Summary
Impaired insulin secretion contributes to the pathogenesis of type 2 diabetes mellitus (T2DM). Treatment with the incretin hormone glucagon like peptide-1 (GLP-1) potentiates insulin secretion and improves metabolic control in humans with T2DM. GLP-1 receptor-mediated signaling leading to insulin secretion occurs via cyclic AMP stimulated protein kinase A (PKA)- as well as guanine nucleotide exchange factor- mediated pathways. However, how these two pathways integrate and coordinate insulin secretion remains poorly understood. Here, we show that these incretin-stimulated pathways converge at the level of snapin, and that PKA-dependent phosphorylation of snapin increases interaction among insulin secretory vesicle-associated proteins, thereby potentiating glucose-stimulated insulin secretion (GSIS). In diabetic islets with impaired GSIS, snapin phosphorylation is reduced, and expression of a snapin mutant, which mimics site-specific phosphorylation, restores GSIS. Thus, snapin is a critical node in GSIS regulation and provides a potential therapeutic target to improve ϐ-cell function in T2DM.
Introduction
Insufficient glucose-stimulated insulin secretion (GSIS) significantly contributes towards hyperglycemia in type 2 diabetes mellitus (T2DM) (Prentki and Nolan, 2006). Insulin is stored in secretory vesicles in pancreatic ϐ-cells and is secreted by exocytosis to precisely control blood glucose homeostasis (Gauthier and Wollheim, 2008; Lang, 1999). Upon stimulus by glucose, ϐ-cells secrete insulin in a biphasic manner, which is considered to be important for optimal glycemic control (Del Prato and Tiengo, 2001; Pimenta et al., 1995). An early, first-phase insulin release occurs during the first few minutes of glucose stimulus, whereas later time points comprise the second phase of GSIS. Humans at risk of developing T2DM or with established T2DM exhibit defective first-phase insulin release well before detectable changes in the second phase (Gerich, 2002; Lillioja et al., 1988; Vaag et al., 1995; Ward et al., 1984), and restoration of first phase insulin secretion corrects glycemic control (Basu et al., 1996).
The incretin hormone glucagon-like peptide-1 (GLP-1) and its peptide analogue exendin-4 (E4) improve metabolic control in T2DM predominantly by restoring first phase and augmenting second phase insulin secretion in humans with T2DM (Egan et al., 2002; Fehse et al., 2005). In addition to their secretagogue effects, GLP-1 and E4 stimulate proliferation and inhibit apoptosis in rodent ϐ-cells (Drucker, 2006). Most, if not all effects of GLP-1 and E4 in ϐ-cells appear to require intracellular activation of the adenosine-3′-5′-cyclic monophosphate (cAMP)- protein kinase A (PKA) signaling pathway by the G-protein coupled receptor of GLP-1, which is highly expressed on pancreatic ϐ-cells (Drucker and Nauck, 2006). A second mechanism of PKA-independent incretin potentiation of GSIS involves cAMP-regulated guanine nucleotide exchange factor (cAMP-GEF) EPAC2 (Seino and Shibasaki, 2005). However, PKA-activity appears to be essential for optimal incretin effects on stimulating insulin vesicle exocytosis (Chepurny et al., 2010; Doyle and Egan, 2007).
In ϐ-cells, insulin exocytosis is regulated in part by specific kinases, which by altering protein phosphorylation modify assembly of proteins associated to secretory vesicles (Foster et al., 1998; Kwan et al., 2006; Shimazaki et al., 1996). Appropriate assembly of vesicle-associated proteins prepare the secretory vesicle for exocytosis. In ϐ-cells, glucose metabolism-induced Ca2+ elevation is required for the final step of vesicle fusion to the cell membrane (Gauthier and Wollheim, 2008; Takahashi et al., 2010). While PKA signaling serves a central role in incretin GSIS potentiation (Kwan et al., 2006; Seino and Shibasaki, 2005), how PKA-dependent and –independent effects of cAMP signaling are coordinated and integrated is unclear. The node at which these two pathways converge, a protein likely to be the target of PKA-dependent phosphorylation and to participate in insulin vesicle exocytosis regulation, remains to be identified.
To examine specifically in vivo effects of PKA signaling in pancreatic ϐ-cells and to identify a PKA target protein important in mediating coordinated incretin effects on GSIS, we have generated a mouse model of disinhibited PKA activity by conditional ablation of the inhibitory PKA regulatory subunit 1A (prkar1a). This mouse exhibits augmented GSIS and improved glucose tolerance, in absence of fasting hyperinsulinemia and hypoglycemia or changes in ϐ-cell proliferation or – mass. We further find that humans that carry inactivating mutations in the PKAR1A encoding gene also exhibit augmented insulin secretion and more rapid glucose disposal in response to an oral glucose load, indicating a trans-species preservation of the central regulatory role of PRKAR1A in ϐ-cell insulin secretion.
Here, we show that PKA mediates incretin action on GSIS and insulin exocytosis via phosphorylation of snapin, an exocytosis modulating protein initially identified in neuronal synapses (Chheda et al., 2001; Ilardi et al., 1999). Snapin phosphorylation is required for its interaction with protein components of the insulin vesicle exocytosis apparatus and for integrating non PKA-dependent incretin effects on insulin secretion (Seino and Shibasaki, 2005). Expression in islets of a mutant snapin (S50D), mimicking site specific phosphorylation, reproduces incretin effects in ϐ-cells, including the restoration of first-phase GSIS in islets of diabetic mice.
Our findings indicate that snapin is a critical mediator of PKA-dependent amplification of glucose-stimulated insulin exocytosis, and suggest its potential as therapeutic target in strategies to improve ϐ-cell function in diabetic mellitus.
Results
Disinhibited PKA activity in ϐ-cells augments glucose stimulated insulin secretion
Mouse model of Prkar1a ablation and disinhibited PKA activity in pancreas
To examine specifically the role of PKA in ϐ-cells without participation of cAMP-stimulated PKA-independent (cAMP-GEF) pathways, we generated a mouse model with tissue-specific disinhibition of PKA activity. The protein kinase A (PKA) holoenzyme is composed of a heterotetramer consisting of two PKA catalytic subunits (Pkac) bound to two PKA regulatory subunits (Prkar). Once generated through ligand G-protein coupled receptor activation of adenylate-cyclase, cellular cyclic AMP binds Prkar, thereby releasing and activating the Pkac.
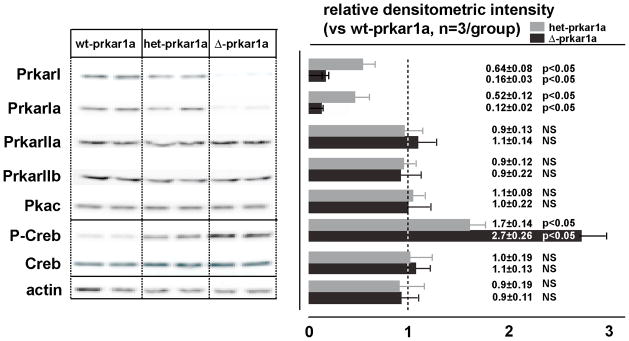
Prkar1a, the most abundant regulatory subtype in ϐ-cells (Petyuk et al., 2008), was conditionally ablated using the CRE/LoxP method by interbreeding floxed prkar1a (prkar1afl/fl) (Kirschner et al., 2005) and pancreas-specific Pdx1-CRE mice (Lammert et al., 2001). Offspring from such breeding carried all expected genotypes at Mendelian frequency. Pdx1-CRE null/prkar1afl/fl (=WT) served as control animals. Body weight of littermates with different genotypes did not vary during an observation period of up to 16 weeks (not shown). Immunoblots of islet extracts from Pdx1-CRE+/prkar1afl/fl (=Δ–prkar1a), as compared to controls, revealed a 90% reduction in Prkar1a protein, while other Prkar isoforms and Pkac levels remained unaltered (Figure 1). Δ–prkar1a mice exhibited increased islet PKA catalytic activity as reflected by elevated phosphorylation at serine 133 of Creb (cyclic AMP response element binding protein), an established Pkac target (Gonzalez and Montminy, 1989) (Figure 1). Islets with heterozygous prkar1a ablation (het-prkar1a) showed the expected 50% reduction in Prkar1a protein (Figure 1).
Figure 1. Prkar1a ablation in pancreatic islets.
Immunoblot (left) with densitometric analysis (right) of total islet protein from wt-prkar1a, het-prkar1a, Δ-prkar1a mice. Specific Prkar1a ablation is detectable, while other prkar subtypes and Pkac remain unchanged. Prkar1a expression is approximately 50% reduced in het-prkar1a islets and 90% reduced in Δ-prkar1a islets. CREB phosphorylation increases with reduced Prkar1a abundance.
Prkar1a ablation does not alter ϐ–cell proliferation or -mass
In 8–9 week old het-/Δ–prkar1a and control littermates, islet size or ϐ-cell mass were similar, as also were proliferation markers Ki67 and EdU incorporation in ϐ-cells. Insulin content was not different between Δ–prkar1a and control islets (Table S1). The exocrine pancreas was unaltered in Δ–prkar1a mice (not shown). These results indicate that disinhibition of PKA catalytic activity in pancreatic islets does not change ϐ-cell mass or insulin content.
Δ–prkar1a mice have improved glucose tolerance and glucose-stimulated insulin secretion
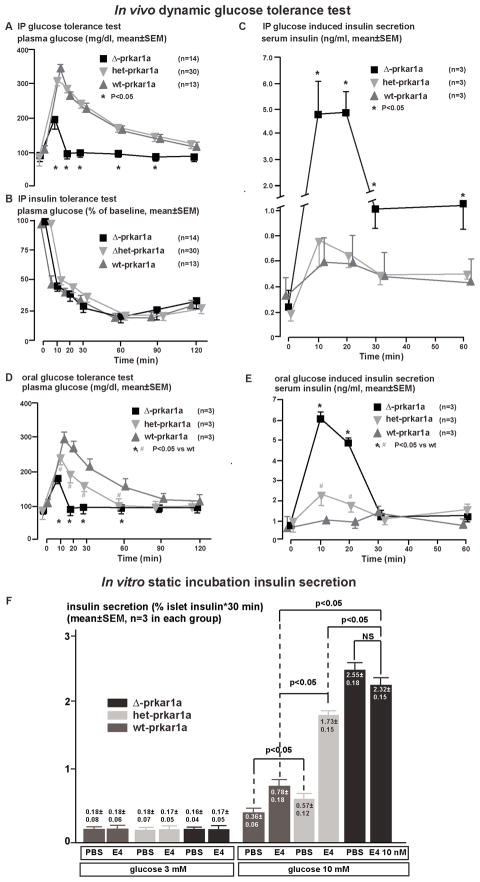
At 8–9 weeks of age, fasting glucose levels in het-/Δ–prkar1a mice were slightly and not significantly reduced when compared to controls (Figure 2A). Prolonged observation up to 20 weeks of age and overnight fasting (18h) did not reveal any differences in glucose levels between Δ–prkar1a and littermates with normal or haploinsufficient prkar1a complement (data not shown). In contrast, during ipGTT, Δ–prkar1a mice exhibited dramatically reduced glucose excursions (Figure 2A, Table S2). Insulin tolerance tests revealed no differences in peripheral insulin sensitivity between Δ–prkar1a and controls (Figure 2B). Δ–prkar1a mice exhibited significantly higher serum insulin levels in response to intraperitoneal glucose administration, while baseline insulin levels were not significantly different (Figure 2C, Table S2). Identical findings were made with ϐ-cell specific prkar1a ablation using RIP2-CRE mice (not shown). These results indicate that in Δ–prkar1a mice, in vivo GSIS is augmented, while basal glucose and insulin levels are unaffected. This finding is reminiscent of incretin-mediated effects on GSIS, albeit under conditions of intra-peritoneal glucose administration, which bypasses intestinal incretin hormone secretion (Drucker, 2006). These observations suggest that islet Pkac disinhibition mimics incretin potentiation of GSIS.
Figure 2. Glucose stimulated insulin secretion in Δ-prkar1a, het-prkar1a, and wt-prkar1a mice in vivo and from respective mouse islets in vitro.
A) Plasma glucose levels during an ipGTT in littermates of indicated genotypes. Δ-prkar1a mice have markedly diminished glucose excursion during ipGTT but do not exhibit baseline or post glucose hypoglycemia. (* p<0.05).
B) Plasma glucose levels during ipITT in littermates of indicated genotypes. No difference is seen in insulin sensitivity among the different genotypes. (* p<0.05).
C) Serum insulin levels during ipGTT in littermates of indicated genotypes. All animals have similar baseline insulin levels. Δ-prkar1a mice have markedly increased glucose stimulated insulin levels, predominantly during the initial phases of ipGTT (* p<0.05).
D) Plasma glucose levels during an oGTT in littermates of indicated genotypes. Baseline glucose levels are similar in all animals. Δ-prkar1a and het-prkar1a mice, respectively, have marked and graded reductions in glucose excursion during oGTT. Neither exhibit baseline or post glucose hypoglycemia. (*, # p<0.05 vs wt).
E) Serum insulin levels during oGTT in littermates of indicated genotypes. All animals have similar baseline insulin levels. Δ-prkar1a mice have marked and graded increases in glucose stimulated insulin levels (*, # p<0.05 vs wt).
F) In vitro static insulin secretion assay of islets cultured in glucose (3 and 10 mM) without and with E4 (10 nM) stimulated insulin secretion (* p<0.05). Accumulated insulin in supernatant was normalized to insulin in corresponding islets and provided in % islet insulin content
Het-prkar1a and Δ–prkar1a islets showed a graded increase in serine 133 Creb phosphorylation (Figure 1), indicating a functional relationship between disinhibited Pkac activity and amount of islet Prkar1a. We reasoned that Prkar1a may also have a gene dose-related effect on incretin action in ϐ-cells. During an oral glucose tolerance test (oGTT), which stimulates intestinal incretin hormone secretion from intestinal L-cells (Drucker, 2006), het-prkar1a and Δ–prkar1a exhibited in an inverse relationship to prkar1a levels, a graded improvement in glucose tolerance (Figure 2D, Table S2) and graded increase in GSIS (Figure 2E, Table S2). Taking advantage of the high expression level on ϐ-cells of the GLP-1 receptor (Doyle and Egan, 2007; Drucker, 2006), we directly tested incretin action on ϐ-cell GSIS in vitro on isolated islets in static incubation. Het-prkar1a islets secreted more insulin in response to glucose (10 mM) than WT islets. E4 further potentiated insulin secretion from het-prkar1a as compared to WT islets (Figure 2F). In addition, Δ-prkar1a islets showed maximal GSIS, which was not augmented by E4. In contrast, insulin secretion at baseline glucose levels (3 mM) was not different among the three genotypes and was not influenced by E4 (Figure 2F). These results suggest that gene dose of prkar1a regulates Pkac function and, hence, ϐ-cell response to incretin. Also, the results indicate that with Pkac disinhibition in complete prkar1a absence, GSIS is maximal with no additive incretin effect by E4.
Humans with inactivating PRKAR1A mutations exhibit increased glucose-stimulated insulin secretion
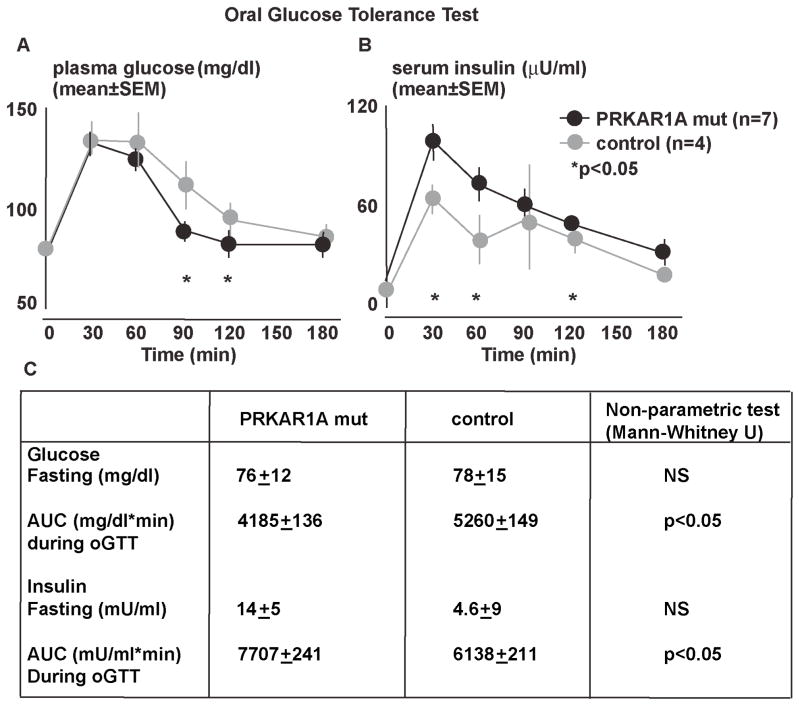
We next examined whether our observations of murine islet PKA disinhibition are reflected in humans carrying inactivating mutations in PRKAR1A. From archival National Institutes of Health (NIH) data of oral glucose tolerance tests, we identified young (17–27 years old) patients with inactivating PRKAR1A mutations (Kirschner et al., 2000) without any confounding endocrine hypersecretion (see Table S3 for patient characteristics). During oGTT, individuals with inactivating PRKAR1A mutations, as compared to matched controls, exhibited significantly higher serum insulin levels combined with more rapid glucose disposal (Figure 3), as also reflected, respectively, by areas under the (AUC) insulin and glucose curves (Figure 3). Remarkably, these results resemble oGTT in het-prkar1a mice (Figure 2D–F).
Figure 3. Oral GTT after in humans with inactivating PRKAR1A mutations and controls.
A) Subjects with a PRKAR1A mutation exhibit normal fasting glucose levels but reduced glucose excursion after an oral glucose load.
B) In subjects with a PRKAR1A mutation serum insulin levels were not different at baseline, and reached a higher peak with increased overall insulin secretion.
C) Table summarizing fasting glucose and insulin levels as well as area under the glucose and insulin curves shown in A and B (*p<0.05)
Taken together, these findings indicate that Prkar1a subunit influences both mouse and human ϐ-cell function. Furthermore, based on the studies in mouse islets (Figure 2F), it is reasonable to conclude that with haploinsufficient prkar1a complement, increased GSIS during an oGTT is at least in part due to increased ϐ-cell response to incretin action (Figure 2F).
Δ-prkar1a ϐ-cells exhibit altered insulin vesicle morphology and location
As increased insulin secretion in Δ-prkar1a mice is not due to changes in ϐ-cell mass or insulin production (Figure 2, Table S1), we reasoned that improved GSIS results from alterations related to insulin vesicle exocytosis. Ultrastructural analyses of Δ-prkar1a ϐ-cells showed significantly more insulin vesicles located along the plasma membrane adjacent to intra-islet capillaries (Figure S2 A–F). In these mice, insulin vesicles located closer than 1 μm to the plasma membrane were larger in size, while the dense core size within the vesicles containing immuno-detectable insulin were not different from controls. (Figure S2 G–I). These findings suggest that in ϐ-cells, disinhibited PKA signaling results in altered morphologic characteristics of insulin vesicles, which may possibly influence GSIS.
PKA-dependent snapin phosphorylation increases GSIS
PKA mediates snapin phosphorylation at serine 50 in ϐ-cells
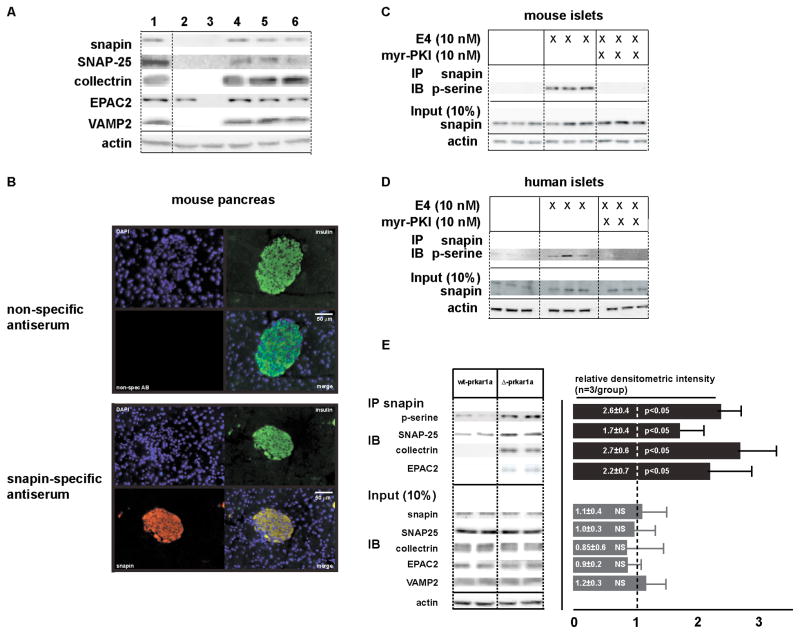
The morphological changes in insulin vesicles in Δ-prkar1a ϐ-cells, prompted us to investigate candidate PKA phosphorylation targets associated with secretory vesicle exocytosis. PKA-dependent snapin phosphorylation modulates vesicle exocytosis in neuronal synapses by direct interaction with the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complex protein SNAP-25 (Ilardi et al., 1999). Snapin is also implicated in calcium-triggered vesicle fusion by regulating the association between the SNARE complex and the Ca2+ -sensor synaptotagmin (Ilardi et al., 1999). In ϐ-cells, snapin interacts with SNAP-25 and collectrin, a protein involved in regulating insulin secretion and a transcriptional target of the maturity onset of diabetes in the young (MODY) 3 gene HNF1β (Fukui et al., 2005). We confirmed the presence of snapin protein in pancreatic ϐ-cell lines and mouse islets (Fukui et al., 2005) (Figure 4A). Immunohistochemical analysis of mouse pancreas showed snapin co-localization with insulin (Figure 4B), indicating high expression levels of snapin protein in ϐ-cells. Despite reports that snapin is expressed ubiquitously (Buxton et al., 2003) we did not find snapin immunoreactivity in exocrine tissue.
Figure 4. Snapin is present in insulinoma cells and in mouse and human islets. E4 stimulates snapin serine-phosphorylation in a PKA-dependent manner.
A) Immunoblot for snapin, SNAP-25, collectrin, EPAC2, VAMP2, and actin in 1: control mouse brain, 2: H4IIE (rat hepatoma) cells, 3: 3T3-L1 cells, 4: INS1 832/13 (rat insulinoma) cells, 5: MIN6 (mouse insulinoma) cells, 6: mouse islet protein extracts. Insulinoma cells and mouse islets express the proteins examined. Hepatoma cells express EPAC2 only, 3T3-L1 cells do not express the examined vesicle associated proteins.
B) Immunohistochemical staining of mouse pancreas sections. Co-immunostaining with insulin (green) and with non-specific antibody (top) or snapin-specific antibody (bottom) (red). Nuclear counterstain with DAPI (blue). Separate pseudocolored images are shown with digitally merged image on bottom right panel, respectively. Snapin immunoreactivity co-localizes with insulin immunoreactivity in pancreatic islets.
C) Co-immunoprecipitation for snapin serine phosphorylation in mouse islets treated with E4 without or with PKA specific inhibition with myr-PKI. E4 stimulates snapin phosphorylation, which is inhibited by adding myr-PKI. Immunoblot for 10% of protein input at bottom.
D) Co-immunoprecipitation for snapin serine phosphorylation in human islets islets treated with E4 (10 nM) without or with PKA specific inhibition with myr-PKI. E4 stimulates snapin phosphorylation, which is inhibited by adding myr-PKI. Immunoblot for 10% of protein input at bottom.
E) Snapin serine phosphorylation is increased in islets of Δ-prkar1a mice. Co-immunoprecipitation for snapin serine phosphorylation in wt-prkar1a and Δ-prkar1a islets.
Mouse (Figure 4C) and human (Figure 4D) islets exposed to E4 (10 nM), followed by snapin-specific immunoprecipitation (IP) and immunoblot (IB) for phospho-serine, revealed E4 induced snapin phosphorylation, which was inhibited by the PKA-specific inhibitor myr-PKI (myristolated PKA inhibitor) (Figure 4C, D) and also by the cAMP antagonist Rp-8-CPT-cAMPs, which blocks PKA activation (Figure S3A). Conversely, increased snapin serine phosphorylation was found in Δ-prkar1a islets as compared to controls (Figure 4E).
Snapin amino-acid sequence in mouse and human are >95% identical with a PKA target phosphorylation site flanking serine 50 (Chheda et al., 2001). To verify the snapin phosphorylation site at serine 50, we transiently expressed C-terminal flag-tagged wild-type (WT), serine 50 to alanine (S50A) and serine 50 to aspartate (S50D) mutant human snapin isoforms in rat insulinoma INS1 832/13 cells (Hohmeier et al., 2000) (Figure 5A). Cells were treated with E4 with and without the PKA inhibitor myr-PKI. In addition, in separate studies to verify whether PKA mediates snapin phosphorylation, we also co-transfected Pkac expression vector with the snapin wt and mutant isoforms. FLAG-specific IP followed by phospho-serine specific IB confirmed serine phosphorylation of WT snapin, which was absent in S50A and S50D mutants (Figure 5A).
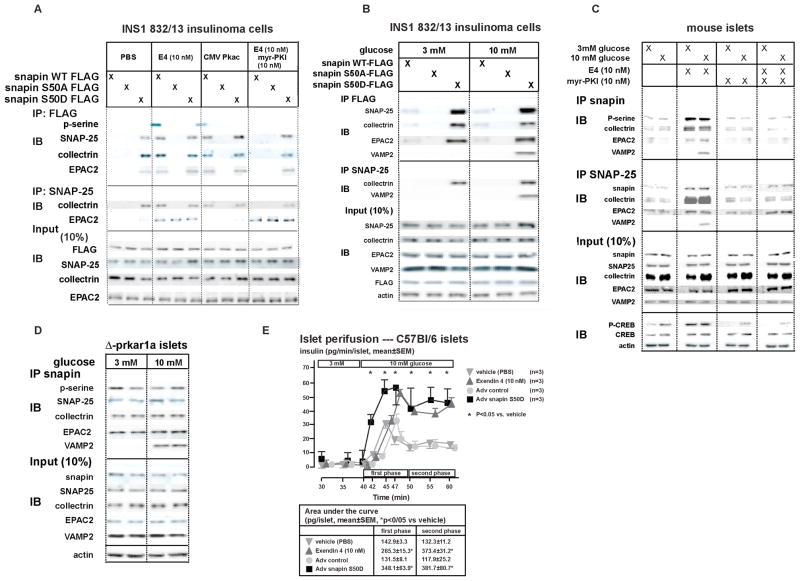
Figure 5. PKA mediated phosphorylation of snapin maps to serine 50. Snapin S50 phosphorylation increases interaction with secretory vesicle-associated proteins SNAP-25, EPAC2 and collectrin. Snapin interaction with VAMP2 is glucose dependent. E4 stimulated SNAP-25 interaction with EPAC2 is not PKA mediated. Overexpression in islets of snapin S50D potentiates GSIS.
A) Transiently transfected INS1 832/13 cells expressing C-terminal FLAG tagged WT, S50A or S50D snapin were treated with PBS (vehicle) E4 (10 nM), myr-PKI (10 nM), treated E4+myr-PKI, or transfected with Pkac followed by IP for FLAG or SNAP-25 and IB for phosphoserine or interacting proteins. Snapin serine phosphorylation occurs in WT snapin and not in S50A and S50D mutants by E4 and Pkac action. E4 effect is inhibited by myr-PKI. Snapin interaction with SNAP-25, collectrin or EPAC2 occurs only with phosphorylated WT snapin (by E4 or Pkac) or with snapin S50D as does SNAP-25 interaction with collectrin. SNAP-25 interaction with EPAC2 occurs with E4 in a PKA-independent manner and not inhibited by myr-PKI.
B) Transiently transfected INS1 832/13 cells expressing C-terminal FLAG tagged WT, S50A or S50D snapin cultured in low (3 mM) or high (10 mM) glucose and co-IP/IB as in A. Snapin S50D mutant binds SNAP-25, collectrin and EPAC2 in both low and high glucose. Snapin interaction with VAMP2 occurs at elevated glucose levels only. SNAP-25- VAMP2 interaction occurs only with snapin S50D and at elevated glucose levels.
C) Isolated C57Bl/6 mouse islets cultured in low (3 mM) or high (10 mM) glucose and treated with PBS, E4 (10 nM), myr-PKI (10 nM) and E4+myr-PKI followed by co-IP/IB as in A. Snapin serine phosphorylation is stimulated by E4 in a PKA dependent manner and increases snapin interaction with SNAP-25, collectrin and EPAC2 independently of glucose levels. Snapin-VAMP2 interaction is stimulated by E4 in PKA dependent manner only with high glucose. E4 stimulates SNAP25-EPAC2 interaction in a glucose- and PKA-independent manner. SNAP-25-VAMP2 interaction is stimulated by E4 in a glucose and PKA-dependent manner. PKA activity is verified by phosphorylation of CREB at serine 133 (p-CREB).
D) Control and Δ-prkar1a islets cultured in low (3 mM) or high glucose (10 mM). IP for snapin or SNAP-25 and IB for phosphoserine and interacting proteins. Snapin phosphorylation and snapin interaction with SNAP-25, collectrin and EPAC2 are increased in Δ-prkar1a islets independently of glucose levels. Snapin-VAMP2 and SNAP-25-VAMP2 interactions are stimulated by high glucose only.
E) Perifusion studies of C57Bl/6 mouse islets in low (3 mM) followed by high (10 mM) glucose concentrations. Islets were treated with either PBS (inverted triangle), E4 (10 nM) (upright triangle) during perifusion, or had been transduced with control (circle) or snapin S50D (square) expressing adenovirus. Table below curve summarizes area under the curves for first and second phase insulin secretion (*P<0.05 vs vehicle).
These results indicate that snapin serine phosphorylation is stimulated by incretin analogue E4 via PKA signaling in both mouse and human islets. Further, the results indicate that within the snapin protein, serine 50 is the sole PKA target serine phosphorylation site.
Snapin phosphorylation increases interaction with insulin exocytosis machinery proteins
We next examined the relation of serine 50 phosphorylation to snapin interaction with SNAP-25, collectrin and EPAC2. Co-immunoprecipitation (IP/IB) studies with FLAG-tagged expression of wt and mutant human snapin isoforms combined with E4 stimulation showed increased wild-type snapin interaction with SNAP-25, collectrin and EPAC2 (Figure 5A). This interaction was absent with the S50A snapin mutant, which lacks the PKA dependent phosphorylation site (Fukui et al., 2005; Ilardi et al., 1999). Conversely, snapin S50D, which mimics S50 phosphorylation, showed binding of snapin with SNAP-25, collectrin and EPAC2 even in the absence of PKA stimulation by E4 (Figure 5A). Stimulation of the PKA independent pathway with the EPAC-selective activator 8-pCPT-2′-O-Me-cAMP-AM (10 μM) increased SNAP-25-EPAC2 interaction, but failed to stimulate snapin-EPAC2 interaction (Figure S4). Taken together, these results suggest that snapin phosphorylation is required for robust interaction between snapin, SNAP25 and EPAC2, and that phosphorylated snapin represents a node where incretin stimulated cAMP-PKA and cAMP-GEF pathways converge.
Vesicle exocytosis requires interaction of plasma membrane bound proteins (i.e. SNAP-25) with proteins bound to the secretory vesicle proper (i.e. synaptotagmin, VAMP2). This protein interaction leading to exocytosis is stimulated by a rise in intracellular Ca2+, which in ϐ-cells occurs as a consequence of a rise in glucose and glucose metabolism (Gauthier and Wollheim, 2008). To assess whether snapin phosphorylation affects interaction among plasma membrane bound (i.e. SNAP-25) with vesicle bound (i.e. VAMP2) proteins of the SNARE complex secretory machinery, we exposed INS 832/13 cells expressing FLAG-tagged snapin isoforms to low (3 mM) and high (10 mM) glucose levels for 30 minutes followed by FLAG-specific co-immunoprecipitation studies (IP/IB) (Figure 5B). The snapin S50D mutant co-immunoprecipitated SNAP-25, collectrin and EPAC2 in both low and high glucose conditions. However, snapin-VAMP2 interaction was detectable only at elevated (10 mM) glucose (Figure 5B). SNAP-25-VAMP2 interaction was detectable only at 10 mM glucose in presence of snapin S50D expression, suggesting that snapin phosphorylation is permissive for SNAP-25-VAMP2 interaction. This finding is consistent with a mechanism involving glucose dependent interaction of the insulin vesicle proteins with partner plasma membrane proteins, which then leads to the final step of exocytosis (Gauthier and Wollheim, 2008; Takahashi et al., 2010).
Consistent with observations in insulinoma cells, co-IP/IB studies in primary cultured mouse islets exposed to E4 at low (3 mM) and high (10 mM) glucose levels showed snapin interaction in a PKA-dependent manner with SNARE complex protein SNAP-25, as well as with collectrin and EPAC2 (Figure 5C). Glucose stimulation (10 mM) did not further increase snapin phosphorylation or snapin interaction with either SNAP25 or EPAC2 above baseline levels. Interaction of both snapin and SNAP-25 with VAMP2 was increased with E4 stimulation under high (10 mM), but not low (3 mM) glucose culture conditions (Figure 5C). PKA inhibition with myr-PKI abolished snapin interaction with SNAP25, collectrin and EPAC2, indicating that snapin interaction with these proteins requires PKA stimulation (Figure 5C). In accordance with these observations, Δ-pkar1a islets showed increased snapin serine phosphorylation and increased interaction between snapin and SNAP-25, collectrin, and EPAC2 (Figure 5C). Further, Δ-pkar1a islets also exhibited snapin-VAMP2 interaction in the presence of high (10 mM) but not low (3 mM) glucose levels (Figure 5D).
Phosphorylation at residue 50 of snapin amplifies GSIS
To determine the importance of snapin phosphorylation on GSIS, we conducted perifusion studies with mouse islets treated with E4 or adenovirus mediated overexpression of snapin S50D, which specifically mimics snapin S50 phosphorylation. As expected, E4 treatment did not alter insulin secretion at low (3 mM) glucose levels, but augmented both first and second phases of insulin secretion at high (10 mM) glucose levels (Figure 5E). Treatment with the cAMP antagonist Rp-8CPT-cAMPs suppressed E4 action (Figure S3B). Snapin S50D over-expression showed no detectable change in insulin secretion at low (3 mM) glucose levels, while at high (10 mM) glucose levels, first and second phase insulin secretion were amplified in a qualitatively similar manner as observed with E4 (Figure 5E).
Snapin is critical for insulin secretion
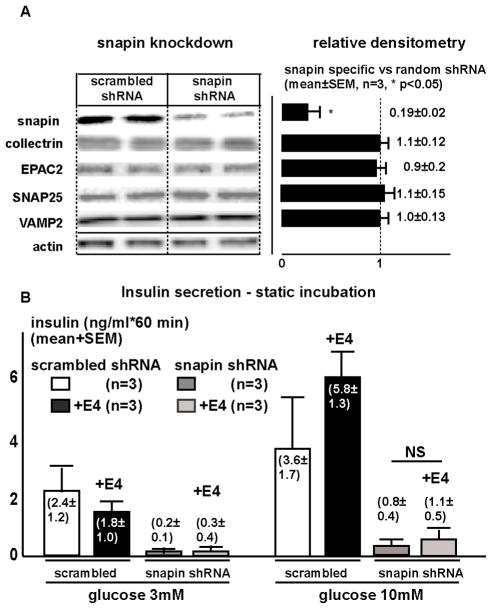
To evaluate whether snapin participates in regulation of insulin secretion, we knocked down snapin using murine sequence specific shRNA lentivirus in cultured mouse islets. Knockdown was specific as reflected on immunoblot by approximately 90% reduction of snapin protein, while SNAP-25, collectrin, VAMP2 and EPAC2 protein levels remained unchanged (Figure 6). Islets with knocked down snapin exhibited diminished insulin secretion at both low and high glucose and no longer responded to E4 stimulation. Adenovirus mediated re-expression of FLAG-tagged human snapin after murine-specific shRNA knockdown rescued GSIS from mouse islets, while re-expression of human snapin S50A mutant had no such effect. Conversely, re-expression of human snapin S50D after murine snapin knockdown augmented GSIS (Figure S5). These observations indicate that snapin is required for insulin secretion from ϐ-cells.
Figure 6. Snapin knockdown in C57Bl/6 mouse islets diminishes insulin secretion. Isolated mouse islets were transduced with lentivirus expressing shRNA with non-specific and snapin specific sequences.
Left: Representative immunoblot of islet extracts shows 80% reduction of snapin protein 48 hours after viral transduction, SNAP25, collectrin, EPAC2 and VAMP2 remain unchanged.
Right: Densitometric analysis of immunoblots from 3 different studies.
B. Insulin secretion in static culture conditions from isolated islets with knocked-down snapin. In islets with reduced snapin, insulin secretion is diminished both at low (3 mM) and high (10 mM) glucose levels. Additional E4 administration does not significantly stimulate insulin secretion at low (3 mM) or high (10 mM) glucose conditions.
Snapin S50 is occupied by O-linked ϐ-N-Acetylglucosamine in diabetic mouse islets
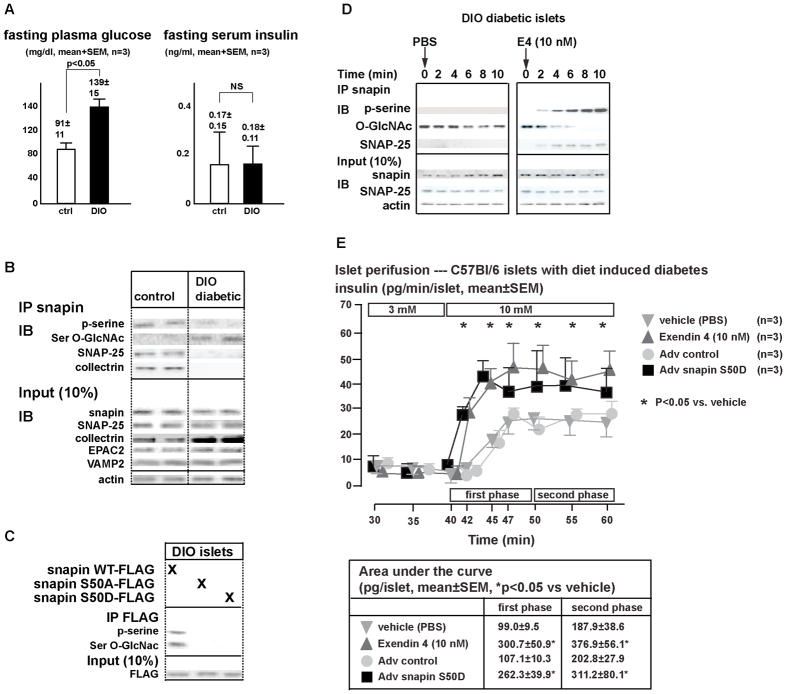
In T2DM dynamic insulin secretion is impaired predominantly resulting from defects in insulin exocytosis (Prentki and Nolan, 2006). We examined whether changes in insulin secretory proteins, especially in snapin, may underlie in part the ϐ-cell secretion defect in T2DM. In a mouse model of diet induced obesity (DIO) and T2DM (=DIO diabetes)(i.e. hyperglycemia and inappropriately low serum insulin levels) (Prentki and Nolan, 2006). (Figure 7A), islet snapin, SNAP-25, EPAC2, and VAMP2 levels were not different as compared to non-diabetic controls (Figure 7B). Collectrin levels were elevated in DIO islets, consistent with previous observations (Figure 7B) (Fukui et al., 2005). Importantly, phosphorylated snapin was reduced in DIO diabetic islets, which also showed reduction in SNAP25-snapin and SNAP-25-collectrin interactions (Figure 7B). In DIO diabetic and control islets, E4-stimulated cAMP production was similar, excluding diminished cAMP generation as a mechanism underlying the reduction in phosphorylated snapin fraction (data not shown).
Figure 7. Impaired GSIS in DIO diabetic mouse islets is corrected with snapin S50D.
A) Glucose and insulin levels of 12 week old C57Bl/6 littermate mice on either normal diet and after 6 weeks of high fat diet. Mice on high fat diet are hyperglycemic and have relative insulin deficiency.
B) Immunoblot of islet proteins from C57Bl/6 mice on normal chow and high fat diet.
C) Mapping of snapin serine-O-GlcNAcylation.
DIO diabetic islets were transduced with adenovirus expressing C-terminal FLAG-tagged wild-type, S50A and S50D snapin isoforms. Immunoprecipitation with FLAG antibody followed by immunoblot was performed. Representative immunoblot is shown. Bar graph indicates densitometric analysis of 3 separate studies.
D) Time course of snapin phosphorylation after E4 treatment of cultured islets of diet induced diabetic mice.
E) Perifusion studies of DIO mouse islets in low (3 mM) followed by high (10 mM) glucose concentrations. Islets were treated with either PBS (inverted triangle), E4 (10 nM) (upright triangle) during perifusion, or had been transduced with control (circle) or snapin S50D (square) expressing adenovirus. Table below curve summarizes area under the curves for first and second phase insulin secretion. (*p<0.05 vs vehicle).
Hyperglycemia and DM are associated with increased post-translational protein modification with O-linked ϐ-N-Acetylglucosamine residues (O-GlcNAcylation) in peripheral tissues as well as in pancreatic islets (Copeland et al., 2008; Slawson et al., 2010). Islet protein O-GlcNAcylation is associated with reduced insulin secretion (Akimoto et al., 2007). Enzymes regulating O-GlycNAcylation, O-GlcNAc transferase and O-GlcNAcase are expressed at high levels in pancreatic islets (Copeland et al., 2008; Wang et al., 2010).
Based on these observations, we reasoned that impaired snapin S50 phosphorylation in DIO islets may result from snapin O-GlcNAcylation. Indeed, DIO islets showed higher serine O-GlcNAcylation than control islets (Figure 7B). Furthermore, over-expression of wild-type, and mutant snapin (S50A & S50D) isoforms in DIO islets showed O-GlcNAcylation of wild-type snapin but not S50A and S50D mutants. This result indicates that the O-GlcNAcylation site in snapin maps to serine 50, which also serves as the PKA target phosphorylation site (Figure 7C).
E4 therapy produces rapid and sustained improvement of insulin secretion in humans with T2DM (Egan et al., 2002; Fehse et al., 2005) and in mouse models of T2DM (Doyle and Egan, 2007). We examined whether rapid E4 effects are reflected by changes in proteins involved in insulin secretion and whether snapin S50 O-GlcNAcylation versus phosphorylation is modified. In freshly isolated DIO diabetic islets, E4 administration acutely increased snapin phosphorylation accompanied by a rapid reduction in O-GlcNAcylated fraction of snapin (Figure 7D). Protein modification at serine and threonine can alternate between phosphorylation and O-GlcNAcylation (Wang et al., 2010), and addition and removal of O-GlcNAc moieties cycle rapidly (Copeland et al., 2008), consistent with the time course of snapin S50 O-GlcNAcylation being replaced by phosphorylation.
Taken together, these results indicate that in hyperglycemic DIO diabetic islets, snapin S50 is O-GlcNAcylated at the expense of being phosphorylated. Snapin S50 O-GlcNAcylation impairs snapin interaction with the SNARE complex protein SNAP-25, and thus impairs insulin secretion. Pharmacologic E4 treatment acutely leads to an increase in snapin S50 phosphorylation, while the S50 O-GlcNAcylated fraction diminishes. These results provide a basis for functional impairment in insulin secretion in DIO diabetic islets, which, consistent with clinical observations, can acutely be reversed with E4-mediated incretin action (Egan et al., 2002; Fehse et al., 2005).
Snapin S50D augments GSIS and restores insulin secretion in diabetic mouse islets
First phase insulin secretion is important in controlling postprandial glycemia (Vaag et al., 1995). In humans with T2DM and rodent models of T2DM, first phase insulin secretion is diminished and can be stimulated with incretin hormone both acutely and during chronic treatment. We reasoned that in DIO islets, expression of snapin S50D, which mimics snapin phosphorylation and is also impervious to O-GlcNAcylation, would affect GSIS in a similar fashion as the incretin analog E4. In perifusion studies, DIO diabetic islets exhibited diminished first phase and reduced second phase insulin secretion, consistent with previous observations (Peyot et al., 2010). In these islets, E4 treatment restored the absent first phase and increased second phase of GSIS. Adenovirus mediated snapin S50D over-expression in DIO diabetic islets similarly resulted in restoration of first phase and potentiation of second phase insulin secretion (Figure 7E). Taken together, these results suggest that in DIO diabetes mellitus, snapin S50 phosphorylation in large part mediates incretin effects on GSIS amplification.
Discussion
The present studies of a genetically defined mouse model highlights the predominant in vivo role of PKA-signaling in potentiating ϐ-cell GSIS, whereas disinhibition of islet PKA activity does not alter ϐ-cell proliferation or -mass. In addition, this model reveals Prkar1a as a functionally important regulatory Prkar subtype for mouse and human insulin secretion. Our studies identify PKA-mediated phospho-S50 snapin where cAMP-PKA and cAMP-GEF pathways converge leading towards GSIS potentiation. In ϐ-cells, snapin appears to have two related, but distinct roles: a) snapin is critical for insulin secretion, as revealed by snapin knockdown in islets (Figure 6); and b) snapin serves in ϐ-cells as the predominant PKA target, which upon phosphorylation at serine 50 promotes interaction and assembly of insulin secretory vesicle-associated proteins SNAP-25, collectrin and EPAC2 (Figure 5).
The studies of het-prkar1a mice (Figure 2D– F) are reflected our observations in humans with inactivating mutations in the PRKAR1A gene, who have higher serum insulin excursions in response to an oral glucose load, indicating higher insulin secretion (Figure 3). In this regard, it will be interesting to examine whether patients with mutations at the PRKAR1A locus are relatively protected from ϐ-cell failure or diabetes mellitus. Importantly patients with inactivating PRKAR1A mutations are not known to develop islet cell tumors, suggesting that, disinhibition of PKA signaling in islets does not cause tumor formation as in other endocrine tissues (Boikos and Stratakis, 2006).
A PKA-independent, cAMP-mediated potentiation of GSIS involves cAMP-regulated GEF EPAC2. Our findings confirm that PKA-independent incretin action promotes SNAP-25 - EPAC2 interaction (Figure 5, S1) (Kwan et al., 2006; Seino and Shibasaki, 2005). However, interaction of SNAP-25 and EPAC2 with snapin requires PKA-dependent snapin phosphorylation. Further, snapin is essential for ϐ-cell insulin secretion (Figure 6). Thus, our findings suggest a model in which phosphorylated snapin serves as a node, where PKA-independent (EPAC2) and PKA-dependent (snapin phosphorylation) incretin effects converge, resulting in increased interaction among protein members important for insulin vesicle exocytosis. While PKA appears to have permissive effects on EPAC2 action (Chepurny et al., 2010), the relative in vivo roles of EPAC2 and snapin in incretin potentiation of GSIS remain unclear. Mouse islets lacking EPAC2 show impaired cAMP analogue potentiation of first phase GSIS (Shibasaki et al., 2007), whilst first phase GSIS also depends on PKA action (Hatakeyama et al., 2006), indicating that first phase insulin secretion may be dually regulated by EPAC2 and PKA. Since mice lacking snapin do not survive the postnatal period (Tian et al., 2005), conditional ϐ-cell-specific models of EPAC2 and snapin ablation will be necessary to evaluate the in vivo roles of these proteins on insulin secretion and incretin action.
Incretins augment insulin secretion only when glucose levels are above a certain threshold beyond normoglycemia (Drucker, 2006). Consistent with this concept of glucose dependent potentiation of GSIS, patients with inactivating PRKAR1A mutations as well as het-/Δ-prkar1a mice do not have fasting hypoglycemia or hyperinsulinemia, but exhibit augmented insulin secretion as glucose levels rise (Figures 2, 3). Similarly, snapin S50D over-expression in mouse islets results in potentiated GSIS, while insulin secretion at low glucose levels is not augmented (Figure 5E). These findings are consistent with the existing model of regulated exocytosis in which proteins bound to the inside surface of the cytoplasmic membrane, on one hand, interact with proteins attached to the secretory vesicle, on the other hand, through a “handshake” of coiled-coil motifs on both sides (Sudhof and Rothman, 2009; Takahashi et al., 2010). Snapin interacts with the plasma membrane proteins (SNAP-25, collectrin) in a S50 phosphorylation-dependent manner and promotes assembly of vesicle-associated proteins, preparing the secretory vesicle for exocytosis. Finally, elevated glucose levels promote snapin interaction with the vesicle-associated protein VAMP2 (Figure 5). While the present studies do not exclude PKA-dependent effects outside of snapin phosphorylation (Lester et al., 2001), the observations suggest that PKA-dependent snapin S50 phosphorylation is an important intracellular mediator of incretin on GSIS, while also permitting a glucose dependency of insulin secretion (Hatakeyama et al., 2006).
Our studies provide a molecular basis for clinical observations made during E4 administration in humans. Type 2 diabetic subjects exhibit defective first phase insulin release, and restoration of first-phase insulin secretion is critical for optimal glycemic control (Basu et al., 1996). Our studies indicate that incretin mediated snapin phosphorylation restores first phase insulin secretion and augments second phase GSIS (Figure 7). In DIO diabetic islets, alternate O-GlcNAcylation and phosphorylation at snapin S50 provide a mechanism for a) impaired insulin secretion in diabetic islets due to snapin O-GlcNAcylation, disturbing snapin-SNAP-25 interaction; and b) rapid incretin mediated replacement of snapin S50 O-GlcNAcylation by phosphorylation, allowing phospho-snapin interaction with SNAP-25 (Figure 7D, E) and likely other protein members of the exocytosis machinery, thereby enabling insulin secretion to proceed.
Our findings suggest that snapin S50 phosphorylation provides unifying mechanistic underpinnings of the following properties of incretin action: 1) glucose dependency in augmenting insulin secretion; 2) rapid action of GLP-1 and E4 in stimulating first and second phase insulin secretion, and 3) rapid effectiveness in DIO diabetic islets in restoring first and second phase insulin secretion, by altering the proportion of phosphorylated to O-GlcNAcylated snapin S50.
In a broader context, for efforts to ameliorate glycemic control and increasing GSIS, both PRKAR1A and snapin are potential drug targets in ϐ-cells for treating diabetes mellitus in humans.
Experimental Procedures
Animals Studies
Prkar1afl/fl, RIP2-CRE, and Pdx-1-CRE mice were previously reported (Kirschner et al., 2005; Lammert et al., 2001; Lee et al., 2006) and were kept in a mixed genetic background (C57Bl/6 x FVB). Animal studies were approved by the institutional animal care and use committee at Johns Hopkins University, where animals were housed in a dedicated vivarium. Dynamic physiologic test details are provided in supplemental material.
Immunofluorescence histology and islet morphometry was conducted as previously described (Hussain et al., 2006; Song et al., 2008). EdU incorporation (100 μg i.p × 5 days) was detected using Click-It (Invitrogen).
Electron microscopy
Standard protocols for transmission and immuno-eelctron microscopy were used. See supplemental material for details.
Isolated Islet studies
Islets isolation, static insulin secretion and perifusion tests were performed as previously described (Chepurny et al., 2010; Hussain et al., 2006; Song et al., 2008) and described in detail in supplemental material. Islet insulin (Alpco ELISA) content was determined after acid ethanol (0.18M HCl in 70% ethanol) extraction (Hussain et al., 2006).
shRNA knockdown studies
Scrambled (random) and murine snapin-specific (TI526219 in pRS, Origene Technologies) shRNA expressing, purified (Virabind, Invitrogen) lentiviral particles (approximately 105 viral particles) were spinoculated (O’Doherty et al., 2000) twice with freshly isolated islets in presence of polybrene (2 μg/ml, Sigma). For re-expression of snapin isoforms after shRNA knockdown, adenovirus expressing FLAG-tagged human snapin isoforms, which are not affected by mouse-specific shRNA, were added within 24 hours after spinoculation and initiation of shRNA knockdown. After a total of 3 days in culture, islet insulin secretion assays were performed followed by protein and RNA extractions for further analyses.
Protein Co-immunoprecipitation studies were performed using standard protocols (Cell Signaling Technologies). Band intensity of interrogated protein was normalized to the corresponding actin intensity (BioRad Chemidoc XRS). Representative immunoblots are shown. Immunoblots (IB) were performed with at least 3 different separately obtained experimental samples. Antibodies used are provided in Table S4.
Human studies
Human subjects had provided informed consent under approved protocol by the NIH institutional review board. Oral glucose tolerance tests (oGTT, 75 gr glucose per os after an overnight fast) were performed at the clinical research center of the NIH on subjects carrying inactivating mutations of the prkar1a gene verified by sequencing of prkar1a exons (Kirschner et al., 2000). Control subjects were selected to match age as well as relevant medical history with patients carrying the prkar1a mutation. Test data were archived at the NIH database until analysis.
Statistics
Results are shown as averages and standard errors of the mean (SEM). Where appropriate, Student’s t-test, non-parametric Mann-Whitney U-test or analysis of variation (ANOVA) were used to calculate differences between groups. A p value of <0.05 was considered significant.
Supplementary Material
References
- Akimoto Y, Hart GW, Wells L, Vosseller K, Yamamoto K, Munetomo E, Ohara-Imaizumi M, Nishiwaki C, Nagamatsu S, Hirano H, et al. Elevation of the post-translational modification of proteins by O-linked N-acetylglucosamine leads to deterioration of the glucose-stimulated insulin secretion in the pancreas of diabetic Goto-Kakizaki rats. Glycobiology. 2007;17:127–140. doi: 10.1093/glycob/cwl067. [DOI] [PubMed] [Google Scholar]
- Basu A, Alzaid A, Dinneen S, Caumo A, Cobelli C, Rizza RA. Effects of a change in the pattern of insulin delivery on carbohydrate tolerance in diabetic and nondiabetic humans in the presence of differing degrees of insulin resistance. J Clin Invest. 1996;97:2351–2361. doi: 10.1172/JCI118678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boikos SA, Stratakis CA. Carney complex: pathology and molecular genetics. Neuroendocrinology. 2006;83:189–199. doi: 10.1159/000095527. [DOI] [PubMed] [Google Scholar]
- Buxton P, Zhang XM, Walsh B, Sriratana A, Schenberg I, Manickam E, Rowe T. Identification and characterization of Snapin as a ubiquitously expressed SNARE-binding protein that interacts with SNAP23 in non-neuronal cells. Biochem J. 2003;375:433–440. doi: 10.1042/BJ20030427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepurny OG, Kelley GG, Dzhura I, Leech CA, Roe MW, Dzhura E, Li X, Schwede F, Genieser HG, Holz GG. PKA-dependent potentiation of glucose-stimulated insulin secretion by Epac activator 8-pCPT-2′-O-Me-cAMP-AM in human islets of Langerhans. Am J Physiol Endocrinol Metab. 2010;298:E622–633. doi: 10.1152/ajpendo.00630.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chheda MG, Ashery U, Thakur P, Rettig J, Sheng ZH. Phosphorylation of Snapin by PKA modulates its interaction with the SNARE complex. Nat Cell Biol. 2001;3:331–338. doi: 10.1038/35070000. [DOI] [PubMed] [Google Scholar]
- Copeland RJ, Bullen JW, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: roles in insulin resistance and glucose toxicity. Am J Physiol Endocrinol Metab. 2008;295:E17–28. doi: 10.1152/ajpendo.90281.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prato S, Tiengo A. The importance of first-phase insulin secretion: implications for the therapy of type 2 diabetes mellitus. Diabetes Metab Res Rev. 2001;17:164–174. doi: 10.1002/dmrr.198. [DOI] [PubMed] [Google Scholar]
- Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther. 2007;113:546–593. doi: 10.1016/j.pharmthera.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- Egan JM, Clocquet AR, Elahi D. The insulinotropic effect of acute exendin-4 administered to humans: comparison of nondiabetic state to type 2 diabetes. J Clin Endocrinol Metab. 2002;87:1282–1290. doi: 10.1210/jcem.87.3.8337. [DOI] [PubMed] [Google Scholar]
- Fehse F, Trautmann M, Holst JJ, Halseth AE, Nanayakkara N, Nielsen LL, Fineman MS, Kim DD, Nauck MA. Exenatide augments first- and second-phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:5991–5997. doi: 10.1210/jc.2005-1093. [DOI] [PubMed] [Google Scholar]
- Foster LJ, Yeung B, Mohtashami M, Ross K, Trimble WS, Klip A. Binary interactions of the SNARE proteins syntaxin-4, SNAP23, and VAMP-2 and their regulation by phosphorylation. Biochemistry. 1998;37:11089–11096. doi: 10.1021/bi980253t. [DOI] [PubMed] [Google Scholar]
- Fukui K, Yang Q, Cao Y, Takahashi N, Hatakeyama H, Wang H, Wada J, Zhang Y, Marselli L, Nammo T, et al. The HNF-1 target collectrin controls insulin exocytosis by SNARE complex formation. Cell Metab. 2005;2:373–384. doi: 10.1016/j.cmet.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Gauthier BR, Wollheim CB. Synaptotagmins bind calcium to release insulin. Am J Physiol Endocrinol Metab. 2008;295:E1279–1286. doi: 10.1152/ajpendo.90568.2008. [DOI] [PubMed] [Google Scholar]
- Gerich JE. Is reduced first-phase insulin release the earliest detectable abnormality in individuals destined to develop type 2 diabetes? Diabetes. 2002;51(Suppl 1):S117–121. doi: 10.2337/diabetes.51.2007.s117. [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Hatakeyama H, Kishimoto T, Nemoto T, Kasai H, Takahashi N. Rapid glucose sensing by protein kinase A for insulin exocytosis in mouse pancreatic islets. J Physiol. 2006;570:271–282. doi: 10.1113/jphysiol.2005.096560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49:424–430. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- Hussain MA, Porras DL, Rowe MH, West JR, Song WJ, Schreiber WE, Wondisford FE. Increased pancreatic beta-cell proliferation mediated by CREB binding protein gene activation. Mol Cell Biol. 2006;26:7747–7759. doi: 10.1128/MCB.02353-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilardi JM, Mochida S, Sheng ZH. Snapin: a SNARE-associated protein implicated in synaptic transmission. Nat Neurosci. 1999;2:119–124. doi: 10.1038/5673. [DOI] [PubMed] [Google Scholar]
- Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- Kirschner LS, Kusewitt DF, Matyakhina L, Towns WH, 2nd, Carney JA, Westphal H, Stratakis CA. A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res. 2005;65:4506–4514. doi: 10.1158/0008-5472.CAN-05-0580. [DOI] [PubMed] [Google Scholar]
- Kwan EP, Xie L, Sheu L, Nolan CJ, Prentki M, Betz A, Brose N, Gaisano HY. Munc13-1 deficiency reduces insulin secretion and causes abnormal glucose tolerance. Diabetes. 2006;55:1421–1429. doi: 10.2337/db05-1263. [DOI] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- Lang J. Molecular mechanisms and regulation of insulin exocytosis as a paradigm of endocrine secretion. Eur J Biochem. 1999;259:3–17. doi: 10.1046/j.1432-1327.1999.00043.x. [DOI] [PubMed] [Google Scholar]
- Lee JY, Ristow M, Lin X, White MF, Magnuson MA, Hennighausen L. RIP-Cre revisited, evidence for impairments of pancreatic beta-cell function. J Biol Chem. 2006;281:2649–2653. doi: 10.1074/jbc.M512373200. [DOI] [PubMed] [Google Scholar]
- Lester LB, Faux MC, Nauert JB, Scott JD. Targeted protein kinase A and PP-2B regulate insulin secretion through reversible phosphorylation. Endocrinology. 2001;142:1218–1227. doi: 10.1210/endo.142.3.8023. [DOI] [PubMed] [Google Scholar]
- Lillioja S, Mott DM, Howard BV, Bennett PH, Yki-Jarvinen H, Freymond D, Nyomba BL, Zurlo F, Swinburn B, Bogardus C. Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indians. N Engl J Med. 1988;318:1217–1225. doi: 10.1056/NEJM198805123181901. [DOI] [PubMed] [Google Scholar]
- O’Doherty U, Swiggard WJ, Malim MH. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J Virol. 2000;74:10074–10080. doi: 10.1128/jvi.74.21.10074-10080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petyuk VA, Qian WJ, Hinault C, Gritsenko MA, Singhal M, Monroe ME, Camp DG, 2nd, Kulkarni RN, Smith RD. Characterization of the mouse pancreatic islet proteome and comparative analysis with other mouse tissues. J Proteome Res. 2008;7:3114–3126. doi: 10.1021/pr800205b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyot ML, Pepin E, Lamontagne J, Latour MG, Zarrouki B, Lussier R, Pineda M, Jetton TL, Madiraju SR, Joly E, et al. Beta Cell Failure in Diet-Induced Obese mice stratified according to body weight gain: secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta cell mass. Diabetes. 2010 doi: 10.2337/db09-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenta W, Korytkowski M, Mitrakou A, Jenssen T, Yki-Jarvinen H, Evron W, Dailey G, Gerich J. Pancreatic beta-cell dysfunction as the primary genetic lesion in NIDDM. Evidence from studies in normal glucose-tolerant individuals with a first-degree NIDDM relative. Jama. 1995;273:1855–1861. [PubMed] [Google Scholar]
- Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Takahashi H, Miki T, Sunaga Y, Matsumura K, Yamanaka M, Zhang C, Tamamoto A, Satoh T, Miyazaki J, et al. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci U S A. 2007;104:19333–19338. doi: 10.1073/pnas.0707054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki Y, Nishiki T, Omori A, Sekiguchi M, Kamata Y, Kozaki S, Takahashi M. Phosphorylation of 25-kDa synaptosome-associated protein. Possible involvement in protein kinase C-mediated regulation of neurotransmitter release. J Biol Chem. 1996;271:14548–14553. doi: 10.1074/jbc.271.24.14548. [DOI] [PubMed] [Google Scholar]
- Slawson C, Copeland RJ, Hart GW. O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci. 2010 doi: 10.1016/j.tibs.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WJ, Schreiber WE, Zhong E, Liu FF, Kornfeld BD, Wondisford FE, Hussain MA. Exendin-4 stimulation of cyclin A2 in beta-cell proliferation. Diabetes. 2008;57:2371–2381. doi: 10.2337/db07-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Hatakeyama H, Okado H, Noguchi J, Ohno M, Kasai H. SNARE conformational changes that prepare vesicles for exocytosis. Cell Metab. 2010;12:19–29. doi: 10.1016/j.cmet.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Tian JH, Wu ZX, Unzicker M, Lu L, Cai Q, Li C, Schirra C, Matti U, Stevens D, Deng C, et al. The role of Snapin in neurosecretion: snapin knock-out mice exhibit impaired calcium-dependent exocytosis of large dense-core vesicles in chromaffin cells. J Neurosci. 2005;25:10546–10555. doi: 10.1523/JNEUROSCI.3275-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaag A, Henriksen JE, Madsbad S, Holm N, Beck-Nielsen H. Insulin secretion, insulin action, and hepatic glucose production in identical twins discordant for non-insulin-dependent diabetes mellitus. J Clin Invest. 1995;95:690–698. doi: 10.1172/JCI117715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Udeshi ND, Slawson C, Compton PD, Sakabe K, Cheung WD, Shabanowitz J, Hunt DF, Hart GW. Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates cytokinesis. Sci Signal. 2010;3:ra2. doi: 10.1126/scisignal.2000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D., Jr Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest. 1984;74:1318–1328. doi: 10.1172/JCI111542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.