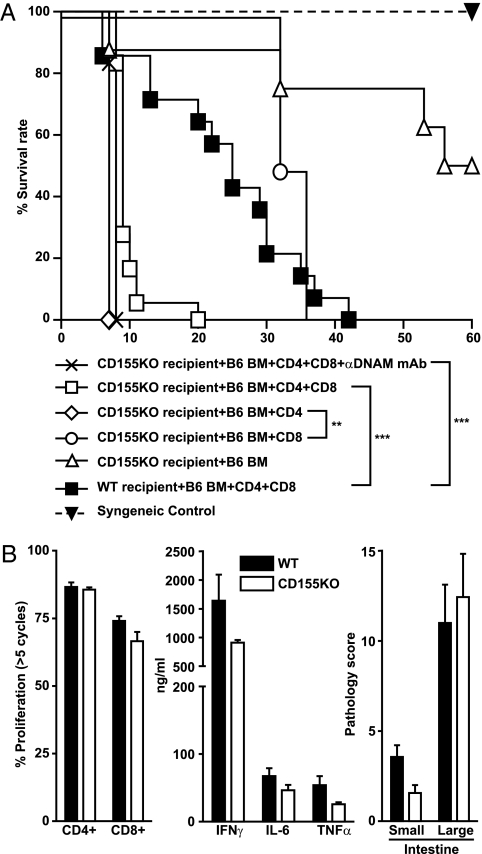
In a recent paper, Nabekura et al. (1) provided compelling evidence that abrogating DNAM-1 (DNAX accessory molecule-1; CD226) activity resulted in milder development of graft-versus-host disease (GVHD). This is expected, because the role of CD226 as an important costimulatory receptor during T-cell activation is well-documented (2, 3). As shown in the paper (1), CD226 present on donor T cells contributed to the typical syndromes of GVHD. A milder course of GVHD was also achieved by treating transplanted mice with an antibody neutralizing CD226. The authors assumed that CD226 signaling is initiated by interaction of the allogeneic T cells with host cells expressing either CD112 or CD155, the two known ligands of CD226 (1). We analyzed the development of GVHD in mice deficient for CD155. Unexpectedly, the mice succumbed to GVHD within 1 wk, whereas WT mice survived for approximately 3 wk (Fig. 1A). Additional experiments suggested that the observed aggravation in the course of disease is mainly caused by CD4+ donor T cells (Fig. 1A). Additional analyses aimed at identifying the cause of premature death of CD155−/− recipients failed to reveal any differences to WT controls (Fig. 1B). Serum cytokine levels of IFNγ, IL-6, and TNFα as well as the extent of T-cell proliferation were virtually identical when investigated 3 d posttransplantation. We also subjected the intestine of recipients to a detailed histological examination 6 d posttransplantation, but again, significant differences in the degree of injury or infiltrating T cells between WT and CD155−/− mice were not detected. Of note, CD155−/− mice receiving T cell-depleted bone marrow only developed lethal GVHD after T cells differentiated from donor marrow. This corroborated that CD155−/− mice died of deleterious T-cell effects and not because of constitutional defects imposed by CD155 deficiency (Fig. 1A).
Fig. 1.
(A) After lethal irradiation, WT BALB/c or CD155−/− (Pvrtm1Gbn) BALB/c (KO) mice received 5 × 106 C57BL/6 T cell-depleted bone marrow cells (BM) alone (n = 8) or BM supplemented with 1 × 106 T cells (CD4+ and CD8+ T cells purified from pooled peripheral lymph nodes using a pan T-cell isolation kit, n = 14; Miltenyi). Alternatively, BM was transferred together with either purified 1 × 106 CD4+ (n = 4) or 1 × 106 CD8+ (n = 4) T cells. One group of CD155−/− mice received i.v. injection of 400 μg anti-DNAM mAb (3B3; n = 6) the day before transplantation. Injection was repeated at day 4 posttransplantation. Mice that received syngeneic grafts are also shown (n = 2). Data are pooled from at least two independent experiments. To analyze survival, Kaplan–Meier estimation and log-rank test for statistical analyses was used. **P < 0.01; ***P < 0.001. (B Left) The proliferation of carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled CD4+ and CD8+ T cells was determined by flow cytometry 3 d after adoptive transfer of 2 × 107 lymph node cells into irradiated WT or CD155−/− recipients. (Center) Serum cytokine levels were analyzed using the Cytometric Bead Array Mouse Inflammation Kit (BD Biosciences). (Right) Epithelial changes, cellular infiltrations, area involved, and cryptonecrosis were classified from 0 (no changes) to 4 (highest effect) each. The sum of all parameters separately determined for proximal small intestine and distal small intestine (Small) as well as colon and cecum (Large) were given as the pathology score. Analysis was done on formalin-fixed, paraffin-embedded intestines that were harvested 6 d posttransplantation. The 4- to 6-μm slides were stained with H&E.
Even if the exact cause of the exacerbated course of GVHD in CD155−/− recipients remains elusive, our results suggest that presence of CD155 may attenuate otherwise devastating consequences of GVHD. Because the study by Nabekura et al. (1) clearly showed that CD226-triggered T-cell coactivation contributes to a worsening course of GVHD, CD155 possibly plays a Janus-faced role in GVHD development: its presence exerts protective effects but at the same time, aggravates GVHD by activating donor cells expressing CD226. Alternatively, as already discussed by Nabekura et al. (1), CD112 expressed by host cells in liver and intestine may be largely responsible for the observed CD226 effects. Future studies involving CD112−/− mice will help to clarify this point. Moreover, the GVHD model in use also matters. Whereas Nabekura et al. (1) chose an experimental strategy eliciting GVHD preferentially by CD8+ T cells, our investigations were based on an MHC fully mismatched model where CD4+ T cells mainly contribute to GVHD development (4). Nevertheless, we were unable to prolong survival time of CD155−/− recipients by treating mice with a CD226 neutralizing antibody (Fig. 1A) (5). Despite its importance in CD4+ T-cell stimulation and differentiation (3), CD226 may, thus, not always play a major role in the complex pathophysiology of GVHD.
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft Grant BE1886/2-2 (to G.B.) and Deutsche Krebshilfe Grant 109451 (to C.K.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Nabekura T, et al. Critical role of DNAX accessory molecule-1 (DNAM-1) in the development of acute graft-versus-host disease in mice. Proc Natl Acad Sci USA. 2010;107:18593–18598. doi: 10.1073/pnas.1005582107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilfillan S, et al. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J Exp Med. 2008;205:2965–2973. doi: 10.1084/jem.20081752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shibuya K, et al. CD226 (DNAM-1) is involved in lymphocyte function-associated antigen 1 costimulatory signal for naive T cell differentiation and proliferation. J Exp Med. 2003;198:1829–1839. doi: 10.1084/jem.20030958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy P, Negrin R, Hill GR. Mouse models of bone marrow transplantation. Biol Blood Marrow Transplant. 2008;14(Suppl 1):129–135. doi: 10.1016/j.bbmt.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seth S, et al. Heterogeneous expression of the adhesion receptor CD226 on murine NK and T cells and its function in NK-mediated killing of immature dendritic cells. J Leukoc Biol. 2009;86:91–101. doi: 10.1189/jlb.1208745. [DOI] [PubMed] [Google Scholar]