Abstract
Geldanamycin and its derivative 17AAG [17-(Allylamino)-17-demethoxygeldanamycin, telatinib] bind selectively to the Hsp90 chaperone protein and inhibit its function. We discovered that these drugs associate with mitochondria, specifically to the mitochondrial membrane voltage-dependent anion channel (VDAC) via a hydrophobic interaction that is independent of HSP90. In vitro, 17AAG functions as a Ca2+ mitochondrial regulator similar to benzoquinone-ubiquinones like Ub0. All of these compounds increase intracellular Ca2+ and diminish the plasma membrane cationic current, inhibiting urokinase activity and cell invasion. In contrast, the HSP90 inhibitor radicicol, lacking a bezoquinone moiety, has no measurable effect on cationic current and is less effective in influencing intercellular Ca2+ concentration. We conclude that some of the effects of 17-AAG and other ansamycins are due to their effects on VDAC and that this may play a role in their clinical activity.
Keywords: benzoquinone ansamycin, calcium regulation
Ansamycin antibiotics, such as Geldanamycin (GA) and its derivative 17AAG, bind to a conserved pocket in the aminoterminal portion of the Hsp90 chaperone protein and inhibit its function. This results in the degradation of Hsp90 client proteins, which include key components of mitogenic signaling pathways and several oncoproteins (1). 17-(Allylamino)-17-demethoxygeldanamycin (17AAG, telatinib) effectively induces degradation of Hsp90 client proteins in vivo and at nontoxic doses, has antitumor activity in a wide variety of murine xenograft and genetically engineered tumor models, and has been shown to have significant clinical activity in patients with HER2 breast cancer and adult refractory AML (2, 3).
17AAG and all GA derivatives have quinone moieties that are metabolized by NAD(P)H: quinone oxidoreductase1 (NQO1) (4, 5). Kelland et al. (6) first reported the relationship between levels of NQO1 and the sensitivity to 17AAG. Others have reported that, in vitro, via quinone catalytic activity, GA leads to superoxide formation without affecting HSP90 (7), suggesting that 17AAG functions through mitochondria. Here, we report that 17AAG can directly bind to mitochondria, specifically to the voltage-dependent anion channel (VDAC) through hydrophobic interaction. We show that HSP90 competes with mitochondria for binding with 17AAG. Moreover, GA derivatives have effects similar to ubiquinone, like Ub0, and within minutes can increase cytoplasmic Ca2+ concentration, as well as diminish membrane cationic current, reduce mitochondrial membrane potential, and interfere with cell invasion. We propose that the benzoquinone ring is responsible for mitochondrial VDAC binding. We conclude that the binding of GA and 17AAG to VDAC inhibits mitochondrial function.
Results
GA Binds to Mitochondria and VDAC Independent of HSP90.
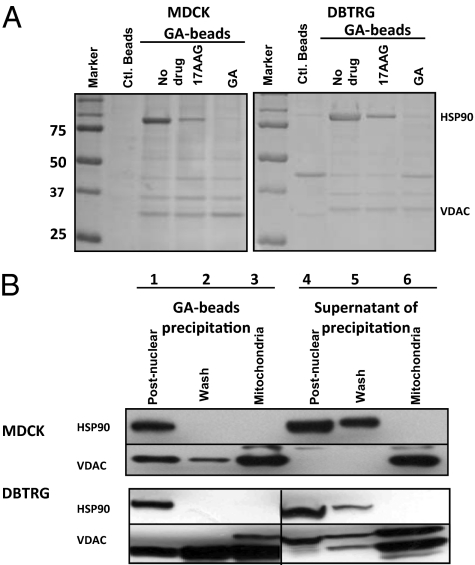
The motility of MDCK and DBTRG cells is known to be sensitive to GA drugs at low concentration (9, 10). Using a modification of the procedure of Whitesell et al. (11), GA-coupled beads were used to precipitate potential GA-binding proteins from canine MDCK and human DBTRG cell lysates. As determined by LC-MS/MS analysis (Table S1), GA-coupled beads precipitate HSP90 as originally described by Whitesell et al. (11); in addition, we identified a 32-kDa protein as the outer membrane mitochondrial voltage-dependent anion channel protein (VDAC) (Fig. 1A). Although the binding of Hsp90 to GA-beads from cell lysates was abolished with high concentrations of either GA or 17AAG (Fig. 1A, lanes indicated as 17AAG or GA), VDAC binding was not affected, suggesting different binding mechanisms for GA-HSP90 and GA-VDAC.
Fig. 1.
Geldanamycin-conjugated affinity beads precipitate mitochondrial VDAC independent of HSP90. (A) Control and GA-bead were used to precipitate MDCK and DBTRG cell lysates. Cells were treated with or without 17AAG or GA at 1 μM as indicated. (B) GA-coupled bead precipitation of HSP90 or VDAC from each fraction collected during the mitochondrial purification. Lanes 1–3 are Western blot analyses of the GA affinity bead precipitates pellet fractions of the postnuclear cell lysate (lane 1), mitochondrial wash supernatant (lane 2), and solubilized mitochondrial fractions (lane 3). Lanes 4–6 are aliquots of supernatants after GA affinity bead precipitation of the three fractions. All preparations were subjected to immunoblotting analysis with antibodies to either HSP90 or VDAC.
To show that VDAC is readily precipitated from mitochondria, MDCK and DBTRG cell-derived mitochondria were purified free of HSP90, treated with GA beads, and then precipitated (Fig. 1B). By Western blot analysis, both HSP90 and VDAC were precipitated from the postnuclear lysate fraction (lane 1). The mitochondrial pellets were also washed repeatedly to further remove the HSP90 until no detectable HSP90 was precipitated (Fig. 1B, lane 2) and only VDAC was detected in the purified mitochondrial fraction (Fig. 1B, lane 3). The supernatant fraction from each precipitated sample was also collected (Fig. 1B, lanes 4–6) to test for remaining HSP90 or VDAC after GA bead precipitation. These results show that VDAC binds directly to GA affinity beads in the mitochondria and that the binding is independent of HSP90.
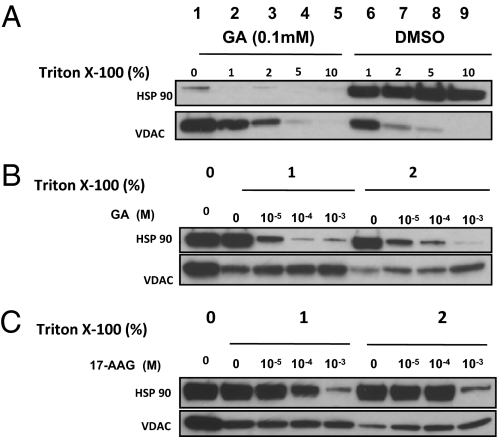
Detergent Micelles Interfere with 17AAG–VDAC Binding.
VDAC-1 is a highly hydrophobic protein that requires detergent to stabilize its structure in vitro by binding with unique hydrophobic sites (12, 13). Different types of detergent can change the secondary structure of recombinant VDAC (14), suggesting that the presence of detergent (e.g., 1% Triton X-100) in cell lysis buffer may influence GA-VDAC binding. To test this, we used GA beads to precipitate both HSP90 and VDAC from total MDCK cell lysates in the presence or absence of Triton X-100 and with or without free GA to see whether detergent would influence either GA-HSP90 or GA-VDAC binding (Fig. 2A). We found that the addition of GA at 0.1 mM efficiently removes HSP90 from the GA-beads whereas VDAC remains tightly associated. By contrast, VDAC can be completely dissociated from the GA-beads by detergent in a dose-dependent manner (Fig. 2A). Although 5–10% Triton X-100 is sufficient to dissociate the VDAC binding (Fig. 2A, VDAC panel), HSP90-GA binding is not affected (Fig. 2A, HSP90 panel). Solutions with 1–2% Triton X-100 and variable concentrations of GA (Fig. 2B) or 17AAG (Fig. 2C) further showed the different binding characteristics of GA and 17AAG to HSP90 and VDAC. The release of VDAC is dependent on the concentration of detergent and is consistent with recent findings that detergent micelles destabilize VDAC structure (12, 13). Our results suggest that VDAC binding to GA occurs through a hydrophobic association that can be regulated by detergent micelle formation, especially since GA drugs are also highly hydrophobic. This is also consistent with the finding of others (15) that detergent may interfere with measuring the binding affinities of GA. A spin column assay demonstrating how detergent micelles influence 17AAG binding is shown in Fig. S1. Although elution buffer without detergent does not interfere with 17AAG binding to HSP90 (Fig. S1A), detergent micelles highly influence 17AAG binding to purified VDAC (Fig. S1 B and C).
Fig. 2.
Detergent micelles interfere with VDAC-GA binding but not HSP90-GA binding. Total MDCK cell lysates were used for the GA-bead precipitation and competition assays with various compounds. (A) After GA-bead precipitation, beads were washed and Triton X-100 was added into cell lysis buffer at different concentrations with or without GA at 0.1 mM to release HSP90 and VDAC. (B and C) After GA bead precipitation, beads were washed and resuspended with lysis buffer with Triton X-100 at either 1% or 2% concentration. GA (B) or 17AAG (C) were then added into the samples at different concentration to release HSP90 or VDAC from the beads.
17AAG Binds to Intact Mitochondria.
VDAC forms a large voltage-gated pore at the planar bilayer of mitochondria and acts as the major channel, allowing the flux of both anions and cations, with selectivity varying with the voltage-dependent “open” or “closed” state of the VDAC channel (16). An intact mitochondrial bilayer is essential for VDAC structure and function (17). We therefore investigated the GA–VDAC binding with purified mitochondria using buffers free of detergent. VDAC was used as a mitochondrial marker and Hsp90 as a marker for contamination by cytosol (Fig. S2A). Mitochondrial viability was determined by Tetramethylrhodamine ethyl ester (TMRE) staining followed by FACS to assess metabolic activity (Fig. S2 B and C). We showed that mitochondria can bind to 3H-17AAG in a dose-dependent fashion, suggesting direct binding between 17AAG and mitochondria (Fig. S2D).
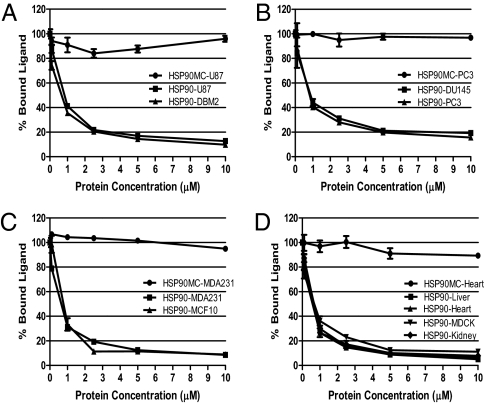
We assessed whether purified full-length HSP90 would compete with mitochondrial VDAC for binding to 3H-17AAG. The VDAC sources included tumor cell lines, such as glioblastoma cells (DBM2, U87), prostate cancer cells (DU145 and PC3), and breast cancer cells (MDA231 and MCF10), as well as normal organs such as murine liver, heart, and kidney, and finally MDCK cells. HSP90-MC, a truncated HSP90 lacking the N-terminal GA binding site, was used as a control (Fig. 3). In all cases, HSP90 could effectively compete for 3H-17AAG binding with mitochondria regardless of source, whereas HSP90-MC did not (Fig. 3). Taken together, we conclude that GA binding to mitochondria and VDAC is independent of HSP90 and occurs with mitochondria from all sources.
Fig. 3.
Competitive binding of HSP90 and purified mitochondria with 3H-17AAG. Recombinant full-length HSP90 or HSP90 lacking the N-terminal (HSP90MC) were used at different concentrations to compete with purified mitochondria isolated from various normal and cancer cells and mouse organs for 3H-17AAG binding. Mitochondria were isolated from the following: (A) glioblastoma U87 and DBM2 cells; (B) prostate cancer DU145 and PC-3 cells; (C) breast cancer MDA231 and MCF10 cells; and (D) canine epithelial MDCK cells as well as mouse organs, including liver, kidney, and heart.
Mitochondrial PTP Inhibition Does Not Correlate with HSP90 Affinity.
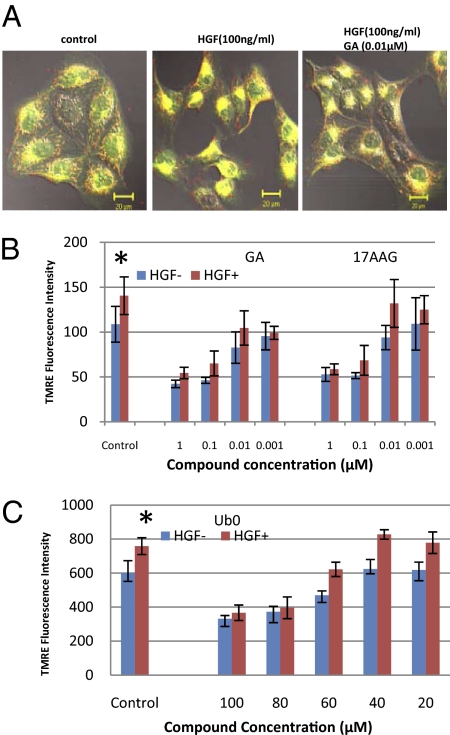
GA compounds are composed of a benzoquinone moiety attached to an ansamycin ring (Fig. S3). Ubiquinone (Ub0) is a benzoquinone molecule known to block the permeability transition pore (PTP) via VDAC (18). Our previous studies showed that hepatocyte growth factor (HGF) mediated MDCK scattering can be blocked with GA compounds (9, 10). Here we showed that HGF-induced cell scattering was associated with mitochondria relocating to a peri-nuclear position (Fig. 4A), indicating that the increased motility is associated with mitochondrial polarization. To test this, we used a TMRE fluorescence quantification assay and showed that HGF induced an increase in mitochondrial membrane potential in MDCK cells that can be blocked by GA and 17AAG at 0.1 μM (Fig. 4B, P < 0.05). Ub0 was active at ∼80 μM (Fig. 4C, P < 0.05), a concentration also required to inhibit the PTP (19). We questioned whether Ub0 acts as an HSP90 inhibitor and treated MDCK cells with GA and 17AAG (Fig. S4). We show that both compounds can markedly up-regulate HSP90 and degrade c-Met. Radicicol, a classic HSP90 inhibitor, also followed the same trend but required log higher concentrations. However, Ub0 did not affect either the HSP90 or the c-Met level, suggesting that Ub0 influences mitochondria directly without affecting HSP90.
Fig. 4.
17AAG reduces mitochondrial membrane potential. (A) Cell scattering and mitochondrial activity were monitored in MDCK cells with JC-1 fluorescence. Cells were either “untreated” or “treated” with HGF (100 ng/mL) or further treated with GA (10−8 M). (B and C) Mitochondrial membrane potential was quantified with TMRE uptake assays. HGF enhances TMRE uptake in MDCK cells, whereas GA and 17AAG (B), or Ub0 (C) block TMRE uptake. *Compared with MDCK control cells without HGF: P < 0.05, Student t test (n = 4).
Geldanamycin and Ub0 inhibit mitochondrial PTP function, but the latter compound does not bind to HSP90. Competitive filter binding assays with 3H-17AAG yielded a Ki of 0.274 ± 0.072 μM for the N-terminal domain of HSP90. This correlated with the Kd of 0.79 ± 0.38 μM, which was determined by filter binding assay for saturation binding and also correlated with that reported value for the Kd of 17AAG for both the N-terminal domain of HSP90 and full-length HSP90. In contrast, we found Ub0 and decyl-Ub have no affinity for the N-terminal domain of HSP90 in filter binding assays at concentrations of 10−4 to 10−11 M. In addition, radicicol, a known inhibitor of HSP90 function, showed strong affinity toward the N-terminal domain of HSP90 by competitive binding experiments with a Ki of 21.5 ± 6.8 nM, in line with that previously reported (20–22). These analyses suggest that GA compounds, due to the benzoquinone moiety, can bind to mitochondrial VDAC and that benzoquinones influence mitochondria directly.
Intracellular [Ca2+] Efflux Influenced by GA and Ub0.
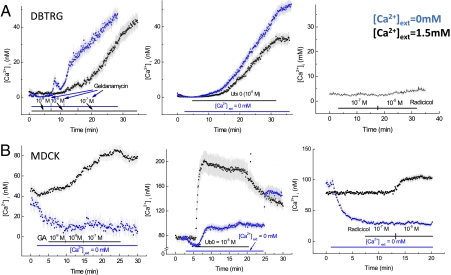
Considering the decrease of mitochondrial membrane potential by both GA and 17AAG, we postulated that drugs with a benzoquinone moiety may increase cytoplasmic or internal calcium concentration, [Ca2+]i, by promoting an efflux from depolarized mitochondria in metabolically compromised cells. We used fluorescence imaging of Fura2-loaded cells to assay the effect of these drugs on [Ca2+]i. Increasing doses of GA to DBTRG cells increased [Ca2+]i at 10−8 M, and further at 10−7 M (Fig. 5A). This effect of GA was comparable whether external [Ca2+] ([Ca2+]ext) equaled 1.5 or 0 mM (Fig. 5A), indicating that an intracellular source was responsible for the increased [Ca2+]i. Thus, we compared effects of the PTP inhibitor Ub0 on [Ca2+]i in DBTRG cells. Ub0 (10−5 M) treatment increased the magnitude of [Ca2+]i, and the rate of increase was comparable to that of GA, regardless of the [Ca2+]ext (Fig. 5A), again indicating an intracellular source for the increased [Ca2+]i. We also measured the effect of radicicol, a specific HSP90 inhibitor lacking the quinone moiety, on [Ca2+]I. Radicicol at either 10−7 or 10−5 M had no effect on [Ca2+]i in DBTRG cells (Fig. 5A), ruling out involvement of HSP90 in this effect.
Fig. 5.
Effect of increasing dose of benzoquinone drugs on intracellular Ca2+, [Ca2+]I with DBTRG and MDCK cells. Black indicates external Ca2+ = 1.5 mM; Blue: external Ca2+ = 0 mM. Data are mean ± SE; at least 25 cells were measured for each group. (A) Effect of Geldanamycin, Ub0, and Radicicol on DBTRG [Ca2+]i. (B) Effect of Geldanamycin, Ub0, and Radicicol on MDCK [Ca2+]i.
We also tested the influence of GA and Ub0 on MDCK [Ca2+] flux (Fig. 5B). GA increased MDCK [Ca2+]i and DBTRG [Ca2+]i equally. Nevertheless, the baseline [Ca2+]i was ∼10-fold greater in MDCK cells than in DBTRG cells, suggesting very different membrane Ca2+ transport properties between the two cell types. Consistent with this difference, switching perfusion solution to 0 mM [Ca2+]ext resulted in a rapid diminution of [Ca2+]i, and under this condition, GA had no appreciable effect on [Ca2+]i (Fig. 5B). Ub0 increased [Ca2+]i comparably to GA when added to 0 mM [Ca2+]ext; however, it markedly increased [Ca2+]i when [Ca2+]ext = 1.5 mM (Fig. 5B). Radicicol slightly increased [Ca2+]i at 10−5 M (which is 100-fold greater than the effective concentration of GA) when [Ca2+]ext = 1.5 mM but had no effect when [Ca2+]ext = 0 mM (Fig. 5B). This indicates that radicicol at 10−5 M stimulates influx of external Ca2+ in MDCK cells, as does Ub0 at this concentration. We have not characterized this CA2+ influx pathway other than its extracellular source.
GA and Ub0 Inhibit Membrane Cationic Current.
The versatility of Ca2+ as a messenger in multiple signaling events requires that the concentration of calcium ions within the cytoplasm be highly regulated. Release of calcium from the endoplasmic reticulum often results in calcium influx across the cell membrane via store operated calcium entry (SOCE) to replenish the depletion. This influx is most evident by an increase in the Ca2+ release-activated Ca2+ current (ICRAC) of the plasma membrane. To test whether the increase in [Ca2+]i by GA and Ub0 occurred by SOCE, we performed whole-cell voltage clamp measurements on both MDCK and DBTRG cells to measure the nonselective cationic current. HGF/SF treatment increased this current (Table 1), consistent with previous findings (23, 24). Dramatically, GA at picomolar levels inhibited cationic currents within 6 min after addition to either MDCK or DBTRG cells (Table 1 and Figs. S5 and S6,). Moreover, the VDAC-PTP inhibitors Ub0 and decyl-ubiquinone both rapidly decreased membrane cationic current in MDCK cells at 10 μM (Table 1 and Fig. S5D), a concentration known to inhibit the PTP (19). We conclude that the benzoquinone moiety of Ub0 and of GA are responsible for rapidly (within minutes) reducing mitochondria membrane potential, increasing cytoplasmic/internal calcium, and inhibiting membrane cationic current. We propose that the inactive or low activity of radicicol in the same assays separates the HSP90 chaperone activity from the mitochondria function associated with either cell motility or toxicity.
Table 1.
Inhibition of HGF/SF-induced cationic currents
| Compound concentration (M) | Slope conductance* (nS/pF) |
| Control (no treatment) | 0.79 ± 0.10† |
| Geldanamycin (10−12) | 0.31 ± 0.06†‡ |
| Ubiquinone (10−5) | 0.50 ± 0.10† |
| Decyl-ubiquinone (10−5) | 0.64 ± 0.12† |
| Radicicol (10−11) | 0.80 ± 0.28† |
| HGF/SF (100 scatter units/mL) | 1.65 ± 0.19 |
| HGF/SF + GA (10−12) | 0.47 ± 0.17† |
| HGF/SF + ubiquinone (10−5) | 0.76 ± 0.22† |
| HGF/SF + decyl-ubiquinone (10−5) | 0.36 ± 0.14† |
| HGF/SF + radicicol (10−11) | 1.29 ± 0.52 |
| HGF/SF + radicicol (10−8) | 1.03 ± 0.03† |
MDCK cells were treated with various conditions as indicated. nS, nanoSiemans; pF, picoFarads.
*Mean ± SEM.
†Differs significantly from HGF/SF treatment, P < 0.05 as determined by Student Newman-Keuls multiple comparison of means.
‡Differs significantly from control, P < 0.05.
Blocking Mitochondrial PTP Inhibits Cell Invasion.
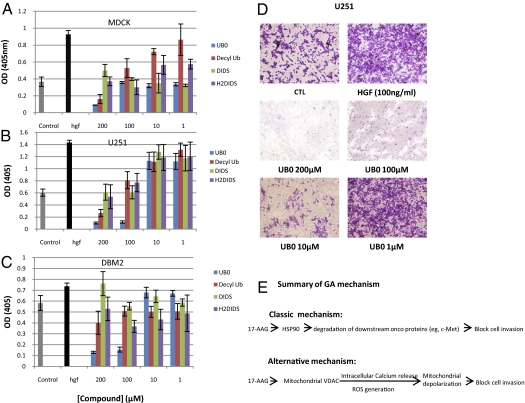
Calcium release from mitochondria can trigger apoptosis and block cell motility (25). Previously we have shown that HGF induces MDCK cell scattering or glioblastoma cell invasion in parallel with up-regulation of urokinase (uPA) activity (9, 10), which can be blocked by GA or 17AAG. Here, we show that GA can diminish HGF-induced membrane cationic current at 10 pM (Table 1). To test whether blocking mitochondrial PTP pores can inhibit cell motility, we tested the following known PTP inhibitors: 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid, disodium salt (DIDS); 4,4′-diisothiocyanatodihydrostilbene-2,2′-disulfonic acid, disodium salt (H2DIDS) (26); Ub0; and Decyl-Ub for their ability to inhibit HGF-mediated uPA-plasmin activation as a surrogate for invasion (9) (Fig. 6A) at concentrations that affect mitochondrial function. DIDS is a nonspecific, voltage-dependent VDAC inhibitor preventing O2·− diffusion from the intermembrane space to the cytoplasm. At 0.5 mM, DIDS inhibits superoxide production in isolated heart mitochondria (26). Both DIDS and H2DIDS at 100 μM inhibited uPA-plasmin activation. Importantly, the concentrations at which Ub0 and Decyl-Ub significantly inhibit HGF-induced uPA-plasmin activation in MDCK, glioblastoma U251, and DBM2 cells (Fig. 6), are the same drug concentrations that block Ca2+ influx in purified mitochondria (18, 19). Again, Ub0 displayed the highest activity. We also performed matrigel invasion assay and showed that Ub0 inhibited HGF induced invasion at the same concentration level as it blocks uPA activity. We conclude that mitochondrial PTP inhibitors, including bezoquinone ansamycin, can block VDAC functions and prevent cell invasion.
Fig. 6.
Blocking mitochondrial PTP inhibits uPA/invasion. Mitochondrial PTP inhibitors block HGF/SF-mediated uPA activity in MDCK (A), U251 (B), and DBTRG-05MG (C) cells. (D) Ub0 inhibits HGF induced cell invasion in U251 cells at same concentration inhibiting uPA activity. (E) Proposed mechanism of action of geldanamycin. Free GA either binds to HSP90 via the specific N-terminal ATP binding site or hydrophobically binds to mitochondrial VDAC. Although binding to HSP90 inactivates client protein chaperone activity, resulting in degradation of various oncoproteins, GA binding, through its benzoquinone moiety, regulates intracellular calcium influx from mitochondria. The increase of intracellular calcium decreases membrane cationic current and reduces mitochondrial metabolism and cell motility (25).
Discussion
HSP90 is the major target of GA and its derivatives, but GA derivatives are also known as antitumor quinones that are both metabolized by NQO1 (27). Ubiquinone compounds reportedly target mitochondria (7, 18), indicating that mitochondria may be an important 17AAG target (4). Our results show that, in a hydrophobic-dependent manner, 17AAG can physically bind to mitochondrial outer membrane protein VDAC, and the binding is HSP90 independent (Figs. 1–3).
VDAC is a mitochondrial outer membrane protein and is the key component of the PTP complex, which is responsible for mitochondrial metabolite flux (28, 29). VDAC regulation of the opening or closing of the PTP pore is a determinant in mitochondria-mediated apoptosis and in the generation of redox potential (25). We show that VDAC in cell lysates is efficiently pulled down with GA beads, but the specific nature of the hydrophobic GA-VDAC binding is unknown. Recently, detergent micelles were shown to be required for the stabilization of VDAC and for determining VDAC crystal structure. The calcium binding site of VDAC has also been mapped to a hydrophobic region (13). Thus, the VDAC-GA binding may occur through hydrophobic regions of VDAC.
Others have reported that 0.01% Nonidet P-40 can increase the binding affinity of HSP90 to GA derivatives 20-fold (15). In a more recent study, a conserved hydrophobic motif in HSP90 β strand has been reported to regulate the secretion and function of HSP90 (30). Thus, the presence of detergent can influence 17AAG binding to HSP90, potentially contributing to the poor correlation between binding affinity and cytotoxicity (31, 32) or to the potency gap between biochemical binding and Her2 degradation (33). We tested detergent by itself and found that 17AAG can significantly bind to detergent micelles. This could be due to the hydrophobicity of GA derivatives, as a highly water-soluble molecule such as thymidine is not affected in these assays (Fig S1). We conclude that GA derivative binding affinity can be affected by solvent and detergent; the presence of detergent in solution should be below the level of micelle formation to provide a more accurate binding affinity or biological activity. Related to this conclusion, our previous study showed that a subgroup of nine GA compounds and derivatives block HGF-induced uPA activity at femtomolar concentration without degrading c-Met oncoprotein (9, 10, 34). More recently, we found that the difference was not as large after we adjusted the method of compound delivery (Table S2). We attribute this difference to the hydrophobic properties of nine of the derivatives that are affected and in some way correlate with the efficacy of inhibiting uPA activity.
That GA and benzoquinone drugs increase [Ca2+]i suggests that they have a common target. It is noteworthy that Ub0 and Decyl-Ub, known inhibitors of the mitochondrial VDAC and PTP (18), also reduce the plasma membrane cationic current similar to GA. Because both the increase of [Ca2+]i and the inhibition of membrane cationic current occurred rapidly, 6–10 min after the addition of GA to the cells (Fig. 5 and Figs. S5 and S6), the mechanism is unlikely to be working through a HSP90 client protein affect; rather, we postulate that the mitochondrial VDAC serves as a target for 17AAG, GA, and Ub0, resulting in depolarization of the mitochondrial membrane potential, a rise in [Ca2+]i, and a corresponding decrease in membrane cationic current (i.e., ICRAC) (35, 36). Collapse of the mitochondrial membrane potential by these drugs disrupts regulation of [Ca2+]i from extracellular, ER, and mitochondrial pools. Our results also suggest that the intracellular Ca2+ influx may be a key step and a potential site for indirectly monitoring the mitochondria VDAC-PTP response to inhibition by Ub0 and GA. Here, dissipation of the mitochondrial membrane potential, by Ub0 and GA, increases [Ca2+]i. Global increases in [Ca2+]i can override intracellular Ca2+ gradients and disrupt flickering Ca2+ microdomains, both of which are necessary to sustain the polarity of migrating cells (37).
GA derivatives all have a quinone moiety and are metabolized by the NQO1 enzyme (6), indicating that they interact with mitochondria. HSP90 has a broad spectrum of client proteins, and any mitochondrial activity could be masked or considered a subset of HSP90 activity. Previously, studies have suggested that GA can regulate ROS generation without affecting HSP90 (7). Here we show that quinones, like Ub0, can increase the membrane cationic current (Table 1) and intracellular Ca2+ influx (Fig. 5), as well as block mitochondrial PTP and inhibit cell invasion (Fig.6). This would be expected to add bioactivity to GA compounds, compared to radicicol, without affecting HSP90. The quinone-mediated activity via mitochondrial VDAC is important in that VDAC is a major component of mitochondrial PTP pore, which regulates cell migration, invasion, and apoptosis via Ca2+ regulation and ROS generation (25). It is for this reason that VDAC has been considered a potential anti-cancer target (38). However, since the ROS generation can also induce cytotoxicity, the safety of targeting VDAC will be an issue. Accordingly, the quinone moiety of the GA derivatives is now being considered for modification to improve both activity and solubility (15). In fact, there are efforts to develop GA derivatives lacking the quinone moiety. Such compounds do not degrade NQO1 but still show HSP90 inhibition (39), and they might be more efficacious with reduced toxicity. It is possible that the benzoquinone moiety binding to mitochondria may explain the liver toxicity of 17AAG observed clinically (5, 40, 41). Others have shown that tumor cells organize an intramitochondrial HSP90 and TRAP-1 chaperone network and regulate mitochondrial permeability transition (8). However, 17AAG and most GA analogs do not permeate mitochondria and, thus, require a cell-penetrating linker added to the quinone moiety (8). Consequently, these mitochondrial HSP90 chaperones cannot account for our findings on the rapid effects of the unmodified, small-molecule HSP90 antagonists.
Collectively, our results suggest a model in which a subset of the activity of the GA drugs originates from targeting mitochondria (Fig. 6E). In this model, the mitochondrial membrane pore protein VDAC would be the novel target that binds to GA. Upon binding, GA, through its benzoquinone moiety, can regulate calcium influx and, in turn, decrease the cell membrane cationic current, inhibiting cell invasion and synergizing with the HSP90 effect to cause an anti-cancer effect on motility (Fig. 6D). However, targeting mitochondria could also account for toxicity, e.g., through ROS generation. Because this activity is independent of inhibiting HSP90 chaperone function, diminishing the benzoquinone moiety's toxicity in future drugs should be considered (39, 40).
Materials and Methods
SI Materials and Methods provides additional information related to the main text on the following topics: cell lines and drugs, GA-immobilized affinity beads and GA bead precipitation assays, release of HSP90 and VDAC from GA-conjugated beads with free GA, mass spectrometry analysis, HSP90, and HSP90MC purification, 3H-17AAG binds to purified mitochondria, competitive binding to 3H-17AAG between purified mitochondria and HSP90, TMRE measurement of mitochondria membrane potential, whole-cell voltage clamp, Ca2+ measurements by fluorescence imaging of Fura2, confocal microscopy, HGF/SF-Met-uPA-plasmin assay, and Matrigel invasion assay.
Supplementary Material
Acknowledgments
We thank David Nadziejka and Julia A. Patzelt for editing the manuscript.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015181108/-/DCSupplemental.
References
- 1.Isaacs JS, Xu W, Neckers L. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell. 2003;3:213–217. doi: 10.1016/s1535-6108(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins LM, Jayanthan AA, Narendran A. Effects of 17-allylamino-17-demethoxygeldanamycin (17-AAG) on pediatric acute lymphoblastic leukemia (ALL) with respect to Bcr-Abl status and imatinib mesylate sensitivity. Pediatr Res. 2005;57:430–437. doi: 10.1203/01.PDR.0000153871.45184.19. [DOI] [PubMed] [Google Scholar]
- 3.Modi S, et al. Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: A phase I dose-escalation study. J Clin Oncol. 2007;25:5410–5417. doi: 10.1200/JCO.2007.11.7960. [DOI] [PubMed] [Google Scholar]
- 4.Guo W, et al. Formation of 17-allylamino-demethoxygeldanamycin (17-AAG) hydroquinone by NAD(P)H:quinone oxidoreductase 1: Role of 17-AAG hydroquinone in heat shock protein 90 inhibition. Cancer Res. 2005;65:10006–10015. doi: 10.1158/0008-5472.CAN-05-2029. [DOI] [PubMed] [Google Scholar]
- 5.Guo W, Reigan P, Siegel D, Ross D. Enzymatic reduction and glutathione conjugation of benzoquinone ansamycin heat shock protein 90 inhibitors: Relevance for toxicity and mechanism of action. Drug Metab Dispos. 2008;36:2050–2057. doi: 10.1124/dmd.108.022004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelland LR, Sharp SY, Rogers PM, Myers TG, Workman P. DT-Diaphorase expression and tumor cell sensitivity to 17-allylamino, 17-demethoxygeldanamycin, an inhibitor of heat shock protein 90. J Natl Cancer Inst. 1999;91:1940–1949. doi: 10.1093/jnci/91.22.1940. [DOI] [PubMed] [Google Scholar]
- 7.Dikalov S, Landmesser U, Harrison DG. Geldanamycin leads to superoxide formation by enzymatic and non-enzymatic redox cycling. Implications for studies of Hsp90 and endothelial cell nitric-oxide synthase. J Biol Chem. 2002;277:25480–25485. doi: 10.1074/jbc.M203271200. [DOI] [PubMed] [Google Scholar]
- 8.Kang BH, et al. Regulation of tumor cell mitochondrial homeostasis by an organelle-specific Hsp90 chaperone network. Cell. 2007;131:257–270. doi: 10.1016/j.cell.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 9.Xie Q, et al. Geldanamycins exquisitely inhibit HGF/SF-mediated tumor cell invasion. Oncogene. 2005;24:3697–3707. doi: 10.1038/sj.onc.1208499. [DOI] [PubMed] [Google Scholar]
- 10.Webb CP, et al. The geldanamycins are potent inhibitors of the hepatocyte growth factor/scatter factor-met-urokinase plasminogen activator-plasmin proteolytic network. Cancer Res. 2000;60:342–349. [PubMed] [Google Scholar]
- 11.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: Essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M, Wagner G. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science. 2008;321:1206–1210. doi: 10.1126/science.1161302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ujwal R, et al. The crystal structure of mouse VDAC1 at 2.3 A resolution reveals mechanistic insights into metabolite gating. Proc Natl Acad Sci USA. 2008;105:17742–17747. doi: 10.1073/pnas.0809634105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shanmugavadivu B, Apell HJ, Meins T, Zeth K, Kleinschmidt JH. Correct folding of the beta-barrel of the human membrane protein VDAC requires a lipid bilayer. J Mol Biol. 2007;368:66–78. doi: 10.1016/j.jmb.2007.01.066. [DOI] [PubMed] [Google Scholar]
- 15.Ge J, et al. Design, synthesis, and biological evaluation of hydroquinone derivatives of 17-amino-17-demethoxygeldanamycin as potent, water-soluble inhibitors of Hsp90. J Med Chem. 2006;49:4606–4615. doi: 10.1021/jm0603116. [DOI] [PubMed] [Google Scholar]
- 16.Pavlov E, et al. The mitochondrial channel VDAC has a cation-selective open state. Biochim Biophys Acta. 2005;1710:96–102. doi: 10.1016/j.bbabio.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Rostovtseva TK, Kazemi N, Weinrich M, Bezrukov SM. Voltage gating of VDAC is regulated by nonlamellar lipids of mitochondrial membranes. J Biol Chem. 2006;281:37496–37506. doi: 10.1074/jbc.M602548200. [DOI] [PubMed] [Google Scholar]
- 18.Fontaine E, Ichas F, Bernardi P. A ubiquinone-binding site regulates the mitochondrial permeability transition pore. J Biol Chem. 1998;273:25734–25740. doi: 10.1074/jbc.273.40.25734. [DOI] [PubMed] [Google Scholar]
- 19.Martinucci S, Szabò I, Tombola F, Zoratti M. Ca2+-reversible inhibition of the mitochondrial megachannel by ubiquinone analogues. FEBS Lett. 2000;480:89–94. doi: 10.1016/s0014-5793(00)01911-6. [DOI] [PubMed] [Google Scholar]
- 20.Roe SM, et al. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem. 1999;42:260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 21.Carreras CW, Schirmer A, Zhong Z, Santi DV. Filter binding assay for the geldanamycin-heat shock protein 90 interaction. Anal Biochem. 2003;317:40–46. doi: 10.1016/s0003-2697(03)00060-5. [DOI] [PubMed] [Google Scholar]
- 22.Howes R, et al. A fluorescence polarization assay for inhibitors of Hsp90. Anal Biochem. 2006;350:202–213. doi: 10.1016/j.ab.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 23.Vriens J, Janssens A, Prenen J, Nilius B, Wondergem R. TRPV channels and modulation by hepatocyte growth factor/scatter factor in human hepatoblastoma (HepG2) cells. Cell Calcium. 2004;36:19–28. doi: 10.1016/j.ceca.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Wondergem R, Ecay TW, Mahieu F, Owsianik G, Nilius B. HGF/SF and menthol increase human glioblastoma cell calcium and migration. Biochem Biophys Res Commun. 2008;372:210–215. doi: 10.1016/j.bbrc.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 25.Gourlay CW, Ayscough KR. The actin cytoskeleton: A key regulator of apoptosis and ageing? Nature Rev. 2005;6:583–589. doi: 10.1038/nrm1682. [DOI] [PubMed] [Google Scholar]
- 26.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 27.Ross D. Quinone reductases multitasking in the metabolic world. Drug Metab Rev. 2004;36:639–654. doi: 10.1081/dmr-200033465. [DOI] [PubMed] [Google Scholar]
- 28.Green DR, Reed JC. Mitochondria and apoptosis . Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 29.Rostovtseva TK, Bezrukov SM. VDAC regulation: Role of cytosolic proteins and mitochondrial lipids. J Bioenerg Biomembr. 2008;40:163–170. doi: 10.1007/s10863-008-9145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsutsumi S, et al. Hsp90 charged-linker truncation reverses the functional consequences of weakened hydrophobic contacts in the N domain. Nat Struct Mol Biol. 2009;16:1141–1147. doi: 10.1038/nsmb.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel K, et al. Engineered biosynthesis of geldanamycin analogs for Hsp90 inhibition. Chem Biol. 2004;11:1625–1633. doi: 10.1016/j.chembiol.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Tian ZQ, et al. Synthesis and biological activities of novel 17-aminogeldanamycin derivatives. Bioorg Med Chem. 2004;12:5317–5329. doi: 10.1016/j.bmc.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 33.Le Brazidec JY, et al. Synthesis and biological evaluation of a new class of geldanamycin derivatives as potent inhibitors of Hsp90. J Med Chem. 2004;47:3865–3873. doi: 10.1021/jm0306125. [DOI] [PubMed] [Google Scholar]
- 34.Shen Y, et al. Geldanamycin derivative inhibition of HGF/SF-mediated Met tyrosine kinase receptor-dependent urokinase-plasminogen activation. Bioorg Med Chem. 2005;13:4960–4971. doi: 10.1016/j.bmc.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 35.Gilabert JA, Parekh AB. Respiring mitochondria determine the pattern of activation and inactivation of the store-operated Ca(2+) current I(CRAC) EMBO J. 2000;19:6401–6407. doi: 10.1093/emboj/19.23.6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilabert JA, Bakowski D, Parekh AB. Energized mitochondria increase the dynamic range over which inositol 1,4,5-trisphosphate activates store-operated calcium influx. EMBO J. 2001;20:2672–2679. doi: 10.1093/emboj/20.11.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei C, et al. Calcium flickers steer cell migration. Nature. 2009;457:901–905. doi: 10.1038/nature07577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Guennec JY, et al. Voltage-gated ion channels, new targets in anti-cancer research. Recent Patents Anticancer Drug Discov. 2007;2:189–202. doi: 10.2174/157489207782497244. [DOI] [PubMed] [Google Scholar]
- 39.Eccles SA, et al. NVP-AUY922: A novel heat shock protein 90 inhibitor active against xenograft tumor growth, angiogenesis, and metastasis. Cancer Res. 2008;68:2850–2860. doi: 10.1158/0008-5472.CAN-07-5256. [DOI] [PubMed] [Google Scholar]
- 40.Breinig M, et al. Targeting heat shock protein 90 with non-quinone inhibitors: A novel chemotherapeutic approach in human hepatocellular carcinoma. Hepatology. 2009;50:102–112. doi: 10.1002/hep.22912. [DOI] [PubMed] [Google Scholar]
- 41.Glaze ER, et al. Preclinical toxicity of a geldanamycin analog, 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG), in rats and dogs: Potential clinical relevance. Cancer Chemother Pharmacol. 2005;56:637–647. doi: 10.1007/s00280-005-1000-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.