Abstract
Hydrogenases catalyze the reversible reaction 2H+ + 2e-↔H2 with an equilibrium constant that is dependent on the reducing potential of electrons carried by their redox partner. To examine the possibility of increasing the photobiological production of hydrogen within cyanobacterial cultures, we expressed the [FeFe] hydrogenase, HydA, from Clostridium acetobutylicum in the non-nitrogen-fixing cyanobacterium Synechococcus elongatus sp. 7942. We demonstrate that the heterologously expressed hydrogenase is functional in vitro and in vivo, and that the in vivo hydrogenase activity is connected to the light-dependent reactions of the electron transport chain. Under anoxic conditions, HydA activity is capable of supporting light-dependent hydrogen evolution at a rate > 500-fold greater than that supported by the endogenous [NiFe] hydrogenase. Furthermore, HydA can support limited growth solely using H2 and light as the source of reducing equivalents under conditions where Photosystem II is inactivated. Finally, we demonstrate that the addition of exogenous ferredoxins can modulate redox flux in the hydrogenase-expressing strain, allowing for greater hydrogen yields and for dark fermentation of internal energy stores into hydrogen gas.
Keywords: biofuel, metabolic engineering
It has long been observed that a number of photosynthetic organisms are capable of producing small quantities of hydrogen gas during the course of normal metabolic activities (1). This process can be driven by hydrogenase enzymes, capable of catalyzing the reversible reaction 2H+ + 2e- → H2 (2). Native cyanobacterial hydrogenases are predominantly thought to function in the recapture of hydrogen that is created as a byproduct of nitrogenase activity within nitrogen-fixing microbes (3). Hydrogenases may further function to provide a terminal electron sink in the absence of oxygen or during changes in flux through the photosynthetic machinery (4, 5).
There are two main classes of hydrogenase enzymes, characterized by the nature of their active site: nickel–iron [NiFe] and iron–iron [FeFe] hydrogenases (2). Whereas [NiFe] hydrogenases are found across a variety of organisms, [FeFe] hydrogenases are typically restricted to algal species and to a few prokaryotes, but are excluded from all cyanobacteria examined to date. Although both hydrogenases catalyze the same reaction, they are structurally unrelated, utilize unique metallocatalytic clusters within their active sites, and are phylogenetically distinct. In addition to differences within the reactive metallocluster, the classes of hydrogenases also interact with different electron carriers for their redox chemistry. Whereas [NiFe]-hydrogenases are typically coupled to NAD(P)H, with a reducing potential of approximately 320 mV, many [FeFe]-hydrogenases are partnered with the electron-carrying protein ferredoxin, which can bear electrons with significantly lower reducing potentials (2). Because ferredoxin proteins may carry electrons with reducing potentials closer to that of the H2/H+ pair (-420 mV) (6), [FeFe]-hydrogenases thermodynamically favor hydrogen production relative to [NiFe] hydrogenases, which are frequently regarded as predominantly H2 uptake enzymes (4, 7).
The hydrogen production capacity of a variety of cyanobacterial and algal species has been surveyed (8–10), and the highest rates of hydrogen evolution are typically observed in algae and some nitrogen-fixing cyanobacteria. Algal species frequently possess [FeFe]-hydrogenases that accept low-potential electrons from ferredoxins that are, in turn, linked to the light reactions of photosynthesis (11). Nitrogen-fixing microorganisms, including many cyanobacteria (12), produce hydrogen gas as a byproduct of nitrogenase activity. Although nitrogenase activity can significantly add to the total hydrogen production of some photosynthetic organisms, the reaction is energetically costly and the nitrogenases’ ATP requirement greatly reduces the theoretical maximal efficiency of the sunlight to hydrogen conversion (13, 14). Due to the oxygen sensitivity of hydrogenases (15), most current biohydrogen production schemes temporally separate the oxygen-generating, water-splitting reaction of photosystem II (PSII) from the hydrogen-generating reactions. In practice, this process involves growing cyanobacteria or algae under normal conditions, to generate internal stores of reductants, then transferring into an anaerobic atmosphere, and inactivating PSII through nutrient depravation or chemical inhibitors (1, 16–18). Anaerobic fermentation in the light (1, 17, 19–21), or dark (22), can then result in elevated hydrogenase activity and hydrogen production.
To examine the possibility of increasing hydrogen production in non-nitrogen-fixing cyanobacteria, we heterologously expressed a Clostridial [FeFe] hydrogenase within Synechococcus elongatus. This cyanobacterium is obligately photoautotrophic, strictly aerobic, does not contain functional nitrogenase homologs, and has no detectable nitrogen-fixing capacity (23, 24), therefore all observed hydrogen production can be attributed to hydrogenase activity. Herein, we report that Clostridial hydrogenase is functionally integrated with the redox machinery of the cell, and is capable of far greater hydrogen production levels than the endogenous [NiFe] hydrogenase, both in vivo and in vitro. Furthermore, we describe rational designs that redirect reducing equivalents toward hydrogenase for increased hydrogen production and dark fermentation of internal carbohydrates into hydrogen.
Results
Expression and in Vitro Activity of [FeFe] Hydrogenase in Cyanobacteria.
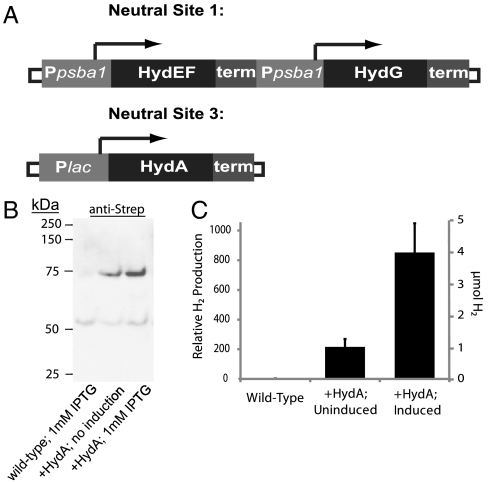
To examine exogenous hydrogenase activity in cyanobacteria, we expressed the [FeFe] hydrogenase (HydA) from Clostridium acetobutylicum in the common cyanobacterial lab strain Synechococcus elongatus sp. 7942. This hydrogenase has previously demonstrated robust activity in vivo and in vitro, and has a relatively small number of maturation factors necessary for activity when expressed heterologously (25–27). Furthermore, in our previous work with a synthetic hydrogen-producing circuit in Escherichia coli, C. acetobutylicum HydA was determined to have the highest level of activity among the tested hydrogenases (28). To express hydrogenase maturation factors HydEF and HydG (29), we cloned these genes separately, placing each of them downstream of the S. elongatus psba1 promoter, which normally constitutively expresses the D1 subunit of photosystem II (PSII). These maturation factors were then combined into a cassette and integrated into the genome at the previously defined neutral site 1 (30) (Fig. 1A). HydA was inserted separately under an IPTG-inducible promoter at neutral site 3 (31) (Fig. 1A). We confirmed the expression a StrepII-tagged HydA by Western blot analysis and observed that expression could be induced (approximately 3–4-fold) by addition of IPTG (Fig. 1B).
Fig. 1.
Expression of HydA in Synechococcus elongatus sp. 7942. (A) Schematic of integrated genes. Maturation factors (HydEF, G) are expressed under the constitutive psba1 promoter. HydA is expressed under the IPTG-inducible PLac. (B) Western blot analysis for His-tagged HydA in wild-type or HydA-expressing strains under varied IPTG concentrations (predicted molecular mass = 65.6 kDa). (C) Representative, same day trial of evolved hydrogen gas from wild-type and HydA-expressing Synechococcus lysates 60 min following methyl viologen treatment under anaerobic conditions.
To confirm that HydA was properly folded and maintained activity, we conducted a standard methyl viologen assay (25) in cell lysates from control and hydrogenase-expressing strains. Methyl viologen is a promiscuous electron donor that can provide low-potential electrons to hydrogenases of both the [FeFe] and [NiFe] classes, thereby providing a measure of hydrogenase activity when methyl viologen is in excess (25, 32). When incubated for 45 min under an anaerobic atmosphere in the presence of methyl viologen, cell lysates from HydA-expressing strains exhibited > 500-fold increase in the amount of hydrogen gas detected in the headspace (Fig. 1C). Furthermore, we note that following extended anaerobic incubation with the same conditions, wild-type strains continued to exhibit little or no hydrogen evolution, suggesting that, even under conditions inducive to native hydrogenase expression, the amount of hydrogenase activity is much greater in HydA-expressing strains.
In Vivo, Light-Dependent Hydrogen Evolution.
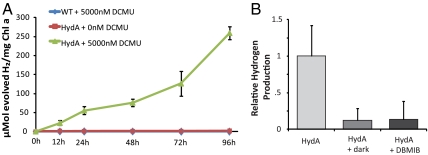
In order for a heterologous hydrogenase to be functional in vivo, it must interact with the endogenous redox machinery and thereby be able to accept reducing equivalents from cellular pools. Due to the extreme oxygen sensitivity of HydA, and most hydrogenases in general, it is typically necessary to spatiotemporally separate the water-splitting reaction of photosystem II from the hydrogen-evolving reaction (8, 19). To assay the capacity of HydA to convert internal stores of reducing equivalents into hydrogen gas, we inhibited PSII using 5 μM diurion [3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU)], induced HydA expression, and sparged the headspace of cyanobacterial cultures with 2.5% CO2 (N2 balance). No hydrogenase activity was detectable within the parent S. elongatus strain, whereas significant quantities of hydrogen were observed in the headspace of HydA-containing cultures only when O2 generation was suppressed (Fig. 2A). On average, the hydrogenase activity of HydA-containing strains over the first 96 h was 2.8 μmol H2 h-1·mg Chl-a-1 (Fig. 2A, where Chl-a is cholorphyll a).
Fig. 2.
In vivo hydrogen production. (A) In vivo hydrogen production under anaerobic conditions (2.5% CO2 in N2) with DCMU treatment. (B) In vivo hydrogen production is dependent on light and electron transport from plastoquinone.
Cyanobacteria and alga are capable of anaerobically metabolizing carbohydrates to provide reducing equivalents for hydrogenase and may do so in either light-dependent or dark reactions (33, 34). In light-dependant reactions, electrons derived from carbohydrate metabolism are donated to the plastoquinone pool and are subsequently reenergized through Photosystem I (PSI) before being transferred to hydrogenase through petF-class plant-type ferredoxins (18, 21, 33). In dark fermentation of starch to hydrogen, electrons from the breakdown of glycogen may be directly donated to the hydrogenase, or may be transferred through intermediate electron carriers, such as NAD(P)H or ferredoxin (35, 36). In HydA-expressing Synechococcus, hydrogen production was greatly reduced in the dark or in the presence of the plastoquinone inhibitor dibromothymoquinone (DBMIB) (Fig. 2B). We therefore conclude that electron transfer to hydrogenase is largely dependent on the activity of the endogenous electron transport chain (ETC).
HydA Activity Supports Limited Growth Utilizing Molecular Hydrogen as a Reductant.
One major function of native hydrogenases in cyanobacteria and algae is presumed to be their ability to recover reducing equivalents from hydrogen that may be generated as a byproduct of nitrogen fixation reactions. In order for the uptake function of hydrogenases to be effective, hydrogenases must be sufficiently interconnected with the redox machinery of the cell such that the hydrogen-derived electrons can be redirected to alternative electron-dependant metabolic processes. The activity of the endogenous bidirectional [NiFe] hydrogenase of S. elongatus to uptake H2 and reduce NAD+ has been examined in cell extracts and determined to be very low (85 nmol h-1·mg protein-1) (37). Although this level of activity is sufficient to fix carbon, increase starch accumulation, or support nitrogenase activity (32, 38) it is thought that this rate is insufficient to support autochemolithic growth using H2 as a reductant, and H2-dependent cell division has not been observed in cyanobacteria (37, 39) (Fig. 3A).
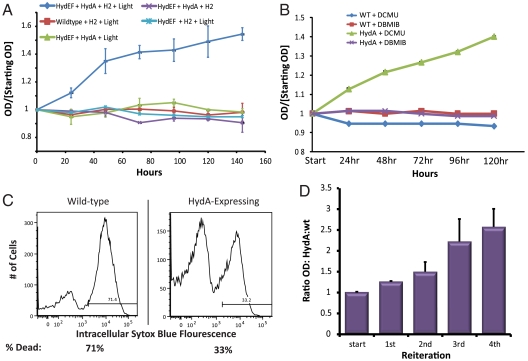
Fig. 3.
Hydrogenase-dependent chemoautotrophic growth. (A) HydA activity supports cell growth when coexpressed with hydrogenase maturation factors under anaerobic conditions in the presence of hydrogen gas. (B) Block of electron transfer from plastoquinone (with DBMIB) prevents hydrogenase-mediated growth. (C) Representative FACS analysis of cell viability in wild-type and HydA-expressing cells as measured by the vital dye Sytox blue. Data shown for cells after 7 d of incubation with DCMU and under a CO2/H2 atmosphere. (D) Reiterative rounds of growth under hydrogen atmosphere (4 d; DCMU/CO2/H2) followed by release of selective pressure (1 d; no DCMU air) enriches for HydA-expressing cells relative to wild type.
To further evaluate the in vivo activity of HydA within S. elongatus, we examined its capacity to consume hydrogen gas as an alternative source of reducing equivalents when PSII water-splitting reactions were inhibited. We found that HydA-expressing strains could support limited cell growth under DCMU inhibition when incubated under illumination in anaerobic, sealed cultures containing 5% CO2 and 5% H2, as assayed by OD750 (Fig. 3A). The majority of growth was restricted to the first 3–4 d following the switch to a hydrogen atmosphere, and HydA-supported growth was dependent on molecular hydrogen, absence of oxygen, HydA maturation factors, and light (Fig. 3A). We used FACS analysis to verify that the observed increase in culture optical density was predominantly due to cell division (50–70%); increases in cell size or density may also contribute slightly to OD increases.
The Calvin cycle requires adequate supplies of both ATP and reducing equivalents in order to fix CO2 and increase cell mass. Under physiological conditions, where cells have an excess of reducing equivalents in comparison to ATP, electrons from ferredoxin can reenter the ETC through the plastoquinone pool in an essential process termed cyclic electron transport (40). Cyclic electron transport increases the proton gradient across thylakoid membranes, stimulating ATP production, but requires additional light absorption at PSI to maintain a reduced ferredoxin pool. Because the oxidation of hydrogen could only provide a source of reducing equivalents and we observed that HydA-dependent growth required light, we suspected that hydrogenase-derived electrons were entering the ETC and undergoing cyclic electron transport to generate ATP. Consistent with this theory, we observed that HydA-dependent growth ceased when electron flow from plastoquinone was blocked by the addition of DBMIB (Fig. 3B).
HydA-expressing strains appeared more robust during extended incubations under restrictive (DCMU/hydrogen) conditions, as determined by culture bleaching and by counts of colony forming units when plated on agar plates. Therefore, we assayed the viability of the culture by FACS analysis using the vital dye SYTOX blue, and observed that HydA-expressing strains maintained greater viability under a hydrogen atmosphere when inhibited by DCMU (Fig. 3C). The selective advantage of HydA-expressing cells allowed for gradual enrichment of HydA-expressing cells relative to wild type in reiterative rounds of incubation under DCMU/CO2/H2 (4 d) followed by incubation under normal conditions (no DCMU, air—24 h; Fig. 3D). Taken together, these experiments demonstrate heterologously expressed HydA is capable of both accepting and donating electrons from/to the endogenous redox machinery of S. elongatus.
Modified Hydrogenase Activity as a Function of Heterologous Ferredoxin Expression.
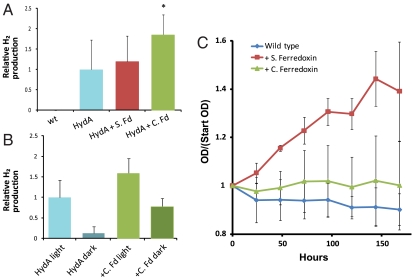
For hydrogen production to be dependent upon light and ETC function (Fig. 2B), it is likely that HydA is interacting with endogenous S. elongatus petF plant-type ferredoxins, which may be only weakly interacting with the heterologous hydrogenase. Previously, research in our lab has utilized a synthetic circuit in E. coli to probe the interactions of HydA with a number of ferredoxin proteins and identified those with the strongest ferredoxin-hydrogenase affinity as a function of hydrogen output from this circuit (28). We sought to utilize these pairings to facilitate increased electron transfer to HydA and increase hydrogen production within S. elongatus. We integrated the strongest ferredoxin pairs, one plant type and one bacterial type (from Spinacia oleracea or C. acetobutylicum, respectively) (28), into the HydA-expressing strain, and expressed them from an IPTG-inducible promoter (30). We found that expression of ferredoxin from C. acetobutylicum (CAC0303) (41), our strongest pairing in our previously reported synthetic circuit (28), could increase the rate of hydrogen evolution by approximately twofold (Fig. 4A), suggesting that expression of this ferredoxin helped to redirect internal reducing equivalents toward HydA. Expression of ferredoxin I from S. oleracea did not significantly increase hydrogen production.
Fig. 4.
Ferredoxin incorporation alters electron flow to/from hydrogenase. (A) In vivo hydrogen production under anaerobic conditions in the presence of exogenous ferredoxins [Spinacea oleracea (S. Fd) and Clostridium acetobutylicum (C. Fd)]. (B) Incorporation of C. Fd allows dark fermentation to generate hydrogen gas. (C) Hydrogenase-bearing strains containing Clostridial ferredoxin are unable to support growth with hydrogen as the sole source of reducing equivalents.
Although the ferredoxin from C. acetobutylicum has previously shown a strong functional interaction with HydA in a synthetic circuit (28), it is a bacterial-type ferredoxin and is unlikely to be capable of direct interactions with PSI in a manner similar to the endogenous S. elongatus plant-type ferredoxins. Therefore, we asked if the observed increase in hydrogen production in strains expressing both HydA and C. acetobutylicum ferredoxin was the result of utilization of reducing equivalents from a distinct source. Indeed, C. acetobutylicum ferredoxin-bearing strains were capable of producing hydrogen in the dark and/or in the presence of DBMIB, whereas these conditions largely abolished hydrogen production when expressing HydA alone (Fig. 4B).
Because expression of exogenous ferredoxin improved the capacity of HydA to support hydrogen evolution (Fig. 4A), we wished to examine if these ferredoxin pairings would also improve and/or alter the ability of HydA to support growth under hydrogen-containing atmospheres (Fig. 3A). However, although we found that strains expressing S. oleracea ferredoxin exhibited HydA-dependent growth in the presence of hydrogen, the presence of C. acetobutylicum ferredoxin attenuated hydrogen-supported growth (Fig. 5C). Collectively, these experiments demonstrate that addition of supplemental ferredoxins or optimization of ferredoxin-hydrogenase interactions can both increase the flux of electrons toward hydrogenase as well as rewire the redox pathway. We suggest that the addition of C. acetobutylicum ferredoxin provides an alternative electron pathway that can partially bypass the need for the light-mediated reduction of HydA by anaerobic fermentation through PSI. However, the presence of an exogenous ferredoxin that is preferred by HydA, but less integrated with endogenous redox pathways may “short circuit” the HydA-dependent redox circuit responsible for hydrogenase-dependent growth under hydrogen (Fig. 5).
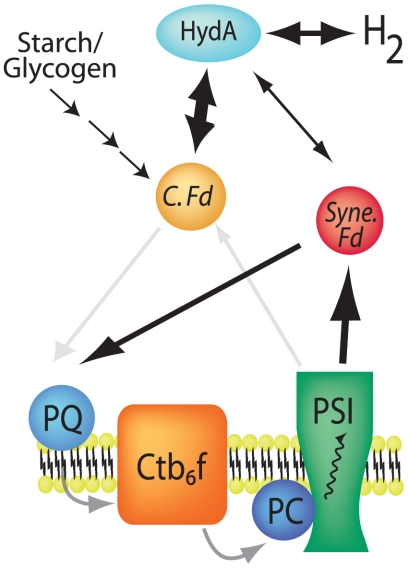
Fig. 5.
Model of ferredoxin-mediated electron transfer to/from hydrogenase. Endogenous S. elongtaus ferredoxins (Syne. Fd) are capable of transferring electrons from PSI to exogenous HydA for the production of hydrogen. When exogenous Clostridial ferredoxin (C. Fd) is expressed, it is the preferred interacting partner for HydA (bold arrows). Although C. Fd can increase the flux of reducing equivalents to HydA under conditions favoring hydrogen evolution, exogenous ferredoxins are inefficient for electron transfer (faded arrows) to the plastoquinone (PQ) pool to allow for hydrogen-mediated growth.
Discussion
In this work, we explore the activity of a heterologously expressed [FeFe] hydrogenase (HydA) that is functional in cyanobacteria in vivo, particularly within the context of the endogenous redox machinery and ETC components. We show a significant boost in the capacity of HydA-expressing cells to evolve hydrogen, especially when compared to the hydrogen evolution rates of wild-type S. elongatus, which rely on native [NiFe] hydrogenases (Figs. 1C and 2A). In this context, it is relevant to note earlier studies on the efficacy of a [FeFe] hydrogenase from Clostridium pasteurianum within cyanobacteria (27), where the hydrogenase exhibited only limited (approximately 2×) activity over that of native hydrogenases and which was only observed when artificially reduced with an exogenous source of methyl viologen. In the present work, we demonstrate that an exogenous hydrogenase is capable of interacting with endogenous redox machinery to evolve hydrogen from internal sources of reducing equivalents at the rate of approximately 2.8 μmol H2 h-1·mg Chl-a-1 (Fig. 2A). We also demonstrate that HydA activity can support limited chemoautotrophic growth of cyanobacteria by uptake of hydrogen gas as a source of reducing equivalents when PSII is chemically inhibited.
Utilizing photosynthetic organisms for the biological production of hydrogen gas or other chemicals is an attractive approach to meet some future energy needs (42). Whereas genetic tools and metabolic engineering have factored heavily in the development of strains of microbes for the production of biodiesel, alcohols, or other combustible compounds, improvement of photobiological hydrogen production has relied heavily upon optimization of culturing conditions and bioprospecting (8). Currently, algal species possessing native [FeFe] hydrogenases are the most favored organisms for biological hydrogen production, with some existing research and plans for taking production to industrial scale. In this work, we have shown that incorporation of [FeFe] hydrogenases into S. elongatus can enhance the hydrogen production rates over that of the wild-type S. elongatus. Strains expressing [FeFe] hydrogenase exhibited a > 500-fold increase of hydrogen evolution in vivo relative to unmodified strains of S. elongatus; wild-type S. elongatus produced hydrogen at rates approximating those previously reported (39). Similarly, our strain produces hydrogen at greater rates (2.8 μmol H2 h-1· mg Chl-a-1) than those reported in most other non-nitrogen-fixing unicellular cyanobacteria (0.02–1 μmol H2 h-1·mg Chl-a-1 (8), with the exception of Synechosystis sp. PCC 6803 when engineered (21) or under nitrogen-depleted conditions (9) (6–30 μmol H2 h-1·mg Chl-a-1), which brings S. elongatus hydrogen production rates nearer to those of many nitrogen-fixing cyanobacteria and algae (approximately 2–70 μmol H2 h-1·mg Chl-a-1 (9, 10, 43). Given the relative ease of transformation of S. elongatus, it may be possible to make further genetic modifications in this strain analogous to those demonstrated in other cyanobacteria or algae that increase starch accumulation (44), reduce cyclic electron transport (20), modify PSII activity (45), or reduce phycobilisome size (46) to allow for enhanced production of hydrogen. Finally, optimal culture conditions or cell immobilization can greatly enhance photobiological hydrogen production (47, 48), which could increase the yield and concentration of hydrogen evolved in this system.
We have previously demonstrated that C. acetobutylicum HydA can functionally interact with a variety of ferredoxin proteins from diverse organisms (28), and herein show that electron carriers within S. elongatus can also donate and/or receive reducing equivalents to/from HydA. Furthermore, our results demonstrate that proper ferredoxin–hydrogenase combinations may optimize hydrogen production and change properties of the reaction, such as allowing for fermentation of internal reducing equivalents without the assistance of light (Figs. 4B and 5). Although the presence of at least two distinct (i.e., light-dependent and light-independent) pathways for electron transfer to hydrogenases have been well documented (9, 36, 49), to our knowledge there have been no previous reports of genetic means to enhance the rate of one pathway relative to the other. Rewiring the flow of reducing equivalents by such a strategy may not only be useful for reducing the cost of bioreactor design (i.e., by reducing the need for illumination, and associated light penetration and heat distribution issues), but also open the possibility of importing parallel redox pathways that are partially insulated from host redox machinery (Fig. 5). Consequently, our data suggest that proposals to characterize and optimize the key residues forming the ferredoxin–hydrogenase interface could significantly improve hydrogen production (11). Finally, the ability of HydA to oxidize hydrogen to support limited growth in S. elongatus also offers a possible method for directed evolution of HydA variants with desirable traits, particularly greater oxygen tolerance. To our knowledge, there have been no previous reports of cyanobacteria capable of chemoautrophic division through the utilization of hydrogen (37).
Although photobiological production of hydrogen is an attractive concept to meet increasing energy demands, it is evident that substantial gains in this technology are necessary to make it economically feasible (34, 50, 51). A successful hydrogen-producing organism would likely combine traits of several species of photoautotrophs (e.g., optimal hydrogen production, growth in environments unsuitable for competing commercial crops, and minimal by-production of toxic biologically active compounds; ref. 52). Discovery or engineering of hydrogenases with increased oxygen tolerance would allow for direct production of hydrogen, which would bypass inefficiencies associated with energy transfer through internal carbohydrate intermediates. The capacity to functionally express [FeFe] hydrogenases in a variety of contexts may open up a range of organisms for development of more efficient biohydrogen processes, and the natural transformability of S. elongatus may facilitate development of hydrogenase-expressing strains to reach target levels of biohydrogen production in cyanobacteria.
Experimental Procedures
Strains, Plasmids, and Culture Conditions.
Wild-type S. elongatus was obtained from the American Type Culture Collection. Cultures and in vivo assays utilized BG11 medium with light illumination of ≤ 4,000 lux at 30° and a 12/12 h day/night cycle. All constructs were cloned in BioBrick format in E. coli (Assembly Standard 21) (53). S. elongatus in log phase (OD750 ∼ 0.4) was transformed with approximately 100 ng plasmid DNA overnight and plated on BG11 plates with antibiotics. Neutral site vectors for genomic integration were obtained from their respective lab of origins [NS1/pAM2314; NS2/pAM1579 (30, 54); NS3/ pHN1-LacUV5 (31)]. Hydrogenase (HydA) from Clostricium acetobutylicum, Clostridium acetobutylicum ferredoxin CAC0303, ferredoxin I from Spinacia oleracea, and hydrogenase maturation factors (HydEF, HydG) from Chlamydomonas reinhardtii were cloned and synthesized as previously described (28).
SDS-PAGE and Western Blotting.
Standard laboratory procedures for SDS-PAGE were used, loading cyanobacterial lysates in Laemmli Buffer on 4–20% gradient gels (Bio-Rad). Proteins were transferred to PVDF membranes (Millipore) and probed with anti-Strep antibodies (Novagen).
Hydrogen Production–Uptake Assays.
S. elongatus was grown under a standard atmosphere with appropriate antibiotics, diluted to OD750 0.1 10/50 mL within 25/240 mL clear glass vials and sealed with rubber septa. Where applicable, the following chemicals were supplemented in the media: 5 μM Diuron (DCMU), 20 μM DBMIB, 0.1–1.0 mM IPTG, 20 mM Hepes pH 8.0. Cultures were sparged with 2.5% CO2, nitrogen balance (Airgas) and placed in 30 °C incubators with light as above (or dark, where appropriate). For in vitro methyl viologen assays, we adapted the method from ref. 25, lysing cyanobacterial sealed cultures with bacterial protein extraction reagents (Pierce) in the presence of 1 mM methyl viologen (Sigma) and 5mM sodium dithionite for 60 min (Fisher). Headspace was measured by gas chromatography (Shimadzu GC-14A). For hydrogen-dependant growth assays, headspace was sparged with 5% H2, 5% CO2, nitrogen balance. Measurement and calculation of chlorophyll a calculated by methanol extraction were conducted as previously described (55).
Fluorescence-Activated Cell Sorting.
FACS was conducted using S. elongatus cell cultures in a BD LSRII with HTS-3 Laser (BD Biosciences). Cell viability was assayed by incorporation of SYTOX blue cell stain (Invitrogen).
Acknowledgments.
We thank Edwin Wintermute, Buz Barstow, Christina Agapakis, and Gerald Grandl for their intellectual input and critical review of the manuscript. The project described was supported by funds from Award F32GM093516 from the National Institute of General Medical Sciences, Army Research Office Award W911NF-09-1-0226, and the Wyss Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Melis A, Zhang L, Forestier M, Ghirardi ML, Seibert M. Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol. 2000;122:127–136. doi: 10.1104/pp.122.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghirardi ML, et al. Hydrogenases and hydrogen photoproduction in oxygenic photosynthetic organisms. Annu Rev Plant Biol. 2007;58:71–91. doi: 10.1146/annurev.arplant.58.032806.103848. [DOI] [PubMed] [Google Scholar]
- 3.Tamagnini P, et al. Hydrogenases and hydrogen metabolism of cyanobacteria. Microbiol Mol Biol Rev. 2002;66:1–20. doi: 10.1128/MMBR.66.1.1-20.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent KA, Parkin A, Armstrong FA. Investigating and exploiting the electrocatalytic properties of hydrogenases. Chem Rev. 2007;107:4366–4413. doi: 10.1021/cr050191u. [DOI] [PubMed] [Google Scholar]
- 5.Appel J, Phunpruch S, Steinmuller K, Schulz R. The bidirectional hydrogenase of Synechocystis sp. PCC 6803 works as an electron valve during photosynthesis. Arch Microbiol. 2000;173:333–338. doi: 10.1007/s002030000139. [DOI] [PubMed] [Google Scholar]
- 6.Ghirardi ML, Dubini A, Yu J, Maness PC. Photobiological hydrogen-producing systems. Chem Soc Rev. 2009;38:52–61. doi: 10.1039/b718939g. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz O, et al. Molecular biological analysis of a bidirectional hydrogenase from cyanobacteria. Eur J Biochem. 1995;233:266–276. doi: 10.1111/j.1432-1033.1995.266_1.x. [DOI] [PubMed] [Google Scholar]
- 8.Dutta D, De D, Chaudhuri S, Bhattacharya SK. Hydrogen production by cyanobacteria. Microb Cell Fact. 2005;4:36. doi: 10.1186/1475-2859-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schutz K, et al. Cyanobacterial H(2) production—a comparative analysis. Planta. 2004;218:350–359. doi: 10.1007/s00425-003-1113-5. [DOI] [PubMed] [Google Scholar]
- 10.Lopes Pinto FA, Troshina O, Lindblad P. A brief look at three decades of research on cyanobacterial hydrogen evolution. Int J Hydrogen Energy. 2002;27:1209–1215. [Google Scholar]
- 11.Stripp ST, Happe T. How algae produce hydrogen-news from the photosynthetic hydrogenase. Dalton Trans. 2009:9960–9969. doi: 10.1039/b916246a. [DOI] [PubMed] [Google Scholar]
- 12.Haselkorn R. Cyanobacteria. Curr Biol. 2009;19:R277–278. doi: 10.1016/j.cub.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Prince RC, Kheshgi HS. The photobiological production of hydrogen: Potential efficiency and effectiveness as a renewable fuel. Crit Rev Microbiol. 2005;31:19–31. doi: 10.1080/10408410590912961. [DOI] [PubMed] [Google Scholar]
- 14.Benemann JR. Feasibility analysis of photobiological hydrogen production. Int J Hydrogen Energy. 1997;22:979–987. [Google Scholar]
- 15.Stripp ST, et al. How oxygen attacks [FeFe] hydrogenases from photosynthetic organisms. Proc Natl Acad Sci USA. 2009;106:17331–17336. doi: 10.1073/pnas.0905343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antal TK, et al. The dependence of algal H2 productionon Photosystem II and O2 consumption activities in sulfur-deprived Chlamydomonas reinhardtii cells. Biochim Biophys Acta. 2003;1607:153–160. doi: 10.1016/j.bbabio.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Happe T, Melis A. Biochemical and morphological characterization of sulfur-deprived and H2-producing Chlamydomonas reinhardtii (green alga) Planta. 2002;214:552–561. doi: 10.1007/s004250100660. [DOI] [PubMed] [Google Scholar]
- 18.Florin L, Tsokoglou A, Happe T. A novel type of iron hydrogenase in the green alga Scenedesmus obliquus is linked to the photosynthetic electron transport chain. J Biol Chem. 2001;276:6125–6132. doi: 10.1074/jbc.M008470200. [DOI] [PubMed] [Google Scholar]
- 19.Melis A, Happe T. Hydrogen production. Green algae as a source of energy. Plant Physiol. 2001;127:740–748. [PMC free article] [PubMed] [Google Scholar]
- 20.Kruse O, et al. Improved photobiological H2 production in engineered green algal cells. J Biol Chem. 2005;280:34170–34177. doi: 10.1074/jbc.M503840200. [DOI] [PubMed] [Google Scholar]
- 21.Cournac L, Guedeney G, Peltier G, Vignais PM. Sustained photoevolution of molecular hydrogen in a mutant of Synechocystis sp. strain PCC 6803 deficient in the type I NADPH-dehydrogenase complex. J Bacteriol. 2004;186:1737–1746. doi: 10.1128/JB.186.6.1737-1746.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansel A, Lindblad P. Towards optimization of cyanobacteria as biotechnologically relevant producers of molecular hydrogen, a clean and renewable energy source. Appl Microbiol Biotechnol. 1998;50:153–160. [Google Scholar]
- 23.Peschek GA. Electron transport reactions in respiratory particles of hydrogenase-induced Anacystis nidulans. Arch Microbiol. 1980;125:123–131. [Google Scholar]
- 24.Eisbrenner G, Distler E, Floener L, Bothe H. The occurrence of the hydrogenase in some blue-green algae. Arch Microbiol. 1978;118:177–184. [Google Scholar]
- 25.King PW, Posewitz MC, Ghirardi ML, Seibert M. Functional studies of [FeFe] hydrogenase maturation in an Escherichia coli biosynthetic system. J Bacteriol. 2006;188:2163–2172. doi: 10.1128/JB.188.6.2163-2172.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veit A, Akhtar MK, Mizutani T, Jones PR. Constructing and testing the thermodynamic limits of synthetic NAD(P)H:H2 pathways. Microb Biotechnol. 2008;1:382–394. doi: 10.1111/j.1751-7915.2008.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asada Y, et al. Heterologous expression of clostridial hydrogenase in the Cyanobacterium synechococcus PCC7942. Biochim Biophys Acta. 2000;1490:269–278. doi: 10.1016/s0167-4781(00)00010-5. [DOI] [PubMed] [Google Scholar]
- 28.Agapakis CM, et al. Insulation of a synthetic hydrogen metabolism circuit in bacteria. J Biol Eng. 2010;4:3. doi: 10.1186/1754-1611-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Posewitz MC, et al. Hydrogen photoproduction is attenuated by disruption of an isoamylase gene in Chlamydomonas reinhardtii. Plant Cell. 2004;16:2151–2163. doi: 10.1105/tpc.104.021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clerico EM, Ditty JL, Golden SS. Specialized techniques for site-directed mutagenesis in cyanobacteria. Methods Mol Biol. 2007;362:155–171. doi: 10.1007/978-1-59745-257-1_11. [DOI] [PubMed] [Google Scholar]
- 31.Niederholtmeyer H, Wolfstadter BT, Savage DF, Silver PA, Way JC. Engineering cyanobacteria to synthesize and export hydrophilic products. Appl Environ Microbiol. 2010;76:3462–3466. doi: 10.1128/AEM.00202-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peschek GA. The role of the Calvin cycle for anoxygenic CO2 photoassimilation in Anacystis nidulans. FEBS Lett. 1979;106:34–38. doi: 10.1016/0014-5793(79)80689-4. [DOI] [PubMed] [Google Scholar]
- 33.Hemschemeier A, Fouchard S, Cournac L, Peltier G, Happe T. Hydrogen production by Chlamydomonas reinhardtii: An elaborate interplay of electron sources and sinks. Planta. 2008;227:397–407. doi: 10.1007/s00425-007-0626-8. [DOI] [PubMed] [Google Scholar]
- 34.Rupprecht J, et al. Perspectives and advances of biological H2 production in microorganisms. Appl Microbiol Biotechnol. 2006;72:442–449. doi: 10.1007/s00253-006-0528-x. [DOI] [PubMed] [Google Scholar]
- 35.Antal TK, Lindblad P. Production of H2 by sulphur-deprived cells of the unicellular cyanobacteria Gloeocapsa alpicola and Synechocystis sp. PCC 6803 during dark incubation with methane or at various extracellular pH. J Appl Microbiol. 2005;98:114–120. doi: 10.1111/j.1365-2672.2004.02431.x. [DOI] [PubMed] [Google Scholar]
- 36.Chochois V, et al. Hydrogen production in Chlamydomonas: Photosystem II-dependent and -independent pathways differ in their requirement for starch metabolism. Plant Physiol. 2009;151:631–640. doi: 10.1104/pp.109.144576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitz O, Bothe H. The diaphorase subunit HoxU of the bidirectional hydrogenase as electron transferring protein in cyanobacterial respiration? Naturwissenschaften. 1996;83:525–527. doi: 10.1007/BF01141957. [DOI] [PubMed] [Google Scholar]
- 38.Bothe H, Tennigkeit J, Eisbrenner G. The utilization of molecular hydrogen by the blue-green alga Anabaena cylindrica. Arch Microbiol. 1977;114:43–49. doi: 10.1007/BF00429628. [DOI] [PubMed] [Google Scholar]
- 39.Peschek GA. Anaerobic hydrogenase activity in Anacystis nidulans H2-dependent photoreduction and related reactions. Biochim Biophys Acta, Bioenerg. 1979;548:187–202. doi: 10.1016/0005-2728(79)90128-2. [DOI] [PubMed] [Google Scholar]
- 40.Shikanai T. Cyclic electron transport around photosystem I: Genetic approaches. Annu Rev Plant Biol. 2007;58:199–217. doi: 10.1146/annurev.arplant.58.091406.110525. [DOI] [PubMed] [Google Scholar]
- 41.Guerrini O, et al. Characterization of two 2[4Fe4S] ferredoxins from Clostridium acetobutylicum. Curr Microbiol. 2008;56:261–267. doi: 10.1007/s00284-007-9072-x. [DOI] [PubMed] [Google Scholar]
- 42.Dismukes GC, Carrieri D, Bennette N, Ananyev GM, Posewitz MC. Aquatic phototrophs: Efficient alternatives to land-based crops for biofuels. Curr Opin Biotechnol. 2008;19:235–240. doi: 10.1016/j.copbio.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Ghirardi ML, Kosourov S, Tsygankov A, Seibert M. Two-phase photobiological algal H2-production system; Proceedings of the 2000 US Department of Energy Hydrogen Program Review; Golden, CO: National Renewable Energy Laboratory; 2000. pp. 282–294. NREL/CP-570-28890. [Google Scholar]
- 44.McNeely K, Xu Y, Bennette N, Bryant DA, Dismukes GC. Redirecting reductant flux into hydrogen production via metabolic engineering of fermentative carbon metabolism in a cyanobacterium. Appl Environ Microbiol. 2010;76:5032–5038. doi: 10.1128/AEM.00862-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Surzycki R, Cournac L, Peltier G, Rochaix JD. Potential for hydrogen production with inducible chloroplast gene expression in Chlamydomonas. Proc Natl Acad Sci USA. 2007;104:17548–17553. doi: 10.1073/pnas.0704205104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melis A, Neidhardt J, Benemann J. Dunaliella salina (Chlorophyta) with small chlorophyll antenna sizes exhibit higher photosynthetic productivities and photon use efficiencies than normally pigmented cells. J Appl Phycol. 1998;10:515–525. [Google Scholar]
- 47.Laurinavichene TV, Fedorov AS, Ghirardi ML, Seibert M, Tsygankov AA. Demonstration of sustained hydrogen photoproduction by immobilized, sulfur-deprived Chlamydomonas reinhardtii cells. Int J Hydrogen Energy. 2006;31:659–667. [Google Scholar]
- 48.Kosourov S, Seibert M, Ghirardi ML. Effects of extracellular pH on the metabolic pathways in sulfur-deprived, H2-producing Chlamydomonas reinhardtii cultures. Plant Cell Physiol. 2003;44:146–155. doi: 10.1093/pcp/pcg020. [DOI] [PubMed] [Google Scholar]
- 49.Winkler M, Kuhlgert S, Hippler M, Happe T. Characterization of the key step for light-driven hydrogen evolution in green algae. J Biol Chem. 2009;284:36620–36627. doi: 10.1074/jbc.M109.053496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brenner MP, et al. Engineering Microorganisms for Energy Production. Washington, DC: US Dept of Energy; 2006. Rept JSR-05-300. [Google Scholar]
- 51.Carrieri D, Kolling D, Ananyev G, Dismukes GC. Prospecting for biohydrogen fuel. Ind Biotechnol. 2006;2:133–137. [Google Scholar]
- 52.US Department of Energy. National Algal Biofuels Technology Roadmap. College Park, MD: US Dept of Energy, Office of Energy Efficiency and Renewable Energy, Biomass Program; 2010. [Google Scholar]
- 53.Registry of Standard Biological Parts—Assembly standard 21. 2009. http://partsregistry.org/Assembly_standard_21.
- 54.Andersson CR, et al. Methods Enzymol. Vol. 305. New York: Academic; 2000. Application of bioluminescence to the study of circadian rhythms in cyanobacteria; pp. 527–542. [DOI] [PubMed] [Google Scholar]
- 55.Porra RJ. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res. 2002;73:149–156. doi: 10.1023/A:1020470224740. [DOI] [PubMed] [Google Scholar]