Abstract
A wide variety of human cancers are associated with injury. Although stem cells participate in tissue regeneration after wounding, it is unclear whether these cells also contribute to epithelial tumors. Human basal cell carcinomas (BCCs) are associated with misactivation of Hedgehog (Hh) signaling, commonly through acquisition of mutations in Smoothened (Smo). We have found that expression of an activated form of Smo by stem cells of the hair-follicle bulge and secondary hair germ does not induce robust Hh signaling or produce BCCs. However, wounding recruits these cells from the follicle to the wound site, where downstream Hh signal transduction is derepressed, giving rise to superficial BCC-like tumors. These findings demonstrate that BCC-like tumors can originate from follicular stem cells and provide an explanation for the association between wounding and tumorigenesis.
Keywords: skin cancer, wound healing, carcinogenesis, reepithelialization
Wound repair is a complicated process involving inflammation, cell migration, proliferation, and tissue remodeling (1–3). Aberrant wound healing with chronic inflammation can promote malignant transformation, and a variety of human cancers, including those arising from the lung, liver, pancreas, bone, and skin, are associated with injury (2, 4). Epithelial stem cells are thought to play critical roles during wound repair, but, under homeostatic conditions, these cells are maintained in quiescent niches that preserve their ability to self-renew and give rise to differentiated progeny (5, 6). Whether stem cells are the cell of origin for most cancers is currently unclear.
Discrete stem-cell populations maintain the hair follicle and interfollicular epidermis (IFE) (7). Basal layer epidermal stem cells replenish the overlying stratified epidermis (6, 8), whereas stem cells located in the follicular bulge and secondary hair germ (SHG) regenerate the lower hair follicle and hair shaft during anagen (9–13). Upon cutaneous wounding, bulge-derived cells migrate into the epidermis, where they lose expression of follicle-specific genes and adopt an epidermal-like phenotype (14, 15). However, the majority of these cells contribute only transiently to the healed wound epithelium (14).
Human basal cell carcinomas (BCCs) are thought to arise from the hair follicle. Indeed, BCCs share certain properties of follicular stem cells, as these tumors are often composed of cells that are slow-growing, poorly differentiated, and, in some cases, capable of differentiating into adnexal structures (16). Recent studies, however, have suggested that cells in the bulge and SHG do not give rise to BCCs, and that these tumors originate instead from the IFE and follicular infundibulum (17). Given that follicular stem cells enter the IFE upon wounding, we examined whether oncogene-expressing bulge and SHG-derived cells also enter sites of injury to form BCCs.
Results
Misactivation of Hh signaling is the underlying cause of BCC (18–22), and mutations in Smo, a central mediator of the Hh pathway, are commonly found in these tumors (23). To assess whether activating the Hh pathway in follicular stem cells causes BCCs, we generated mice that express a conditional oncogenic allele of Smo previously isolated from human BCCs (SmoM2) (24) under the control of an RU486-inducible Cre recombinase driven by the Keratin-15 promoter (K15-CrePR1, hereafter referred to as K15:SmoM2) (25). Keratin-15 is expressed postnatally in the bulge and SHG, locations where follicular stem cells reside (25). We induced SmoM2 expression in telogen skin at 7.5 wk of age and assessed tumor formation 11 wk later. Similar to previously reported results (17), we observed that K15:SmoM2 skin developed few tumors (Fig. 1A). Most hair follicles remained in telogen and did not exhibit overt abnormalities, although occasional focal hyperplasias were detected in the bulge and SHG (Fig. 1B). Thus, follicular stem cells appear protected against tumorigenesis.
Fig. 1.
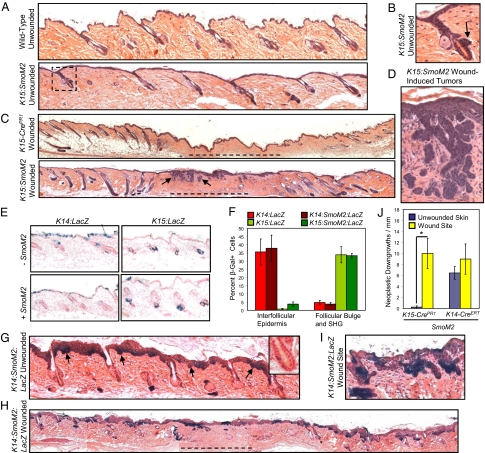
SmoM2-expressing bulge or SHG-derived cells form superficial BCC-like tumors at sites of injury. (A) Intact K15:SmoM2 dorsal skin, 11 wk after oncogene induction, displays largely normal IFE and telogen hair follicles, similar to wild-type skin. (B) Magnified view of the boxed area in A, showing a follicle with a focally hyperplastic bulge (arrow). (C) RU486-induced K15:SmoM2 skin, 10 wk after wounding, possesses superficial BCC-like tumors (arrows) at the site of the healed wound (dashed line). Control skin does not form tumors after wounding. (D) Magnified view of BCC-like tumors in C. (E) β-Gal staining indicating that K14-CreERT and K15-CrePR1 exhibit largely reciprocal domains of activity, irrespective of SmoM2 expression, 3 d post induction. (F) Quantitation of β-Gal+ cell location 3 d after induction. (G) Intact K14:SmoM2:LacZ dorsal skin, 11 wk after oncogene induction, contains extensive IFE hyperplasia and BCC-like downgrowths (arrows), without obvious involvement of the bulge (Inset). (H) K14:SmoM2:LacZ skin, 10 wk after wounding, exhibits BCC-like downgrowths equally abundant at the healed wound site (dashed line) and in intact skin. (I) Magnified view of tumors from wounded K14:SmoM2:LacZ skin. (J) Quantitation of BCC-like neoplastic downgrowths, either at the healed wound site or in intact skin (*P = 0.017). G–I were stained for LacZ expression. In non–LacZ-expressing control animals, no consistent β-Gal staining was detected (data not shown).
As expression of SmoM2 in the bulge and SHG did not lead to BCCs, we examined whether cutaneous injury can collaborate with oncogene expression to promote tumor formation. We induced SmoM2 expression as described above and, 3 d later, generated 0.25-cm2 full-thickness excisional wounds in the dorsal skin. In contrast to the paucity of tumors in unwounded K15:SmoM2 skin, clusters of superficial BCC-like tumors formed at the wound site (Fig. 1 C and D). These tumors were composed of nests of Keratin-5-, Keratin-14-, and Keratin-17-positive cells that radiated downward from the healed epidermis and exhibited high nuclear-to-cytoplasmic ratios and histopathological features of BCC, such as nuclear palisading (Fig. S1). The small size of the wound did not induce follicular neogenesis within the healed epithelium, although intact follicles adjacent to the wound reentered anagen and were hyperplastic (Fig. 1C and Fig. S1). In the absence of SmoM2 expression, wounding did not induce tumorigenesis (Fig. 1C). These findings indicate that wounding stimulates the formation of BCC-like lesions at sites of injury in K15:SmoM2 mice.
Unlike K15:SmoM2 skin, expression of SmoM2 using a tamoxifen-inducible Keratin-14 promoter-driven Cre recombinase (K14-CreERT) induces widespread formation of BCC-like tumors (17, 24, 26). To understand how K14-CreERT-driven expression of SmoM2 promotes tumorigenesis in intact skin, but K15-CrePR1 fails to do so, we examined the specificity of K14-CreERT, reported to be expressed in basal layer cells of the IFE and follicular outer root sheath (27). In tamoxifen-treated K14-CreERT mice harboring a Cre-inducible LacZ reporter (K14:LacZ), we observed efficient recombination in the IFE but surprisingly little activity in the bulge and SHG, irrespective of oncogene expression (Fig. 1 E and F and Fig. S2).
Given that the K15:SmoM2 wound-induced tumors formed outside of the bulge and SHG, we assessed whether K15-CrePR1 induces recombination outside of these domains by using mice possessing the Cre-inducible LacZ reporter (K15:LacZ). In agreement with previous results (25), we detected β-galactosidase-positive (β-Gal+) cells in RU486-induced K15:LacZ skin overwhelmingly in the bulge and SHG (Fig. 1 E and F and Fig. S2). In rare instances, we observed β-Gal+ cells in the IFE for both K15:LacZ and K15:SmoM2:LacZ skin (Fig. 1F). Therefore, K15-CrePR1 and K14-CreERT exhibit largely reciprocal domains of activity in the skin, inducing recombination primarily in cells of the bulge and SHG, or of the IFE, respectively.
Because K15-CrePR1-mediated recombination occurred in rare cells in the IFE, it is possible that these SmoM2-expressing IFE cells gave rise to the wound-induced BCC-like tumors in K15:SmoM2 mice. To test the possibility that wounding promotes IFE cell formation of tumors, we took advantage of K14:SmoM2:LacZ mice that express SmoM2 primarily in IFE cells. In the absence of wounding, K14:SmoM2:LacZ skin developed widespread BCC-like tumors throughout the IFE, as expected (Fig. 1G). In nearly all cases, the follicular bulge appeared normal. After wounding, K14:SmoM2:LacZ skin developed superficial BCC-like tumors in the healed epithelium (Fig. 1 H and I). Unlike K15:SmoM2 skin, however, wounding did not increase the frequency of tumorigenesis (Fig. 1J). Importantly, this result indicates that wounding does not increase the tumorigenic capacity of SmoM2-expressing IFE cells. Thus, IFE cells are unlikely to account for the injury-induced enhancement in tumorigenesis displayed by K15:SmoM2 mice. In addition, the absence of increased tumorigenesis in K14:SmoM2:LacZ mice suggests that wounding in SmoM2-expressing skin does not promote oncogenesis through a general mechanism, such as an injury-associated inflammatory response.
Because wounding induces the migration of follicular stem cells to sites of injury, we determined whether SmoM2-expressing bulge and SHG cells also migrate into the wound site. At 3 d after wounding, SmoM2-expressing follicular stem cells had migrated from the bulge or SHG into the epidermal leading edge of the wound margin (Fig. 2A and Fig. S3). After 10 wk, lineage analysis revealed that bulge or SHG-derived cells could still be detected at the healed wound site (Fig. 2B). SmoM2 expression did not affect the radial spoke cell-migration pattern previously reported (14). β-Gal staining revealed that bulge or SHG-derived cells at the wound site contributed normally to the simple stratified epithelium in K15:LacZ skin (Fig. 2B). In contrast, bulge or SHG-derived cells formed tumors at wound sites of K15:SmoM2:LacZ skin (Fig. 2B), and these tumors displayed increased proliferation over reepithelialized control skin (Fig. S4). However, we also observed that some tumors were negative for β-Gal, suggesting that β-Gal staining underestimates the number of tumor cells within the wound. Nonetheless, all wound-induced neoplastic downgrowths expressed SmoM2 and Keratin-5 (Fig. S4), indicating that these tumors were of epithelial origin. These data reveal that oncogene-expressing follicular stem cells migrate into sites of injury to seed BCC-like tumors.
Fig. 2.
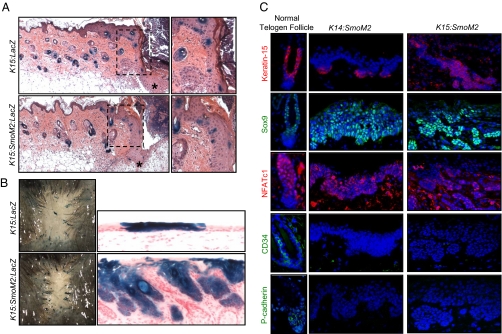
Wounding induces the migration of bulge and SHG-derived cells into sites of injury. (A) β-Gal+ bulge and SHG-derived cells from K15:LacZ and K15:SmoM2:LacZ skin migrate into the epithelial tongue (asterisk) at the leading edge of the contracting wound, 3 d after injury. (Right) Magnified views of the boxed areas. (B) β-Gal staining of healed dorsal skin, 10 wk after wounding, indicates that labeled cells infrequently persist in K15-CrePR1 skin. (Right) Infrequently retained bulge or SHG-derived β-Gal+ cells contribute normally to the healed epithelium in the absence of oncogene expression but form tumors in the presence of SmoM2. (C) Immunofluorescence staining of normal telogen follicles (Left), IFE-derived tumors of K14:SmoM2 skin (Center), and wound-induced tumors of K15:SmoM2 skin (Right).
We next examined whether SmoM2-expressing bulge or SHG-derived cells, similar to normal follicular stem cells, lose expression of follicular markers and adopt an epidermal-like phenotype upon wound-induced movement into the IFE (14). We assessed whether tumors in K15:SmoM2 wounded skin express bulge-enriched proteins Keratin-15, Sox9, NFATc1, and CD34 (25, 28–31). These tumors strongly expressed Keratin-15 and Sox9, heterogeneously expressed NFATc1, and did not express CD34 (Fig. 2C). In addition, these lesions did not express P-cadherin, which is normally enriched in the SHG (13). Interestingly, BCC-like lesions originating from the IFE of K14:SmoM2 mice expressed similar markers as the wound-induced tumors of K15:SmoM2 animals (Fig. 2C). These results indicate that bulge or SHG-derived tumors lose some, but not all, markers of their follicular origin and are similar to tumors that arise from the IFE independent of wounding.
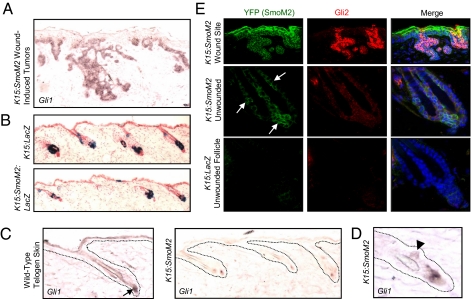
To determine how SmoM2 expression by follicular stem cells promotes the formation of BCC-like tumors only upon wounding, we compared the activation of signaling pathways between wounds from wild-type skin and tumorigenic wounds from K15:SmoM2 mice. In normal skin, we found no evidence of up-regulated Hh or Wnt signaling subsequent to wounding, as assessed by in situ staining for the target genes, Gli1 and Axin2, respectively (Fig. S5). Similarly, in mice expressing LacZ under the control of a Hh-responsive Patched1 promoter (32), there was no evidence of pathway activation after wounding (Fig. S5). In wound-induced tumors, Axin2 was modestly up-regulated, and most tumor cells did not display β-catenin nuclear localization (Fig. S6). In contrast, Gli1 expression was strongly up-regulated in wound-induced tumors (Fig. 3A), indicating that misactivation of Hh signaling by bulge and SHG-derived cells specifically at the wound site may contribute to tumorigenesis.
Fig. 3.
SmoM2-expressing bulge and SHG cells fail to activate robust Hh signaling. (A) In situ staining for Gli1 in wound-induced tumors from K15:SmoM2 skin. (B) β-Gal staining reveals that cells that had undergone K15-CrePR1-mediated recombination are retained in the bulge and SHG of unwounded skin for at least 10 wk, irrespective of SmoM2 expression. (C) In situ staining for Gli1 in wild-type telogen skin (Left) and unwounded K15:SmoM2 skin (Right). Arrow indicates the site of normal Gli1 expression near the SHG and dermal papilla. (D) Gli1 is modestly up-regulated at a site of focal hyperplasia in the bulge of unwounded K15:SmoM2 skin (arrowhead). (E) Immunofluorescence staining for the YFP tag of SmoM2 and Gli2 in K15:SmoM2 wound-induced tumors (Top), in unwounded K15:SmoM2 follicles (Middle), and in non-SmoM2-expressing control follicles (Bottom). Arrows indicate the SmoM2-expressing cells from unwounded K15:SmoM2 follicles (blue, DAPI).
Why does SmoM2 expression induce tumors in the IFE and upon wounding but not in the intact bulge or SHG? One possibility may be that follicular stem cells must leave their niche to activate tumorigenic signaling pathways. To begin testing this possibility, we assessed whether oncogene-expressing cells from K15:SmoM2:LacZ mice were retained in the bulge and SHG in intact skin but leave upon wounding. Indeed, staining for SmoM2 or β-Gal in unwounded skin at 11 wk after induction revealed that all follicles retained oncogene-expressing cells within the bulge and SHG (Fig. 3 B and E). Interestingly, neither Axin2 nor Gli1 was strongly up-regulated by SmoM2-expressing cells in the bulge and SHG (Fig. 3C and Fig. S6). In undamaged K15:SmoM2 telogen skin, Gli1 expression was almost exclusively localized near the SHG and dermal papilla, similar to control telogen skin (Fig. 3C). In contrast, wound-induced tumors expressed elevated levels of Gli1, even compared with sites of focal hyperplasia in the follicular bulges of unwounded K15:SmoM2 skin (Fig. 3 A and D).
As with Gli1, we observed strong expression of Gli2, previously associated with increased Hh signaling in the skin (24, 26), in wound-induced lesions but not in control skin (Fig. 3E). Importantly, Gli2 up-regulation was not detected in SmoM2-expressing cells in the bulge and SHG (Fig. 3E). These differences in Gli1 and Gli2 up-regulation are likely not attributable to regional differences in the abundance of primary cilia, which are necessary for Smo-mediated signaling, because Smo localization to cilia was frequently observed in SmoM2-induced bulge and SHG cells as well as in wound-induced lesions (Fig. S7). Together, our findings suggest that a block in downstream Hh pathway activation may contribute to protecting against SmoM2-mediated oncogenesis in the bulge and SHG, and that migration of SmoM2-expressing stem cells from their follicular niches is associated with derepressed Hh signaling and tumorigenesis.
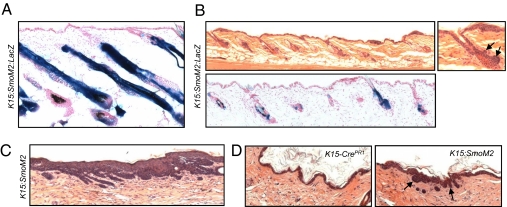
During anagen, stem cells in the bulge and SHG proliferate and migrate from their niche to regenerate the lower follicular bulb and hair shaft (9, 11, 13, 15). To determine whether anagen-induced proliferation and migration of stem cells also promotes tumorigenesis, we induced SmoM2 expression in K15:SmoM2 mice during telogen and, 3 d later, depilated the skin to induce anagen reentry. At 20 d after depilation, lineage analysis revealed that SmoM2-expressing cells contributed to the matrix, hair shaft, and lower anagen bulb (Fig. 4A). Similar to normal anagen follicles, these follicles activated the Wnt and Hh pathways in the lower follicle (Fig. S8). Although follicular hyperplasia was observed, severe aberrations in follicular morphology did not occur in skin that had exited anagen and subsequently reentered telogen, despite the fact that nearly all follicles retained SmoM2-expressing cells (Fig. 4B). These results indicate that, unlike wounding, anagen induction does not promote tumorigenesis in K15:SmoM2 mice.
Fig. 4.
Bulge or SHG-derived BCC-like tumors are induced by incisional or excisional wounding but not by depilation. (A) β-Gal staining indicates that bulge and SHG-derived cells that had undergone K15-CrePR1-mediated recombination contribute to cells of the lower anagen bulb in hyperplastic follicles from depilated K15:SmoM2:LacZ skin. (B Upper) Follicles of K15:SmoM2 skin are grossly normal 10 wk after depilation. Note that anagen hair follicles had subsequently progressed to telogen without developing aberrations, aside from occasional focal hyperplasia (Right, arrows). (Lower) β-Gal staining demonstrates that bulge and SHG cells that had undergone K15-CrePR1-mediated recombination are retained in K15:SmoM2 skin 10 wk after depilation. (C) BCC-like tumors arise from healed excisional wound sites of K15:SmoM2 skin injured 5 wk after induction of SmoM2. (D) Tumors (arrows) arise at the healed incisional wound junction of K15:SmoM2 mice 10 wk after injury but do not form in healed control K15-CrePR1 skin.
To examine whether the oncogene-expressing bulge and SHG cells that persist in the follicle remain competent long term to form tumors in response to wounding, we performed excisional wounding 5 wk, rather than 3 d, after SmoM2 induction. As before, we observed numerous tumor nests and aberrant downgrowths at the healed wound site (Fig. 4C). To assess whether less severe wounds can promote formation of superficial BCC-like tumors in K15:SmoM2 mice, we induced expression of SmoM2 and subsequently performed incisional wounding. Indeed, we observed that bulge and SHG-derived cells were recruited to sites of injury and formed superficial tumors at the healed wound junction (Fig. 4D and Fig. S9). Together, these findings indicate that SmoM2-expressing follicular stem cells persist and remain competent for tumorigenesis for at least several weeks, and that less severe incisional wounds also promote formation of BCC-like tumors.
Discussion
Rudolf Virchow postulated a relationship between injury and cancer in 1863, noting that both the frequency and extent of injury seemed to contribute to tumor formation (33). Indeed, human BCCs have been observed at sites of vaccination (34, 35), surgery (36, 37), burns (38), and other physical trauma (39, 40). Our findings suggest that, although BCCs likely originate from IFE cells in undamaged skin, as has been previously reported by Youssef et al. (17), oncogene-expressing follicular stem cells can give rise to tumors within sites of cutaneous injury. Ito et al. previously demonstrated that normal bulge-derived cells are recruited to sites of wounding (14), and our findings indicate that the wound-induced recruitment of SmoM2-expressing follicular stem cells releases these cells to form tumors. This paradigm may also apply to BCCs that display misactivation of Hh signal transduction due to loss-of-function mutations in Patched1, which normally suppresses Smo. However, it remains unclear whether the amplitude of downstream Hh activation is similar in tumors possessing loss-of-function mutations in Patched1 and those with gain-of-function mutations in Smo. In addition, Patched1 may contribute to tumorigenesis independently of Hh signaling, as suggested by a study of skin squamous carcinoma (41).
We have also shown here that, in the absence of wounding, SmoM2-expressing follicular stem cells persist without forming tumors. However, these cells remain competent for wound-induced oncogenesis, even long after the onset of SmoM2 expression. Similarly, in a model of squamous cell carcinoma, oncogene-expressing cells can form tumors up to a year after an initial exposure to a chemical carcinogen (42, 43). These findings are concordant with step-wise models of cancer development, where long-lived progenitor cells residing in quiescent niches accumulate oncogenic insults before a final event exposes their tumorigenic phenotype. Our work suggests that this final event need not be an additional mutation but can be wound-induced mobilization of oncogenically initiated stem cells. Similarly, work by Kasper et al., has recently shown that wounding can recruit follicular keratinocytes to form tumors at sites of injury (44).
Importantly, our observation that wounding does not promote IFE cell-mediated tumorigenesis suggests that the rare IFE cells that had undergone K15-CrePR1-mediated recombination likely do not account for the enhanced tumorigenesis observed upon wounding in K15:SmoM2 skin. Instead, migration into the wound may allow follicular stem cells or their progeny to acquire epidermal properties, as has been previously reported (14), and our findings suggest that these properties may include the ability to form tumors. In support of this hypothesis, wound-induced tumors derived from bulge or SHG cells display morphologies and marker expression similar to those of IFE-derived tumors.
Because SmoM2 expression in the IFE, but not in the bulge or SHG, is sufficient to induce tumors, we hypothesize that the bulge microenvironment inhibits SmoM2-mediated oncogenesis. Similar to our findings, recent studies have reported that expression of SmoM2 or truncated β-catenin in the bulge or SHG does not cause tumor formation (17, 45). Inefficient activation of downstream Hh signaling, as reflected by the absence of Gli1 and Gli2 up-regulation, may explain the inability of SmoM2 to induce tumors in the bulge and SHG. This may be due to the activity of negative regulators of the Hh pathway, such as Gli3 or Sufu, whose expression was recently reported to be up-regulated in Lgr5-expressing bulge and SHG cells (10). In addition, proteolytic degradation of Gli transcription factors may attenuate Hh signaling in the bulge (46, 47). Because the bulge is thought to maintain a quiescent microenvironment through the action of BMPs (48, 49) and the suppression of Wnt signaling (15), these pathways may also interfere with transduction of downstream Hh signals and contribute to tumor suppression.
In addition to eliciting stem-cell escape from growth-restrictive niches, wounding also promotes transient epidermal hyperproliferation (2). In the wound microenvironment, fibroblasts and immune cells produce cytokines and extracellular matrix that promote cell division (4, 50). Indeed, inflammation accelerates tumorigenesis in a variety of cancer models, including those for skin (51), and is associated with increased formation of reactive oxygen species and genomic stress (4). Thus, wounding may promote oncogenic mutations that contribute to wound-induced tumorigenesis in some contexts. However, our finding that SmoM2-expressing IFE cells form numerous BCC-like tumors in K14:SmoM2 skin in a manner unaffected by wounding suggests that injury-associated inflammation does not play a general role in SmoM2-mediated BCC formation. Nonetheless, we cannot rule out the possibility that inflammation may act specifically on bulge or SHG-derived cells to promote tumorigenesis by inducing mutations and increasing Hh signaling at the wound site.
Our study demonstrates that the cell of origin for BCC can depend on extrinsic factors such as wounding. Given the slow-growing nature of these tumors and the fact that even small incisional wounds can recruit oncogene-expressing stem cells from the follicle, it is possible that the formation of human BCCs may be temporally removed from their provocative injuries or that the associated injuries may go unnoticed. In future studies, it will be interesting to determine whether mobilization of oncogene-expressing stem cells underlies the development of other cancers whose pathogenesis has been associated with injury.
Materials and Methods
Mice.
Mice expressing K15-CrePR1, K14-CreERT, SmoM2, ROSA26-LacZ reporter, and Patched1-LacZ have been previously described (SI Materials and Methods) (24, 25, 27, 32, 51). Animals harbored single copies of the transgenes and were of a mixed background. At 7.5 wk of age, dorsal skin from K15-CrePR1 mice was shaved and treated with 200 μL of 10 mg/mL mifepristone (Sigma-Aldrich) diluted in 50% DMSO/50% ethanol, applied topically for 5 d to induce Cre-mediated recombination. K14-CreERT mice were induced with tamoxifen (Sigma-Aldrich) (26). At 3 d after the final treatment, a 0.5 × 0.5-cm full-thickness excisional wound was generated 3.5 cm from the base of the tail and 1 cm to the left of the spine. At 10 wk after wounding, biopsies spanning the healed wound site were removed, as were contralateral biopsies from the unwounded side (Fig. S10). For incisional wounds, a 0.5-cm incision was made 3.5 cm from the base of the tail and 1 cm to the left of the spine. For depilation, dorsal skin was treated with Nair (Church & Dwight) for 3 min. All mouse procedures were approved by the University of California San Francisco Institutional Animal Care and Use Committee.
Histology, Immunohistochemistry, and In Situ Hybridization.
Skin biopsies were collected and stained by using standard protocols. Fixation conditions, staining procedures, and antibodies are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank A. Dlugosz for insightful discussions; K. Thorn and the University of California San Francisco Nikon Imaging Center for microscopy; C. Miller and J. D. Fish for histology; A. Hristov and S. Kogan for pathology; M. Grachtchouk for advice on in situ staining; T. Caspary for Arl13b antibody; and Y. Choe for Axin2 probe. This work was funded by grants from the National Institutes of Health (RO1AR054396), the Burroughs Wellcome Fund, the Packard Foundation, and the Sandler Family Supporting Foundation (to J.F.R.), and by a grant from the American Cancer Society and National Institutes of Health Grant 1K99AR059796 (to S.Y.W.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013098108/-/DCSupplemental.
References
- 1.Coulombe P-A. Wound epithelialization: Accelerating the pace of discovery. J Invest Dermatol. 2003;121:219–230. doi: 10.1046/j.1523-1747.2003.12387.x. [DOI] [PubMed] [Google Scholar]
- 2.Schäfer M, Werner S. Cancer as an overhealing wound: An old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 3.Dvorak H-F. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 4.Lin W-W, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owens D-M, Watt F-M. Contribution of stem cells and differentiated cells to epidermal tumours. Nat Rev Cancer. 2003;3:444–451. doi: 10.1038/nrc1096. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs E. Skin stem cells: Rising to the surface. J Cell Biol. 2008;180:273–284. doi: 10.1083/jcb.200708185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy V, Lindon C, Harfe B-D, Morgan B-A. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell. 2005;9:855–861. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Ghazizadeh S, Taichman L-B. Multiple classes of stem cells in cutaneous epithelium: A lineage analysis of adult mouse skin. EMBO J. 2001;20:1215–1222. doi: 10.1093/emboj/20.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotsarelis G, Sun T-T, Lavker R-M. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 10.Jaks V, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 11.Blanpain C, Lowry W-E, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Taylor G, Lehrer M-S, Jensen P-J, Sun T-T, Lavker R-M. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 13.Greco V, et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito M, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 15.Tumbar T, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotsarelis G. Epithelial stem cells: A folliculocentric view. J Invest Dermatol. 2006;126:1459–1468. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- 17.Youssef K-K, et al. Identification of the cell lineage at the origin of basal cell carcinoma. Nat Cell Biol. 2010;12:299–305. doi: 10.1038/ncb2031. [DOI] [PubMed] [Google Scholar]
- 18.Oro A-E, et al. Basal cell carcinomas in mice overexpressing sonic hedgehog. Science. 1997;276:817–821. doi: 10.1126/science.276.5313.817. [DOI] [PubMed] [Google Scholar]
- 19.Johnson R-L, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 20.Hahn H, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 21.Dahmane N, Lee J, Robins P, Heller P, Ruiz i Altaba A. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature. 1997;389:876–881. doi: 10.1038/39918. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson M, et al. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc Natl Acad Sci USA. 2000;97:3438–3443. doi: 10.1073/pnas.050467397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie J, et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 24.Mao J, et al. A novel somatic mouse model to survey tumorigenic potential applied to the Hedgehog pathway. Cancer Res. 2006;66:10171–10178. doi: 10.1158/0008-5472.CAN-06-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris R-J, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 26.Wong S-Y, et al. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med. 2009;15:1055–1061. doi: 10.1038/nm.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: Genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci USA. 1999;96:8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horsley V, Aliprantis A-O, Polak L, Glimcher L-H, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trempus C-S, et al. CD34 expression by hair follicle stem cells is required for skin tumor development in mice. Cancer Res. 2007;67:4173–4181. doi: 10.1158/0008-5472.CAN-06-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vidal V-P, et al. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol. 2005;15:1340–1351. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 31.Nowak J-A, Polak L, Pasolli H-A, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodrich L-V, Milenković L, Higgins K-M, Scott M-P. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 33.Virchow R, Virchow R. Aetiologie der neoplastischen Geschwulste/Pathogenie der neoplastischen Geschwulste. Berlin, Germany: Verlag von August Hirschwald; 1863. [Google Scholar]
- 34.Rich J-D, Shesol B-F, 3rd, Horne D-W., 3rd Basal cell carcinoma arising in a smallpox vaccination site. J Clin Pathol. 1980;33:134–135. doi: 10.1136/jcp.33.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zelickson A-S. Basal cell epithelioma at site and following smallpox and vaccination. Arch Dermatol. 1968;98:35–36. [PubMed] [Google Scholar]
- 36.Ozyazgan I, Kontaçs O. Basal cell carcinoma arising from surgical scars: A case and review of the literature. Dermatol Surg. 1999;25:965–968. doi: 10.1046/j.1524-4725.1999.99192.x. [DOI] [PubMed] [Google Scholar]
- 37.Jorquero E, Moreno J-C, Díaz-Cano S-J, Rodríguez-Adrados F, Camacho F. Basal cell carcinoma arising in a surgical scar: Reconstructive surgical treatment. J Dermatol Surg Oncol. 1994;20:846–847. doi: 10.1111/j.1524-4725.1994.tb03720.x. [DOI] [PubMed] [Google Scholar]
- 38.Koga Y, Sawada Y. Basal cell carcinoma developing on a burn scar. Burns. 1997;23:75–77. doi: 10.1016/s0305-4179(96)00077-0. [DOI] [PubMed] [Google Scholar]
- 39.Ozyazgan I, Kontaş O. Previous injuries or scars as risk factors for the development of basal cell carcinoma. Scand J Plast Reconstr Surg Hand Surg. 2004;38:11–15. doi: 10.1080/02844310310005883. [DOI] [PubMed] [Google Scholar]
- 40.Rustin M-H, Chambers T-J, Munro D-D. Post-traumatic basal cell carcinomas. Clin Exp Dermatol. 1984;9:379–383. doi: 10.1111/j.1365-2230.1984.tb00820.x. [DOI] [PubMed] [Google Scholar]
- 41.Wakabayashi Y, Mao J-H, Brown K, Girardi M, Balmain A. Promotion of Hras-induced squamous carcinomas by a polymorphic variant of the Patched gene in FVB mice. Nature. 2007;445:761–765. doi: 10.1038/nature05489. [DOI] [PubMed] [Google Scholar]
- 42.Van Duuren B-L, Sivak A, Katz C, Seidman I, Melchionne S. The effect of aging and interval between primary and secondary treatment in two-stage carcinogenesis on mouse skin. Cancer Res. 1975;35:502–505. [PubMed] [Google Scholar]
- 43.Loehrke H, et al. On the persistence of tumor initiation in two-stage carcinogenesis on mouse skin. Carcinogenesis. 1983;4:771–775. doi: 10.1093/carcin/4.6.771. [DOI] [PubMed] [Google Scholar]
- 44.Kasper M, et al. Wounding enhances epidermal tumorigenesis by recruiting hair follicle keratinocytes. Proc Natl Acad Sci USA. 2011;108:4099–4104. doi: 10.1073/pnas.1014489108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker C-M, Verstuyf A, Jensen K-B, Watt F-M. Differential sensitivity of epidermal cell subpopulations to β-catenin-induced ectopic hair follicle formation. Dev Biol. 2010;343:40–50. doi: 10.1016/j.ydbio.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huntzicker E-G, et al. Dual degradation signals control Gli protein stability and tumor formation. Genes Dev. 2006;20:276–281. doi: 10.1101/gad.1380906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huntzicker E-G, Oro A-E. Controlling hair follicle signaling pathways through polyubiquitination. J Invest Dermatol. 2008;128:1081–1087. doi: 10.1038/sj.jid.5700957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, et al. Bone morphogenetic protein signaling inhibits hair follicle anagen induction by restricting epithelial stem/progenitor cell activation and expansion. Stem Cells. 2006;24:2826–2839. doi: 10.1634/stemcells.2005-0544. [DOI] [PubMed] [Google Scholar]
- 49.Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc Natl Acad Sci USA. 2007;104:10063–10068. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martins-Green M, Boudreau N, Bissell M-J. Inflammation is responsible for the development of wound-induced tumors in chickens infected with Rous sarcoma virus. Cancer Res. 1994;54:4334–4341. [PubMed] [Google Scholar]
- 51.Moore R-J, et al. Mice deficient in tumor necrosis factor-α are resistant to skin carcinogenesis. Nat Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.