Abstract
Chemotherapy-induced broad toxicities are the leading cause of the drug-induced mortality in cancer patients. Antiangiogenic drugs (ADs) in combination with chemotherapy are widely used as front-line therapy for the treatment of various human cancers. However, the beneficial mechanisms underlying combination therapy are poorly understood. Here we show that, in several murine tumor models, administration of sunitinib markedly reduced chemotherapy-induced bone marrow toxicity. Intriguingly, in a sequential treatment regimen, delivery of ADs followed by chemotherapy demonstrated superior survival benefits compared with simultaneous administration of two drugs. In murine tumor models, we show that VEGF increased chemotoxicity by synergistically suppressing bone marrow hematopoiesis with cytostatic drugs. These findings shed light on molecular mechanisms by which ADs in combination with chemotherapy produce survival benefits in cancer patients and provide conceptual information guiding future designs of clinical trials, current practice, and optimization of ADs for the treatment of cancer.
Keywords: antiangiogenic therapy, angiogenesis, angiogenic factors, cancer therapy, anemia
Antiangiogenic drugs (ADs) are one of the key components of front-line therapy in current combination regimens for the treatment of various human cancers (1–3). Clinical experiences gained from different types of cancers demonstrate that ADs such as bevacizumab, sunitinib, and sorafenib in combination with chemotherapy often produce significant but modest survival benefits (4, 5). These clinical findings have raised several important issues regarding the beneficial mechanisms of antiangiogenic therapy in cancer patients. For example, what is the fundamental mechanism underlying the clinical benefits of combination therapy? What are the molecular targets of ADs in combinatorial settings? Could ADs modulate the efficacy of chemotherapy or vice versa? If so, what kind of chemotherapeutic drugs (CDs) should be chosen for optimizing therapeutic effects with ADs? What is the optimal sequence of delivery of combinatorial drugs? To date, these important and clinically relevant issues remain unresolved.
We have previously shown that circulating VEGF significantly impairs hepatic functions, the endocrine system, and bone marrow (BM) hematopoiesis, leading to hemorrhagic ascites, weight loss, and early death in mice that manifest a cancer-associated systemic syndrome (CASS) (6). The VEGF-induced CASS resembles cancer cachexia and a paraneoplastic syndrome frequently seen in cancer patients (6, 7). In fact, CASS is responsible for ∼20% of mortality in all cancer patients (8). CDs such as cyclophosphamide (CTX), platins, and taxanes usually produce a broad spectrum of toxicity in multiple tissues and organs that undergo renewal and regeneration, often leading to severe anemia, cardiovascular failure, liver dysfunction, hair loss, and gastrointestinal disorders (9, 10). Owing to chemotoxicity, a substantial number of cancer patients die of therapy-related toxicities rather than disease. Because tumor-derived VEGF impairs physiological functions of multiple tissues and organs, and because CDs produce similar toxicities in a set of overlapping organs, we hypothesize that ADs might reduce chemotoxicity-related mortality by improving tissue functions such as BM hematopoiesis. In the present study, we provide experimental evidence to support this hypothesis.
Results
Survival Benefit of Combination Therapy in a Murine Melanoma Model.
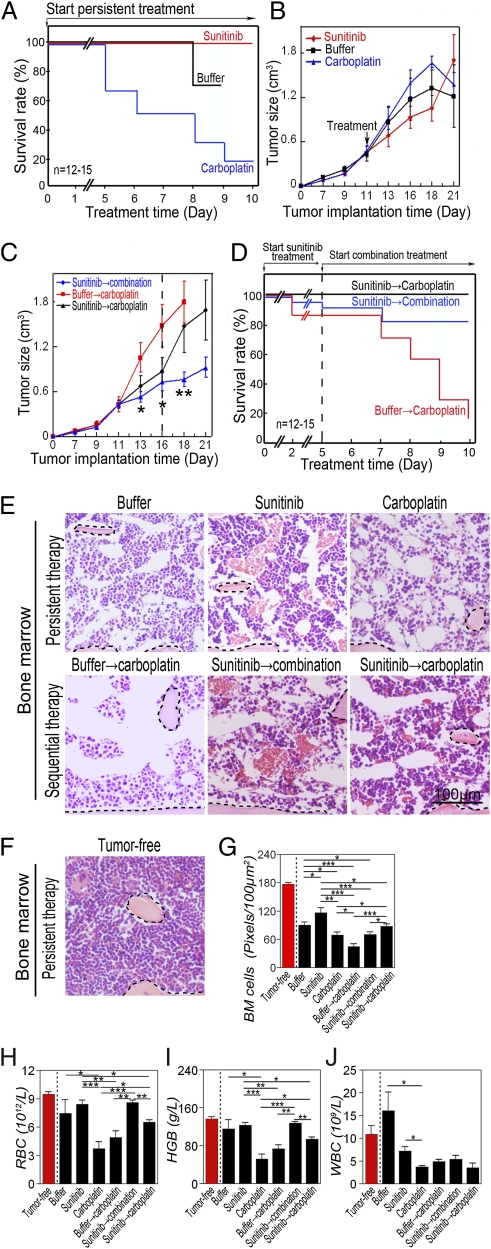
To study the beneficial mechanisms of combinations of chemotherapy and antiangiogenic therapy, we chose sunitinib and carboplatin, which are widely used in cancer therapy (5, 9). In a murine B16 melanoma model, we showed that administration of carboplatin at a clinically relevant dose of 50 mg/kg per day (11) to tumor-bearing mice for 10 d resulted in broad toxicities, leading to 80% of animal deaths (n = 12–15 mice/group) (Fig. 1A). Conversely, delivery of sunitinib (60 mg/kg per day) alone did not result in death. Our findings reconciled with the clinical safety profiles of these well-characterized ADs and CDs (5, 9). It appeared that chemotherapy-induced mortality was not associated with tumor volume because carboplatin did not exhibit a significant antitumor effect in this melanoma model (Fig. 1B).
Fig. 1.
Improvement of survival in a murine melanoma model. (A) Treatment of B16 melanoma-bearing mice (n = 12–15 mice/group) with sunitinib, carboplatin, or vehicle was started when the average tumor size reached 0.4 cm3. Survivals of mice were closely monitored several times per day. (B) Tumor growth rates of sunitinib-, carboplatin-, or vehicle-treated groups (n = 12–15 mice/group). (C) When the average tumor size reached 0.4 cm3, tumor-bearing mice (n = 12–15 mice/group) were treated with sunitinib. At day 6 after treatment, sunitinib-treated mice received carboplatin or carboplatin plus sunitinib until the end of experiments. Vehicle-pretreated group followed by carboplatin was used as a control. (D) Kaplan–Meier survival curve of various treated groups under the regimen described in C. Dashed line marks pretreatment endpoint. (E and F) BM histology of various treated groups described in A–D. Dashed lines enclose bone matrix. (Bar = 100 μm.) (G) Quantification of density of BM cells (20× magnification, eight randomized fields per group). (H–J) RBC (H), hemoglobin (I), and WBC (J) in peripheral blood. *P < 0.05, **P < 0.01, ***P < 0.001. Data are shown as mean ± SEM.
Before combination therapy, pretreatment of tumor-bearing mice with sunitinib for 5 d followed by sunitinib plus carboplatin significantly improved tumor suppression relative to the effect observed with carboplatin alone (Fig. 1C). Surprisingly, the degree of tumor suppression by sunitinib plus carboplatin did not correlate with significant improvement of animal survival compared with the group successively treated with carboplatin alone. In fact, animals treated with sunitinib followed by carboplatin alone showed slightly better survival relative to the group treated with sunitinib followed by sunitinib plus carboplatin (Fig. 1D). These findings demonstrate that survival benefits of ADs and chemotherapy are not associated with suppression of tumor growth, and that sequential delivery of an AD followed by chemotherapy produces superior survival effects.
Sunitinib Protects Chemotherapy-Induced BM Destruction in a Melanoma Model.
Chemotherapy is known to display broad adverse effects, which include BM suppression, hair loss, gastrointestinal disorders, immunosuppression, and cardiotoxicity (9, 10). Among these drug-induced global toxicities, suppression of BM hematopoiesis is probably the most severe clinical issue that is often associated with chemotherapy-induced mortality in cancer patients. Among commonly used CDs, carboplatin is known to induce severe anemia and myelogenic defects. We and others have recently shown that tumor-derived VEGF also significantly impairs BM hematopoiesis, and that ADs including sunitinib significantly improve survival in mice by a mechanism of off-tumor targets (6, 12, 13). These data suggest that tumor-derived VEGF and chemotherapeutic drug might synergistically impair hematopoiesis and myelogenesis in tumor-bearing hosts.
To study the impact of sunitinib and carboplatin on BM hematopoiesis and myelogenesis, BM from tumor-bearing mice treated with monotherapy or combinatorial therapy were histologically analyzed. Interestingly, BM from B16 tumor mice showed scattered distribution of hematopoietic islets relative to BM from healthy mice (Fig. 1 E–G), suggesting a tendency of an anemic phenotype, which was validated by measurement of hematocrits and hemoglobin (Fig. 1 H and I). However, white blood cell (WBC) values in the B16 tumor group were not decreased, and slightly elevated levels were detected (Fig. 1J).
As expected, carboplatin alone significantly reduced hematopoietic islets by showing increased areas of noncellular structures (Fig. 1 E–G). Conversely, treatment with sunitinib alone did not result in significant suppression of hematopoiesis (Fig. 1 E–G). Pretreatment of tumor-bearing mice with sunitinib significantly improved carboplatin-induced hematopoietic impairments but not myelogenic defects (Fig. 1 E–J). Consistent with the reported myelosuppression profile (14), sunitinib alone also displayed a marked effect on repression of myelogenesis by a possible mechanism of inhibiting c-kit and flt-3 kinases (Fig. 1J).
Analysis of tumor angiogenesis confirmed that sunitinib significantly inhibited tumor neovascularization and normalized the remaining vasculature (Fig. S1 A and B). By contrast, carboplatin alone did not significantly inhibit tumor angiogenesis (Fig. S1 A and B). Similarly, the addition of carboplatin to the sunitinib-treated group did not further increase the antiangiogenic effect of sunitinib (Fig. S1 A and B). Intriguingly, carboplatin did not seem to maintain the antiangiogenic activity of sunitinib when these drugs were sequentially delivered (Fig. S1 A and B). These findings demonstrate that pretreatment of tumor-bearing mice with sunitinib protects the host from chemotherapy-induced hematopoietic defects, and provides a possible mechanism underlying survival benefits of combination therapy.
Tumor-Derived VEGF Impairs BM Hematopoiesis and Shortens Survival.
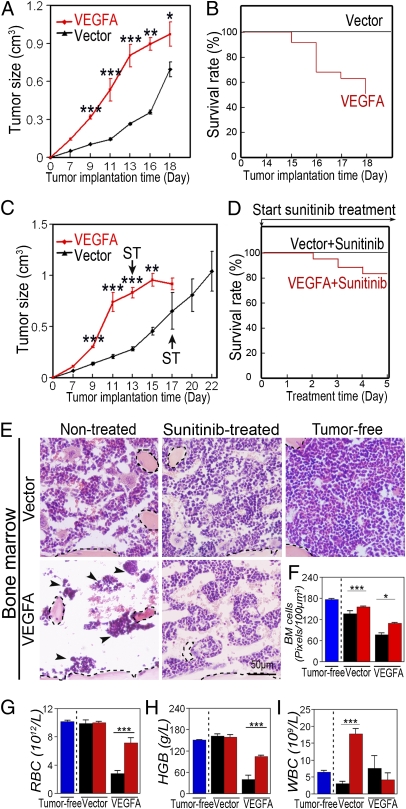
The protective effect of chemotoxicity by sunitinib suggests that tumor-derived VEGF might augment the toxic adverse effect of CDs. To study this possibility, we transfected a murine T241 fibrosarcoma cell line with human VEGF165 cDNA to reach a relative high level of expression. In agreement with our previously published findings (15–17), implantation of VEGF-T241 tumors into syngeneic mice resulted in an accelerated tumor growth rate (Fig. 2A). Similarly, VEGF-T241 tumor-bearing mice showed significantly shortened lifespans relative to those in the control group (Fig. 2B). By day 18 after tumor implantation, ∼50% of VEGF-T241 tumor-bearing mice died of CASS (Fig. 2B). It appeared that sunitinib did not significantly affect the tumor mass during this short time of treatment (Fig. 2A and C). However, treatment of VEGF-T241 tumor-bearing mice with sunitinib significantly improved survival (Fig. 2D), further validating that VEGF plays a causal role in murine death. By contrast, none of the mice in the control group, i.e., vector-T241 tumor-bearing mice, died during this experimental period.
Fig. 2.
Tumor growth rate and survival of sunitinib-treated or nontreated VEGF or vector-transfected T241 fibrosarcoma. (A) Growth rates of VEGF-T241 and vector tumors. (B) Kaplan–Meier survival curve of VEGF-T241 and vector-T241 tumor-bearing mice (n = 8–10 mice/group). (C) Growth rates of sunitinib-treated VEGF-T241 and vector tumors. (D) Kaplan–Meier survival curve of sunitinib-treated VEGF-T241 and vector-T241 tumor-bearing mice (n = 8–10 mice/group). (E) BM histology of sunitinib-treated and nontreated groups described in A–D. Dashed lines enclose bone matrix. Arrowheads point to residual hematopoietic islets attached to bone matrix. (Bar = 50 μm.) (F) Quantification of density of BM cells (20× magnification, eight randomized fields per group). (G–I) RBC (G), hemoglobin (H), and WBC (I) in peripheral blood. Blue bars represent tumor-free, black bars represent non-treated, and red bars represent sunitinib-treated. *P < 0.05, ***P < 0.001. Data are shown as mean ± SEM.
Histological analysis showed that, in addition to enhanced tumor angiogenesis VEGF-T241 tumor-bearing mice had severe hematopoietic defects in their BMs relative to controls (Fig. 2 E and F). In virtually all tumor-bearing mice, BM hematopietic islets had almost completely disappeared, and only a small number of remaining hematopoietic niches were associated with the bone matrix (Fig. 2E). Conversely, vector tumor-bearing mice exhibited a relatively healthy BM hematopoietic phenotype. To further validate the role of VEGF-induced hematopoietic defects, tumor-bearing mice were treated with sunitinib, which largely restored the VEGF-induced BM impairments (Fig. 2 E and F).
Consistent with hematopoietic defects in BM, peripheral red blood cell (RBC) and hemoglobin values were markedly lower in VEGF-T241 tumor-bearing mice relative to vector control tumor-bearing mice (Fig. 2 G and H). However, myelogenesis remained largely unaffected by tumor-derived VEGF (Fig. 2I). These findings demonstrate that tumor-derived VEGF induced severe anemia in mice by selectively destroying BM-dependent hematopoiesis. As expected, in this VEGF tumor model sunitinib demonstrates marked antiangiogenic effects (Fig. S2 A and B).
Tumor-Derived VEGF Increases Chemotoxicity and Chemotherapy-Induced Mortality.
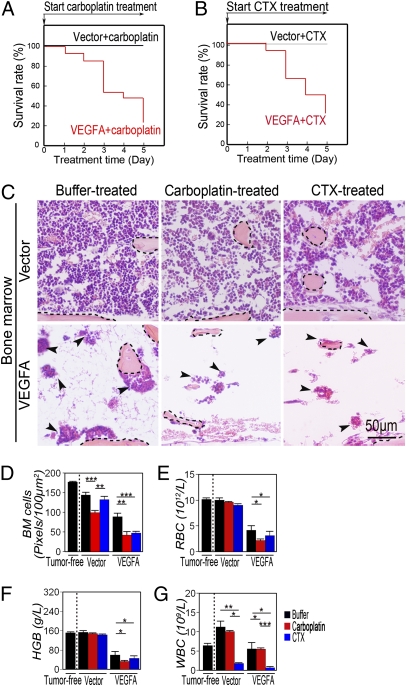
As shown above, both CDs and VEGF induced severe hematopoietic defects in BM, although the underlying mechanisms might be different. We hypothesized that administration of CDs to VEGF-producing tumors might produce a synergistic destructive activity on BM hematopopiesis. Surprisingly, treatment of VEGF-T241 tumor-bearing mice with carboplatin at a relatively low dose (25 mg/kg per day) resulted in death in 80% of animals, suggesting that VEGF and chemotherapy synergistically abbreviate survival in this tumor model (Fig. 3A). To further study whether the synergistic antisurvival effects of VEGF and chemotherapy were particularly limited to carboplatin, CTX at a dose of 62.5 mg/kg per day, a clinically relevant dose (18), was used for treatment. Like carboplatin, CTX induced a similarly high death rate in VEGF tumor-bearing mice (Fig. 3B), suggesting that a common mechanism underlying high death rates caused by various CDs might exist.
Fig. 3.
Treatment of VEGF- or vector-T241 tumors with chemotherapeutic drugs. (A) Kaplan–Meier survival curve of carboplatin-treated VEGF-T241 and vector-T241 tumor-bearing mice (n = 8–10 mice/group). Carboplatin exhibited a marked lethal effect on VEGF-tumor bearing mice. (B) Kaplan–Meier survival curve of CTX-treated VEGF-T241 and vector-T241 tumor-bearing mice (n = 8–10 mice/group). (C) BM histology of carboplatin-, CTX-treated, and vehicle-treated groups described in A and B. Dashed lines encircle bone matrix. Arrowheads point to the residue hematopoietic islets attached to the bone matrix. (Bar = 50 μm.) (D) Quantification of density of BM cells (20× magnification, eight randomized fields per group). (E–G) RBC (E), hemoglobin (F), and WBC (G) in peripheral blood. *P < 0.05, **P < 0.01, ***P < 0.001. Data are shown as mean ± SEM.
Consistent with synergistic effects on antisurvival effects, administration of CTX or carboplatin to VEGF tumor-bearing mice led to almost complete eradication of hematopoietic cells in the BM (Fig. 3 C and D). These data demonstrate that VEGF and CDs synergistically suppress BM hematopoiesis. In concordance with the rigorously defective phenotype in BM, peripheral values of RBC and hemoglobin in CTX- or carboplatin-treated groups were markedly low (Fig. 3 E and F), demonstrating that these treated mice has severe anemia. In addition, in the CTX-treated group, the WBC value was markedly reduced (Fig. 3G). However, carboplatin did not significantly reduce the myeloid value in VEGF tumor-bearing mice. These data support clinical experience with the safety profiles of these two drugs. Analysis of the tumor vasculature showed that, whereas CTX exhibited antiangiogenic activity in VEGF tumor tissues, carboplatin produced only a modest effect on suppression of tumor angiogenesis (Fig. S3 A and B).
Anti-VEGF Therapy Improves Survival by Reducing Carboplatin-Induced Chemotoxicity.
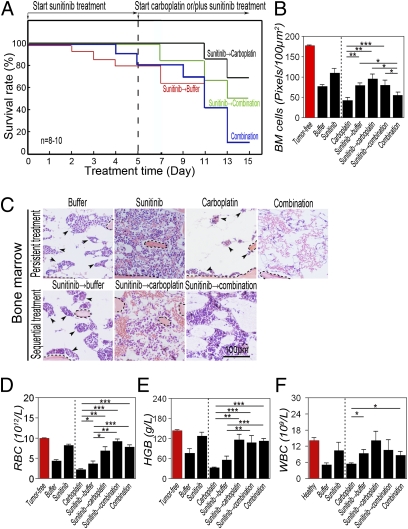
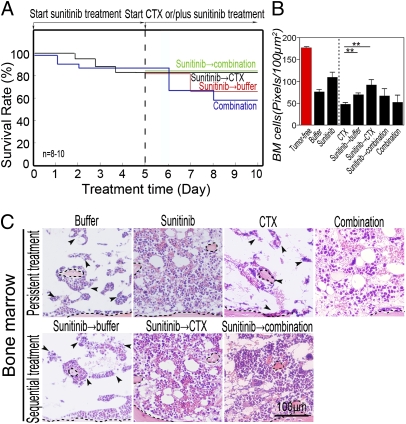
Synergistic suppression of BM hematopoiesis and shortened survival by tumor-derived VEGF and CDs suggests that anti-VEGF drugs might improve survival by a mechanism reducing chemotoxicity. If so, sequential delivery of anti-VEGF drugs before chemotherapy to normalize BM hematopoiesis would produce greater beneficial effects than simultaneous delivery of both drugs. To rationalize this experiment, we pretreated VEGF-T241 tumor-bearing mice with sunitinib followed by chemotherapy. As a control, simultaneous delivery of both drugs was initiated from the beginning of the experiment. Intriguingly, sequential delivery of sunitinib followed by carboplatin was associated with superior survival relative to that in the group (n = 8–10 mice/group) simultaneously treated with both drugs from the beginning (Fig. 4A). Similarly, pretreatment of tumor-bearing mice with sunitinib followed by a combination of sunitinib plus carboplatin was also significantly associated with improved survival. In contrast, carboplatin induced wide-ranging mortality in VEGF tumor-bearing mice that did not have prior treatment with sunitinib (Fig. 4 A and C). These findings demonstrate that, when administered before chemotherapy, sunitinib significantly improves survival in tumor-bearing mice.
Fig. 4.
Sequential treatment of T241 tumor-bearing mice with sunitinib followed by carboplatin significantly improves survival. (A) Kaplan–Meier survival curve of VEGF-T241 tumor-bearing mice (n = 8–10 mice/group) that received sequential therapy of sunitinib followed by carboplatin or by combination. Simultaneous delivery of both drugs and sunitinib followed by vehicle were used as controls. Sequential regimen of delivery sunitinib followed carboplatin markedly improved survival rates relative to rates in the group that received simultaneous combination therapy. (B) Quantification of density of BM cells (20× magnification, eight randomized fields per group). (C) BM histology of various treated groups described in A. Dashed lines enclose bone matrix. Arrowheads point to residual hematopoietic islets attached to bone matrix. (Bar, 100 μm.) (D–F) RBC (D), hemoglobin (E), and WBC (F) in peripheral blood. *P < 0.05, **P < 0.01, ***P < 0.001. Data are shown as mean ± SEM.
To elucidate the mechanism underlying superior survival effects of sequential delivery of anti-VEGF therapy followed by chemotherapy, BM from tumor-bearing mice was histologically analyzed. As expected, pretreatment with sunitinib significantly normalized the BM cell population in VEGF tumor-bearing mice (Fig. 4 B and C). Surprisingly, sunitinib in combination with carboplatin did not significantly improve the hematopoietic population in BM (Fig. 4 B and C). Conversely, pretreatment with sunitinib followed by carboplatin markedly rescued BM cells relative to those in the group simultaneously treated with both drugs. Similarly, pretreatment with sunitinib significantly protected carboplatin-induced BM toxicity in the group receiving combination therapy (Fig. 4 B and C). In agreement with the protective effect of sunitinib on BM in VEGF tumor-bearing mice, values of peripheral hematocrit and hemoglobin were increased in groups pretreated with sunitinib (Fig. 4 D and E). Likewise, WBC values were elevated in groups pretreated with sunitinib. These data demonstrate that sunitinib significantly protects BM against chemotoxicity if this anti-VEGF drug is administrated before chemotherapy.
Analysis of the tumor vasculature showed that sunitinib significantly inhibited tumor neovascularization and normalized the remaining vascular networks (Fig. S4 A and B). Notably, termination of sunitinib treatment resulted in revascularization in groups followed by either the vehicle or carboplatin (Fig. S4 A and B). These results also show that carboplatin cannot maintain the sunitinib-induced antiangiogenic activity. Surprisingly, cessation of sunitinib treatment in these tumor-bearing mice led to higher degrees of tumor neovascularization in both groups successively treated with buffer or carboplatin (Fig. S4 A and B), suggesting that a rebound tumor angiogenesis occurred in this model.
Taken together, our data show that the antiangiogenic effect of sunitinib administered in a sequence followed by chemotherapy does not correlate with improved survival. On the contrary, improved survival is well correlated with the sunitinib-induced protective effect against chemotoxicity in BM.
Anti-VEGF Therapy Improves Survival by Reducing Cyclophosphomide-Induced Chemotoxicity.
To determine whether the AD sunitinib had a broad protective effect against chemotoxicity, sunitinib and CTX were simultaneously or sequentially delivered to tumor-bearing mice. Consistent with the findings from carboplatin, sequential administration of sunitinib followed by CTX produced marked improvement in survival relative that to mice treated simultaneously with both drugs (Fig. 5A). In agreement with survival improvement, sunitinib significantly protected against CTX-induced BM toxicity, whereas simultaneous delivery of the combination of both drugs did not show increased numbers of nucleated cell population in the BM relative to animals treated with CTX alone (Fig. 5 B and C). These data further demonstrate that sunitinib can protect against chemotherapy-induced BM toxicity and can improve survival if it is delivered before chemotherapy.
Fig. 5.
Survival improvement by sequential treatment of T241 tumor-bearing mice with sunitinib followed by CTX. (A) Kaplan–Meier survival curve of VEGF-T241 tumor-bearing mice (n = 8–10 mice/group) that received sequential therapy of sunitinib followed by CTX or by a combination. Simultaneous delivery of both drugs and sunitinib followed by vehicle was used as control. (B) Quantification of density of BM cells (20× magnification, eight randomized fields per group). (C) BM histology of various treated groups described in A. Dashed lines enclose bone matrix. Arrowheads point to residual hematopoietic islets attached to bone matrix. (Bar, 100 μm.) **P < 0.01. Data are shown as mean ± SEM.
Unlike carboplatin, CTX was able to maintain sunitinib-induced antiangiogenic activity (Fig. S5 A and B), suggesting that CTX displayed substantial antiangiogenic activity. These findings demonstrate that various CDs may have different antiangiogenic activities, which should be selectively used in antiangiogenic maintenance therapy.
Discussion
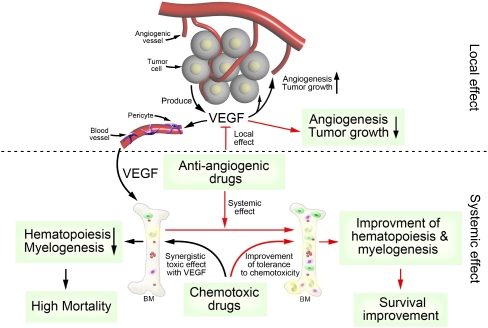
The mechanisms underlying the clinical benefits of ADs plus chemotherapy are far from clear, although several preclinical evidence-based hypotheses have been put forward to explain the possible mechanisms, including AD-induced vascular normalization allowing more efficient delivery of CDs in tumors (19) and improved antitumor activity by combination therapy. However, the survival benefit of ADs may not necessarily correlate with tumor suppression in cancer patients (6, 7, 20), suggesting alternative mechanisms underlying combination therapy. In the present study, we provide compelling evidence that tumor-derived VEGF and chemotherapy synergistically suppress BM hematopoiesis and myelogenesis, leading to poor survival in the animals studied. Notably, pretreatment with VEGF blockades markedly protects chemotherapy-induced systemic toxicity and improves survival, supporting the beneficial advantage of sequential delivery of ADs followed by chemotherapy.
Off-tumor targets of anticancer drugs are conventionally associated with adverse effects. For example, CD-induced systemic effects on multiple organs including suppression of BM hematopoiesis, gastrointestinal tract-related disorders, and dysfunction of the endocrine system are the foremost reason for drug-induced mortality in cancer patients (9, 10). Similar to chemotherapy, systemic delivery of ADs to cancer patients in currently routine clinical practice may also affect vascular structures and functions in multiple tissues and organs. The fundamental principle underlying currently available ADs is the objective of blocking functions of one or more angiogenic pathways in tumors (21). However, the excessive amounts of tumor-derived angiogenic factors are often accumulated in the circulation to display broad biological functions on healthy tissues and organs. Thus, systemic delivery of ADs in cancer patients may also block angiogenic factor-induced systemic effects (6, 12). Paradoxical to the adverse effects, emerging preclinical and clinical evidence suggests that off-tumor targets of ADs might be potentially beneficial sites for survival improvement (7). Supportive data include the following: (i) High levels of circulating angiogenic factors in cancer patients have been correlated with poor prognosis; (ii) high levels of circulating VEGF have been found to induce a systemic paraneoplastic syndrome manifesting severe anemia, endocrine disorders, and splenohepatomegaly (6, 12, 13); (iii) clinical benefits of ADs are often not associated with tumor suppression (6, 7, 20); (iv) beneficial responses of ADs have positively been correlated with systemic responses including hypertension and skin rashes (7); (v) Genetic polymorphisms of VEGF or VEGFR2 in cancer patients have been associated with drug responses and survival benefit (7); and (vi) in preclinical tumor models, delivery of a low dose of an anti-VEGF agent to tumor-bearing mice has led to improved survival without affecting tumor growth (6).
In designing clinical trials, patients with advanced malignancies are frequently recruited for antiangiogenic therapy. and these patients often have CASS manifesting different degrees of paraneoplastic syndrome and cachexia. Although several inflammatory cytokines such as TNF-α, IL-1β, and IL-6 have been linked to cancer cachexia (22), circulating VEGF may significantly contribute to the development of CASS (6, 12). In particular, high levels of VEGF significantly suppress BM hematopoiesis (6, 13). Although the mechanisms underlying VEGF-induced BM impairment remain uncharacterized, it is plausible that VEGF may mobilize BM stem cells to peripheral tissues, leading to decreased numbers of hematopoietic islets. Alternatively, VEGF may mobilize specific lineages of hematopoietic cells that express a specific type of VEGFR. Our current data support the first possibility. This interesting issue, in relation to chemotherapy-induced BM damage and recovery, warrants further investigation.
In fact, a substantial number of cancer patients die of chemotherapy-induced toxicity rather than disease. In light of the existence of hematopoietic defects in a substantial number of cancer patients at the advanced stage their disease, systemic administration of CDs would further suppress hematopoiesis and increase the risk of high mortality. In this study, we have validated, in murine tumor models, this concept that VEGF and CDs synergistically suppress hematopoiesis and myelogenesis, leading to BM crisis and high mortality. Our findings have raised another interesting issue related to optimal combination regimens of ADs and CDs. Based on our preclinical findings, it is reasonably speculated that selection of less BM-toxic CDs for patients whose circulating levels of VEGF are high would be a better choice. Optimal combination regimens involving ADs and different CDs need to be further validated in clinical settings. Sequential delivery of anti-VEGF drugs before chemotherapy significantly improves BM hematopoiesis and tolerance to chemotherapy-induced toxicity and lethality. Our findings provide a rationalized mechanism supporting the superior beneficial effects by sequential delivery of antiangiogenic therapy followed by chemotherapy (Fig. 6). Given the small incremental benefits of patient survivals by simultaneous delivery of ADs in combination with CDs as routinely prescribed during clinical practice, sequential delivery of ADs followed by chemotherapy would probably markedly increase the survival benefits by rescuing the population of chemotherapy-induced death. Although this concept warrants further validation in clinical settings, it at least provides a valuable rationale for designing future clinical trials to improve therapeutic outcomes.
Fig. 6.
Schematic diagram of mechanisms underlying antiangiogenic and cytostatic drugs.
Although sunitinib was used in the present study, we speculate that our findings could be extended to other anti-VEGF drugs. Indeed, our previously published findings demonstrate that a VEGFR2 blockade could completely prevent VEGF-induced BM damage, and these data thus allows us to generalize our findings to other ADs targeting the VEGF–VEGFR2 axis (6). Because preclinical studies show that cessation of antiangiogenic therapy may produce a rebound effect of tumor neovascularization, long-term delivery of ADs is desirable for maximizing clinical benefits. Owing to the high costs of currently available ADs, long-term antiangiogenic therapy remains a challenging issue (1). If CTX and other CDs were able to sustain AD-induced antiangiogenic activity, long-term or metronomic chemotherapy after antiangiogenic therapy would be an alternative option for optimizing clinical regimens.
Materials and Methods
Animals, Murine Tumor Model, Therapy, and Blood Chemistry.
All animal experiments were approved by the North Stockholm Animal Board (Stockholm, Sweden). Survival studies were performed according to the ethical permit in which humane endpoint was the criteria to sacrifice each mouse. This is done using an established score sheet. Details are provided in SI Materials and Methods.
Histological Studies and Whole-Mount Staining.
Blood Chemistry.
Statistical Analysis.
Supplementary Material
Acknowledgments
This work was supported through research grants from the Swedish Research Council, the Swedish Cancer Foundation, the Karolinska Institute Foundation, the Karolinska Institute distinguished professor award, the Torsten Söderberg’s foundation, the European Union Integrated Project of Metoxia (Project 222741), and the European Research Council Advanced Grant ANGIOFAT (Project 250021) (all to Y.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016220108/-/DCSupplemental.
References
- 1.Cao Y, Langer R. Optimizing the delivery of cancer drugs that block angiogenesis. Sci Translat Med. 2010;2:15ps13. doi: 10.1126/scitranslmed.3000399. [DOI] [PubMed] [Google Scholar]
- 2.Cao Y, Zhong W, Sun Y. Improvement of antiangiogenic cancer therapy by understanding the mechanisms of angiogenic factor interplay and drug resistance. Semin Cancer Biol. 2009;19:338–343. doi: 10.1016/j.semcancer.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Sarmiento R, D'Andrea MR, Cacciamani F, Salerno F, Gasparini G. Antiangiogenic therapies in breast cancer. Curr Opin Investig Drugs. 2009;10:1334–1345. [PubMed] [Google Scholar]
- 4.Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 6.Xue Y, et al. Anti-VEGF agents confer survival advantages to tumor-bearing mice by improving cancer-associated systemic syndrome. Proc Natl Acad Sci USA. 2008;105:18513–18518. doi: 10.1073/pnas.0807967105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao Y. Off-tumor target-beneficial site for antiangiogenic cancer therapy? Nat Rev Clin Oncol. 2010;7:604–608. doi: 10.1038/nrclinonc.2010.118. [DOI] [PubMed] [Google Scholar]
- 8.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862–871. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 9.Lokich J, Anderson N. Carboplatin versus cisplatin in solid tumors: An analysis of the literature. Ann Oncol. 1998;9:13–21. doi: 10.1023/a:1008215213739. [DOI] [PubMed] [Google Scholar]
- 10.Powis G. Dose-dependent metabolism, therapeutic effect, and toxicity of anticancer drugs in man. Drug Metab Rev. 1983;14:1145–1163. doi: 10.3109/03602538308991425. [DOI] [PubMed] [Google Scholar]
- 11.Egorin MJ. Population pharmacokinetc/pharmacodynamic modeling of paclitaxel and carboplatin in ovarian cancer. Clin Cancer Res. 2008;14:2517. doi: 10.1158/1078-0432.CCR-07-5049. author reply 2517–2518. [DOI] [PubMed] [Google Scholar]
- 12.Wong AK, et al. Excessive tumor-elaborated VEGF and its neutralization define a lethal paraneoplastic syndrome. Proc Natl Acad Sci USA. 2001;98:7481–7486. doi: 10.1073/pnas.121192298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue Y, Chen F, Zhang D, Lim S, Cao Y. Tumor-derived VEGF modulates hematopoiesis. J Angiogenes Res. 2009;1:9. doi: 10.1186/2040-2384-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar R, et al. Myelosuppression and kinase selectivity of multikinase angiogenesis inhibitors. Br J Cancer. 2009;101:1717–1723. doi: 10.1038/sj.bjc.6605366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedlund EM, Hosaka K, Zhong Z, Cao R, Cao Y. Malignant cell-derived PlGF promotes normalization and remodeling of the tumor vasculature. Proc Natl Acad Sci USA. 2009;106:17505–17510. doi: 10.1073/pnas.0908026106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eriksson A, et al. Placenta growth factor-1 antagonizes VEGF-induced angiogenesis and tumor growth by the formation of functionally inactive PlGF-1/VEGF heterodimers. Cancer Cell. 2002;1:99–108. doi: 10.1016/s1535-6108(02)00028-4. [DOI] [PubMed] [Google Scholar]
- 17.Cao R, et al. VEGFR1-mediated pericyte ablation links VEGF and PlGF to cancer-associated retinopathy. Proc Natl Acad Sci USA. 2010;107:856–861. doi: 10.1073/pnas.0911661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes DF, et al. Cancer and Leukemia Group B (CALGB) Investigators. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496–1506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 19.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 20.Ledford H. Drug markers questioned. Nature. 2008;452:510–511. doi: 10.1038/452510a. [DOI] [PubMed] [Google Scholar]
- 21.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Todorov P, et al. Characterization of a cancer cachectic factor. Nature. 1996;379:739–742. doi: 10.1038/379739a0. [DOI] [PubMed] [Google Scholar]
- 23.Björndahl M, et al. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci USA. 2005;102:15593–15598. doi: 10.1073/pnas.0507865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.