle and a separate box around the lens placode. The average pixel intensity within each box was recorded and the ratio of fluorescence in the two tissues was computed. Differences in pixel intensity between wild type and conditional knockout eyes were evaluated using Student’s T-test.
BrdU staining
Pregnant females were injected with 50 mg/kg of body weight of 10 mM BrdU (Roche, Indianapolis, IN) and 1 mM 5-fluoro-5′-deoxyuridine (Sigma, St. Louis, MO) and sacrificed after 1 hour. Staining was performed on sections of paraffin-embedded embryos using a monoclonal anti-BrdU antibody (1:250) (Dako, Carpinteria, CA) with a Vectastain Elite Mouse IgG ABC kit. Sections were counterstained with hematoxylin.
Measuring placode thickness
This was performed as described previously. Briefly, in frontal sections through the middle of the lens placode, the thickness of the placode was measured at most dorsal and ventral points of contact between the optic vesicle and the lens-forming ectoderm and at three equally-spaced locations between these extremes at mid-placode stage (E9.5).
TUNEL labeling
Terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) was done with an Apoptag kit (Chemicon, Temecula, CA). The deparaffinized slides were treated with 3% H2O2 in methanol for 30 min, followed by proteinase K treatment (20 μg/ml) for 15 min. Slides were incubated with TdT enzyme in equilibration buffer for 1 hour at 37° C. The reaction was terminated with wash buffer provided by the manufacturer for 10 min at room temperature. Anti-digoxigenin-peroxidase conjugate was added for 30 min at room temperature, followed by DAB plus H202 treatment. Slides were counterstained with hematoxylin.
RESULTS
The FGFs and FGF receptors expressed in the ectoderm during lens induction
Twenty-two ligands and four receptors containing cytoplasmic tyrosine kinase domains mediate FGF signaling in mammals (Itoh and Ornitz, 2008; Ornitz and Itoh, 2001; Ornitz et al., 1996). Previous studies implicated FGF signaling in lens formation in the mouse, although the ligands and receptors required are not known (Faber et al., 2001; Gotoh et al., 2004; Pan et al., 2006; Smith et al., 2010).
To identify the FGF ligands and receptors expressed in the lens placode, placode cells were laser microdissected from E9.5 embryos, the RNA was reverse transcribed and amplified, and transcripts encoding FGF ligands and receptors were identified by microarray and RT-PCR analysis. These were compared to the transcripts detected by RT-PCR in adult lens epithelial cells. Table 1 lists the FGFs and FGF receptors and shows the number that were detected above background levels in 18 microarrays of RNA isolated from wild type E9.5 or E10.0 lens placode cells. RT-PCR analysis was used to confirm the expression of FGF transcripts detected on the microarray or detected previously in mammalian lens placodes. Of the FGFs identified by microarray, transcripts encoding Fgf1, 9, 10 and 15 were readily detected by RT-PCR (Fig. 1A). A PCR product of the approximate size of the Fgf18 PCR product from the adult lens epithelial cells was present at trace levels. Although Fgf2 was detected on seven of the microarrays and in adult lens epithelial cells, Fgf2 transcripts were not detectable by RT-PCR in lens placode cDNA after 40 cycles of RT-PCR, suggesting that the transcripts detected by the microarray probes were due to off-target hybridization. Except for Fgf10, each of the PCR products tested was detected in adult lens epithelial cells. Fgf7 was included as a negative control for the PCR analyses, since it was not detected at E9.5 by microarray. Although Fgf7 transcripts were present in adult lens epithelial cells, only a trace was detected at E9.5 after 40 cycles of amplification. Although Fgf13 was detected on all microarrays, we did not test for this transcript by RT-PCR, since Fgf13 functions intracellularly and does not interact with FGF receptors (Goetz et al., 2009; Goldfarb et al., 2007; Itoh and Ornitz, 2008)
Table 1.
Number of FGF and FGF receptor transcripts detected (p<0.05) in 18 microarray studies that used RNA extracted from E9.5 (15 arrays) or E10.0 (3 arrays) wild type lens placode cells.
| FGF Ligands | FGF Receptors | ||
|---|---|---|---|
| Transcript | Number Detected (of 18) | Transcript | Number Detected (of 18) |
| Fgf1 | 4 | Fgfr1 | 18 |
| Fgf2 | 7 | Fgfr2 | 18 |
| Fgf3 | 1 | Fgfr3 | 1 |
| Fgf4 | 2 | Fgfr4 | 0 |
| Fgf5 | 4 | ||
| Fgf6 | 2 | ||
| Fgf7 | 0 | ||
| Fgf8 | 0 | ||
| Fgf9 | 2 | ||
| Fgf10 | 5 | ||
| Fgf11 | 1 | ||
| Fgf12 | 1 | ||
| Fgf13 | 18 | ||
| Fgf14 | 0 | ||
| Fgf15 | 15 | ||
| Fgf16 | 1 | ||
| Fgf17 | 4 | ||
| Fgf18 | 7 | ||
| Fgf20 | 0 | ||
| Fgf21 | 2 | ||
| Fgf22 | 0 | ||
| Fgf23 | 0 | ||
Figure 1. Gel showing PCR products for FGF and FGF receptor transcripts.
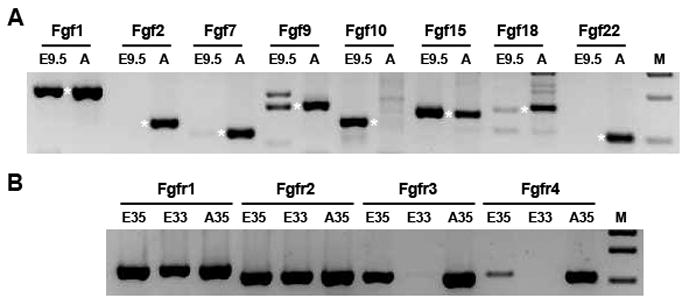
A. FGFs amplified using cDNA from E9.5 lens placodes (E9.5) and adult lens epithelial cells (A). Products of the expected size are denoted by asterisks. B. FGF receptor transcripts amplified using cDNA from E9.5 lens placodes for 35 (E35) or 33 (E33) PCR cycles or from adult lens epithelial cells amplified for 35 PCR cycles (A35). M – molecular weight markers
Transcripts encoding Fgfr1 and 2 were readily detected by microarray and RT-PCR in the lens placode (Fig. 1B). At 35 PCR cycles, Fgfr3 and 4 were also detectable, but decreasing the PCR cycles to 33 reduced the Fgfr3 PCR product to trace level and made Fgfr4 undetectable (Fig. 1B).
Conditional deletion of FGF receptors lens placode cells
Conditional deletion of Fgfr2 from lens placode cells did not prevent lens formation, although it resulted in the formation of smaller lenses that had high levels of cell death and defects in fiber cell differentiation (Garcia et al., 2005). In this earlier study, the effects of deleting Fgfr2 were not examined at the lens placode stage. Since signaling through Fgfr1 could have moderated the effects of Fgfr2 deletion, we deleted floxed alleles of Fgfr1 and 2 from the lens placode using Le-Cre, which is expressed in the lens-forming ectoderm on E9 (Ashery-Padan et al., 2000). Littermate embryos homozygous for the floxed alleles, but not expressing the Cre transgene, served as controls. For simplicity, embryos that expressed the Cre transgene in the lens-forming ectoderm are referred to as Fgfr1/2CKO, while those with no transgene expression are denoted as Fgfr1/2WT.
Most Fgfr1/2CKO embryos did not have a lens at E12.5. In a few cases, rudimentary lens material was present soon after birth, but most eyes examined did not have detectable lens tissue. Fig. 2A–C shows a wild type eye and two Cre-positive eyes at postnatal day 1 (P1). One of the Cre-positive eyes had a rudimentary lens. The other had no recognizable lens tissue. Fig. 2D–E shows sections through WT and Fgfr1/2CKO eyes at E12.5. In the Fgfr1/2WT eye, a normal-appearing lens is stained with antibody against the lens protein, αA-crystallin. In the Fgfr1/2CKO eye, the retina was folded and a no morphologically-identifiable lens was detected. A few αA-crystallin-positive cells, presumably remnants of the lens, were embedded in the mesenchyme that would normally form the corneal stroma.
Figure 2. The effect of deleting FGF receptors on lens morphology and αA-crystallin expression.
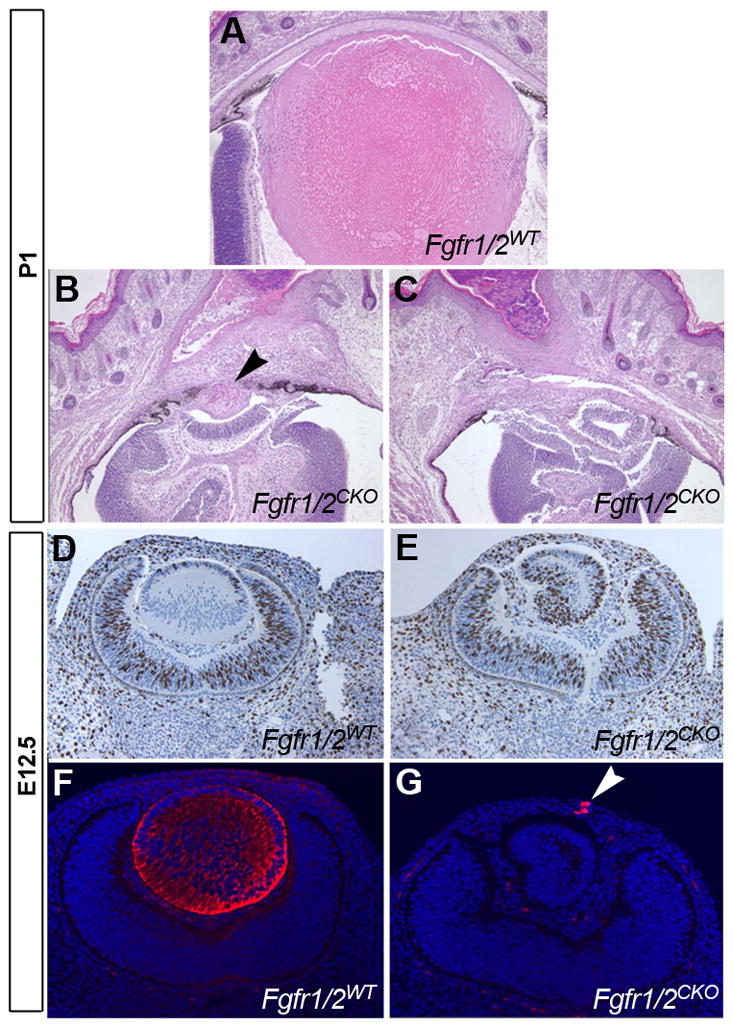
Wild type (A) and conditional knockouts (B, C) at P1. In rare cases, a lens rudiment was detectable (arrowhead in B). In most cases, no lens was detected. At E12.5, the wild type lens had normal morphology (D) and stained for αA-crystallin (F). In conditional knockouts, the retina folded, sometimes appearing like an abnormal lens (E). However, only a few αA-crystallin-positive cells were present. These were embedded in the mesenchyme that will later form the corneal stroma (G).
Examination of embryos on E9.5 revealed that the lens placodes of Fgfr1/2CKO embryos appeared thinner than Fgfr1/2WT placodes. Measurement of thickness at five locations along the dorsal-ventral extent of the placode confirmed this impression (Fig. 3). Previous studies showed that interfering with FGF signaling reduced the BrdU labeling index in the prospective lens cells at E9.5 and E10.5 (Faber et al., 2001; Pan et al., 2006). We performed BrdU labeling at E9.5 to determine whether reduced cell proliferation could be responsible for the thinner placodes in Fgfr1/2CKO embryos. The BrdU labeling index was reduced in Fgfr1/2CKO lens placodes, but the difference between the knockout and Fgfr1/2WT placodes was not statistically significant (38.1 ± 5.6 and 33.3 ± 3.4 [SEM], respectively; N = 6–8 lenses, p = 0.49). Since deletion of Fgfr2 increased cell death later in lens development (Garcia et al., 2005), we measured the TUNEL labeling index in early (20–24 somite [s]) and late (28–34s) Fgfr1/2WT and Fgfr1/2CKO lens placode cells. At both stages, the TUNEL labeling index was significantly increased in the Fgfr1/2CKO placodes (Fig 4). The increase in apoptosis was confirmed by labeling with an antibody against active caspase3 (Fig. 4G–I). Since deletion of Fgfr2 alone did not prevent lens formation at E12.5 (Garcia et al., 2005), we compared the TUNEL labeling index in Fgfr1/2WT, Fgfr2CKO and Fgfr1/2CKO placodes. Deletion of Fgfr1 and 2 increased the level of cell death beyond that seen in Fgfr2CKO placodes (Fig. 4J).
Figure 3. Effect of deleting FGF receptors on the thickness of the lens placode.
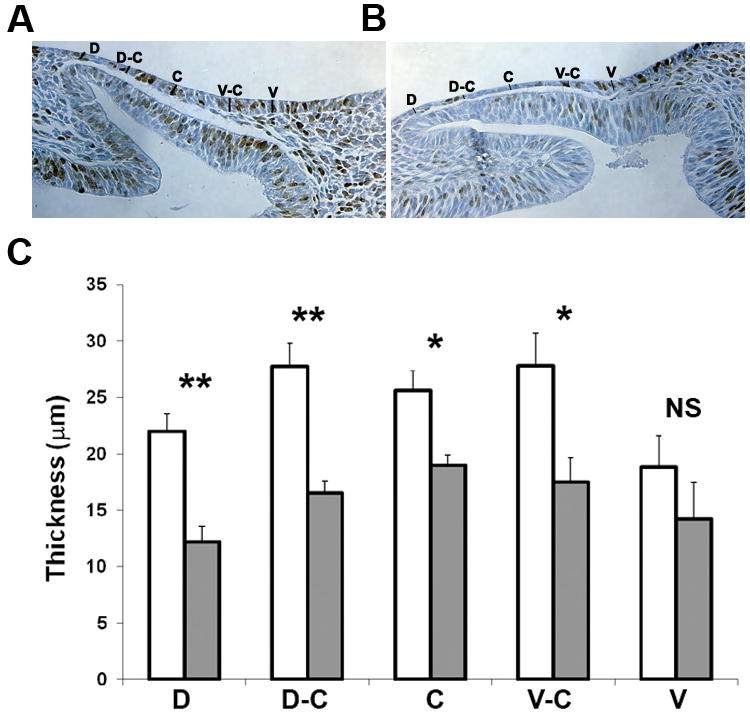
(A, B) Placode thickness was measured at five dorso-ventral locations at E9.5 (mid-placode stage) in wild type (A) and Fgfr1/2CKO (B) placodes. The brown nuclei in these sections are BrdU-labeled. (B) Fgfr1/2CKO placodes (shaded bars) were significantly thinner than wild type placodes (open bars) at most locations. * p<0.05; ** p<0.01; NS, not significantly different
Figure 4. Effect of deleting FGF receptors on apoptosis in the lens placode.
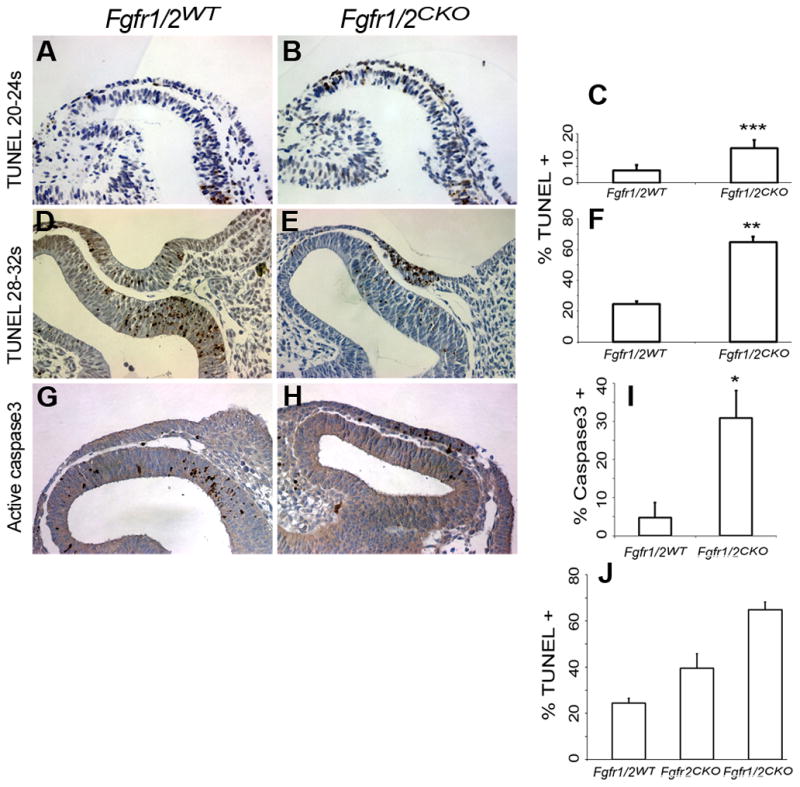
TUNEL staining was greater in conditional knockout lens placodes from 20-24-somite and 28-32-somite embryos than in wild type littermates (A, B, D, E). The TUNEL labeling index is shown in (C) for 20-24-somite embryos and in (F) for 28-32-somite embryos. Antibody to activated caspase3 was also greater in conditional knockout than in wild type embryos (G–I). Compared to wild type, the TUNEL labeling index increased in a dose-dependent manner in Fgfr2CKO and Fgfr1/2CKO embryos (J).
Effect of Fgfr1/2 deletion on known targets of FGF receptor signaling
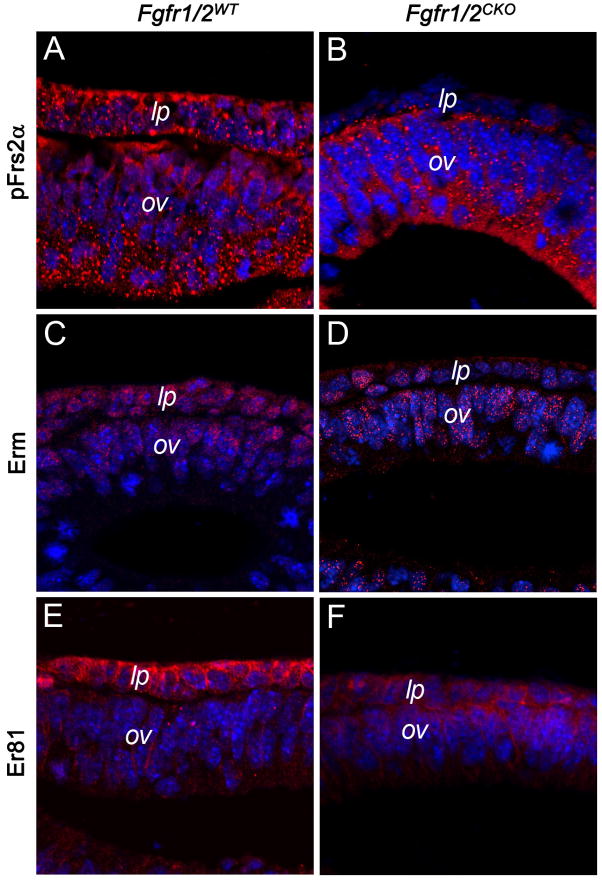
FGF receptor activation leads to the recruitment of the adaptor protein, Frs2α. Frs2α is phosphorylated by the FGF receptor tyrosine kinase, permitting the assembly of a signaling complex that includes the phosphatase, Shp2, and the adapter protein, Grb2 (Ong et al., 2000). “Knock in” constructs of Frs2α that lack the two phosphorylation sites required for binding Shp2 resulted in defects in retina and lens formation (Gotoh et al., 2004). We used antibodies to phosphorylated Frs2α (pFrs2α) to measure its activation in the lens placode and optic vesicle. Antibodies to pFrs2α stained Fgfr1/2WT E9.5 lens placode cells at least as strongly as optic vesicle cells (Fig. 5). Staining for pFrs2α decreased markedly in Fgfr1/2CKO placodes, compared to the optic vesicle (Fig. 5A–B). Staining for phosphorylated ERK (pERK), another downstream target of FGF receptor signaling, was also greatly reduced early in placode formation (Supplementary Data, Fig. S1). FGF signaling often increases the expression of members of the Ets family of transcription factors. Staining for two of these, Erm and Er81, was markedly reduced in Fgfr1/2CKO lens placode cells, when compared to Fgfr1/2WT placode and adjacent optic vesicle cells (Fig. 5C–F).
Figure 5. The effect of FGF receptor deletion on the levels in the lens placode of the adapter protein, pFrs2α and the Ets family members, Erm and Er81.
Staining for the phosphorylated (activated) form of Frs2α (A, B), Erm (C, D) and Er81 (E, F) decreased markedly in Fgfr1/2CKO lens placode cells.
Deletion of Fgfr1/2 did not prevent the increased expression of Pax6 that occurs during placode formation, or reduce the expression of Pax6 downstream targets
Germline deletion of Ndst1 or mutation of phosphorylation sites in Frs2α disrupted lens and retina differentiation and decreased Pax6 protein levels in the lens placode, at least in the most severely affected embryos (Gotoh et al., 2004; Pan et al., 2006). Antibody staining detected no obvious difference in Pax6 levels between wild type and Fgfr1/2CKO lens placodes (Fig. 6A–D). Since the results for Pax6 expression were different from those obtained in previous studies of FGF signaling in lens induction, they were repeated in two labs, using different litters of embryos, different antibodies to Pax6 and different methods of antibody detection (immunofluorescence or immunohistochemistry). Quantification of immunofluorescence intensity confirmed that Pax6 staining was not different in wild type and Fgfr1/2CKO lens placodes at E9.5. Quantification was performed by using staining in the adjacent, wild type optic vesicle cells as an internal standard (placode/optic vesicle [P/O] ratio: 1.27 (WT) and 1.21 (CKO); p = 0.37). Similar analysis showed that staining for Er81 was significantly reduced in the Fgfr1/2CKO lens placodes (P/O ratio: 1.37 (WT) and 0.42 (CKO); p = 3.8 × 10−7). Pax6 levels did decrease in the Fgfr1/2CKO ectoderm cells remaining at E10.5.
Figure 6. Conditional deletion of Fgfr1/2 in the lens placode did not significantly decrease Pax6 levels.
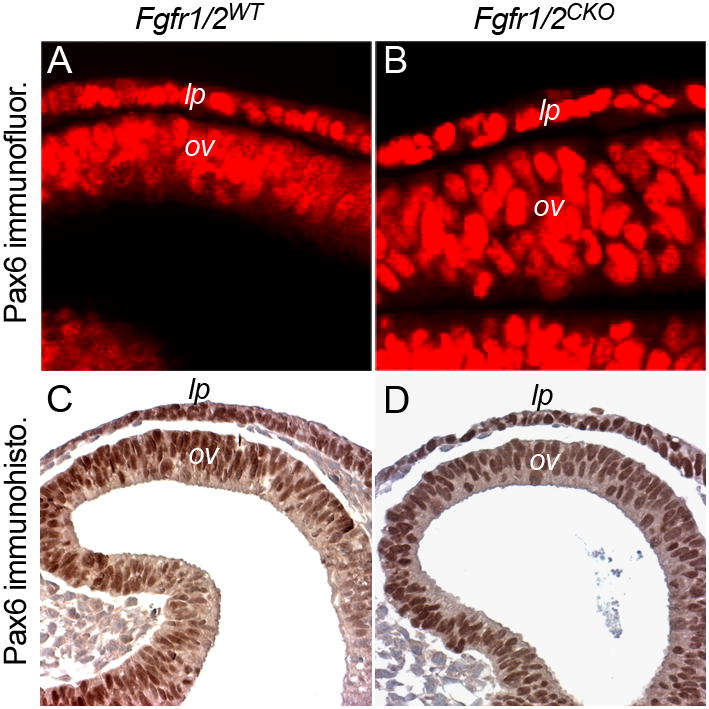
Sections of E9.5 lens placodes (lp) and optic vesicles (ov) were stained with antibodies to Pax6. Antibody staining was detected by immunofluorescence (A, B) or immunohistochemistry (C, D).
Deletion of Pax6 in the lens-forming ectoderm prevents lens formation and the expression of several genes that are required for later stages of lens cell differentiation (Ashery-Padan et al., 2000). The transcription factor, Sox2, is downstream of and genetically interacts with Pax6 in the lens placode to promote lens development (Smith et al., 2009). As with Pax6, germline mutation or deletion of Frs2α or Ndst1 reduced Sox2 expression in the lens placode of the most severely affected embryos (Gotoh et al., 2004; Pan et al., 2006). In the present study, antibodies to Sox2 stained nuclei in the retina and lens placode to a similar degree in Fgfr1/2WT and Fgfr1/2CKO eyes at E9.5 (Fig. 7A, B).
Figure 7. The effect of deleting FGF receptors on the expression of the Pax6 targets, Sox3, FoxE3 and αA-crystallin.
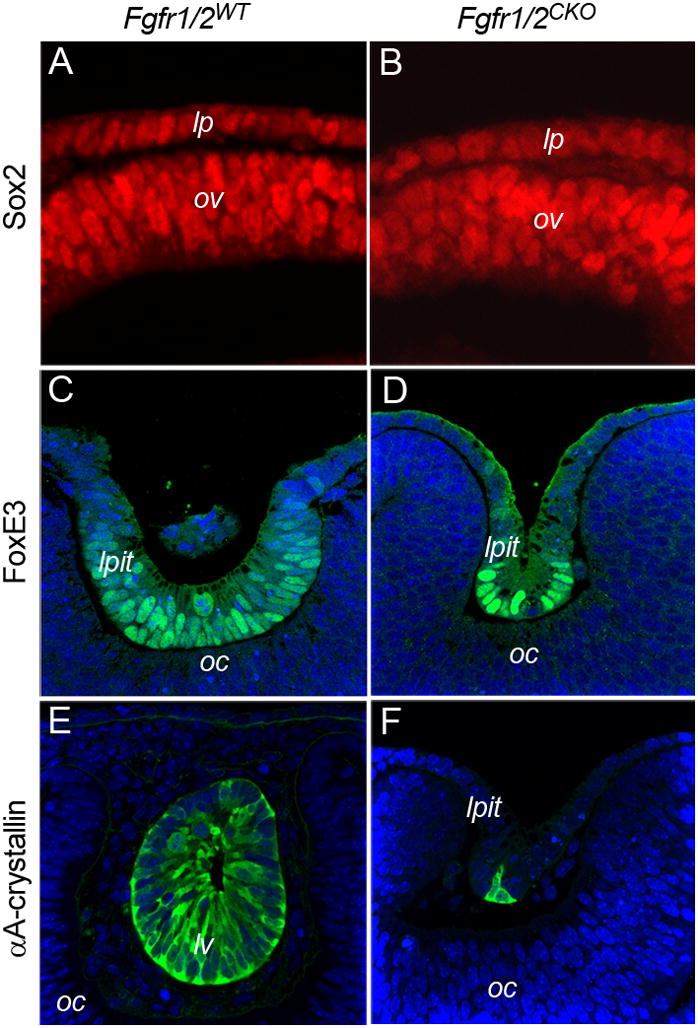
Conditional deletion of Fgfr1/2 in the lens placode did not reduce the expression of Sox2 (A, B), FoxE3 (C, D), or αA-crystallin in lens placode (lp) or lens pit (lpit) cells. The lens vesicle shown in E is from an older embryo (36 somites) than the lens pit shown in F (33 somites).
Pax6 is required for expression of the transcription factor, FoxE3, and the lens-preferred protein, αA-crystallin (Blixt et al., 2007; Brownell et al., 2000; Yang and Cvekl, 2005; Yang et al., 2006). These proteins are first detectable at the late placode/lens pit stage. Due to the extensive cell death in Fgfr1/2CKO eyes, few cells remained at the onset of lens invagination. However, cells located deep in the small lens pits stained intensely with antibodies to FoxE3 and αA-crystallin (Fig. 7C–F), showing that Pax6 function was preserved in these cells.
Interactions between BMP and FGF receptors in the lens placode
The morphogens Bmp4 and 7 are required for lens formation (Furuta and Hogan, 1998; Jena et al., 1997; Wawersik et al., 1999). Conditional deletion of the type I BMP receptors, Bmpr1a and Acvr1 in the lens placode prevented lens formation, demonstrating that BMP signaling is required in the ectoderm cells for lens induction (Rajagopal et al., 2009). Removal of one allele of Bmp7 enhanced the phenotype of a dominant-negative Fgfr1 transgene in the lens placode, suggesting that BMP and FGF signaling pathways interact in lens development (Faber et al., 2001). Deletion of either Fgfr2 or Bmpr1a significantly increased cell death, resulting in the formation of smaller lenses (Garcia et al., 2005; Rajagopal et al., 2009). To explore the nature of the interaction between BMP and FGF signaling during placode formation, we conditionally deleted Fgfr2 and Bmpr1a using Le-Cre. Cre-positive mice were born with small eyes resulting from absence of the lens (Fig. 8A–B). At E10.5, Fgfr2/Bmpr1aCKO lens placodes were thin, with numerous TUNEL-positive cells (Fig. 8C–D). The TUNEL labeling index in the Fgfr2/Bmpr1aCKO placodes increased 2.5 fold, compared to Fgfr2/Bmpr1aWT (Fig. 8E). These results confirm that inhibition of FGF and BMP signaling can have additive effects on lens formation and show that these defects involve increased cell death.
Figure 8. Deleting Fgfr2 and Bmpr1a increased apoptosis and prevented lens formation.
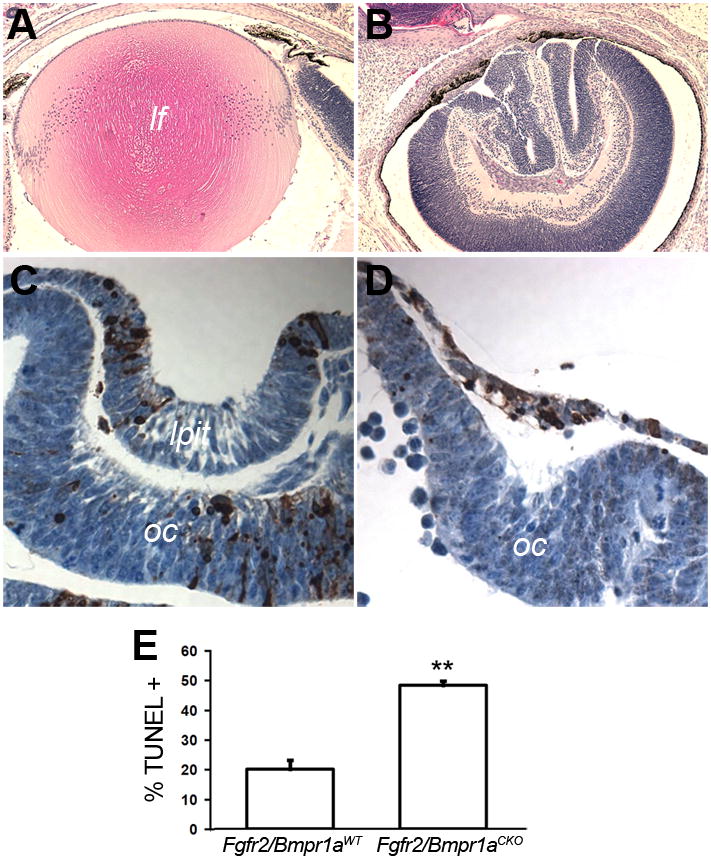
Conditional deletion of Fgfr2 and Bmpr1a resulted in eyes with no detectable lens at P1 (A, B). At E10.5, extensive apoptosis, decreased placode thickness and failure of lens pit formation was evident (C, D). At E10.5, the TUNEL labeling index increased greatly in double knockout lens placodes, compared to wild type (E).
DISCUSSION
The tissue interactions required for lens formation have been studied for over a century (Grainger et al., 1988; Lang, 2004; Spemann, 1901; Swindell et al., 2008). The requirement for the optic vesicle to promote lens placode thickening and invagination is one of the most thoroughly-studied examples of embryonic induction. Prior to lens placode formation, the prospective lens ectoderm is exposed to signals that permit it to respond to the final inductive stimulus from the optic vesicle and must be shielded from influences that inhibit lens formation (Bailey et al., 2006; Grainger et al., 1992; Sullivan et al., 2004). In the analysis that follows, we reserve the classical term “lens induction” for the effects of signals or other influences that originate from the optic vesicle.
The major phenotype observed after deleting of the two most abundant FGF receptors that are expressed in the lens placode was extensive cell death, which, in most cases, resulted in loss of a morphologically-identifiable lens. Although deletion of Fgfr1 and Fgfr2 decreased the levels of several proteins that depend on FGF signaling in the lens placode cells, the transcription factor Pax6 and its downstream targets, Sox2, FoxE3 and αA-crystallin were expressed in the remaining lens cells at normal levels. In the following sections, we reconcile these observations with the results obtained in previous studies of FGF signaling during lens induction. We then consider the implications of the experiments performed in this and other studies as they relate to the role of FGF signaling in lens induction.
Germline mutations and FGF receptor function
Two previous studies used germline genetic modifications to obtain information about the role of FGF signaling in lens formation. In one study, Frs2α was mutated to prevent Frs2α from recruiting the Shp2 phosphatase to FGF receptors (Gotoh et al., 2004). In the other, Ndst1, an enzyme involved in the production of heparan sulfate proteoglycans, was genetically inactivated (Pan et al., 2006). Heparan sulfate proteoglycans are important co-factors for the efficient binding of FGFs to their receptors (Ornitz, 2000). Because these genes were inactivated in all cells, it was difficult to determine whether their deleterious effects on lens and eye formation occurred prior to or during lens induction and in which tissues FGF receptor function was most important.
Information relevant to these questions can be surmised from the phenotypes of the knockout embryos. In both studies, the authors classified the mutant phenotypes as “mild” or “severe” (Gotoh et al., 2004; Pan et al., 2006). In embryos with mild phenotypes, a smaller lens vesicle formed, with few or no abnormalities noted in the optic vesicle, the tissue responsible for lens induction. Some of these mildly-affected embryos went on to form normal-appearing eyes with small lenses. Severe phenotypes were associated with disruption of the morphogenesis or differentiation of the optic vesicle and showed little or no lens formation. These embryos later became severely microphthalmic or anophthalmic. It seems possible that the severe phenotypes were due to defects in the differentiation or morphogenesis of the optic vesicle that occurred before lens induction.
In the Ndst1 mutants, mildly affected eyes had normal levels of Pax6 in the lens placode, while more severely affected eyes had reduced Pax6 in the nuclei of placode cells (Pan et al., 2006). Since loss of Ndst1 would be expected to impair FGF signaling in all cells of every embryo, it seems likely that the lower levels of Pax6 in severely-affected embryos were due to defects in formation of the optic vesicle, rather than defects in the ability of placode cells to respond to FGFs. Pax6 expression was also reduced in the placode cells of severely-affected Frs2α mutants (Gotoh et al., 2004). Pax6 levels were not reported for mildly-affected eyes, making it unclear whether Pax6 levels were normal or reduced in the lens placodes of these less severely-affected embryos. Mutation of Frs2α also had significant effects on the expression of several genes in the optic vesicle; levels of the transcription factors, Six3 and Chx10 (Vsx2) and the morphogen, Bmp4 were greatly reduced. Bmp4 from the optic vesicle is required for lens induction (Furuta and Hogan, 1998) and Six3 and Chx10 may regulate the expression of other genes needed for lens induction. Therefore, although the results of both studies are consistent with some role for FGF signaling in lens formation, they raise questions about when FGFs act and in what tissues.
Similar concerns apply to the use of the FGF receptor antagonist, SU9597 to inhibit lens formation (Faber et al., 2001). Embryo heads cultured in this inhibitor showed decreased Pax6 expression in the lens placode and formed smaller lens pits. However, the inhibitor also decreased Pax6 levels in the optic vesicle, such that Pax6 immunostaining was always greater in the lens placode cells. Therefore, its effects on lens differentiation could have been due to its effects on lens placode cells, optic vesicle cells, or both.
Targeted interference with FGF receptor signaling in the lens placode
Transgenic over expression in the lens placode of a kinase-deficient form of Fgfr1 delayed lens invagination, reduced lens size, decreased αA-crystallin mRNA and delayed the accumulation of α-crystallin, β-crystallin and MIP, markers of lens cell differentiation (Faber et al., 2001). In the transgenic embryos, the lens placode appeared thinner and the BrdU labeling index was decreased at the lens pit stage (E10.5) and in the lens epithelial cells at E13.5. Cell death was not measured. The strength of this study is that the transgenic construct was targeted to the lens placode, minimizing concerns that it might have effects before lens induction or on other tissues required for lens formation, like the optic vesicle. However, the transgene only modestly reduced the level of Pax6 in the lens placode, if at all. In the figures shown, levels of Pax6 were always greater in the lens placode than in the adjacent, non-transgenic optic vesicle and the extent of any decrease in Pax6 levels in the lens placode was not quantified (Faber et al., 2001). The persistence of Pax6 expression in the lens placode in the present study is consistent with continued Pax6 expression several days after deletion of Fgfr1/2/3 at a the lens vesicle stage (Zhao et al., 2008). An additional concern about this experimental design is that, to be effective, the “dominant-interfering” construct must be expressed at levels in excess of the endogenous receptors. This can result in “gain-of-function” phenotypes. For example, over expression of a “dominant interfering” construct encoding kinase-dead form of the TGFβ type 2 receptor (Tgfbr2) caused abnormal lens fiber cell differentiation and eventual degeneration, but deletion of Tgfbr2 from the lens produced no lens phenotype. These results showed that TGFβ signaling is not essential for lens development and that over expression of a mutant receptor can interfere with normal development.
In the present study, FGF receptors were removed by conditional deletion using the Le-Cre transgene, which is targeted to the lens placode by the Pax6 P0 enhancer (Ashery-Padan et al., 2000). The lens placode does not form when Pax6 is deleted using Le-Cre (Smith et al., 2009). This indicates that Pax6 deletion is efficient and occurs before placode formation commences. Consistent with this observation, we found that cell death increased significantly in the lens-forming ectoderm of 20–24 somite Fgfr1/2CKO embryos, which is before or at the onset of placode formation. This early increase in apoptosis and the decrease in FGF target genes, like Erm and Er81, in the lens placode show that FGF signaling was functionally absent throughout the time when lens induction was occurring.
Increased cell death occurred throughout the placode stage, such that, by the time of invagination, few cells remained to form a lens. Deletion of FGF receptors had a dose-dependent effect on cell death; deletion of Fgfr1 and 2 resulted in greater cell death than when only Fgfr2 was deleted. Similarly, conditional deletion of Bmpr1a enhanced the cell death seen in Fgfr2CKO placodes and prevented subsequent lens formation. In spite of the severe cell death phenotype and decreased expression of FGF targets in Fgfr1/2CKO embryos, Pax6 and Sox2 were expressed at normal levels in the remaining placode cells and expression of Foxe3 and αA-crystallin was activated in the small number of lens pit cells remaining on E10.5. No morphological defects were observed in the optic vesicles in Fgfr1/2CKO embryos at the lens placode or pit stages.
It is important to consider whether the results obtained in the present study can be explained by mechanisms other than the loss of FGF receptors. For example, the Le-Cre transgene might increase placode cell death, independent of the inhibition of FGF signaling. This is unlikely, since Le-Cre did not increase the TUNEL-labeling index above that seen in Cre-negative placodes when used to delete the BMP receptor Acvr1 or the mediator of BMP and TGFβ signaling, Smad4 (Rajagopal et al., 2009). Therefore, the presence of Cre recombinase in lens placode cells, independent of its effects on the floxed FGF receptors, is unlikely to be the cause of the cell death seen in this study.
Although smaller lens size was a common phenotype in all studies in which FGF signaling was impaired, only one other study of the role of FGFs in lens induction measured apoptosis in the lens placode or vesicle. In contrast to results obtained in the present study, Ndst1 null lens vesicles showed no obvious increase in TUNEL labeling (Pan et al., 2006). Although heparan sulfate proteoglycans are important co-receptors for FGF signaling, deletion of Ndst1 must not completely block FGF receptor activation, since germline deletion of Fgfr1 causes developmental arrest at gastrulation, while Ndst1 null embryos survive until after birth (Fan et al., 2000). Therefore, the cell death that occurs when FGF receptors are deleted in the lens placode may be avoided in Ndst1 null embryos by the partial activation of FGF receptors.
The role of FGF signaling in the lens placode
Previous investigations found that FGFs are expressed in the lens placode. These include Fgf1 and Fgf2 in rats (de Iongh and McAvoy, 1993) and Fgf19 in chickens and zebrafish (Fgf19 in humans and chickens is the ortholog of Fgf15 in the mouse, designated in this report as Fgf15/19) (Kurose et al., 2004; Nakayama et al., 2008; Wright et al., 2004). The present study extends this number to at least 5. Therefore, it is reasonable that FGF receptors in lens placode cells are activated in an autocrine or paracrine manner by FGFs produced within the ectoderm.
FGF signaling is required to establish the preplacodal region that gives rise to all cranial placodes (Bailey et al., 2006). However, later FGF signaling inhibited lens placode differentiation and promoted olfactory fate in the chicken embryo (Bailey et al., 2006). Fgf8 transcripts were detected in the optic vesicle of chicken embryos (Karabagli et al., 2002; Tian et al., 2002; Vogel-Hopker et al., 2000) and ectopic addition of Fgf8 prior to lens induction promoted the formation of cells that expressed L-maf, a marker of lens formation in chicken embryos (Vogel-Hopker et al., 2000). However, in situ hybridization analysis did not detect Fgf8 in the mouse optic vesicle at E9.0 (Crossley and Martin, 1995), a result consistent with our microarray studies of optic vesicle cells at E9.5 (data not shown). Fgf15/19 is important for lens formation in the zebrafish and may be involved in lens formation in chicken embryos (Kurose et al., 2005; Nakayama et al., 2008). Previous in situ hybridization studies showed that Fgf15/19 was abundant in the distal optic vesicle of the mouse embryo (McWhirter et al., 1997). However, germline deletion of Fgf15/19 in the mouse was not reported to affect eye development (Wright et al., 2004). Fgf15/19 binds selectively to Fgfr4 (Kurose et al., 2005), which was barely detectable in the mouse lens placode and germline deletion of Fgfr4 did not result in an obvious eye or lens phenotype (Weinstein et al., 1998), a result that we have confirmed (data not shown). Therefore, a role for FGFs from the optic vesicle in lens induction remains unclear.
Conclusions
The data from the present study suggest that the primary role of FGF signaling in the mouse lens placode is to activate or maintain survival signaling pathways. Since other investigators did not detect increased cell death or extensive loss of placode cells when FGF signaling was impaired in the ectoderm, our results represent the most severe lens phenotype resulting from interference with FGF signaling in early lens development. Since no increase in TUNEL labeling was detected when the optic vesicle was severely disrupted in Ndst1 knockout embryos, FGFs from the optic vesicle are unlikely to be required for cell survival in the lens placode (Pan et al., 2006). Endogenous FGFs may serve this function (Faber et al., 2001; Pan et al., 2006).
Apoptosis was 4–6 fold higher in Fgfr1/2CKO ectoderm at the beginning of placode formation, the time when lens induction is initiated. This indicates that Fgfr1 and 2 were functionally absent during the period of induction and could not have contributed to induction. In spite of the functional absence of FGF signaling, the increased expression of Pax6 that occurs during induction occurred normally and the expression levels of Pax6 downstream targets was unaffected. We conclude from these data that FGFs are not involved in lens induction by the optic vesicle.
Supplementary Material
Staining for phosphorylated ERK (pERK) in Fgfr1 and Fgfr2 wild type and conditional knockout lens placodes. In this figure, staining in the Fgfr1/2CKO eye is over exposed (compare pERK staining in the optic vesicles), yet staining in the ectoderm is significantly reduced at this early stage of lens placode formation.
Table 2.
PCR primer sequences
| Transcript | Primer Sequences | Product Size (bp) |
|---|---|---|
| Fgfr1 | F 5′-CCCTCAGGAAACAGAAAACG-3′ | 238 |
| R 5′-GAAGCAGCCCTAACCCCTAC-3′ | ||
| Fgfr2 | F 5′-CTCTCGAGGGATGGCAAAAG-3′ | 216 |
| R 5′-AGCAAAGTGAGTGGGCGTAT-3′ | ||
| Fgfr3 | F 5′-CCCTGCAAGAAGGTTCAGAT-3′ | 209 |
| R 5′-CCTAGGGCCCAGTGACAGTA-3′ | ||
| Fgfr4 | F 5′-CCCTTGGACTCATCCTCAGA-3′ | 214 |
| R 5′-TCACCAAGATGCTGGAACAA-3′ | ||
| Fgf1 | F 5′-TGTGTACGAAGTCCCAAGACC3′ | 223 |
| R 5-GTCTTCAATGGCAGCTGATGT3′ | ||
| Fgf2 | F 5′-CTTACCGGTCACGGAAATACTC-3′ | 128 |
| R 5′-TCAGCTCTTAGCAGACATTGGA-3′ | ||
| Fgf7 | F 5′-CCAGTGAGAACTATAATCCGGAAA-3′ | 107 |
| R 5′-CAGTACAGGCATGTTCCAAGC-3′ | ||
| Fgf9 | F 5′-CTGTGTATCACCCTGGGAAGT-3′ | 165 |
| R 5′-GAGTGGTTTGGCTATCTGAGC-3′ | ||
| Fgf10 | F 5′-TTCTGCCTCCGTGGGAAGT-3′ | 130 |
| R 5′-TGAGGATTAGGAGGAGGGAAG-3′ | ||
| Fgf15 | F 5′-CCAGGAGCTTGTCTCTGTCC-3′ | 154 |
| R 5′-ACCAGAACTGAGAGCCAGGA-3′ | ||
| Fgf18 | F 5′-CACAGTCACCAAGCGATCC-3′ | 184 |
| R 5′-CCCCTCCTCCCAAGACTTTA-3′ | ||
| Fgf22 | F 5′-TTCTCCTCCACTCACTTTTTCC-3′ | 103 |
| R 5′-GGACAGAACGGATCTCCACTAT-3′ |
Acknowledgments
Research was supported by NIH Grants EY04853 (DCB) and E012995 (MLR), an unrestricted grant from Research to Prevent Blindness, and Core Grant EY02687 to the Department of Ophthalmology and Visual Sciences, Washington University. The authors are indebted to Juha Partanen for providing the Fgfr1flox mice, David Ornitz for Fgfr2flox, Yuji Mishina for Bmpr1aflox, Ruth Ashery-Padan for Le-Cre, Usha Andley for the αA-crystallin antibody and to Peter Carlsson for the FoxE3 antibody.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey AP, Bhattacharyya S, Bronner-Fraser M, Streit A. Lens Specification Is the Ground State of All Sensory Placodes, from which FGF Promotes Olfactory Identity. Developmental Cell. 2006;11:505–517. doi: 10.1016/j.devcel.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Blixt A, Landgren H, Johansson BR, Carlsson P. Foxe3 is required for morphogenesis and differentiation of the anterior segment of the eye and is sensitive to Pax6 gene dosage. Developmental Biology. 2007;302:218–229. doi: 10.1016/j.ydbio.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Brownell I, Dirksen M, Jamrich M. Forkhead Foxe3 maps to the dysgenetic lens locus and is critical in lens development and differentiation. Genesis. 2000;27:81–93. doi: 10.1002/1526-968x(200006)27:2<81::aid-gene50>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- de Iongh R, McAvoy JW. Spatio-temporal distribution of acidic and basic FGF indicates a role for FGF in rat lens morphogenesis. Dev Dyn. 1993;198:190–202. doi: 10.1002/aja.1001980305. [DOI] [PubMed] [Google Scholar]
- Donner AL, Lachke SA, Maas RL. Lens induction in vertebrates: Variations on a conserved theme of signaling events. Seminars in Cell & Developmental Biology. 2006;17:676–685. doi: 10.1016/j.semcdb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Faber SC, Dimanlig P, Makarenkova HP, Shirke S, Ko K, Lang RA. Fgf receptor signaling plays a role in lens induction. Development. 2001;128:4425–4438. doi: 10.1242/dev.128.22.4425. [DOI] [PubMed] [Google Scholar]
- Fan G, Xiao L, Cheng L, Wang X, Sun B, Hu G. Targeted disruption of NDST-1 gene leads to pulmonary hypoplasia and neonatal respiratory distress in mice. FEBS Lett. 2000;467:7–11. doi: 10.1016/s0014-5793(00)01111-x. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Hogan BLM. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CM, Yu K, Zhao H, Ashery-Padan R, Ornitz DM, Robinson ML, Beebe DC. Signaling through FGF receptor-2 is required for lens cell survival and for withdrawal from the cell cycle during lens fiber cell differentiation. Dev Dyn. 2005;233:516–527. doi: 10.1002/dvdy.20356. [DOI] [PubMed] [Google Scholar]
- Goetz R, Dover K, Laezza F, Shtraizent N, Huang X, Tchetchik D, Eliseenkova AV, Xu CF, Neubert TA, Ornitz DM, Goldfarb M, Mohammadi M. Crystal structure of a fibroblast growth factor homologous factor (FHF) defines a conserved surface on FHFs for binding and modulation of voltage-gated sodium channels. J Biol Chem. 2009;284:17883–17896. doi: 10.1074/jbc.M109.001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M, Schoorlemmer J, Williams A, Diwakar S, Wang Q, Huang X, Giza J, Tchetchik D, Kelley K, Vega A, Matthews G, Rossi P, Ornitz DM, D’Angelo E. Fibroblast growth factor homologous factors control neuronal excitability through modulation of voltage-gated sodium channels. Neuron. 2007;55:449–463. doi: 10.1016/j.neuron.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh N, Ito M, Yamamoto S, Yoshino I, Song N, Wang Y, Lax I, Schlessinger J, Shibuya M, Lang RA. Tyrosine phosphorylation sites on FRS2{alpha} responsible for Shp2 recruitment are critical for induction of lens and retina. PNAS. 2004:0407577101. doi: 10.1073/pnas.0407577101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger RM, Henry JJ, Saha MS, Servetnick M. Recent progress on the mechanisms of embryonic lens formation. Eye. 1992;6:117–122. doi: 10.1038/eye.1992.26. [DOI] [PubMed] [Google Scholar]
- Grainger RM, Herry JJ, Henderson RA. Reinvestigation of the role of the optic vesicle in embryonic lens induction. Development. 1988;102:517–526. doi: 10.1242/dev.102.3.517. [DOI] [PubMed] [Google Scholar]
- Grindley JC, Hargett LK, Hill RE, Ross A, Hogan BL. Disruption of PAX6 function in mice homozygous for the Pax6Sey-1Neu mutation produces abnormalities in the early development and regionalization of the diencephalon. Mech Dev. 1997;64:111–126. doi: 10.1016/s0925-4773(97)00055-5. [DOI] [PubMed] [Google Scholar]
- Huang J, Dattilo LK, Rajagopal R, Liu Y, Kaartinen V, Mishina Y, Deng CX, Umans L, Zwijsen A, Roberts AB, Beebe DC. FGF-regulated BMP signaling is required for eyelid closure and to specify conjunctival epithelial cell fate. Development. 2009;136:1741–1750. doi: 10.1242/dev.034082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Functional evolutionary history of the mouse Fgf gene family. Dev Dyn. 2008;237:18–27. doi: 10.1002/dvdy.21388. [DOI] [PubMed] [Google Scholar]
- Jena N, Martin-Seisdedos C, McCue P, Croce CM. BMP7 null mutation in mice: developmental defects in skeleton, kidney, and eye. Exp Cell Res. 1997;230:28–37. doi: 10.1006/excr.1996.3411. [DOI] [PubMed] [Google Scholar]
- Karabagli H, Karabagli P, Ladher RK, Schoenwolf GC. Survey of fibroblast growth factor expression during chick organogenesis. Anat Rec. 2002;268:1–6. doi: 10.1002/ar.10129. [DOI] [PubMed] [Google Scholar]
- Kurose H, Bito T, Adachi T, Shimizu M, Noji S, Ohuchi H. Expression of Fibroblast growth factor 19 (Fgf19) during chicken embryogenesis and eye development, compared with Fgf15 expression in the mouse. Gene Expression Patterns. 2004;4:687–693. doi: 10.1016/j.modgep.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Kurose H, Okamoto M, Shimizu M, Bito T, Marcelle C, Noji S, Ohuchi H. FGF19-FGFR4 signaling elaborates lens induction with the FGF8-L-Maf cascade in the chick embryo. Dev Growth Differ. 2005;47:213–223. doi: 10.1111/j.1440-169X.2005.00795.x. [DOI] [PubMed] [Google Scholar]
- Lang RA. Pathways regulating lens induction in the mouse. Int J Dev Biol. 2004;48:783–791. doi: 10.1387/ijdb.041903rl. [DOI] [PubMed] [Google Scholar]
- McWhirter JR, Goulding M, Weiner JA, Chun J, Murre C. A novel fibroblast growth factor gene expressed in the developing nervous system is a downstream target of the chimeric homeodomain oncoprotein E2A-Pbx1. Development. 1997;124:3221–3232. doi: 10.1242/dev.124.17.3221. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR. Generation of Bmpr/Alk3 conditional knockout mice. Genesis. 2002;32:69–72. doi: 10.1002/gene.10038. [DOI] [PubMed] [Google Scholar]
- Nakayama Y, Miyake A, Nakagawa Y, Mido T, Yoshikawa M, Konishi M, Itoh N. Fgf19 is required for zebrafish lens and retina development. Developmental Biology. 2008;313:752–766. doi: 10.1016/j.ydbio.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Ogino H, Fisher M, Grainger RM. Convergence of a head-field selector Otx2 and Notch signaling: a mechanism for lens specification. Development. 2008;135:249–258. doi: 10.1242/dev.009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong SH, Guy GR, Hadari YR, Laks S, Gotoh N, Schlessinger J, Lax I. FRS2 proteins recruit intracellular signaling pathways by binding to diverse targets on fibroblast growth factor and nerve growth factor receptors. Mol Cell Biol. 2000;20:979–989. doi: 10.1128/mcb.20.3.979-989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM. FGFs, heparan sulfate and FGFRs: complex interactions essential for development. Bioessays. 2000;22:108–112. doi: 10.1002/(SICI)1521-1878(200002)22:2<108::AID-BIES2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2:reviews 3005.3001–reviews 3005.3012. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- Pan Y, Woodbury A, Esko JD, Grobe K, Zhang X. Heparan sulfate biosynthetic gene Ndst1 is required for FGF signaling in early lens development. Development. 2006:4933–4944. doi: 10.1242/dev.02679. [DOI] [PubMed] [Google Scholar]
- Rajagopal R, Huang J, Dattilo LK, Kaartinen V, Mishina Y, Deng CX, Umans L, Zwijsen A, Roberts AB, Beebe DC. The type I BMP receptors, Bmpr1a and Acvr1, activate multiple signaling pathways to regulate lens formation. Dev Biol. 2009;335:305–316. doi: 10.1016/j.ydbio.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha M, Spann C, Grainger R. Embryonic lens induction: More than meets the optic vesicle. Cell Differ. 1989;28:153–172. doi: 10.1016/0922-3371(89)90001-4. [DOI] [PubMed] [Google Scholar]
- Smith AN, Miller LA, Radice G, Ashery-Padan R, Lang RA. Stage-dependent modes of Pax6-Sox2 epistasis regulate lens development and eye morphogenesis. Development. 2009;136:2977–2985. doi: 10.1242/dev.037341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AN, Radice G, Lang RA. Which FGF ligands are involved in lens induction? Developmental Biology. 2010;337:195–198. doi: 10.1016/j.ydbio.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spemann H. Uber Correlationen in der Entwickelung des Auges. Verh Anat Ges. 1901;15:61–79. [Google Scholar]
- Sullivan CH, Braunstein L, Hazard-Leonards RM, Holen AL, Samaha F, Stephens L, Grainger RM. A re-examination of lens induction in chicken embryos: in vitro studies of early tissue interactions. Int J Dev Biol. 2004;48:771–782. doi: 10.1387/ijdb.041894cs. [DOI] [PubMed] [Google Scholar]
- Swindell EC, Liu C, Shah R, Smith AN, Lang RA, Jamrich M. Eye formation in the absence of retina. Developmental Biology. 2008;322:56–64. doi: 10.1016/j.ydbio.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian E, Kimura C, Takeda N, Aizawa S, Matsuo I. Otx2 Is Required to Respond to Signals from Anterior Neural Ridge for Forebrain Specification. Developmental Biology. 2002;242:204–223. doi: 10.1006/dbio.2001.0531. [DOI] [PubMed] [Google Scholar]
- Trokovic R, Trokovic N, Hernesniemi S, Pirvola U, Vogt Weisenhorn DM, Rossant J, McMahon AP, Wurst W, Partanen J. FGFR1 is independently required in both developing mid- and hindbrain for sustained response to isthmic signals. EMBO J. 2003;22:1811–1823. doi: 10.1093/emboj/cdg169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel-Hopker A, Momose T, Rohrer H, Yasuda K, Ishihara L, Rapaport DH. Multiple functions of fibroblast growth factor-8 (FGF-8) in chick eye development. Mech Dev. 2000;94:25–36. doi: 10.1016/s0925-4773(00)00320-8. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R. BMP7 acts in murine lens placode development. Dev Biol. 1999;207:176–188. doi: 10.1006/dbio.1998.9153. [DOI] [PubMed] [Google Scholar]
- Weinstein M, Xu X, Ohyama K, Deng CX. FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development. 1998;125:3615–3623. doi: 10.1242/dev.125.18.3615. [DOI] [PubMed] [Google Scholar]
- Wright TJ, Ladher R, McWhirter J, Murre C, Schoenwolf GC, Mansour SL. Mouse FGF15 is the ortholog of human and chick FGF19, but is not uniquely required for otic induction. Developmental Biology. 2004;269:264–275. doi: 10.1016/j.ydbio.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Yang Y, Cvekl A. Tissue-specific regulation of the mouse alphaA-crystallin gene in lens via recruitment of Pax6 and c-Maf to its promoter. J Mol Biol. 2005;351:453–469. doi: 10.1016/j.jmb.2005.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Stopka T, Golestaneh N, Wang Y, Wu K, Li A, Chauhan BK, Gao CY, Cveklova K, Duncan MK, Pestell RG, Chepelinsky AB, Skoultchi AI, Cvekl A. Regulation of alphaA-crystallin via Pax6, c-Maf, CREB and a broad domain of lens-specific chromatin. Embo J. 2006;25:2107–2118. doi: 10.1038/sj.emboj.7601114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, Towler DA, Ornitz DM. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- Zhao H, Yang T, Madakashira BP, Thiels CA, Bechtle CA, Garcia CM, Zhang H, Yu K, Ornitz DM, Beebe DC, Robinson ML. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol. 2008;318:276–288. doi: 10.1016/j.ydbio.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Staining for phosphorylated ERK (pERK) in Fgfr1 and Fgfr2 wild type and conditional knockout lens placodes. In this figure, staining in the Fgfr1/2CKO eye is over exposed (compare pERK staining in the optic vesicles), yet staining in the ectoderm is significantly reduced at this early stage of lens placode formation.