Abstract
Background
Guanylyl cyclase-B (GC-B; NPR-B), the receptor for C-type natriuretic peptide (CNP) is rapidly and effectively desensitized by a factor(s) in serum. Given the potential importance of this receptor in remodeling after tissue injury, identification of the serum factor(s) is of significant medical importance.
Results
Partial purification of desensitization activity in serum by DEAE-Sepharose and reverse phase C18 chromatography, followed by mass spectroscopy, identified peptide sequences identical to those of apolipoprotein A2 (Apo A2), a known component of high density lipoprotein (HDL). Apo A2, however, could be eliminated as the active desensitization factor. Never the less, substantial desensitization activity was associated with purified preparations of bovine or human HDL. Since HDL is a well-known transporter of various lipids and phospholipids, we extracted either HDL or partially purified serum preparations with butanol and all activity extracted into the solvent. Of various lipophilic signaling molecules known to be associated with HDL, a prominent component is sphingosine-1-phosphate (S1P). We therefore tested authentic S1P as well as other known components of HDL (sphingosylphosphorylcholine; platelet activating factor) for activity; only S1P caused desensitization of GC-B. S1P was relatively potent, causing one-half maximal desensitization of GC-B at concentrations of 5–10 nM. These effects were seen within a few minutes after addition. Lysophosphatidic acid, another component of serum capable of desensitizing GC-B, was only effective at Micromolar concentrations. The pathway by which serum or S1P desensitizes GC-B seems unique in that pertussis toxin failed to inhibit GC-B desensitization, and yet blocked serum or S1P activation of extracellular signal-regulated kinase (ERK) or Akt/protein kinase B (Akt/PKB).
Conclusion
Since the concentrations of S1P that desensitize GC-B are well within serum physiological ranges, this mitogenic signaling molecule likely functions as a strong adversary of the CNP/GC-B signaling pathway in the regulation of cell proliferation and other growth factor-induced phenotypes. The mechanism by which S1P desensitizes GC-B appears different than the known S1P signaling pathways.
Background
Atrial natriuretic peptide (ANP1), C-type natriuretic peptide (CNP), and cell-permeant guanosine-3',5'-monophosphate (cGMP) analogs antagonize growth factor-stimulated proliferation and chemotaxis in cultured fibroblasts and vascular smooth muscle cells, and CNP also slows coronary artery restenosis in vivo [1-3]. Conversely, serum and certain peptide and phospholipid growth factors suppress CNP-elevations of cGMP in primary dermal fibroblasts, BALB-c/3T3 fibroblasts, or NIH/3T3 fibroblasts that over-express the receptor for CNP, guanylyl cyclase B (GC-B) [1,4]. Therefore, it has been suggested that CNP/GC-B and various mitogens oppose each other in the regulation of tissue remodeling after injury [1,3,4]. Adult cardiac fibroblasts, for example, synthesize CNP and express GC-B and CNP or cell permeant analogs of cGMP antagonize increases in DNA and collagen synthesis promoted by serum [5,6]. In previous studies, a number of growth factors and other agents have been shown to cause desensitization of guanylyl cyclase A (GC-A) or GC-B, possibly through the induction of receptor dephosphorylation [1,7,8]. As we compared the relative potencies of various serum growth factors (platelet-derived growth factor, PDGF; lysophosphatidic acid, LPA; basic fibroblast growth factor, bFGF) to desensitize GC-B, we realized that serum acted at dilutions where the above factors could not represent the most active desensitization factor. We then identified a serum factor (sphingosine-1-phosphate; S1P) that causes desensitization of GC-B at concentrations well within the range of those found in serum.
Results
Identification of a Protein Component in Purified Inhibitory Fractions
Mass spectroscopy identified a tryptic fragment (KAGTDLLNFLSSFIDPK) in the purified fractions from the final HPLC column (Fig 1, fraction 17.5 min) as identical to bovine ApoA2 [9]. Apolipoprotein A2 (ApoA2), a peptide of 76 amino acids, constitutes about 20% of the lipoprotein in HDL and functions primarily to modulate lipid binding of HDL [10]. ApoA2 is also found in bovine serum free of association with HDL [9]. However, that it represented a desensitization factor seemed unlikely after we compared the activity of serum from many different species (Fig 2). The serum of all species tested caused desensitization of GC-B at about the same relative serum concentration, and yet chicken serum has been reported to express only barely detectable levels of ApoA2 [11]. Thus, Apo A2 was not the active factor.
Figure 1.
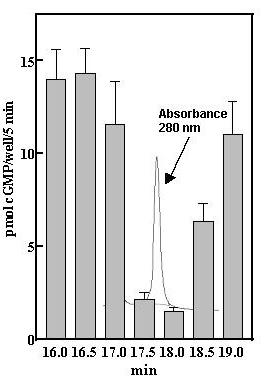
Isolation of desensitization activity by reverse-phase chromatography. Inhibition of CNP-induced elevations of cGMP levels in GC-B/3T3 cells by fractions from the second analytic C18 column. Fifty-μl aliquots of the 0.5 ml fractions were dried and dissolved in 50 μl PBS and 5 μl added to the cells in a final volume of 0.5 ml. IBMX (0.25 mM) and CNP (20 nM) were added and cGMP levels were determined after 5 min incubation with CNP as described under Methods. Error bars indicate the range of duplicate determinations.
Figure 2.
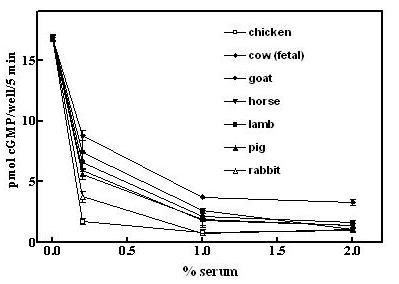
Low serum from a variety of species desensitizes GC-B to CNP. GCB/3T3 cells were incubated with heat-inactivated serum from the indicated species for 30 min prior to adding 0.25 mM IBMX and 20 nM CNP and cGMP contents were determined after 5 min of incubation with CNP as described under Methods. Error bars indicated the range of duplicate determinations.
Desensitization of GC-B by HDL
Given that ApoA2 is a constituent of HDL, we compared purified preparations of human and bovine HDL for desensitization activity and both displayed similarly high activities based on protein content (Fig 3). The results also established that serum desensitization activity is directly associated with HDL from both human plasma and bovine serum, and is not an artifact of the purification procedure.
Figure 3.
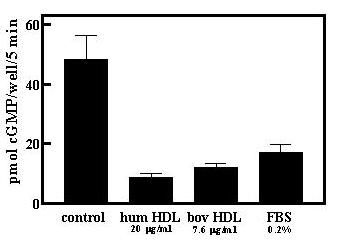
Human and bovine HDL desensitize GC-B in GCB/3T3 cells. Human and bovine HDL preparations were added to GC-B/3T3 cells for 30 min and desensitization of GC-B was then determined as described under Methods. Fetal bovine serum (FBS) was diluted in PBS and cells treated as described for the HDL preparations. Results are the mean -/+ S.D. (n = 4).
Extraction of lipid components into Butanol
HDL transports various phospholipids and sphingolipids [12,13], and so we asked whether the active component was lipophilic. HDL preparations were subjected to butanol extraction and essentially all activity was extracted into the organic solvent phase (Fig 4A). In a separate series of experiments on the partially purified factor preparations from bovine serum (first C18 column), butanol also extracted the desensitization activity (Fig 4B).
Figure 4.
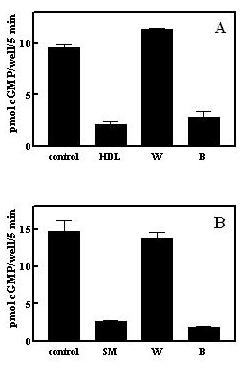
Desensitization activity is extracted by butanol. A. Butanol extraction of human HDL. One mg human HDL protein was extracted with 33% n-butanol/20 mM acetic acid as described under Methods and desensitization activity in the upper ("B") and lower ("W") phases determined. HDL represents the starting material for the butanol extraction and was included with cells at 10 μg/ml. B. Butanol extraction of a partially purified serum preparation. Active fractions (about 4 ml) from the initial C18 column were dried and extracted with 33% n-butanol/20 mM acetic acid as described under Methods and diluted 1000-fold into the cell incubation for measurement of desensitization activity in the upper ("B") and lower ("W") phases. SM represents the pooled active C18 column fractions prior to butanol extraction diluted 1000-fold into the cell incubation.
Identification of Serum Factor
Lysophosphatidic acid (LPA), is also present in serum at concentrations of 2–10 μM [14], but was not found as a component of the HDL butanol extract (not shown). Both S1P and LPA are released into serum by activated platelets [15,14] and therefore represent potential regulators of tissue remodeling at sites of injury. S1P has been suggested to bind to G-protein coupled cell surface receptors (S1PR1–5) homologous to LPA receptors (LPAR1–3); it is mitogenic when added to fibroblasts and smooth muscle cells in culture [16]. S1P also has been suggested to serve as an intracellular second messenger for the mitogenic actions of serum or PDGF in fibroblasts or smooth muscle cells [17]. S1P therefore became a primary candidate as the serum and HDL desensitization factor. Sphingosine-1-phosphate rapidly desensitized GC-B (Fig 5) and at the same time induced rapid increases of pERK and pAkt in the GCB/3T3 cell line (Fig 5, inset). The S1P concentration used (100 nM) desensitized with a half-time of about 5–10 min and was maximal by 20 min prior to CNP addition. S1P was also very potent, being effective at low nM concentrations, and was much more potent than LPA (Fig 6A). The approximate 100-fold shift in the CNP concentration-response curve (Fig 6B) would effectively desensitize GC-B over the physiological concentration range for CNP (Fig 6B). In addition to S1P, we also tested two other known components of HDL, sphingosylphosphorylcholine and platelet activating factor, and neither demonstrably desensitized GC-B (Fig 7). The IC50 of 5–10 nM for S1P, similar to the nM Kd's reported for all of the S1P receptors, including Gi-promoted inhibition of adenylate cyclase [17], together with the rapid increases in pERK and pAkt (Fig 5, inset) initially suggested it acted through cell surface receptors to desensitize GC-B. The proposed intracellular pathway for S1P seems to require μM amounts for detectable responses [17,18].
Figure 5.
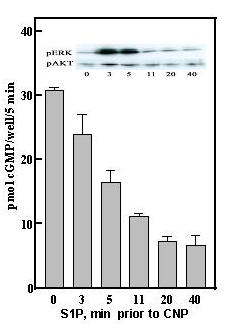
Sphingosine-1-phosphate rapidly desensitizes GC-B. S1P (100 nM) was added to GCB/3T3 cells at the indicated times before addition of 20 nM CNP and 5 min later the reaction was terminated with 0.5 N perchloric acid and cGMP accumulation determined as in Methods. Inset; S1P (100 nM) was added to cells for the indicated times and the levels of pERK and pAkt determined by Western blot as described in Methods.
Figure 6.
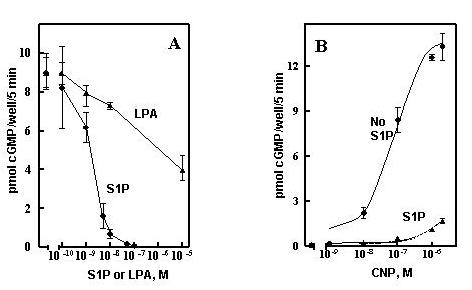
S1P is a potent desensitization agent. A, GC-B/3T3 cells were incubated with the indicated concentrations of S1P or LPA for 30 min and the responses to 20 nM CNP determined as described under Methods. B, GC-B/3T3 cells were incubated with 100 nM S1P 30 min and responses to the indicated amounts of CNP determined as described under Methods. Results are given as the mean of duplicate wells -/+ the range of duplicates, error bars.
Figure 7.
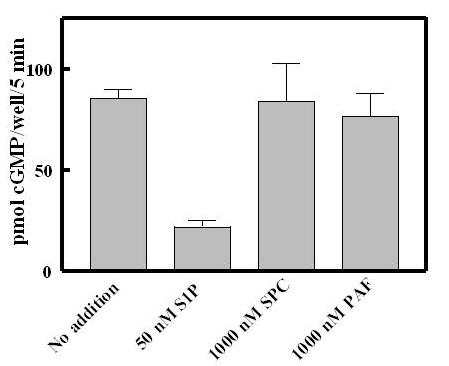
Sphingosylphosphorylcholine and platelet activating factor fail to desensitize GC-B. S1P, sphingosylphosphorylcholine (SPC) or platelet activating factor (PAF) were added to the final concentrations indicated. The assays for GC-B desensitisation were as described in Methods.
Desensitization in other Cells
The potent effects of S1P on GC-B signaling are not confined to the immortalized murine fibroblasts over-expressing GC-B in the above studies. S1P significantly suppressed CNP/GCB signaling in a human aortic smooth muscle primary cell line with an IC50 of 5–10 nM (Fig 8A) and shifted the dose response curve for CNP about 100-fold higher (Fig 8B) responses similar to those of the GCB/3T3 cell line (Fig 6). LPA also suppressed cGMP elevations in this cell line but was far less effective than S1P. The suppression of cGMP levels in these cells by the mitogens, S1P or LPA, is consistent with our earlier observations that the antimitogenic effects of cGMP can be antagonized by mitogen-mediated inhibition of cell surface guanylyl cyclase signaling [1]. In fact, it has been postulated that S1P contributes to restenosis following coronary artery angioplasty through the promotion of vascular smooth muscle proliferation [19]. CNP, in contrast, inhibits restenosis [2]. Thus an adversarial relationship between CNP and S1P signaling pathways may be operative in event such as vascular smooth muscle remodeling.
Figure 8.
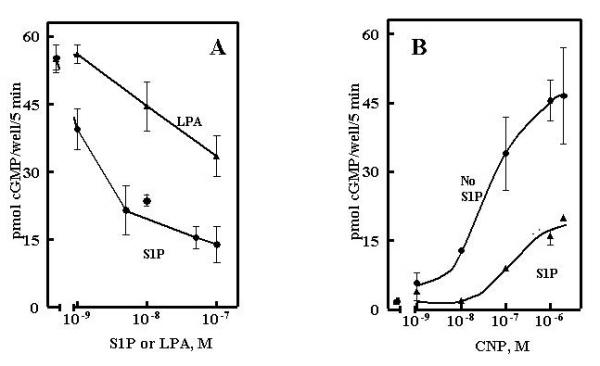
S1P potently desensitizes GC-B in human aortic smooth muscle cells. A, cells were incubated with 100 nM S1P for 30 min and the responses to the indicated concentrations of CNP determined as described under Methods. B, cells were incubated for 30 min with the indicated concentrations of S1P or LPA as indicated and the responses to 20 nM CNP determined as described under Methods. Results are expressed as the mean of duplicate observations -/+ the range of duplicates.
Uniqueness of the Desensitization Pathway
Dependent on the specific receptors expressed, S1P stimulates diverse cell functions such as proliferation, differentiation, and motility. The most widely distributed receptors, S1PR1–3, are coupled to pertussis toxin-sensitive Gi/0 [17]. However, pertussis toxin was completely ineffective in blocking desensitization of GC-B by S1P, while it completely prevented increases in pERK and pAkt (Fig 9). Thus, the pathway of S1P desensitization of GC-B is apparently not coupled to Gi/0. Likewise, serum-desensitization of GC-B was unaltered by pertussis toxin, even though pAkt activation was completely blocked; likewise, pERK activation was substantially reduced under the same conditions. Partial inhibition of serum-elevated pERK by pertussis toxin is consistent with the presence of both peptide growth factors (e.g.; bFGF and PDGF) that do not signal through G-protein coupled receptors, and phospholipid mitogen-activated G-protein coupled receptors. The complete inhibition of serum-induced pAkt activation by pertussis toxin suggests that pAkt regulation is tied exclusively to G-protein coupled receptors (GPCRs) in the fibroblast 3T3 cell line. However, pertussis toxin, Wortmannin, and C3-exotoxin, agents that block G-protein coupled receptors, PI3-kinase, and Rho kinase, respectively, failed to inhibit the desensitization of GC-B induced by serum in primary human dermal fibroblasts (not shown). Furthermore, ATP and ATPγS, G-protein coupled P2 receptor agonists [20], activated the MAP kinase pathway without altering GC-B signaling (not shown). These results clearly dissociate many of the known mitogen-activated pathways from the pathway of GC-B desensitization, and therefore seem to also eliminate a number of the known receptors from consideration as mediators of the desensitization.
Figure 9.
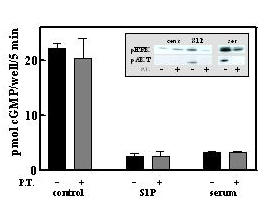
Pertussis toxin does not block GC-B desensitization by 100 nM S1P or by 1% fetal bovine serum. GC-B/3T3 cells were incubated where indicated with 100 ng/ml pertussis toxin for 24 h prior to a 30 min incubation with 100 nM S1P or 1% fetal bovine serum as indicated. CNP responses following these treatments were determined as described under Methods. Results are the mean of duplicate observations -/+ the range of duplicates. Inset, cells were incubated for 10 min following pertussis treatment as described above and the levels of pERK and pAkt were determined as described under Methods.
Conclusions
A unifying model for the actions of various mitogens and S1P to desensitize GC-B appears to require the existence of receptors that are not coupled to Gi. These could be cell surface or intracellular receptors. In the model of Spiegel and colleagues [17,21] PDGF or serum activate sphingosine kinase, which then catalyzes increased formation of S1P. S1P may then act on intracellular receptors and bypass the G-coupled receptors on the plasma membrane. Such a pathway would provide a unifying model to explain the common action of various mitogens to desensitize the CNP receptor independent of ERK or Akt activation. On the other hand, possibly the various mitogens, including S1P, act through a unique pathway not involving Gi or the activation of pathways such as those of ERK or Akt.
Methods
Reagents
The contents of cGMP in cultured cell lines were determined as previously described. Human aortic smooth muscle cells were from Clonetics and cultured as instructed by the supplier. NIH/3T3 cells over-expressing rat GC-B (GCB/3T3) were as previously described [22]; continuous passaging of this cell line can result in decreased expression of GCB (Fig. 1vs Fig 7). Human HDL was from Biotechnology Industries and was diluted in phosphate-buffered saline (PBS) just prior to experimentation. Peptide sequencing was by the Mass Spectroscopy Facility at UT Southwestern Medical Center in Dallas. Pertussus toxin was from List Biochemicals and fetal bovine serum (FBS) was from Atlanta Biologicals.
Desensitization Assay
Desensitization of GC-B to the ligand CNP was determined in duplicate or quadruplicate as described previously [1]. Confluent cells in 24-well tissue culture plates were placed in serum-deficient DMEM for 24 to -48 h and were then transferred to fresh serum-deficient medium 2 h prior to experimental treatments. Stock solutions of CNP (Peninsula Biochemicals), LPA and S1P (Sigma Aldrich) were diluted in fatty acid-free bovine serum albumin (Sigma Aldrich) at 100-times the concentration in the cell reactions. Samples were added to the cells for 30 min prior to the addition of 0.25 mM 3-isobutyl-1-methylxanthine for 10 min; this was followed by the addition of CNP to a 20 nM final concentration for 5 min. Addition of perchloric acid to a final concentration of 0.5 N terminated the cell reactions. cGMP content of each well was determined in duplicate by radioimmunoassay as described [1]. Bovine HDL (d = 1.063–1.21) was isolated from heat-inactivated fetal bovine serum (FBS) by centrifugation into KBr as described for isolation of human plasma HDL [23]. The resulting preparations were at least 90% pure as judged by protein staining (GelCode Blue Stain Reagent (Pierce)) following sodium dodecylsulfate(SDS)-polyacrylamide gel electrophoresis.
Butanol extraction of HDL or partially purified serum factor
One mg human HDL in 0.8 ml PBS/20 mM acetic acid was mixed with 0.4 ml n-butanol/20 mM acetic acid and the phases separated by centrifugation as described by English et al. [15]. Twenty-μl aliquots of the upper ("butanol') and lower ("water") phases were dried and dissolved in 50 μl PBS and 5 μl used for cell incubation. For the partially purified serum factor preparation, active fractions from the initial C18 column were dried and the residue dissolved in 4 ml PBS/20 mM acetic acid and 2 ml n-butanol/20 mM acetic acid added. Further processing was as described above except that the 50-μl aliquots were dried and dissolved in 500 μl PBS.
Western Blots
The phosphorylated forms of extracellular signal-regulated kinases 1 and 2 (pERK1/2) were determined by Western blot using rabbit antisera to pERKs (Promega) diluted 1:20,000 as previously described [1] with the modification that the horseradish peroxidase-linked secondary antibody (goat anti-rabbit IGG) was diluted 1:500,000 and chemiluminescence detected by Pierce SuperSignal West Femto reagent. Phosphorylated Akt/PKB (pAkt) was detected using rabbit antisera to phosphoserine 473 (Cell Signaling Technology) diluted 1:1000 and the secondary antibody diluted 1:250,000 and detected as above.
Purification of Desensitization Activity
Isolation of a desensitization component of heat-inactivated FBS was accomplished using a combination of anion exchange chromatography (DEAE) and C18-reverse-phase chromatography. Generally, three hundred ml serum was diluted with 4 volumes 25 mM Tris, pH 7.8–50 mM NaCl and mixed with 200 ml DEAE-Sepharose, equilibrated in the same buffer overnight at 8°C. The mixture was filtered, washed with 1000 ml 25 mM Tris, pH 7.8–100 mM NaCl and the washed DEAE Sepharose placed in a 5 cm diameter column. Protein was eluted from the DEAE-Sepharose with 500 ml 25 mM Tris, pH 7.8–200 mM NaCl and 10-ml fractions were collected. Fractions with an A280 greater than 1.0 were pooled (about 100 ml) and acetic acid and triflouroacetic acid (TFA) were added to final concentrations of 10% and 0.1%, respectively. The acidified fractions were applied to 3 C18 cartridges (Sep-Pak Plus C18, Waters) equilibrated in 0.1% TFA and each washed with 20 ml 40% acetonitrile-0.1% TFA prior to elution of retained material with 10-ml 60% acetonitrile-0.1% TFA per cartridge. The pooled eluates were diluted approximately 3-fold with 0.1% TFA and applied to a C18 column (Vydac 218TP510) equilibrated in 35% acetonitrile-0.1% TFA at a flow rate of 2-ml/min. The column was washed for 10 min with 35%acetonitrile-0.1% followed by 49% acetonitrile-0.1% TFA for 10 min and then a linear 49%–53% acetonitrile gradient over 40 min. Inhibitory activity in the 2-ml fractions was monitored using inhibition of CNP-elevated cGMP in the GC-B/3T3 cell line. The fractions eluting at approximately 51% acetonitrile contained significant desensitization activity. The active fractions were pooled, diluted 3-fold in 0.1% TFA and applied to an analytic C18 column (Vydac 218TP54) equilibrated in 5% acetonitrile-0.1% TFA followed with 48.8% % acetonitrile-0.1% TFA for 10 min and then with a linear 48.8%–50.3% acetonitrile-0.1% TFA gradient for 65 min. Active fractions eluting at approximately 50 % acetonitrile were diluted with 2 volumes 0.1% TFA and reapplied to the analytic C18 column equilibrated in 5% acetonitrile-0.1% TFA, washed for 5 min with 5% acetonitrile-0.1% TFA and then for 5 min with 48% acetonitrile-0.1% TFA. Desensitization activity was eluted with a linear 48–49% acetonitrile-0.1% TFA gradient obtained over 60 min at 1 ml/min. Active fractions contained a single symmetrical peak of absorbance at 280 nm that co-eluted with inhibitory activity (Fig 1).
List of Abbreviations
ANP, atrial natriuretic peptide; CNP, C-type natriuretic peptide; GC-B, guanylyl cyclase B; GC-A, guanylyl cyclase A; FBS, fetal bovine serum; ERK, extracellular regulated kinase; Akt/PKB, Akt/protein kinase B; GCB/3T3, guanylyl cyclase B/NIH 3T3 fibroblasts; S1P, sphingosine-1-phosphate; LPA, lysophosphatidic acid; HDL, high density lipoprotein; ApoA2, apolipoprotein A2; cGMP, guanosine-3',5'-monophosphate
Competing interests
None declared.
Authors' contributions
TDC designed and conceived most of the experiments and drafted the manuscript. DTP performed most of the radioimmunoassays and other experimental procedures. DLG participated in the experimental design and coordination of the research.
Acknowledgments
Acknowledgements
This work was supported in part by an award to DLG from the Sandler Program for Asthma Research, the Howard Hughes Medical Institute and the Cecil H. and Ida Green Center for Reproductive Biology Sciences.
Contributor Information
Ted D Chrisman, Email: Ted.Chrisman@utsouthwestern.edu.
Dorenda T Perkins, Email: Dorenda.perkins@utsouthwestern.edu.
David L Garbers, Email: David.Garbers@utsouthwestern.edu.
References
- Chrisman TD, Garbers DL. Reciprocal antagonism coordinates C-type natriuretic peptide and mitogen-signaling pathways in fibroblasts. J Biol Chem. 1999;274:4293–4299. doi: 10.1074/jbc.274.7.4293. [DOI] [PubMed] [Google Scholar]
- Ueno H, Haruno A, Morisaki N, Furuya M, Kangawa K, Takeshita A, Saito Y. Local expression of C-type natriuretic peptide markedly suppresses neointimal formation in rat injured arteries through an autocrine/paracrine loop. Circulation. 1997;96:2272–2279. doi: 10.1161/01.cir.96.7.2272. [DOI] [PubMed] [Google Scholar]
- Tamura N, Chrisman TD, Garbers DL. The regulation and physiological roles of the guanylyl cyclase receptors. Endocr J. 2001;48:611–634. doi: 10.1507/endocrj.48.611. [DOI] [PubMed] [Google Scholar]
- Abbey SE, Potter LR. Lysophosphatidic acid inhibits C-type natriuretic peptide activation of guanylyl cyclase-B. Endocrinology. 2003;144:240–246. doi: 10.1210/en.2002-220702. [DOI] [PubMed] [Google Scholar]
- Horio T, Tokudome T, Maki T, Yoshihara F, Suga S, Nishikimi T, Kojima M, Kawano Y, Kangawa K. Gene expression, secretion, and autocrine action of C-type natriuretic peptide in cultured adult rat cardiac fibroblasts. Endocrinology. 2003;144:2279–2284. doi: 10.1210/en.2003-0128. [DOI] [PubMed] [Google Scholar]
- Cao L, Gardner DG. Natriuretic peptides inhibit DNA synthesis in cardiac fibroblasts. Hypertension. 1995;25:227–234. doi: 10.1161/01.hyp.25.2.227. [DOI] [PubMed] [Google Scholar]
- Potter LR, Garbers DL. Dephosphorylation of the guanylyl cyclase-A receptor causes desensitization. J Biol Chem. 1992;267:14531–14534. [PubMed] [Google Scholar]
- Potter LR. Phosphorylation-dependent regulation of the guanylyl cyclase-linked natriuretic peptide receptor B: dephosphorylation is a mechanism of desensitization. Biochemistry. 1998;37:2422–2429. doi: 10.1021/bi972303k. [DOI] [PubMed] [Google Scholar]
- Motizuki M, Itoh T, Yamada M, Shimamura S, Tsurugi K. Purification, primary structure, and antimicrobial activities of bovine apolipoprotein A-II. J Biochem (Tokyo) 1998;123:675–679. doi: 10.1093/oxfordjournals.jbchem.a021990. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Innerarity TL, Rall SC Jr., Weisgraber KH. Plasma lipoproteins: apolipoprotein structure and function. J Lipid Res. 1984;25:1277–1294. [PubMed] [Google Scholar]
- Swaney JB. Characterization of the high-density lipoprotein and its major apoprotein from human, canine, bovine and chicken plasma. Biochim Biophys Acta. 1980;617:489–502. doi: 10.1016/0005-2760(80)90015-6. [DOI] [PubMed] [Google Scholar]
- Sachinidis A, Kettenhofen R, Seewald S, Gouni-Berthold I, Schmitz U, Seul C, Ko Y, Vetter H. Evidence that lipoproteins are carriers of bioactive factors. Arterioscler Thromb Vasc Biol. 1999;19:2412–2421. doi: 10.1161/01.atv.19.10.2412. [DOI] [PubMed] [Google Scholar]
- Okajima F. Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an anti-atherogenic mediator? Biochim Biophys Acta. 2002;1582:132–137. doi: 10.1016/S1388-1981(02)00147-6. [DOI] [PubMed] [Google Scholar]
- Moolenaar WH. Lysophosphatidic acid, a multifunctional phospholipid messenger. J Biol Chem. 1995;270:12949–12952. doi: 10.1074/jbc.270.22.12949. [DOI] [PubMed] [Google Scholar]
- English D, Welch Z, Kovala AT, Harvey K, Volpert OV, Brindley DN, Garcia JG. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. Faseb J. 2000;14:2255–2265. doi: 10.1096/fj.00-0134com. [DOI] [PubMed] [Google Scholar]
- Watterson K, Sankala H, Milstien S, Spiegel S. Pleiotropic actions of sphingosine-1-phosphate. Prog Lipid Res. 2003;42:344–357. doi: 10.1016/S0163-7827(03)00015-8. [DOI] [PubMed] [Google Scholar]
- Van Brocklyn JR, Lee MJ, Menzeleev R, Olivera A, Edsall L, Cuvillier O, Thomas DM, Coopman PJ, Thangada S, Liu CH, Hla T, Spiegel S. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J Cell Biol. 1998;142:229–240. doi: 10.1083/jcb.142.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceyka M, Payne SG, Milstien S, Spiegel S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta. 2002;1585:193–201. doi: 10.1016/S1388-1981(02)00341-4. [DOI] [PubMed] [Google Scholar]
- Goetzl EJ, An S. Diversity of cellular receptors and functions for the lysophospholipid growth factors lysophosphatidic acid and sphingosine 1-phosphate. Faseb J. 1998;12:1589–1598. [PubMed] [Google Scholar]
- Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22:364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- Payne SG, Milstien S, Spiegel S. Sphingosine-1-phosphate: dual messenger functions. FEBS Lett. 2002;531:54–57. doi: 10.1016/S0014-5793(02)03480-4. [DOI] [PubMed] [Google Scholar]
- Chrisman TD, Schulz S, Potter LR, Garbers DL. Seminal plasma factors that cause large elevations in cellular cyclic GMP are C-type natriuretic peptides. J Biol Chem. 1993;268:3698–3703. [PubMed] [Google Scholar]
- Havel RJ, Eder HA, Bragton JH. The distribution and chemical composition of ultracentrifugally seperated lipoproteins in human serum. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]


