Abstract
Although some DNA methylation patterns are altered by steroid hormone exposure in the developing brain, less is known about how changes in steroid hormone levels influence DNA methylation patterns in the adult brain. Steroid hormones act in the adult brain to regulate gene expression. Specifically, the expression of the socially relevant peptide vasopressin (AVP) within the bed nucleus of the stria terminalis (BST) of adult brain is dependent upon testosterone exposure. Castration dramatically reduces and testosterone replacement restores AVP expression within the BST. As decreases in mRNA expression are associated with increases in DNA promoter methylation, we explored the hypothesis that AVP expression in the adult brain is maintained through sustained epigenetic modifications of the AVP gene promoter. We find that castration of adult male rats resulted in decreased AVP mRNA expression and increased methylation of specific CpG sites within the AVP promoter in the BST. Similarly, castration significantly increased estrogen receptor α (ERα) mRNA expression and decreased ERα promoter methylation within the BST. These changes were prevented by testosterone replacement. This suggests that the DNA promoter methylation status of some steroid responsive genes in the adult brain is actively maintained by the presence of circulating steroid hormones. The maintenance of methylated or demethylated states of some genes in the adult brain by the presence of steroid hormones may play a role in the homeostatic regulation of behaviorally relevant systems.
Epigenetic modification of chromatin (e.g., changes in DNA methylation status), by either steroid hormone exposure or changes in the social environment, can create changes in transcription rates of a number of genes within the developing brain, and in some cases these changes extend into adulthood (1–6). In adulthood, steroid hormones act to regulate the expression of steroid receptors as well as other factors in a fairly transient manner (7). Recent findings in vitro have shown that DNA methylation patterns on some genes cycle back and forth from methylated to unmethylated states within minutes (8, 9). This supports the emerging concept that not all methylation patterns are stable. Whether a change in methylation status plays a role in the regulation of gene expression in the adult brain is not well understood. We begin to elucidate this possibility by examining the hormonal maintenance of expression and DNA promoter methylation status of the socially relevant neuropeptide vasopressin (AVP) in the adult brain.
The extrahypothalamic AVP system in the rat brain is highly sexually dimorphic and steroid responsive (10). Adult male rats have two times more AVP-expressing cells in the bed nucleus of the stria terminals (BST) compared with females (11, 12). AVP expression within this area is dependent upon gonadal hormones, as castration results in a significant decrease in AVP mRNA and protein expression, and testosterone replacement restores AVP expression (13–15). The AVP cells within the BST express receptors for androgens (16), estrogens (17), and progestins (18). Although testosterone reinstates AVP expression in the BST following castration (10), it is essentially estradiol, a metabolite of testosterone, that is the major factor regulating AVP expression. Estradiol regulates AVP expression mainly by acting upon neuronal estrogen receptors. Interestingly, castration is known to increase estrogen receptor α (ERα) mRNA (14, 19). Therefore, castration decreases AVP but increases ERα expression. As ER activation by estradiol is known to down-regulate ERα expression (14, 19), it is not surprising that castration increases ERα due to lowered ERα activation. This suggests that testosterone inversely regulates these two genes in the adult male brain, and possibly within the same cell.
As AVP expression is exquisitely responsive to steroid hormones within the BST of the adult brain (14, 20), and as changes in DNA methylation patterns can influence gene expression, we hypothesized that the steroid dependence of AVP expression within the adult brain is maintained through epigenetic modification (i.e., DNA methylation) of the AVP promoter region. In additionally, we expect that ERα promoter methylation will follow a pattern opposite to that of AVP promoter methylation. Therefore, we assessed whether testosterone removal by castration differentially altered AVP and ERα promoter methylation patterns. The data here suggest that steroid hormones actively maintain DNA methylation patterns in the adult brain, and that changes in steroid hormone levels result in changes in mRNA expression and DNA methylation promoter patterns of AVP and ERα in the adult brain.
Results
Castration Effect on AVP mRNA Expression and Methylation.
AVP mRNA in BST.
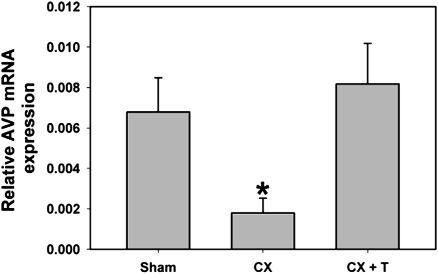
Our data replicate previous findings on testosterone regulation of AVP mRNA levels in adult male rats. That is, castration (CX) significantly reduces AVP expression within the BST of male rats compared with sham controls. We also confirmed that testosterone replacement (CX+T) can reverse this effect in adult male rats [F (2, 18) = 5.380, P = 0.015] (Fig. 1).
Fig. 1.
Castration effect on AVP mRNA in BST. Relative AVP mRNA expression within the BST in each treatment group. As expected, AVP is significantly reduced by CX and replacement with testosterone-filled capsules reinstates AVP expression in the BST (*P = 0.015; n ≥ 6). Error bars represent SEM.
AVP methylation in BST.
We then examined the methylation profile of the AVP promoter within the BST using two different methylation sensitive restriction enzymes (MSREs) based on the specific CpG sites bound by these enzymes on the AVP promoter (Fig. 2, gray-shaded bases in each sequence). Using four different primer sets, we were able to target four individual CpG sites on the AVP promoter. We used the HpaII enzyme to bind three distinct CCGG sequences on the AVP promoter. For the first and third CCGG sequence that we targeted, located at bases 1392 and 3895, using AVP promoter primer-targeted CpG site 1 and 3, respectively, we did not detect any change in relative methylation in any of the groups [F (2, 26) = 0.0987, P = 0.906; Figs. 2A and 3A; F (2, 26) = 0.251, P = 0.780; Figs. 2C and 3C]. With the second CCGG sequence that we targeted, located at base 2243, using AVP promoter primer-targeted CpG site 2, we found that our treatment had a significant effect on methylation status. Methylation at this site was significantly increased in response to long-term castration, and returned to control levels in animals that were castrated and 2 wk later treated with testosterone [F (2, 24) = 4.681, P = 0.019; Figs. 2B and 3B]. Using the BstUI enzyme, which binds to a CGCG sequence at base 4970 on the AVP promoter, we found a similar methylation pattern to the second CCGG site. Using AVP promoter primer-targeted CpG site 4, we found that relative methylation was significantly increased following castration, and importantly, this increase was prevented by replacement with testosterone 2 wk after castration [F (2, 25)=17.26, P ≤ 0.001; Figs. 2D and 3D]. Increased methylation of the AVP promoter region is associated with decreased AVP mRNA levels.
Fig. 2.
Primer sequences used to assess relative methylation levels. For all primers forward and reverse primers are underlined, targeted CpG sites are highlighted and bolded. Base numbering from GenBank accession no. AF112363.1. HpaII restriction enzyme was used to bind highlighted CCGG site encompassed by primers in A–C. BstUI restriction enzyme was used to bind highlighted CGCG site encompassed by primer in D. (A) AVP promoter primer-targeted CpG site 1. (B) AVP promoter primer-targeted CpG site 2. (C) AVP promoter primer-targeted CpG site 3. (D) AVP promoter primer- targeted CpG site 4. This CGCG site is near a number of transcriptional regulators. Bases in bold lower-case type signify an ERE-half site (*ggtca*), GRE/PRE (*tgtcacaactgtcct*), and CRE (*tcccgtca*). (E) Forward and reverse primers for ERα promoter region are underlined; and the CGCG site at which the BstUI restriction enzyme binds is highlighted and bold. Base numbering from GenBank accession no. NM_012689.1.
Fig. 3.
Effects of CX and testosterone replacement on methylation of the AVP gene promoter in BST. (A) Relative methylation a region of the AVP promoter. Real-time PCR using primer, AVP promoter target site 1, did not reveal any difference in relative methylation levels between any of the treatment groups. (*P = 0.906; n ≥ 9). (B) Primer that encompassed the second target site did reveal a difference between groups, with relative methylation at low levels in control animals (Sham), relative methylation levels increasing with testosterone removal (CX), and relative methylation levels returning back to control levels when hormones were replaced (CX+T) (*P = 0.019; n ≥ 7). (C) Much like the methylation profile using the primer to target site 1, with the AVP promoter primer that encompassed a different target site, target site 3, no differences in relative methylation levels between groups were observed (*P = 0.780; n ≥ 9). (D) With the primer, AVP promoter-target site 4, robust differences in relative methylation between the controls (Sham), castrated (CX), and CX animals replaced with testosterone (CX+T) were observed. Relative methylation levels appeared to be low in control animals, but removal of hormones caused these levels to increasel replacement with hormones caused a decrease in relative methylation levels, which could be suggestive of possible demethylase activity increasing with hormonal replacement (*P < 0.001; n ≥ 8). Error bars represent SEM.
AVP mRNA in paraventricular nucleus of hypothalamus (PVN), and AVP methylation in PVN.
As AVP in the BST is highly steroid responsive, we examined AVP mRNA in another brain area that is less steroid responsive than the BST. We chose to examine AVP expression within the PVN; in this area, there was no statistically significant effect of castration or testosterone replacement on AVP mRNA (P > 0.05).
As expected from the mRNA data, we did not find any significant changes in methylation profiles of the AVP gene (P > 0.05; Fig, S1). We used the same restriction enzymes in the PVN as we did in the BST, and we also used the same primer sets corresponding to each enzyme cut site. There was no difference in the methylation status of the AVP gene in the PVN using primers for AVP promoter primer-targeted CpG site 1, 2, 3, or 4 (P > 0.05 for each primer set).
Castration Effect on ERα mRNA Expression and Methylation.
ERα mRNA in BST.
Previous studies have reported that castration of male rats, results in a significant increase in ER mRNA levels within the BST (19). Also, treatment with testosterone (21) or estradiol (19) reduces ER mRNA expression in the hypothalamus and BST, respectively. Our data replicate these previous findings. Castration results in significantly higher levels of ERα mRNA expression within the BST. Treatment with testosterone 2 wk after castration lowers ERα mRNA expression back to control levels [F (2, 25) = 4.683, P = 0.019) (Fig. 4A)].
Fig. 4.
Castration (CX) effects on ERα expression and methylation in the BST. (A) Relative expression of ERα mRNA is significantly increased by CX and reduced with testosterone (CX+T) treatment (*P = 0.019; n ≥ 8). (B) Relative methylation of ERα promoter. Methylation of ERα promoter mirrors pattern observed in mRNA levels. CX reduces methylation, and testosterone replacement reinstates methylation to sham CX levels (*P = 0.028; n ≥ 9). Error bars represent SEM.
ERα methylation in BST.
Methylation of the ERα promoter was examined using the MSRE BstUI. The BstUI enzyme binds to a CGCG site, located at base 2215, on the ERα promoter that is near the Stat 5 binding region. As opposed to the increase in methylation we observed for the AVP promoter, we found in the same samples that castration decreased ERα promoter methylation. This decrease in ERα promoter methylation was prevented in animals by testosterone replacement 2 wk after castration [F (2, 24) = 4.14, P = 0.028; Fig. 4B for primer sequence, and Fig. 2E]. The decrease in CGCG ERα promoter methylation following castration was associated with increased ERα mRNA levels.
ERα mRNA in PVN, and ERα methylation in PVN.
In contrast to the changes in ERα mRNA levels and ERα promoter methylation within the BST following castration and testosterone replacement, we did not detect any statically significant changes in ERα mRNA levels or ERα promoter methylation within the PVN (P > 0.05).
Discussion
We report that the methylation status of some CpG sites on the AVP gene promoter within the BST can be altered in the adult brain in response to changes in testosterone levels. Specifically, testosterone withdrawal following castration, which leads to a decrease in AVP expression (14), results in an increase in methylation of CpG sites within the AVP promoter. These data are in agreement with the repressive function of methylation on gene expression (22). The increase in methylation of the AVP gene in the BST can be prevented in castrated animals by testosterone replacement. This suggests that the methylation status of the AVP gene is maintained by the hormonal environment, and that changes in hormonal status lead to changes in AVP mRNA expression and methylation patterns. These data also support the emerging concept that, in some cases, DNA methylation patterns in the brain may not be stable, and further suggest that some methylation patterns may need to be actively maintained. There was no significant change in mRNA levels or methylation status of the AVP gene within the PVN following castration. As the AVP cells in the PVN are less steroid-responsive compared with AVP cells in the BST, and that the PVN is not rich in ERα (23), the lack of hormone responsiveness in AVP mRNA or promoter methylation status is not surprising. However, this suggests that steroid hormone maintenance of AVP promoter methylation patterns and expression is region specific in the adult brain.
We also report that ERα is epigenetically regulated by castration in the adult brain; however the pattern is opposite to that of AVP. Previous studies show that gonadectomy results in increased ER expression and hormone treatment decreases ERα expression (19, 21, 24, 25), as activation of ER leads to a subtle down-regulation of its own receptor. We extend these findings to show that the increase in ERα mRNA expression in response to adult castration corresponds to a reduction in ERα promoter methylation, supporting previous findings indicating that ERα promoter methylation correlates inversely with changes in ERα mRNA levels (2, 3, 26). Interestingly, the directional change in promoter methylation of AVP and ERα are opposing, as castration increases AVP promoter methylation and decreases ERα promoter methylation, and testosterone replacement prevents these effects. While ERα is coexpressed in AVP cells, and is an important regulator of AVP expression, it is unknown if ERα plays a direct role in AVP promoter methylation. Nevertheless, this suggests that testosterone can act to either increase or decrease gene expression within the same brain region and potentially within the same cell by altering DNA methylation patterns.
We recently suggested a model by which the same hormone signal can have opposing outcomes on the epigenetic regulation of gene expression during development to program juvenile and adult behavior (27). We now report a bidirectionality in the methylation status of the ERα and AVP gene promoters in response to changes in gonadal hormones in adulthood. When the major source of testosterone is removed, the methylation and gene expression profile of both these genes are reversed. It is intriguing that hormonal withdrawal can elicit methylation and demethylation of these steroid responsive genes. As to how this occurs is unclear; however, it may suggest that different methylating or demethylating factors are being directed to ERα promoter regions versus those being directed to AVP promoter regions in response to changes in steroid hormone levels. Alternatively, the activity of the same methylating or demethylating factor may be increased or decreased at the different promoter regions by steroid hormones. It remains to be elucidated how this specificity in promoter targeting is regulated. While studies have identified several methylating factors within the brain, it is less clear how demethylation occurs within the brain.
Previous studies have suggested that transitions of histone acetylation and decactylation as well as DNA methylation and demethylation states are important for gene expression as well as for physiological function (28, 29). Indeed, recent studies report that memory enhancing genes must be turned on and memory inhibiting genes must be turned off for proper memory consolidation to occur in adult brain (30). Our current data suggest that the presence of testosterone actively maintains the promoter methylation status of AVP and ERα in the adult male brain, and that testosterone withdrawal leads to increased methylation of the AVP promoter and demethylation of the ERα promoter. Therefore, the maintenance of methylation patterns by the presence of testosterone may be part of a potential homeostatic mechanism by which testosterone sustains gene specific expression profiles and subsequent behavior. As decreases in testosterone and AVP expression occur with normal aging in male rats (31), it is possible that decreases in testosterone levels also coincide with altered DNA methylation profiles during aging.
Although the number of AVP cells is a small minority of all cells within the BST, AVP expression is highly regulated by steroid hormones (13–15) and therefore shows dramatic changes in mRNA levels and promoter methylation in response to changes in steroid hormones. In contrast, steroid hormones appear to have a less dramatic impact on ERα mRNA levels and promoter methylation relative to the large population of ERα cells within the BST. As castration results in a near elimination of AVP within the BST, it is possible that most AVP cells in this area undergo a change in promoter methylation and a near silencing of that gene. As castration results in less dramatic changes in ERα levels (19), changes in ERα promoter methylation in response to castration may occur in a subset of ERα cells or on CpG sites that only decrease ERα transcription rates. These data suggest a difference in the homeostatic maintenance of DNA promoter methylation of AVP versus ERα in response to a decline in hormone levels.
It is important to note that the locations of the CpG sites being altered are near transcriptional response elements within the AVP promoter region. For example, changes in AVP promoter methylation were confirmed in two of the four CpG sites that we targeted. One of these sites is located near a number of binding sites for transcriptional regulators (i.e., response elements for CREB, glucocorticoid/progestin receptors, and an estrogen response element half site) within the AVP promoter (Fig. 2D) (32, 33). Although the consequences of methylation on gene expression are being investigated (34), it is possible that methylation at this site would have a higher probability of suppressing AVP gene transcription, as methylation at this site could reduce the accessibility of the DNA to several transcription factors.
Much of the study of epigenetic phenomena in the mammalian systems focus on methylation events that occur early on in development, and have lasting effects well into adulthood [i.e., early-life stress (35) and sexual differentiation (6, 27, 36)]. Indeed, early-life stress in mouse pups results in an increased expression of AVP in the parvocellular cells of the PVN. This up-regulation of AVP mRNA is associated with hypomethylation of the AVP promoter, an effect that is likely achieved through phosphorylation of methyl CpG-binding protein 2 (MeCP2) (5). This suggests that the epigenetic control of AVP cells in the PVN is at least sensitive to stress hormones during early development, it is unclear if stress hormones alter DNA methylation patterns in adulthood within these cells. Other examples of long term epigenetic modification of chromatin also come from studies examining sexual differentiation of the brain. Indeed, sexual differentiation of the BST in mice appears to require histone acetylation, as inhibiting histone acetylation blocks the masculinization of the BST, which is typically larger in males contrasted to females (37). Furthermore, sexually dimorphic ERα promoter methylation appears to be partly responsible for the sexually dimorphic expression of this receptor (2, 3). Although DNA methylation patterns may change during the perinatal period, methylation of some genes in the brain is considered a somewhat stable event that may require maintenance throughout the life span. It is assumed that the maintenance of some methylation patterns occur through relatively passive processes; however, our data suggest that in the adult brain, steroid hormones play an active role in maintaining methylation profiles.
In conclusion, the present study suggests a homeostatic nature of AVP and ERα promoter methylation patterns that are maintained by steroid hormones in the adult brain, and that reducing this signal leads to altered DNA methylation patterns of these two steroid responsive genes in opposing directions. This indicates that steroid hormones actively maintain the methylation or demethylation status of these two genes.
Materials and Methods
Animals and Treatment.
Adult male Sprague–Dawley rats were obtained from our breeding colony and group housed (usually two animals per cage) until sacrifice. At ∼3 mo of age, all animals underwent surgery. We assessed if castration altered AVP promoter methylation in 3 groups of adult male rats. One (n = 10) was castrated (CX) for 5 wk until sacrifice, another (n = 9) was sham castrated for the same amount of time, and a third (n = 11) was castrated for 2 wk and then implanted with testosterone-filled Silastic capsules (Dow Corning; 2.5 cm long, 1.5 mm i.d., 2.4 mm o.d; CX+T) for 3 wk before animals were killed (Fig. 1A). All animals were killed at the same time following the initial surgery.
Tissue Processing.
Brains were collected, snap frozen, and sectioned at 250μm using a cryostat at around -10 °C. Micropunches were taken from the appropriate sections for BST and PVN, rapidly refrozen and stored at -80 °C. BST punches were taken from an area of the BST covered by AVP expressing cells (18). These areas encompass portions of the lateral and medial portions of BST, ventral to the stria terminalis (plate 21 for the BST and plate 25 for the PVN in the atlas of Paxinos and Watson(38)). DNA and RNA were extracted from each individual animal's micropunch using the AllPrep DNA/RNA Mini Kit (Qiagen). Individual samples were maintained throughout the experiment. Concentrations of DNA and RNA in each sample were measured using the Qubit quantification platform (Invitrogen).
Primers and qPCR for AVP and ERα mRNA.
Extracted RNA was converted to cDNA using the ImProm-II Reverse Transcription System (Promega U.S., Madison, WI). The relative levels of AVP and estrogen receptor α (ERα) mRNA in the samples was assessed using primers that were designed using the OligoPerfect tool (Invitrogen), and were purchased from Invitrogen or synthesized with standard purity by the Biotechnology center at the University of Wisconsin. Primers are as follows: AVP, accession number NM_016992.2, forward TGCCTGCTACTTCCAGAACTGC, reverse AGGGGAGACACTGTCTCAGCTC; ERα, accession number NM_012689.1, forward TCCGGCACATGAGTAACAAA, reverse TGAAGACGATGAGCATCCAG. Expression of HPRT was measured within the same samples and was used as a control. The Real-Time PCR protocol was as follows: an initial denaturing step at 95 °C for 25 min followed by 40–45 cycles of a 95 °C denaturing step for 15 s, a 55 °C annealing step for 30 s, and a 72 °C elongation step for 30 s. Relative mRNA levels were calculated using the 2-∆∆CT method (39).
Methylation-Sensitive Restriction Enzyme Assay.
Methylation of samples was assessed using the methylation sensitive restriction enzyme (MSRE) process (40). This method uses a restriction enzyme that will bind a specific CpG site and cut DNA unless it has been methylated. Therefore, designing primers surrounding the targeted CpG site will produce a PCR product only if that site has been methylated. This is a powerful technique as it can assess the relative methylation of all DNA in isolated from the tissue sample. To examine DNA methylation, 2 μg of DNA from each rat was equally divided into two tubes: an enzyme treated + buffer and a no-enzyme + buffer control tube. These tubes were then processed using the same primers surrounding the targeted CpG site with the no-enzyme control serving as the normalizer control during Real-Time PCR. Of the two enzymes used in this study, HpaII cleaves unmethylated DNA at any CCGG sequence and BstUI cleaves unmethylated DNA at any CGCG sequence. For examining methylation at CCGG sites, 1 μg of DNA from each animal was diluted in 1 μl of NEB buffer 1 in separate tubes, and was incubated at 37 °C for three hours with 2 μl of HpaII restriction enzyme (New England Biolabs, Ipswich, MA). For examining methylation at CGCG sites, 1 μg of DNA from each animal was diluted in 1 μl of buffer R in separate tubes, and was incubated at 37 °C for one hour with 1 μl of BstUI restriction enzyme (Fermentas Inc., Glen Burnie, MD). No-enzyme controls for each sample were also run, as well as no-DNA with enzyme and no-DNA and no-enzyme controls. Omission of DNA resulted in no PCR product. HpaII and BstUI were inactivated by incubation at 65 °C for 20 min.
To assess relative levels of methylation in the promoter regions of the AVP and ERα genes, all enzyme-treated and no-enzyme controls were subjected to Real-Time PCR. The primers were designed to encompass each one of three CCGG sites on the AVP gene promoter (Fig. 2A–C), and to encompass the CGCG site on the AVP gene promoter that is near a CRE binding site, a GRE and an ERE (Fig. 2D). Primers were also designed to amplify a portion encompassing a CGCG site near the Stat 5 binding region of the ERα gene promoter (2) (Fig. 2E). All primers were found to have efficiencies of near 100%, both dissociation curve and amplification plot analysis was used to confirm purity of products. Real-Time PCR was optimized and conducted using the Stratagene Mx3000P system (Cedar Creek) with primers designed using the OligoPerfect tool (Invitrogen) and purchased from Invitrogen or synthesized with standard purity by the Biotechnology Center at the University of Wisconsin. Real-time PCR was carried out as described previously (41). The following procedure was used for amplification of DNA samples: an initial denaturing step at 95 °C for 25 min followed by 40–45 cycles of a 95 °C denaturing step for 15 s, a 55 °C annealing step for 30 s, and a 72 °C elongation step for 30 s. Relative DNA levels were calculated using the 2-∆∆CT method (39). The ∆CT for each sample was determined by obtaining the difference between the average CT of the non enzyme-treated gene and the average CT of the enzyme-treated sample. The ∆CT of the calibrator was subtracted from the ∆CT of each of the samples to determine the ∆∆CT. This number was then used to determine the amount of DNA relative to the calibrator, or the n-fold difference. The n-fold difference was calculated by the equation 2-∆∆CT.
Statistical Analysis.
N-fold differences between the groups were compared by one-way ANOVA, using the statistical software package Sigma-Stat (Systat). ANOVAs that produced significant results were followed by analysis with the Student–Neuman–Keuls post hoc method. Significance was set at P < 0.05.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Mental Health Grant R01-MH072956 (to A.P.A.) and the Psychology Department and Graduate School at the University of Wisconsin-Madison.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100314108/-/DCSupplemental.
References
- 1.Weaver IC, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 2.Kurian JR, Olesen KM, Auger AP. Sex differences in epigenetic regulation of the estrogen receptor-alpha promoter within the developing preoptic area. Endocrinology. 2010;151:2297–2305. doi: 10.1210/en.2009-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Westberry JM, Trout AL, Wilson ME. Epigenetic regulation of estrogen receptor alpha gene expression in the mouse cortex during early postnatal development. Endocrinology. 2010;151:731–740. doi: 10.1210/en.2009-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwarz JM, Nugent BM, McCarthy MM. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology. 2010;151:4871–4881. doi: 10.1210/en.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murgatroyd C, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy MM, et al. The epigenetics of sex differences in the brain. J Neurosci. 2009;29:12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tetel MJ, Auger AP, Charlier TD. Who's in charge? Nuclear receptor coactivator and corepressor function in brain and behavior. Front Neuroendocrinol. 2009;30:328–342. doi: 10.1016/j.yfrne.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Métivier R, et al. Cyclical DNA methylation of a transcriptionally active promoter. Nature. 2008;452:45–50. doi: 10.1038/nature06544. [DOI] [PubMed] [Google Scholar]
- 9.Kangaspeska S, et al. Transient cyclical methylation of promoter DNA. Nature. 2008;452:112–115. doi: 10.1038/nature06640. [DOI] [PubMed] [Google Scholar]
- 10.De Vries GJ, Wang Z, Bullock NA, Numan S. Sex differences in the effects of testosterone and its metabolites on vasopressin messenger RNA levels in the bed nucleus of the stria terminalis of rats. J Neurosci. 1994;14:1789–1794. doi: 10.1523/JNEUROSCI.14-03-01789.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Leeuwen FW, Caffe AR, De Vries GJ. Vasopressin cells in the bed nucleus of the stria terminalis of the rat: Sex differences and the influence of androgens. Brain Res. 1985;325:391–394. doi: 10.1016/0006-8993(85)90348-8. [DOI] [PubMed] [Google Scholar]
- 12.Miller MA, Vician L, Clifton DK, Dorsa DM. Sex differences in vasopressin neurons in the bed nucleus of the stria terminalis by in situ hybridization. Peptides. 1989;10:615–619. doi: 10.1016/0196-9781(89)90152-6. [DOI] [PubMed] [Google Scholar]
- 13.Brot MD, De Vries GJ, Dorsa DM. Local implants of testosterone metabolites regulate vasopressin mRNA in sexually dimorphic nuclei of the rat brain. Peptides. 1993;14:933–940. doi: 10.1016/0196-9781(93)90069-s. [DOI] [PubMed] [Google Scholar]
- 14.DeVries GJ, Buijs RM, Van Leeuwen FW, Caffé AR, Swaab DF. The vasopressinergic innervation of the brain in normal and castrated rats. J Comp Neurol. 1985;233:236–254. doi: 10.1002/cne.902330206. [DOI] [PubMed] [Google Scholar]
- 15.Miller MA, Urban JH, Dorsa DM. Steroid dependency of vasopressin neurons in the bed nucleus of the stria terminalis by in situ hybridization. Endocrinology. 1989;125:2335–2340. doi: 10.1210/endo-125-5-2335. [DOI] [PubMed] [Google Scholar]
- 16.Zhou L, Blaustein JD, De Vries GJ. Distribution of androgen receptor immunoreactivity in vasopressin- and oxytocin-immunoreactive neurons in the male rat brain. Endocrinology. 1994;134:2622–2627. doi: 10.1210/endo.134.6.8194487. [DOI] [PubMed] [Google Scholar]
- 17.Axelson JF, Leeuwen FW. Differential localization of estrogen receptors in various vasopressin synthesizing nuclei of the rat brain. J Neuroendocrinol. 1990;2:209–216. doi: 10.1111/j.1365-2826.1990.tb00852.x. [DOI] [PubMed] [Google Scholar]
- 18.Auger CJ, De Vries GJ. Progestin receptor immunoreactivity within steroid-responsive vasopressin-immunoreactive cells in the male and female rat brain. J Neuroendocrinol. 2002;14:561–567. doi: 10.1046/j.1365-2826.2002.00809.x. [DOI] [PubMed] [Google Scholar]
- 19.Handa RJ, Kerr JE, DonCarlos LL, McGivern RF, Hejna G. Hormonal regulation of androgen receptor messenger RNA in the medial preoptic area of the male rat. Brain Res Mol Brain Res. 1996;39:57–67. doi: 10.1016/0169-328x(95)00353-t. [DOI] [PubMed] [Google Scholar]
- 20.Miller MA, DeVries GJ, al-Shamma HA, Dorsa DM. Decline of vasopressin immunoreactivity and mRNA levels in the bed nucleus of the stria terminalis following castration. J Neurosci. 1992;12:2881–2887. doi: 10.1523/JNEUROSCI.12-08-02881.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simerly RB, Young BJ. Regulation of estrogen receptor messenger ribonucleic acid in rat hypothalamus by sex steroid hormones. Mol Endocrinol. 1991;5:424–432. doi: 10.1210/mend-5-3-424. [DOI] [PubMed] [Google Scholar]
- 22.Razin A, Riggs AD. DNA methylation and gene function. Science. 1980;210:604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- 23.Shughrue PJ, Scrimo PJ, Merchenthaler I. Evidence for the colocalization of estrogen receptor-beta mRNA and estrogen receptor-alpha immunoreactivity in neurons of the rat forebrain. Endocrinology. 1998;139:5267–5270. doi: 10.1210/endo.139.12.6525. [DOI] [PubMed] [Google Scholar]
- 24.Meredith JM, Auger CJ, Blaustein JD. Down-regulation of estrogen receptor immunoreactivity by 17B-estradiol in the guinea pig forebrain. J Neuroendocrinol. 1994;6:639–648. doi: 10.1111/j.1365-2826.1994.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 25.Cintra A, et al. Rapid important paper on the cellular localization and distribution of estrogen receptors in the rat tel- and diencephalon using monoclonal antibodies to human estrogen receptor. Neurochem Int. 1986;8:587–595. doi: 10.1016/0197-0186(86)90196-8. [DOI] [PubMed] [Google Scholar]
- 26.Westberry JM, Prewitt AK, Wilson ME. Epigenetic regulation of the estrogen receptor alpha promoter in the cerebral cortex following ischemia in male and female rats. Neuroscience. 2008;152:982–989. doi: 10.1016/j.neuroscience.2008.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auger AP, Auger CJ. Epigenetic turn ons and turn offs: Chromatin reorganization and brain differentiation. Endocrinology. 2011;152:349–353. doi: 10.1210/en.2010-0793. [DOI] [PubMed] [Google Scholar]
- 28.Miller CA, Campbell SL, Sweatt JD. DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol Learn Mem. 2008;89:599–603. doi: 10.1016/j.nlm.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Métivier R, et al. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell. 2003;115:751–763. doi: 10.1016/s0092-8674(03)00934-6. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Z, Fan L, Frick KM. Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc Natl Acad Sci USA. 2010;107:5605–5610. doi: 10.1073/pnas.0910578107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fliers E, De Vries GJ, Swaab DF. Changes with aging in the vasopressin and oxytocin innervation of the rat brain. Brain Res. 1985;348:1–8. doi: 10.1016/0006-8993(85)90351-8. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro RA, Xu C, Dorsa DM. Differential transcriptional regulation of rat vasopressin gene expression by estrogen receptor alpha and beta. Endocrinology. 2000;141:4056–4064. doi: 10.1210/endo.141.11.7796. [DOI] [PubMed] [Google Scholar]
- 33.Mohr E, Richter D. Sequence analysis of the promoter region of the rat vasopressin gene. FEBS Lett. 1990;260:305–308. doi: 10.1016/0014-5793(90)80130-b. [DOI] [PubMed] [Google Scholar]
- 34.Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- 35.Murgatroyd C, Wu Y, Bockmühl Y, Spengler D. Genes learn from stress: How infantile trauma programs us for depression. Epigenetics. 2010;5:194–199. doi: 10.4161/epi.5.3.11375. [DOI] [PubMed] [Google Scholar]
- 36.Auger AP, Jessen HM, Edelmann MN. Epigenetic organization of brain sex differences and juvenile social play behavior. Horm Behav. 2010. 10.1016/j.yhbeh.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray EK, Hien A, de Vries GJ, Forger NG. Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology. 2009;150:4241–4247. doi: 10.1210/en.2009-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Orlando: Academic Press; 1986. [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Hashimoto K, Kokubun S, Itoi E, Roach HI. Improved quantification of DNA methylation using methylation-sensitive restriction enzymes and real-time PCR. Epigenetics. 2007;2:86–91. doi: 10.4161/epi.2.2.4203. [DOI] [PubMed] [Google Scholar]
- 41.Auger CJ, Jessen HM, Auger AP. Microarray profiling of gene expression patterns in adult male rat brain following acute progesterone treatment. Brain Res. 2006;1067:58–66. doi: 10.1016/j.brainres.2005.10.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.