Abstract
A fundamental aspect of climate change is the potential shifts in flowering phenology and pollen initiation associated with milder winters and warmer seasonal air temperature. Earlier floral anthesis has been suggested, in turn, to have a role in human disease by increasing time of exposure to pollen that causes allergic rhinitis and related asthma. However, earlier floral initiation does not necessarily alter the temporal duration of the pollen season, and, to date, no consistent continental trend in pollen season length has been demonstrated. Here we report that duration of the ragweed (Ambrosia spp.) pollen season has been increasing in recent decades as a function of latitude in North America. Latitudinal effects on increasing season length were associated primarily with a delay in first frost of the fall season and lengthening of the frost free period. Overall, these data indicate a significant increase in the length of the ragweed pollen season by as much as 13–27 d at latitudes above ~44°N since 1995. This is consistent with recent Intergovernmental Panel on Climate Change projections regarding enhanced warming as a function of latitude. If similar warming trends accompany long-term climate change, greater exposure times to seasonal allergens may occur with subsequent effects on public health.
Keywords: aerobiology, allergies, global warming
Allergic disorders represent an important group of chronic diseases in the United States, with estimated costs at approximately $21 billion per year (1). Aeroallergen exposure is associated with two principal allergic diseases: allergic rhinitis (hayfever) and asthma. For much of geographic North America, there are three distinct plant-based aeroallergen seasons; tree pollen in the spring; grass pollen in the early summer, and, weed pollen, including ragweed (Ambrosia spp.) in the summer and fall. Pollen from the genus Ambrosia which includes A. artemisiifolia (short or common ragweed), A. trifida (giant ragweed), A. psilostachya (western ragweed), and A. bidentata (lanceleaf ragweed) has long been acknowledged to be a significant cause of allergic disease (2). An extensive skin test survey demonstrated that at least 10% of the US population is ragweed sensitive; the prevalence of ragweed sensitivity among atopic individuals was 27% in two large case series (3, 4). It has been reported that Ambrosia may cause more seasonal allergic rhinitis than all other plants combined (5).
Although there is unequivocal evidence that the prevalence of allergic disease has increased in the United States and elsewhere during the last 30 y (6), the reasons for this increase are uncertain. One possibility is an overall increase in exposure to significant aeroallergens such as ragweed pollen. An increase in ragweed pollen exposure, in turn, may be due to a number of factors including anthropogenic land use and climate change, although the connection between aeroallergens and climate change remains elusive.
There are several potential mechanisms by which climate change might affect allergic disease. First, longer pollen seasons may increase the duration of human exposure to aeroallergens and may thus increase allergic sensitization. Second, longer pollen seasons may increase the duration of allergy symptoms in individuals with allergic disease. Finally, higher atmospheric pollen counts may increase the severity of allergic symptoms (6).
To evaluate actual exposure to ragweed over time, a series of temporal measurements of ragweed pollen production is being determined by members of the National Allergy Bureau of the American Academy of Allergy, Asthma and Immunology. Although at present almost all US counting stations associated with this monitoring network (7) use Burkard Samplers, other volumetric devices (e.g., Rotorod Sampler) and gravimetric methods (e.g., Durham Sampler) have been used in recent decades. Unfortunately, quantitative comparisons between these various sampling methods are not possible (8). This confounds some analyses involving climate change, pollen counts, and allergy epidemiology.
Longer pollen seasons have been suggested (9) based on previous reconstructions of phenology networks and analysis of anthropogenic warming. However, other long-term temporal studies investigating possible anthropogenic changes in aeroallergen load or seasonality have been inconclusive, with several studies indicating no consistent change in duration of a pollen season for a given location (10–13).
Prior struggles relating aeroallergen season length to climatic warming may reflect geographical variation. The Intergovernmental Panel on Climate Change (IPCC) assessments have emphasized that the current and projected increases in global warming are not uniform, and enhanced land-surface temperatures (relative to the global average) are more probable with poleward and altitudinal increases (14, 15). If this is true, then longer aeroallergen seasons associated with anthropogenic warming could reflect elevational or latitudinal changes and may not be indicative of a given location per se.
Results
For this study, we apply this hypothesis regarding the differential rise in global surface temperatures to ragweed pollen data obtained by the National Allergy Bureau in the United States and Aerobiology Research Laboratories in Canada. By evaluating locations across central North America, a region of high spatial and altitudinal coherence, we could test the effects of latitude on season length of aeroallergen production for ragweed in response to climate warming as projected by the IPCC.
The National Allergy Bureau has eight locations with at least 15 y of ragweed data ranging from a latitude of 30.63°N (Austin, TX) to 46.88°N (Fargo, ND) (Table 1). A software program developed by Texas A&M University (16) was used to locate the nearest US weather station to obtain daily temperatures that corresponded to the pollen record. Ragweed data from two additional sites in Canada (Winnipeg, 50.1°N; Saskatoon, 52.1°N) were obtained from Aerobiology Research Laboratories. Corresponding weather data for these latter sites was obtained from Environment Canada, National Climate Data and Information Archive (17).
Table 1.
Change in length (day of year, days) of ragweed pollen season as a function of latitude for National Allergy Bureau and Aerobiology Research Laboratories sites along a south–north latitudinal gradient
| Start |
End |
Start |
End |
||||
| Location | Latitude | Years of data | 1995 | 2009 | Change | ||
| Georgetown, TX | 30.63°N | 17 | 198 ± 7 | 320 ± 7 | 195 ± 7 | 313 ± 7 | −4 d |
| Oklahoma City, OK | 35.47°N | 19 | 212 ± 7 | 300 ± 10 | 227 ± 9 | 316 ± 15 | +1 d |
| Rogers, AR | 36.33°N | 15 | 231 ± 7 | 295 ± 8 | 227 ± 6 | 296 ± 8 | −3 d |
| Papillion, NE | 41.15°N | 21 | 212 ± 3 | 281 ± 6 | 208 ± 4 | 288 ± 10 | +11 d |
| Madison, WI | 43.00°N | 27 | 208 ± 2 | 272 ± 4 | 205 ± 3 | 281 ± 6 | +12 d |
| LaCrosse, WI | 43.80°N | 22 | 213 ± 3 | 271 ± 3 | 205 ± 5 | 276 ± 5 | +13 d* |
| Minneapolis, MN | 45.00°N | 19 | 208 ± 5 | 270 ± 6 | 206 ± 7 | 284 ± 7 | +16 d* |
| Fargo, ND | 46.88°N | 15 | 216 ± 4 | 252 ± 8 | 217 ± 4 | 269 ± 8 | +16 d* |
| Winnipeg, MB, Canada | 50.07°N | 16 | 207 ± 7 | 264 ± 6 | 197 ± 7 | 279 ± 7 | +25 d* |
| Saskatoon, SK, Canada | 52.07°N | 16 | 206 ± 12 | 250 ± 6 | 197 ± 13 | 268 ± 7 | +27 d* |
Years represent the number of years for which pollen data were available. Regression analysis was used to determine the “best-fit” line for all years for a given location. This analysis was then used to determine the start and end day of each year (±95% confidence interval) for the duration of the ragweed pollen season in 1995 and again in 2009.
*Significant increase in the length (days) of the ragweed pollen season.
Pollen counting stations along this south–north latitudinal transect from east Texas to Saskatoon extended ~2,200 km (Table 1). Although the number of years of collection data varied, comparisons were made for a common temporal period (from 1995 through 2009) for each location. Simple regressions (± 95% confidence intervals) were used to determine changes in the start and end dates of the ragweed season over this period for each location. There was a highly significant correlation between latitude and increase in the length (days) of the ragweed pollen season over the period from 1995 to 2009 (r2 = 0.95).
Seasonal changes in temperature, particularly the number of frost-free days and delays in the onset of the first fall frost were plotted for each location and compared with the duration of the ragweed pollen season for each location (Fig. 1). There was a clear increase in frost-free days and a temporal shift in the delay of fall frosts that were associated with an increase in the ragweed season length during the last two decades (Fig. 1). Other weather phenomena, most notably annual seasonal precipitation, did not change in any systematic fashion as a function of latitude, and no correlation was observed with pollen season length for this same time period (Fig S1).
Fig. 1.
Change in the length (days) of ragweed pollen season from 1995 to 2009 as a function of frost-free days, and delays in the time of first frost during the fall, for 10 central North American locations (eight in the United States and two in Canada) as a function of latitude. Data were determined as a function of simple regression for each location. Additional details are provided in text.
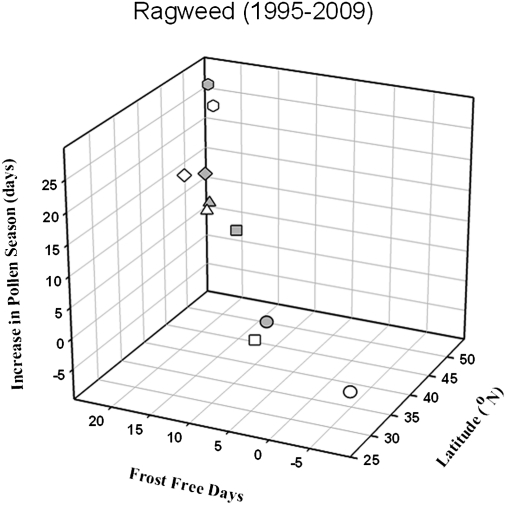
For each pollen collection location, latitude was compared with both the number of frost-free days and changes in the length of the ragweed pollen season (Fig. 2). These data demonstrate a clear correlation between frost-free days and ragweed pollen season as a function of latitude. This finding is consistent with both IPCC projections regarding climate impacts (14), and with greater shifts in the plant hardiness zones for the upper midwestern United States (18).
Fig. 2.
Change in the length (days) of ragweed pollen season as a function of frost-free days with latitude for the period 1995–2009. Data were determined as a function of simple regression for each location. Additional details are provided in text. Legend is the same as in Fig. 1.
Discussion
A number of studies have made compelling arguments that plant phenology is shifting in response to global environmental change (19). These shifts in timing of plant activity provide valuable confirmation that species as well as ecosystems are being affected by global change. However, a clear association between such shifts and aeroallergen exposure times has been unavailable.
Perhaps the most studied plant species in the context of earlier temperature shifts has been birch (Betula spp.), a known aeroallergen and cause of allergic disease in both North America and Europe. Emberlin (12, 20) observed earlier start dates for Betula by 6 d, but ranging up to 30 d. Yli-Panula et al. (21) demonstrated that warming temperatures contributed to early phenological development and greater pollen concentrations over a 31-y period for Betula in Turku, Finland, however no change in season length was reported. Research with Betula is complicated by differential responses among birch species to low winter temperatures (22), and often difficulties in distinguishing birch pollen from pollen of similar species (23). Although trees release aeroallergens during the spring, warmer winters may result in earlier flowering, or delays in flowering and floral numbers, depending on the tree species’ specific need for vernalization.
Multiyear pollen season analysis has also been determined in a few cases for other known aeroallergen species (10, 24, 25). Over a 21-y period, an analysis of 11 different plant taxa demonstrated that 71% of the taxa flowered earlier each year (10); however, no pollen type demonstrated any increase in season length. A recent Italian study (26) did report increased seasonal floral durations and pollen counts for Parietaria (prob. judaica) as well as olive and cypress, but only for western Liguria (approximately) 47°N. It is unclear whether this increase is a result of greater relative impact of warming at this latitude or of urbanization per se (27).
Outside of anthropogenic changes related to land use, for example, the importation or destruction of tree species due to changing architectural and landscape preferences (28), it has been thought that aeroallergen exposure times have remained consistent in relation to human activity (6). Because of its well-recognized association with allergic disease, a number of studies have demonstrated a probable role between climate change (i.e., rising CO2 and temperature), phenology, and pollen production of common ragweed (29–31). However, these links were established at the laboratory level (30) and as a function of urbanization (27). The current study illustrates, on a continental scale, a clear association between recent warming, and an increase in the duration of ragweed pollen season, a major aeroallergen. Furthermore, this finding regarding surface temperatures and allergy season length is consistent with the IPCC projections of disproportionate warming at higher latitudes (14).
To more accurately assess the intensity and duration of the pollen season in response to anthropogenic warming, standardized local pollen collection should be expanded. Pollen data, relevant meteorological variables, carbon dioxide concentrations, and local land use variables as well as clinical data could address this need, particularly in regard to health-relevant outcomes (32, 33). In this way, it will be possible to better determine the contribution of climate change on aeroallergen concentrations in the United States and the resultant public health impacts, and to derive appropriate scientific and policy solutions.
Materials and Methods
The American Academy of Allergy, Asthma and Immunology (AAAAI) administers the National Allergy Bureau (NAB; http://www.aaaai.org/nab/index.cfm), a network for monitoring clinically relevant outdoor aeroallergens in the United States. Composed primarily of physician's offices, volunteer members meet various quality standards for pollen sampling and counting proficiency. For the Aerobiology Research Laboratory sites in Canada, postgraduates are trained in pollen and spore identification using optical microscopes and a standardized, computer-aided counting methodology (http://www.aerobiology.ca/company/profile.php). All data used to determine pollen season length for ragweed was obtained by certified pollen counters at the stations listed in this study.
Counting stations were selected based on two criteria: geographic position along a South-North transect, and at least 15 y of ragweed pollen data. A search of pollen records among the NAB collection sites in the central United States indicated eight locations with 15+ y of data collection on site. These data were obtained directly for the location, or if available, supplemented from the American Academy of Allergy and Immunology, Aeroallergen Monitoring Network Pollen and Spore Reports that were published from 1965 through 1993 by the AAAAI. With the exception of Minneapolis, care was chosen to consider counting stations that were not near major metropolitan (i.e., +500,000) centers. Collection data were met, in part, by combining previously published reports by the American Academy of Allergy and Immunology, Aeroallergen Monitoring Network for Ambrosia pollen start and end dates (if available) as well as post 1993 data obtained from the same counting locations.
Two additional criteria were applied to ragweed pollen records based on plant physiological parameters: First, ragweed is a short-day plant, meaning that it will not flower before June 21st; as such, if pollen was recorded on or before this date, it was not considered; Second, any pollen reported for ragweed after average daily minimum temperatures fell at or below 0 °C were not recorded. This is because ragweed is frost sensitive and does not survive below this temperature (34). Pollen counts outside this range generally did not occur over the time period examined. Within these parameters, start and end dates of the pollen season were defined as the days of year when 1% and 99% of the cumulative season ragweed pollen total were reached.
A stepwise regression program (Statview; SAS Institute) was used to determine the best-fit regression line for each location with respect to pollen season, year, frost-free days, and day of year for first fall frost. Regressions of frost-free days, pollen season, and latitude were significant using a 3D mesh curve with Sigmaplot (version 10.0; SAS Institute). In this analysis predictive intervals were used to determine a 95% confidence limit for the start and end of ragweed pollen season for each location for a 15-y period from 1995 through 2009.
Weather data, including precipitation, was downloaded from the nearest available station that matched all years of pollen data collection as described here. Data were then examined to determine first and last days of the year when average daily minimum temperatures fell to 0 °C or below, and this interval was recorded as frost-free days. In addition, the day of year for the initial fall frost was documented. Precipitation indicated no consistent effect on pollen season with latitude (Fig. S1 and Dataset S1).
Supplementary Material
Acknowledgments
The authors thank Dennis Gebhard for acquisition of pollen records and Seyi Fayanju of the Environmental Defense Fund for comments. We are grateful to Ted Wilson at Texas A&M for his software program and Dr. Paul Beggs and Dr. Kris Ebi for their advice. We also thank Dr. Wayne Polley of US Department of Agriculture-Agricultural Research Service, and Dr. Stella Coakley of Oregon State University for reviewing the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014107108/-/DCSupplemental.
References
- 1.Centers for Disease Control and Prevention Allergies and Hayfever. 2005. Available at www.cdc.gov/nchs/fastabs/allergies.htm. Accessed February 3, 2011.
- 2.Frenz DA. Interpreting atmospheric pollen counts for use in clinical allergy: allergic symptomology. Ann Allergy Asthma Immunol. 2001;86:150–158. doi: 10.1016/S1081-1206(10)62683-X. [DOI] [PubMed] [Google Scholar]
- 3.Chapman JA. Aeroallergens of southeastern Missouri. Grana. 1986;25:235–246. [Google Scholar]
- 4.Gergen PJ, Turkeltaub PC, Kovar MG. The prevalence of allergic skin test reactivity to eight common aeroallergens in the U.S. population: results from the second National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 1987;80:669–679. doi: 10.1016/0091-6749(87)90286-7. [DOI] [PubMed] [Google Scholar]
- 5.Wodehouse RP. Hayfever Plants. 2nd Ed. New York: Hafner; 1971. [Google Scholar]
- 6.United States Environmental Protection Agency . Washington, DC: US EPA; 2008. A Review of the Impact of Climate Variability and Change in Aeroallergens and Their Associated Effects (Final Report) EPA/600/R-06/164F. [Google Scholar]
- 7.American Academy of Allergy, Asthma and Immunology Available at pollen.aaaai.org/nab/index.cfm. Accessed February 3, 2011.
- 8.Frenz DA. Comparing pollen and spore counts collected with the Rotorod Sampler and Burkard spore trap. Ann Allergy Asthma Immunol. 1999;83:341–347. doi: 10.1016/S1081-1206(10)62828-1. quiz 348–349. [DOI] [PubMed] [Google Scholar]
- 9.Beggs PJ. Impacts of climate change on aeroallergens: Past and future. Clin Exp Allergy. 2004;34:1507–1513. doi: 10.1111/j.1365-2222.2004.02061.x. [DOI] [PubMed] [Google Scholar]
- 10.Clot B. Trends in airborne pollen: An overview of 21 years of data in Neuchatel (Switzerland) Aerobiologia. 2003;19:227–234. [Google Scholar]
- 11.Dvorin DJ, Lee JJ, Belecanech GA, Goldstein MF, Dunsky EH. A comparative, volumetric survey of airborne pollen in Philadelphia, Pennsylvania (1991-1997) and Cherry Hill, New Jersey (1995-1997) Ann Allergy Asthma Immunol. 2001;87:394–404. doi: 10.1016/S1081-1206(10)62921-3. [DOI] [PubMed] [Google Scholar]
- 12.Emberlin J, et al. Responses in the start of Betula (birch) pollen seasons to recent changes in spring temperatures across Europe. Int J Biometeorol. 2002;46:159–170. doi: 10.1007/s00484-002-0139-x. [DOI] [PubMed] [Google Scholar]
- 13.Kosisky SE, Carpenter GB. Predominant tree aeroallergens of the Washington, DC area: A six year survey (1989-1994) Ann Allergy Asthma Immunol. 1997;78:381–392. doi: 10.1016/S1081-1206(10)63200-0. [DOI] [PubMed] [Google Scholar]
- 14.Intergovernmental Panel on Climate Change . Summary for policymakers. In: Solomon S, et al., editors; Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- 15.Hansen J, et al. Global temperature change. Proc Natl Acad Sci USA. 2006;103:14288–14293. doi: 10.1073/pnas.0606291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Wilson LT, Wang J. Development of an automated climatic data scraping, filtering and display system. Comp Electron Ag. 2010;71:77–87. [Google Scholar]
- 17.Environment Canada 2011. National Climate Data and Information Archive, www.climate.weatheroffice.gc.ca/prods_servs/index_e.html#cdcd. Accessed February 3, 2011.
- 18.Arbor Day Foundation 2006. Zone changes: USDA plant hardiness zones. www.arborday.org/media/mapchanges.cfm. Accessed February 3, 2011.
- 19.Cleland EE, Chuine I, Menzel A, Mooney HA, Schwartz MD. Shifting plant phenology in response to global change. Trends Ecol Evol. 2007;22:357–365. doi: 10.1016/j.tree.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Emberlin J, et al. The trend to earlier birch pollen seasons in the U.K.: A biotic response to changes in weather conditions? Grana. 1997;36:29–33. [Google Scholar]
- 21.Yli-Panula E, Fekedulegn DB, Green BJ, Ranta H. Analysis of airborne betula pollen in Finland; a 31-year perspective. Int J Environ Res Public Health. 2009;6:1706–1723. doi: 10.3390/ijerph6061706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller-Rushing AJ, Primack RB. Effects of winter temperatures on two birch (Betula) species. Tree Physiol. 2008;28:659–664. doi: 10.1093/treephys/28.4.659. [DOI] [PubMed] [Google Scholar]
- 23.Dahl A, Strandhede S-O. Predicting the intensity of the Birch pollen season. Aerobiol. 1996;12:97–106. [Google Scholar]
- 24.Emberlin J. The effects of patterns in climate and pollen abundance on allergy. Allergy. 1994;49(18, Suppl):15–20. doi: 10.1111/j.1398-9995.1994.tb04233.x. [DOI] [PubMed] [Google Scholar]
- 25.Van Vilet AJH, et al. The influence of temperature and climate change on the timing of pollen release in the Netherlands. Int J Climatol. 2002;22:1757–1767. [Google Scholar]
- 26.Ariano R, Canonica GW, Passalacqua G. Possible role of climate changes in variations in pollen seasons and allergic sensitizations during 27 years. Ann Allergy Asthma Immunol. 2010;104:215–222. doi: 10.1016/j.anai.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Ziska LH, et al. Cities as harbingers of climate change: Common ragweed, urbanization, and public health. J Allergy Clin Immunol. 2003;111:290–295. doi: 10.1067/mai.2003.53. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Rajo F, Diego F-S, Alicja S, Victoria J. Assessments between pollen seasons in areas with different urbanization level related to local vegetation sources and differences in allergen exposure. Aerobiologia. 2010;26:1–14. [Google Scholar]
- 29.Wayne P, Foster S, Connolly J, Bazzaz FA, Epstein PR. Production of allergenic pollen by ragweed (Ambrosia artemisiifolia L.) is increased in CO2-enriched atmospheres. Ann Allergy Asthma Immunol. 2002;88:279–282. doi: 10.1016/S1081-1206(10)62009-1. [DOI] [PubMed] [Google Scholar]
- 30.Ziska LH, Caulfield FA. Rising carbon dioxide and pollen production of common ragweed, a known allergy-inducing species: Implications for public health. Aust J Plant Physiol. 2000;27:893–898. [Google Scholar]
- 31.Rogers CA, et al. Interaction of the onset of spring and elevated atmospheric CO2 on ragweed (Ambrosia artemisiifolia L.) pollen production. Environ Health Perspect. 2006;114:865–869. doi: 10.1289/ehp.8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shea KM, Truckner RT, Weber RW, Peden DB. Climate change and allergic disease. J Allergy Clin Immunol. 2008;122:443–453. doi: 10.1016/j.jaci.2008.06.032. quiz 454–455. [DOI] [PubMed] [Google Scholar]
- 33.Levetin E, Van de Water P. Changing pollen types/concentrations/distribution in the United States: fact or fiction? Curr Allergy Asthma Rep. 2008;8:418–424. doi: 10.1007/s11882-008-0081-z. [DOI] [PubMed] [Google Scholar]
- 34.Deen W, Hunt LA, Swanton CJ. Photothermal time describes common ragweed (Ambrosia artemisiifolia L.) phenological development and growth. Weed Sci. 1998;46:561–568. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.