Abstract
Apolipoprotein (apo) E4 is the major known genetic risk factor for Alzheimer's disease (AD). We have shown in vitro and in vivo that apoE4 preferentially undergoes aberrant cleavage in neurons, yielding neurotoxic C-terminal-truncated fragments. To study the effect of these fragments on amyloid-β (Aβ) clearance/deposition and their potential synergy with Aβ in eliciting neuronal and behavioral deficits, we cross-bred transgenic mice expressing apoE3, apoE4, or apoE4(Δ272–299) with mice expressing human amyloid protein precursor (APP) harboring familial AD mutations (hAPPFAD). At 6–8 mo of age, hAPPFAD mice expressing apoE3 or apoE4 had lower levels of hippocampal Aβ (94% and 89%, respectively) and less Aβ deposition (89% and 87%) than hAPPFAD mice without apoE, whereas hAPPFAD mice expressing mouse apoE had higher Aβ levels. Thus, human apoE stimulates Aβ clearance, but mouse apoE does not. Expression of apoE4(Δ272–299) reduced total Aβ levels by only 63% and Aβ deposition by 46% compared with hAPPFAD mice without apoE. Unlike apoE3 and apoE4, the C-terminal-truncated apoE4 bound poorly with Aβ peptides, leading to decreased Aβ clearance and increased Aβ deposition. Despite their lower levels of Aβ and Aβ deposition, hAPPFAD/apoE4(Δ272–299) mice accumulated pathogenic Aβ oligomers and displayed neuronal and behavioral deficits similar to or more severe than those in hAPPFAD mice. Thus, the C-terminal-truncated apoE4 fragment inefficiently clears Aβ peptides and acts in concert with low levels of Aβ to elicit neuronal and behavioral deficits in mice.
Keywords: animal model, neurodegeneration
Alzheimer's disease (AD) is a complex neurodegenerative disorder likely caused by interactions among multiple genetic and environmental factors. Mutations in amyloid protein precursor (APP), presenilin-1 (PS1), and PS2 have been linked to early-onset familial AD (1–3), which accounts for <5% of AD cases. All mutations affect APP processing and alter the production of amyloid-β(Aβ) peptides (1, 2). Several transgenic mouse models have been developed to study the effects of Aβ on neuronal and behavioral deficits (4). The J20 line of transgenic mice expressing human APP harboring familial AD mutations (hAPPFAD) accumulates neurotoxic Aβ peptides and display synaptic and cognitive deficits at 5–6 mo of age (5–7).
Apolipoprotein (apo) E4, one of three isoforms of apoE (apoE2, apoE3, and apoE4), is the major genetic risk factor for late-onset AD, which accounts for >95% of AD cases (8–10). ApoE4 carriers account for 65–80% of all AD cases, highlighting the importance of apoE4 in AD pathogenesis (11). Emerging data suggest that apoE4 contributes to AD by interacting with different pathogenic factors through various pathways (12–15).
Several mouse models have been established to study the roles of apoE4 in AD pathogenesis. Neuron-specific apoE4 transgenic mice (NSE-apoE4) develop neuronal and spatial learning and memory deficits (16–18). Astrocyte-specific apoE4 transgenic mice (GFAP-apoE4) display working memory deficits without significant neuronal deficits (19). ApoE4 knock-in (apoE4-KI) mice also develop neuronal and behavioral deficits (20). When cross-bred with hAPPFAD mice, both apoE3 and apoE4, regardless of the cellular source, efficiently clear Aβ from the brain in young mice; however, at older ages, apoE4 mice have more Aβ deposits than apoE3 mice, again regardless of the cellular source of apoE (21–24).
We demonstrated that neurons express apoE in response to brain insults and injury (25, 26). Neuronal apoE undergoes aberrant proteolysis, with apoE4 being more susceptible to cleavage than apoE3, generating C-terminal-truncated fragments that are more abundant in human AD brains than in controls (27–29). The apoE4 fragments are neurotoxic in neuronal cultures (27, 30) and cause AD-like neuronal and behavioral deficits in transgenic mice expressing high levels of the fragment at young ages (6–7 mo) (28) or low levels of the fragment at old ages (12–13 mo; ref. 31).
Here, we tested the effect of C-terminal-truncated apoE4 on Aβ clearance and deposition and its potential synergy with Aβ in eliciting neuronal and behavioral deficits. For this purpose, we cross-bred J20 hAPPFAD mice and apoE4(Δ272–299) transgenic mice, in which a major apoE4 fragment found in AD brains was expressed in neurons at low levels (31).
Results
Generation of hAPPFAD Mice Expressing Human apoE3, apoE4, apoE4(Δ272–299), or Mouse apoE.
To determine the effect of the apoE4 fragment on Aβ levels in brains, we crossbred transgenic mice expressing the C-terminal-truncated human apoE4 [apoE4(Δ272–299)] at low levels (31) with the J20 line of hAPPFAD mice (5). To avoid the potential combined effect of human and mouse apoE (mE), both parental lines were first bred onto the mouse apoE knockout (mEKO) background. The mice used in this study were hAPPFAD/apoE4(Δ272–299)/mEKO, hAPPFAD/mEKO, apoE4(Δ272–299)/mEKO, and mEKO. To generate hAPPFAD/apoE3/mEKO and hAPPFAD/apoE4/mEKO mice as additional controls, we crossed NSE-apoE3/mEKO and NSE-apoE4/mEKO mice (16) with hAPPFAD/mEKO mice. hAPPFAD mice expressing endogenous mouse apoE (hAPPFAD/mE) were also included in the study as additional controls. Expression of different forms of apoE at similar levels did not alter the levels of hAPPFAD in transgenic mice (Fig. S1). Because we were interested in studying the potentially additive or synergistic effect of the apoE4 fragment with Aβ on neuronal and behavioral deficits, we used young mice at 6–8 mo of age, before hAPPFAD mice display extensive Aβ deposition (5). We focused our neuropathological studies on the hippocampus because it is preferentially affected in AD.
Higher Hippocampal Aβ Levels in hAPPFAD/apoE4(Δ272–299) Mice than in hAPPFAD Mice Expressing apoE3 or apoE4.
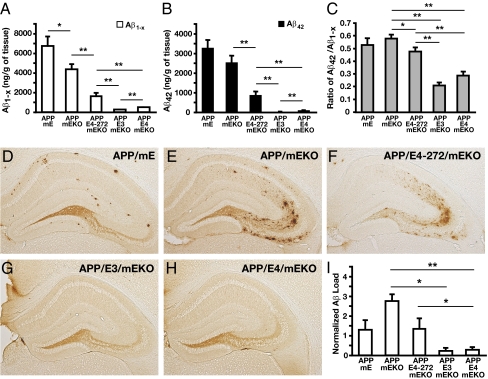
As determined by ELISA, Aβ(1–x) and Aβ42 levels in the hippocampus were 94% lower in hAPPFAD/apoE3/mEKO and 89% lower in hAPPFAD/apoE4/mEKO mice than in hAPPFAD/mEKO mice (Fig. 1 A and B), consistent with the strong stimulation of Aβ clearance by apoE3 and apoE4 (21–24). However, the Aβ(1–x) and Aβ42 levels were significantly higher in hAPPFAD/apoE4/mEKO than in hAPPFAD/apoE3/mEKO mice, suggesting that apoE4 is less able to clear Aβ or has a higher tendency to retain Aβ than apoE3. Total Aβ and Aβ42 levels were 3.5-fold and 6.8-fold higher in hAPPFAD/apoE4(Δ272–299)/mEKO than in hAPPFAD/apoE4/mEKO mice, but 63% and 65% lower than in hAPPFAD/mEKO mice (Fig. 1 A and B). Thus, the apoE4 fragment is less able to clear Aβ or has a higher tendency to retain Aβ than the full-length apoE4. hAPPFAD mice with endogenous mouse apoE (hAPPFAD/mE) had higher total Aβ levels than hAPPFAD/mEKO mice and similar levels of Aβ42, suggesting that mouse apoE does not significantly stimulate Aβ clearance or strongly retains Aβ in the brain.
Fig. 1.
Aβ levels in the hippocampus of different mice at 6–8 mo of age. (A and B) Levels of Aβ1-x (A) and Aβ42 (B) were determined by a sandwich ELISA in hippocampal lysates. (C) Aβ42/Aβ1-x ratios for each genotype. In A–C, values are mean ± SEM; n = 11–17 per genotype. *P < 0.05, **P < 0.01. (D–H) Serial sections (30 μm thick, collected 300 μm apart) from APP/mE (D), APP/mEKO (E), APP/E4-272/mEKO (F), APP/E3/mEKO (G), and APP/E4/mEKO (H) mice were immunostained with 3D6 monoclonal antibody. (I) Percent area of Aβ deposition determined by densitometry. Values are mean ± SEM n = 4–17 per genotype. *P < 0.05, **P < 0.01.
The ratio of Aβ42 to total Aβ is frequently used to indicate the fibrillogenic potential of a mixture of Aβ species (5). This ratio was about twofold higher in hAPPFAD/apoE4(Δ272–299)/mEKO mice than in hAPPFAD/apoE3/mEKO and hAPPFAD/apoE4/mEKO mice, although significantly lower than in hAPPFAD/mEKO mice (Fig. 1C). Thus, apoE4 fragment preferentially affects Aβ42 clearance and/or retention, compared with the full-length apoE3 and apoE4. The ratio of Aβ42 to total Aβ was similar in hAPPFAD/mE and hAPPFAD/mEKO mice (Fig. 1C), suggesting that mouse apoE affects the clearance and/or retention of Aβ42 and other Aβ species equally.
Greater Hippocampal Aβ Deposition in hAPPFAD/apoE4(Δ272–299) Mice than in hAPPFAD Mice Expressing apoE3 or apoE4.
To assess Aβ deposition, we immunostained brain sections with the 3D6 monoclonal antibody and quantified the area of Aβ deposits in the hippocampus (5). Aβ accumulation in hAPPFAD/apoE3/mEKO and hAPPFAD/apoE4/mEKO mice was mostly in the hilus of the dentate gyrus (Fig. 1 G and H) and only about 11% and 13% of the area, respectively, of those in hAPPFAD/mEKO mice (Fig. 1I), which were more widespread (Fig. 2E), as reported (32). The Aβ deposits in hAPPFAD/mE mice were dense-core like (Fig. 1D) and Thioflavin S positive (Fig. S2), whereas the deposits in hAPPFAD/mEKO mice were Thioflavin S-negative diffuse plaques (Fig. 1E and Fig. S2). The area of Aβ deposition was fivefold greater in hAPPFAD/apoE4(Δ272–299)/mEKO mice than in hAPPFAD/apoE3/mEKO or hAPPFAD/apoE4/mEKO mice (Fig. 1I). The location and morphology of Aβ deposits were similar in hAPPFAD/apoE4(Δ272–299)/mEKO mice and hAPPFAD/mEKO mice (Fig. 1 E and F), although the area was 46% smaller in hAPPFAD/apoE4(Δ272–299)/mEKO mice (Fig. 1I). There were no Thioflavin S-positive plaques in hAPPFAD/apoE4(Δ272–299)/mEKO mice (Fig. S2). Thus, mouse apoE stimulated dense-core plaque formation (32), whereas human apoE4 fragment led to more diffuse Aβ accumulation in the brain parenchyma.
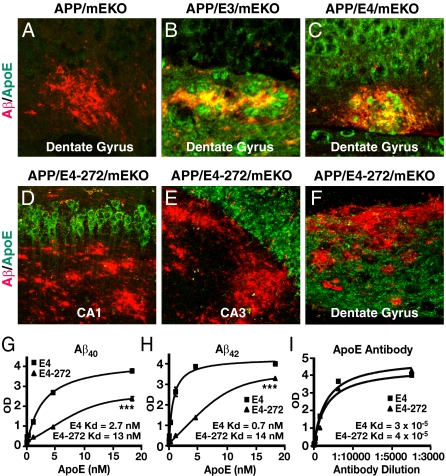
Fig. 2.
Fluorescence immunostaining for Aβ and apoE in the hippocampus of different mice at 6–8 mo of age. (A) ApoE immunoreactivity is absent in APP/mEKO mice. (B and C) ApoE colocalizes with Aβ in Aβ deposits in hAPPFAD mice expressing apoE3 (B) or apoE4 (C). (D–F) The apoE4 fragment does not colocalize with Aβ in APPFAD mice expressing apoE4-272. (G and H) Aβ interaction with apoE4 or apoE4-272 detected by ELISA. Aβ40 (G) or Aβ42 (H) was coated onto 96-well microtiter plates (330 ng per well) and allowed to bind to decreasing concentrations of recombinant apoE4 or apoE4-272 (starting amount was 62.5 ng with fourfold dilutions thereafter). Bound apoE was detected with a polyclonal apoE antibody. (I) The polyclonal anti-apoE reacts equally well with apoE4 and apoE4-272 in an ELISA. ApoE4 or apoE4-272 was coated at 50 ng/well onto a 96-well ELISA plate. Polyclonal anti-apoE was diluted serially starting at 1:4,000 and fourfold thereafter and applied as the detection antibody. Data points are mean ± SD; ***P < 0.001.
ApoE4(Δ272–299) Is Not Present in Aβ Deposits and Has a Lower Binding Affinity for Aβ than apoE4.
Double immunofluorescence staining for apoE and Aβ revealed that apoE3 and apoE4 localized within Aβ deposits in the dentate gyrus of the hippocampus in hAPPFAD/apoE3/mEKO and hAPPFAD/apoE4/mEKO mice (Fig. 2 B and C). In hAPPFAD/mEKO mice, as expected, there was no apoE staining in neurons or Aβ deposits (Fig. 2A). Surprisingly, apoE4(Δ272–299) was not detected within any Aβ deposits in the CA1, CA3, and dentate gyrus of hAPPFAD/apoE4(Δ272–299)/mEKO mice (Fig. 2 D–F). Thus, apoE4(Δ272–299) may not interact as effectively with Aβ in vivo as apoE3 and apoE4.
To test this possibility, we performed an in vitro binding assay in which different forms of apoE were incubated in 96-well plates coated with Aβ peptides. Although the apoE detection antibody had equal affinity for apoE4 and apoE4(Δ272–299) (Fig. 2I), apoE4(Δ272–299) bound poorly to both Aβ40 and Aβ42 compared with apoE4 (Fig. 2 G and H), suggesting that the C-terminal region of apoE (aa 272–299) is critical for its interaction with Aβ. Thus, the higher Aβ levels in hAPPFAD/apoE4(Δ272–299)/mEKO mice than in hAPPFAD/apoE4/mEKO mice (Fig. 1 A and B) are likely due to the decreased ability of apoE4(Δ272–299) to bind with Aβ, leading to decreased Aβ clearance.
ApoE4 Fragment Acts in Concert with Aβ to Elicit Neuronal Deficits in Mice.
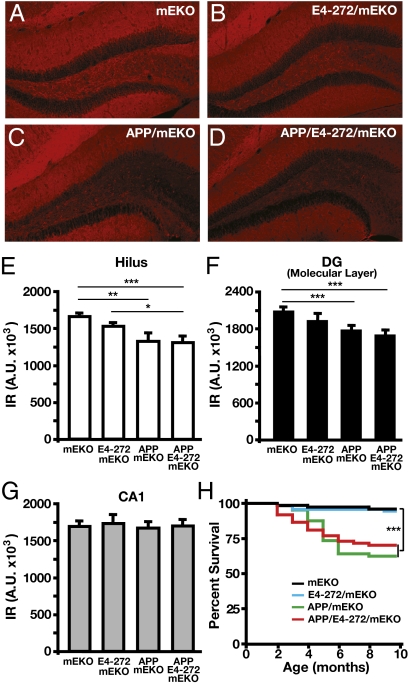
We next determined whether the apoE4 fragment and Aβ act in concert to elicit neuronal deficits. We first immunostained for MAP2, a dendritic marker (16). ApoE4(Δ272–299)/mEKO mice, which had no Aβ accumulation in the hippocampus, showed a trend toward lower MAP2 immunoreactivity (IR) in the hilus and molecular layer of the dentate gyrus compared with mEKO mice (Fig. 3 A, B, E, and F), which had similar MAP2 IR to apoE3 transgenic mice, as we reported (33). hAPPFAD/mEKO mice, which had high levels of Aβ in the hippocampus (Fig. 1 A, B, E, and I), had a significant reduction in MAP2 IR in the hilus and molecular layer of the dentate gyrus (Fig. 3 A, C, E, and F). Importantly, the MAP2 reduction in both areas was similar in hAPPFAD/apoE4(Δ272–299)/mEKO mice, which had 63% lower Aβ levels and 46% less Aβ deposition than hAPPFAD/mEKO mice (Fig. 1 A, B, F, and I), and in hAPPFAD/mEKO mice (Fig. 3 A, C, E, and F). There was no significant difference in MAP2 IR in the CA1 area of the hippocampus among the various groups of mice (Fig. 3G). Thus, the apoE4 fragment appears to act in concert with low levels of Aβ to cause a pronounced decrease in MAP2 levels, probably a reflection of dendritic impairment specifically in the hilus and molecular layer of the dentate gyrus. Interestingly, the premature death of hAPPFAD mice, as reported (17, 28, 34), was also similar in hAPPFAD/mEKO and hAPPFAD/apoE4(Δ272–299)/mEKO mice (Fig. 3H), although the latter had significantly lower Aβ levels and Aβ deposition.
Fig. 3.
Immunostaining of MAP2 in different mice at 6–8 mo of age. (A–D) MAP2 staining in representative sections from mEKO (A), E4-272/mEKO (B), APP/mEKO (C), and APP/E4-272/mEKO (D) mice. (E–G) MAP2 IR determined by densitometry in the hilus (E), the molecular layer of the dentate gyrus (DG; F), and the CA1 region (G) of the hippocampus. Values are mean ± SEM; n = 7–22 per genotype. *P < 0.05, **P < 0.01, ***P < 0.001. (H) The differences in mouse survival were assessed by Kaplan–Meier analysis. n = 73 for mEKO, n = 92 for E4-272/mEKO, n = 64 for APP/mEKO, n = 74 for APP/E4-272/mEKO; ***P < 0.001. IR, immunoreactivity; A.U., arbitrary units.
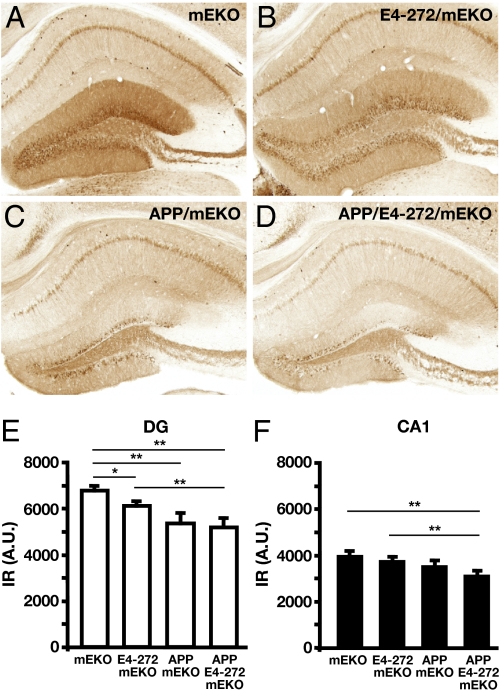
We then immunostained for calbindin, an activity-dependent calcium binding protein that is significantly decreased in the dentate gyrus of mouse models of AD and whose levels correlate with cognitive impairment (6). ApoE4(Δ272–299)/mEKO mice, which had no Aβ accumulation in the hippocampus, displayed moderately less calbindin IR in the molecular layer of the dentate gyrus than mEKO mice (Fig. 4 A, B, E). This suggests that the apoE4 fragment alone can cause a moderate decrease in calbindin. hAPPFAD/mEKO mice, which had high levels of Aβ in the hippocampus (Fig. 1 A, B, E, and I), had a greater reduction in calbindin IR (Fig. 4 A, C, and E). Importantly, the calbindin reduction was similar in hAPPFAD/apoE4(Δ272–299)/mEKO mice, which had 63% lower Aβ levels and 46% less Aβ deposition than hAPPFAD/mEKO mice (Fig. 1 A, B, F, and I), and in hAPPFAD/mEKO mice (Fig. 4 C–E). Thus, the apoE4 fragment appears to act in concert with low levels of Aβ to cause a pronounced decrease in calbindin levels. Furthermore, hAPPFAD/apoE4(Δ272–299)/mEKO mice had significantly reduced calbindin IR in the CA1 stratum radiatum layer of the hippocampus, unlike mice expressing either apoE4(Δ272–299) or hAPPFAD alone (Fig. 4F), supporting a synergistic effect of the apoE4 fragment and Aβ in this subregion.
Fig. 4.
Immunostaining of calbindin in different mice at 6–8 mo of age. (A–D) Calbindin staining in representative sections from mEKO (A), E4-272/mEKO (B), APP/mEKO (C), and APP/E4-272/mEKO (D) mice. (E and F) Calbindin IR determined by densitometry in the dentate gyrus (E) and the CA1 region (F) of the hippocampus. Values are mean ± SEM; n = 7–22 per genotype. *P < 0.05, **P < 0.01. IR, immunoreactivity; A.U., arbitrary units.
We also analyzed granule cells of the dentate gyrus for expression of Fos, an immediate-early gene encoding a synaptic activity-dependent protein. A reduction in the number of Fos-positive granule cells indicates neuronal impairment (6). hAPPFAD/mEKO mice, which had high levels of Aβ accumulation, and hAPPFAD mice expressing apoE4(Δ272–299), which had significantly lower Aβ accumulation, had similar reductions in the number of Fos-positive granule cells to levels much lower than those in mice not expressing any apoE or only apoE4(Δ272–299) (Fig. S3). These results further support a concerted neurotoxic effect of the apoE4 fragment and low levels of Aβ.
ApoE4 Fragment Acts in Concert with Aβ to Elicit Behavioral Deficits in Mice.
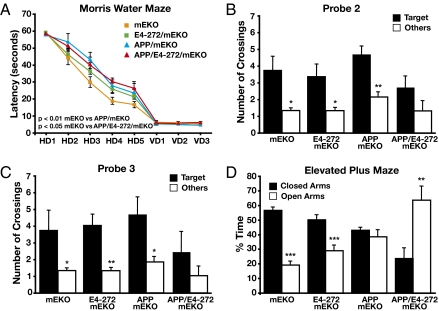
We also determined whether the apoE4 fragment and Aβ act in concert to induce behavioral deficits. Spatial learning and memory were assessed by the Morris water maze test. At 5–9 mo of age, mEKO mice quickly learned to find the hidden platform (Fig. 5A). It has been reported that mEKO mice at this age learn as well as wild-type mice in the Morris water maze (35). However, hAPPFAD/mEKO mice showed a mild, but significant deficit in spatial learning (Fig. 5A). ApoE4(Δ272–299)/mEKO mice did not differ significantly from mEKO mice in the hidden platform trial at this young age (Fig. 5A). hAPPFAD/apoE4(Δ272–299)/mEKO and hAPPFAD/mEKO mice had similar impairments in spatial learning (Fig. 5A), although the former had 63% lower Aβ levels and 46% less Aβ deposition (Fig. 1 A, B, and I). Swim speeds did not differ among various groups (Fig. S4), indicating that the impairment was not due to motor deficits. All mice performed equally well in visible platform trials (Fig. 5A). In the probe trials 72 h (Fig. 5B) and 120 h (Fig. 5C) after the last hidden platform trial, hAPPFAD/apoE4(Δ272–299)/mEKO mice had impaired memory retention, whereas apoE4(Δ272–299)/mEKO and hAPPFAD/mEKO mice did not (Fig. 5 B and C), suggesting a concerted or synergistic detrimental effect of apoE4 fragment and Aβ on memory retention.
Fig. 5.
Learning and memory impairments and abnormal anxiety in hAPPFAD mice expressing apoE4(Δ272–299). (A–C) Spatial learning and memory were tested in the Morris water maze in female mice at 5–9 mo of age. (A) Learning curves. One-way ANOVA and Tukey/Bonferroni multiple comparison tests were performed. (B and C) Memory in probe trials 72 h (B) and 120 h (C) after the last session of the hidden platform trial. (D) Anxiety was assessed in the elevated plus maze test. Values are mean ± SEM; n = 6–10 per genotype. *P < 0.05, **P < 0.01, ***P < 0.001. HD, hidden day; VD, visible day.
In the elevated plus maze, both mEKO and apoE4(Δ272–299)/mEKO mice had normal levels of anxiety at 5–9 mo of age (Fig. 5D). However, the abnormalities in anxiety were observed in hAPPFAD/mEKO mice and further increased in hAPPFAD/apoE4(Δ272–299)/mEKO mice (Fig. 5D).
Accumulation of Pathogenic Aβ Oligomers in hAPPFAD/apoE4(Δ272–299)/mEKO Mice with Low Levels of Total Aβ.
In searching for mechanisms underlying the concerted effects of apoE4 fragment and low levels of Aβ on neuronal and behavioral deficits, we measured Aβ*56, a pathogenic Aβ oligomer that correlates with learning and memory deficits in different lines of hAPPFAD mice (36–38). Aβ*56 isolated from hAPPFAD mouse brains also elicits memory deficits when injected into the brains of wild-type rats (36). Interestingly, hAPPFAD/apoE4(Δ272–299)/mEKO mice had a trend toward significantly increased Aβ*56 levels (P = 0.05) compared with hAPPFAD/mEKO mice (Fig. S5), although the former had 63% lower Aβ levels (Fig. 1A and B). The overall hAPP levels were comparable in the two groups of mice (Figs. S1 and S5). Thus, apoE4 fragments enhance the accumulation of pathogenic Aβ*56 in the presence of low levels of Aβ, possibly contributing to neuronal and behavioral deficits.
Discussion
This study shows that hAPPFAD/apoE4(Δ272–299)/mEKO mice had much higher levels of total Aβ and Aβ42 and more Aβ deposits than hAPPFAD/apoE3/mEKO and hAPPFAD/apoE4/mEKO mice at 6–8 mo of age. ApoE4(Δ272–299) did not colocalize with Aβ in deposits and had a lower binding affinity for Aβ42 and Aβ40. Thus, it likely has a reduced ability to clear Aβ than full-length apoE3 and apoE4, rather than a greater tendency to stimulate Aβ deposition. Furthermore, the C-terminal-truncated apoE4 fragment acts in concert with lower levels of Aβ to elicit neuronal and behavioral deficits in mice at 5–9 mo of age. Thus, apoE4 fragments and Aβ may act in concert to contribute to AD pathogenesis.
Importantly, our data demonstrate that the C-terminal 28 amino acids (amino acids 272–299) in apoE are critical in mediating its interaction with Aβ, and thus Aβ clearance, at least in mice. ApoE has two structural domains—a N-terminal domain (amino acids 1–191) containing the receptor binding region (amino acids 135–150), and a C-terminal domain (amino acids 222–299) containing the lipid-binding region (amino acids 241–272), which are linked by a hinge region (amino acids 192–221; ref. 39). In vitro studies of the interaction between apoE and Aβ identified the lipid-binding domain as the binding partner for Aβ peptides (40, 41). Our findings suggest that the C-terminal 28 amino acids (amino acids 272–299) affect the conformation of this domain, altering its interaction with Aβ. Consistent with this possibility, biophysical studies suggest that the lipid-binding domain has a less organized structure in C-terminal-truncated apoE4 than in apoE4 (42, 43). We reported that C-terminal-truncated apoE4 fragments are more abundant in AD brains than in age-matched controls (27, 28). Others reported lower levels of apoE4 in AD brains than in controls (44). Thus, an increase in the ratio of C-terminal-truncated apoE4 to apoE4, which conveys a decreased ability to clear Aβ, might contribute to increased Aβ accumulation and amyloid plaque formation in AD patients with apoE4.
Our data confirm that both apoE3 and apoE4 stimulate Aβ clearance in young mice, whereas mouse apoE stimulates Aβ deposition, compared with the absence of apoE (22, 24, 32). This observation has implications for understanding the effect of apoE on Aβ metabolism and for validating and interpreting clinical trials of anti-Aβ therapy. Almost all preclinical drug development studies related to Aβ are performed in hAPPFAD mice with mouse apoE (45). If mouse apoE differs significantly from human apoE in regulating Aβ metabolism (mouse apoE stimulates Aβ deposition, whereas human apoE stimulates Aβ clearance), as demonstrated in the current and previous studies (22, 24, 32), drugs that work well in hAPPFAD mice with mouse apoE might not work well in AD patients with human apoE. This might explain, at least to some extent, the unsatisfactory outcome of many clinical trials targeting Aβ (45). Thus, hAPPFAD mice expressing different forms of human apoE are more reliable models for preclinical studies of drugs targeting Aβ. However, in hAPPFAD mice expressing human apoE3 or apoE4, significant Aβ accumulation usually appears after 12–16 mo of age. Thus, hAPPFAD/apoE4(Δ272–299)/mEKO mice, which develop significant Aβ accumulation and neuronal and behavioral deficits at 6–8 mo of age, represent an alternative mouse model for studying anti-AD drugs targeting both Aβ and apoE4.
Previously, we observed neuronal and behavioral deficits in transgenic mice expressing high levels of C-terminal-truncated apoE4 fragments at a young age (6–7 mo; ref. 28) or low levels in old age (12–13 mo; ref. 31). Here, we show that low levels of apoE4 fragments elicit marginal neuronal and behavioral deficits in young mice (5–9 mo), but in combination with low levels of Aβ, which alone do not cause deficits at low levels (46, 47), lead to significant premature death and more pronounced neuronal and behavioral deficits in mice at a young age. Thus, although Aβ is not necessary for apoE4 fragments to be involved in neuropathology, low levels of both cause early-onset neuronal and behavioral deficits in mice. Importantly, apoE4 fragments enhanced the accumulation of pathogenic Aβ*56 in the presence of low levels of Aβ; however, it is not clear whether this was due to increased formation or decreased clearance of Aβ*56 in the presence of apoE4 fragments. The greater abundance of apoE4 fragments in AD brains than in age-matched controls (27, 28) might facilitate Aβ*56 accumulation, contributing to learning and memory deficits. Thus, in mice, apoE4 fragments alone can elicit neuronal and behavioral deficits, and the additional presence of low levels of Aβ or Aβ*56 accelerates the deficits. ApoE4 fragments may act in the same way to contribute to the pathogenesis and lower the age of onset of AD in humans. Consequently, apoE4 cleavage should also be considered a target for anti-AD drug development (12–15).
Materials and Methods
J20 hAPPFAD mice expressing hAPP harboring the Swedish (K670N,M671L) and Indiana (V717F) mutations (5) were backcrossed with murine apoE knockout mice (mEKO) and subsequently cross-bred with NSE-apoE3, NSE-apoE4 (16), or low expresser Thy1-apoE4(Δ272–299) mice (31) on the mEKO background. Aβ(1-x) and Aβ42 levels in the hippocampus were measured by ELISA (6). Aβ*56 oligomers in the cortex and hippocampus were measured as reported (36). Aβ load and MAP2 fluorescence intensities were quantified using Image J software (NIH; ref. 48). The calbindin immunoreactivity (IR) and the number of Fos-positive granule cells were quantified as described (6). Morris water maze test was used to determine the spatial learning and memory (17, 28, 31, 34). The elevated plus maze was performed to assess anxiety (31, 34). Data are presented as mean ± SEM or SD. Detailed methods can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Lennart Mucke for providing hAPPFAD transgenic mice (J20 line). We also thank Gui-Qiu Yu and Kaitlyn Ho for assistance on Aβ measurements, Nino Devidze for assistance on behavioral tests, John Carroll for graphics, Stephen Ordway and Gary Howard for editorial assistance, and Linda Turney for manuscript preparation. This work was supported by the J. David Gladstone Institutes and National Institutes of Health Grants P01 AG022074 and C06RR18928.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018381108/-/DCSupplemental.
References
- 1.Selkoe DJ. Deciphering the genesis and fate of amyloid β-protein yields novel therapies for Alzheimer disease. J Clin Invest. 2002;110:1375–1381. doi: 10.1172/JCI16783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardy J. Amyloid, the presenilins and Alzheimer's disease. Trends Neurosci. 1997;20:154–159. doi: 10.1016/s0166-2236(96)01030-2. [DOI] [PubMed] [Google Scholar]
- 3.Sherrington R, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 4.Ashe KH, Zahs KR. Probing the biology of Alzheimer's disease in mice. Neuron. 2010;66:631–645. doi: 10.1016/j.neuron.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mucke L, et al. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palop JJ, et al. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer's disease-related cognitive deficits. Proc Natl Acad Sci USA. 2003;100:9572–9577. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng IH, et al. Aggressive amyloidosis in mice expressing human amyloid peptides with the Arctic mutation. Nat Med. 2004;10:1190–1192. doi: 10.1038/nm1123. [DOI] [PubMed] [Google Scholar]
- 8.Corder EH, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 9.Tang M-X, et al. The APOE-ε4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279:751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- 10.Saunders AM, et al. Association of apolipoprotein E allele ε 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 11.Farrer LA, et al. APOE and Alzheimer Disease Meta Analysis Consortium Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 12.Huang Y, Weisgraber KH, Mucke L, Mahley RW. Apolipoprotein E: diversity of cellular origins, structural and biophysical properties, and effects in Alzheimer's disease. J Mol Neurosci. 2004;23:189–204. doi: 10.1385/JMN:23:3:189. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y. Molecular and cellular mechanisms of apolipoprotein E4 neurotoxicity and potential therapeutic strategies. Curr Opin Drug Discov Devel. 2006;9:627–641. [PubMed] [Google Scholar]
- 14.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc Natl Acad Sci USA. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y. Abeta-independent roles of apolipoprotein E4 in the pathogenesis of Alzheimer's disease. Trends Mol Med. 2010;16:287–294. doi: 10.1016/j.molmed.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Buttini M, et al. Expression of human apolipoprotein E3 or E4 in the brains of Apoe-/- mice: isoform-specific effects on neurodegeneration. J Neurosci. 1999;19:4867–4880. doi: 10.1523/JNEUROSCI.19-12-04867.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raber J, et al. Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: increased susceptibility of females. Proc Natl Acad Sci USA. 1998;95:10914–10919. doi: 10.1073/pnas.95.18.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raber J, et al. Apolipoprotein E and cognitive performance. Nature. 2000;404:352–354. doi: 10.1038/35006165. [DOI] [PubMed] [Google Scholar]
- 19.Hartman RE, et al. Behavioral phenotyping of GFAP-apoE3 and -apoE4 transgenic mice: apoE4 mice show profound working memory impairments in the absence of Alzheimer's-like neuropathology. Exp Neurol. 2001;170:326–344. doi: 10.1006/exnr.2001.7715. [DOI] [PubMed] [Google Scholar]
- 20.Bour A, et al. Middle-aged human apoE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks. Behav Brain Res. 2008;193:174–182. doi: 10.1016/j.bbr.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Buttini M, et al. Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid β peptides but not on plaque formation. J Neurosci. 2002;22:10539–10548. doi: 10.1523/JNEUROSCI.22-24-10539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fagan AM, et al. Human and murine ApoE markedly alters A β metabolism before and after plaque formation in a mouse model of Alzheimer's disease. Neurobiol Dis. 2002;9:305–318. doi: 10.1006/nbdi.2002.0483. [DOI] [PubMed] [Google Scholar]
- 23.Fryer JD, et al. Human apolipoprotein E4 alters the amyloid-β 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J Neurosci. 2005;25:2803–2810. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bales KR, et al. Human APOE isoform-dependent effects on brain beta-amyloid levels in PDAPP transgenic mice. J Neurosci. 2009;29:6771–6779. doi: 10.1523/JNEUROSCI.0887-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Q, et al. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci. 2006;26:4985–4994. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Q, et al. Intron-3 retention/splicing controls neuronal expression of apolipoprotein E in the CNS. J Neurosci. 2008;28:1452–1459. doi: 10.1523/JNEUROSCI.3253-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Y, et al. Apolipoprotein E fragments present in Alzheimer's disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc Natl Acad Sci USA. 2001;98:8838–8843. doi: 10.1073/pnas.151254698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris FM, et al. Carboxyl-terminal-truncated apolipoprotein E4 causes Alzheimer's disease-like neurodegeneration and behavioral deficits in transgenic mice. Proc Natl Acad Sci USA. 2003;100:10966–10971. doi: 10.1073/pnas.1434398100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brecht WJ, et al. Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci. 2004;24:2527–2534. doi: 10.1523/JNEUROSCI.4315-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang S, et al. Lipid- and receptor-binding regions of apolipoprotein E4 fragments act in concert to cause mitochondrial dysfunction and neurotoxicity. Proc Natl Acad Sci USA. 2005;102:18694–18699. doi: 10.1073/pnas.0508254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrews-Zwilling Y, et al. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J Neurosci. 2010;30:13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irizarry MC, et al. Apolipoprotein E affects the amount, form, and anatomical distribution of amyloid β-peptide deposition in homozygous APP(V717F) transgenic mice. Acta Neuropathol. 2000;100:451–458. doi: 10.1007/s004010000263. [DOI] [PubMed] [Google Scholar]
- 33.Buttini M, et al. Cellular source of apolipoprotein E4 determines neuronal susceptibility to excitotoxic injury in transgenic mice. Am J Pathol. 2010;177:563–569. doi: 10.2353/ajpath.2010.090973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberson ED, et al. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 35.Grootendorst J, Enthoven L, Dalm S, de Kloet ER, Oitzl MS. Increased corticosterone secretion and early-onset of cognitive decline in female apolipoprotein E-knockout mice. Behav Brain Res. 2004;148:167–177. doi: 10.1016/s0166-4328(03)00188-8. [DOI] [PubMed] [Google Scholar]
- 36.Lesné S, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 37.Cheng IH, et al. Accelerating amyloid-beta fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J Biol Chem. 2007;282:23818–23828. doi: 10.1074/jbc.M701078200. [DOI] [PubMed] [Google Scholar]
- 38.Meilandt WJ, et al. Neprilysin overexpression inhibits plaque formation but fails to reduce pathogenic Abeta oligomers and associated cognitive deficits in human amyloid precursor protein transgenic mice. J Neurosci. 2009;29:1977–1986. doi: 10.1523/JNEUROSCI.2984-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weisgraber KH. Apolipoprotein E: structure-function relationships. Adv Protein Chem. 1994;45:249–302. doi: 10.1016/s0065-3233(08)60642-7. [DOI] [PubMed] [Google Scholar]
- 40.Strittmatter WJ, et al. Binding of human apolipoprotein E to synthetic amyloid β peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pillot T, et al. β-amyloid peptide interacts specifically with the carboxy-terminal domain of human apolipoprotein E: relevance to Alzheimer's disease. J Neurochem. 1999;72:230–237. doi: 10.1046/j.1471-4159.1999.0720230.x. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka M, et al. Effect of carboxyl-terminal truncation on structure and lipid interaction of human apolipoprotein E4. Biochemistry. 2006;45:4240–4247. doi: 10.1021/bi060023b. [DOI] [PubMed] [Google Scholar]
- 43.Chou C-Y, Jen W-P, Hsieh Y-H, Shiao M-S, Chang G-G. Structural and functional variations in human apolipoprotein E3 and E4. J Biol Chem. 2006;281:13333–13344. doi: 10.1074/jbc.M511077200. [DOI] [PubMed] [Google Scholar]
- 44.Poirier J. Apolipoprotein E, cholesterol transport and synthesis in sporadic Alzheimer's disease. Neurobiol Aging. 2005;26:355–361. doi: 10.1016/j.neurobiolaging.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Zahs KR, Ashe KH. ‘Too much good news’ - are Alzheimer mouse models trying to tell us how to prevent, not cure, Alzheimer's disease? Trends Neurosci. 2010;33:381–389. doi: 10.1016/j.tins.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Chin J, et al. Fyn kinase modulates synaptotoxicity, but not aberrant sprouting, in human amyloid precursor protein transgenic mice. J Neurosci. 2004;24:4692–4697. doi: 10.1523/JNEUROSCI.0277-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chin J, et al. Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2005;25:9694–9703. doi: 10.1523/JNEUROSCI.2980-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li G, et al. GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell. 2009;5:634–645. doi: 10.1016/j.stem.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.