Abstract
This paper presents research on the genetic structure and diversity of populations of a common marine protist and their changes over time. The bloom-forming diatom Skeletonema marinoi was used as a model organism. Strains were revived from anoxic discrete layers of a 210Pb-dated sediment core accumulated over more than 100 y, corresponding to >40,000 diatom mitotic generations. The sediment core was sampled from the highly eutrophic Mariager Fjord in Denmark. The genetic structure of S. marinoi was examined using microsatellite markers, enabling exploration of changes through time and of the effect of environmental fluctuations. The results showed a stable population structure among and within the examined sediment layers, and a similar genetic structure has been maintained over thousands of generations. However, established populations from inside the fjord were highly differentiated from open-sea populations. Despite constant water exchange and influx of potential colonizers into the fjord, the populations do not mix. One fjord population, accumulated in 1980, was significantly differentiated from the other groups of strains isolated from the fjord. This differentiation could have resulted from the status of Mariager Fjord, which was considered hypereutrophic, around 1980. There was no significant genetic difference between pre- and posteutrophication groups of strains. Our data show that dispersal potential and generation time do not have a large impact on the genetic structuring of the populations investigated here. Instead, the environmental conditions, such as the extreme eutrophication of the Mariager Fjord, are deemed more important.
Keywords: microevolution, microsatellites
Most planktonic protists sink to the bottom of the sea, die, and subsequently degenerate; however, some cells have the ability to survive in the sediment as resting stages. These resting stages can act as short- or long-term survival mechanisms, with cells remaining viable in the sediment for several decades (1). The formation of resting stages usually is initiated by adverse conditions. Resting stages in the sediment are of ecological importance, because they provide genetic material for future years when resuspended in the water column (2). They are well preserved in anoxic sediment, and in the absence of bioturbation and subsequent laminated sediment the resting stage-forming species are suitable for microevolutionary studies.
Different local or regional factors, such as climate, nutrient concentrations, and oceanographic conditions, have relative importance for the sources of sedimentation. Changes in these factors, including anthropogenic disturbances, sometimes are reflected in the sediment. Aquatic organisms in anoxic sediment cores have been used extensively to assess changes in species composition associated with environmental changes, i.e., eutrophication, shifts in salinity, or changes in oxygen concentration (e.g., refs. 3, 4). However, very little is known about changes in population genetic structure during periods of natural shift or anthropogenic-caused changes. Diapausing eggs produced by the water flea Daphnia are an exception. Subfossil resting eggs, often viable for up to 100 y and containing sufficient quality DNA for genetic analysis (5), have permitted studies of the effects of eutrophication on genetic structure that are important for the ecology and evolution of freshwater bodies (6). However, there are no equivalent studies in the marine environment, and the effects of environmental changes on intraspecific genetic structure and microevolution of eukaryote protists are poorly understood.
Aquatic protists are considered to have high dispersal ability because of their small size and high abundance. Consequently, they are thought to display low genetic diversity and to lack biogeographic patterns (7). Because of predominant asexual division, it also has been assumed that populations consist of only a few clones. This assumption has been challenged recently by an increasing number of studies demonstrating high genetic and phenotypic diversity in populations of eukaryotic microorganisms (8–10).
We use the chain-forming marine diatom Skeletonema marinoi as a model organism. This species is abundant during the spring bloom and often dominates the plankton community in temperate waters (11). S. marinoi is an important primary producer and constitutes a valuable food source for higher trophic levels. It usually reproduces asexually, but the formation of auxospores and sexual reproduction has been documented (12). Generation time is short, with approximately one division per day under laboratory conditions (13). It has a benthic resting stage, and in Scandinavian sediments up to 50 000 propagules per gram of sediment can be found (1). S. marinoi is easy to collect, isolate, and maintain in culture, and the survival of monoclonal cultures after single-cell isolation is almost 100% (14).
Laminated sediment cores from the Mariager Fjord in Denmark have been analyzed previously for ecosystem structural changes over time. Anthropogenic loadings of phosphorus (P) and nitrogen (N) have increased in Danish estuarine waters, especially since the 1950s (15). During the 1970s the input of P in the Mariager Fjord reached 80 tons per year, and an annual average phytoplankton biomass of 60 milligrams of chlorophyll a per liter was recorded. In the 1980s, several coastal areas faced oxygen depletion, and mass mortality of lobsters in the Kattegat was associated with low oxygen concentration (16). This scenario led to legislative action with the goal of reducing nutrient loading, P by 80% and N by 50%. P loads have decreased by 75% in the Mariager Fjord, and the chlorophyll a concentration has dropped to 4–12 μg/L (17). However, despite efforts to reduce nutrient levels in the area, the Mariager Fjord still is highly eutrophic, and N and P levels and pH are considered high (18). The main biological changes in the fjord, such as shifts in abundance of phytoplankton groups and species, occurred from 1915 to the 1940s (19), suggesting that the fjord has been highly eutrophic since the beginning of the 20th century. However, the exact consequences are unknown, especially because regular plankton monitoring was initiated only a few decades ago.
Here, we present an approach for examining the genetic structure of a marine protist and its changes over time. We germinated S. marinoi strains from temporally discrete layers of a sediment core spanning a period of more than 100 y and investigated how groups of strains from Mariager Fjord related to the open-sea population. We hypothesized that changes in environmental conditions, in particular the nutrient loads, would influence the genetic structure and the intrasample genetic diversity of temporally discrete populations of S. marinoi from the fjord.
Results
The analyzed groups of strains 1–7 established from the discrete sediment layers of the core represent a time span from 2 y ago to more than 100 y ago. The proportion of survival and successful genotyping of the clonal cultures was, on average, 66% but varied among sediment layers. The highest percentage of survival and genotyping (100%) was documented in the group of strains isolated from the sediment originating from 1980, and the lowest (39%) was in the oldest sediment layer (Table 1). In total, 158 monoclonal isolates from the sediment core were genotyped. Additionally, 87 clones from the open sea (Kattegat; Anholt and Vinga; Fig. S1) were established and genotyped.
Table 1.
Chronology of the sediment core MF08/ll with the depths of the analyzed sediment layers, approximate ages, proportions of survival, and genotyped Skeletonema marinoi clonal isolates
| Depth (cm) | Age (y) | Error age (y) | Date | Sediment accumulation rate (kg·m−2·y−1) | Error rate (kg·m−2·y−1) | Label of strains established | Proportion of genotyped isolates (%) |
| 0.0 | 2008 | ||||||
| 1.0 | 2 | 2 | 2006 | 0.11 | 0.02 | 7 | 68 |
| 2.5 | 8 | 2 | 2000 | 0.11 | 0.02 | 6 | 79 |
| 4.5 | 19 | 3 | 1989 | 0.09 | 0.02 | 5 | 64 |
| 5.5 | 28 | 3 | 1980 | 0.07 | 0.01 | 4 | 100 |
| 6.5 | 41 | 4 | 1967 | 0.06 | 0.01 | ||
| 7.5 | 57 | 5 | 1951 | 0.05 | 0.01 | ||
| 8.5 | 72 | 5 | 1936 | 0.06 | 0.01 | ||
| 9.5 | 87 | 7 | 1921 | 0.06 | 0.01 | 3 | 62 |
| 15–16 | >87 | 2 | 50 | ||||
| 21–22 | >87 | 1 | 39 |
Full details of the two deepest layers are not shown because of uncertain dating but are >87 y old.
All eight microsatellite loci were polymorphic. The most variable was S.mar5, with 8–21 alleles, and the least variable was S.mar3. Significant (P < 0.05) departure from the Hardy–Weinberg equilibrium (HWE) was observed for all loci except S.mar2 and S.mar4, at varying numbers of samples. Loci S.mar1, S.mar5, and S.mar7 displayed heterozygote deficiency in all samples (Table S1). No evidence was found for large allele dropout or stuttering effects, but null alleles might be present in some loci in selected samples. Based on the method by Brookfield (20), estimates of null allele frequencies were low or nonexistent in S.mar2, S.mar3, S.mar4, and S.mar6, moderate in S.mar7 and S.mar8, and highest in S.mar1 and S.mar5 (Table S1). The indications of null allele coincided with the loci displaying heterozygote deficiencies (χ2 test, n = 64; P = 0.002). There was no significant correlation between samples and null allele frequencies (pair-sampled two-tailed t-test, P > 0.05). No pairs of microsatellite loci were linked significantly across all samples.
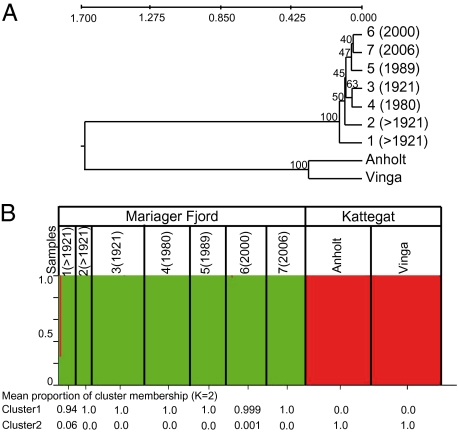
Genetic differentiation was confirmed for the samples from the open sea (Anholt and Vinga) and all of the samples from the Mariager Fjord (Fig. 1 A and B and Table 2). The magnitude of fixation index (FST) values of the open-sea populations and fjord populations indicated great genetic differentiation. The unweighted pair-grouping method with arithmetic means (UPMGA) dendrogram based on Nei's genetic distance identified two distinct clusters with 100% bootstrap support: (i) the strains established from the open sea, i.e., Anholt and Vinga; and (ii) the strains established from germinated resting stages from discrete sediment layers within the Mariager Fjord. From five independent simulations of the Bayesian clustering method implemented in STRUCTURE, the calculated ΔK (Materials and Methods) indicated that two clusters best explained the uppermost hierarchical level of genetic structuring in the S. marinoi samples. The genotyped strains were divided into fjord samples as Cluster 1 and Kattegat samples as Cluster 2 (Fig. 1B), consistent with the results of the UPMGA (Fig. 1A). All individuals were assigned with high probability (1.0) to one of the clusters. The exception was one outlier from in the oldest Mariager sample, which was assigned to Cluster 1 with a probability of 0.3.
Fig. 1.
Population structure of S. marinoi based on eight microsatellite loci. Samples 1–7 are groups of strains established from resting stages germinated from discrete layers of a sediment core collected in the Mariager Fjord (the approximate years when resting stages accumulated are given in parentheses). Samples Anholt and Vinga are groups of strains established from recent sediment collected in the Kattegat. (A) An unweighted pair grouping method using an arithmetic average (UPGMA) of clonal cultures established from the nine different groups of strains based on Nei's genetic distance (49). Bootstrap values are indicated on each node. (B) Genetic clustering estimated by STRUCTURE. Assignment of 245 individuals to K = 2 genetically distinguishable groups. Each individual is represented by a vertical bar colored according to the assigned group, and the average proportions of membership of each sample to the two clusters are shown below.
Table 2.
Genetic differentiation between pairs of samples (FST) from the Mariager Fjord and the open sea (Kattegat; Anholt and Vinga)
| Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 | Sample 7 | Anholt | Vinga | |
| Sample 1 | −0.02 | 0.030 | 0.038 | −0.003 | −0.01 | 0.007 | 0.225 | 0.224 |
| Sample 2 | – | 0.005 | 0.007 | −0.014 | −0.01 | 0.002 | 0.241 | 0.241 |
| Sample 3 | – | 0.005 | 0.018 | 0.013 | 0.007 | 0.247 | 0.242 | |
| Sample 4 | – | 0.022 | 0.016 | 0.018 | 0.268 | 0.260 | ||
| Sample 5 | – | −0.01 | −0.004 | 0.224 | 0.224 | |||
| Sample 6 | – | −0.011 | 0.224 | 0.224 | ||||
| Sample 7 | – | 0.224 | 0.227 | |||||
| Anholt | – | 0.071 |
Bold numbers indicate significant differentiation after Bonferroni correction (P < 0.05).
Sequences of the internal transcriber spacer (ITS) region displayed a maximum of 0.007 differences per site that was attributed to intraspecific variation and could not be assigned to the open-sea or Mariager Fjord individuals. The ITS secondary structure of the 12 individuals examined folded similarly, and the 30-nt motif of the 5′ side of the internal transcriber spacer 2 (ITS2) helix III were identical in all clones investigated.
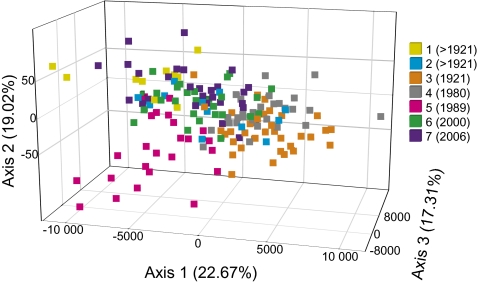
Analyses of genetic differentiation among the Mariager Fjord samples showed significant separations of some groups of strains. Strains established from the 28-y-old sediment layer (sample 4) were significantly differentiated (P < 0.05) from the strains originating from germinated resting stages older than 87 y (sample 1), 19 y, 8 y, and 2 y (samples 5–7, respectively). Strains established from the 87-y-old sediment (sample 3) were significantly differentiated from the strains established from the oldest sediment and from the more recent strains (samples 5 and 6; Table 2). Factorial correspondence analysis (FCA) identified six axes with the eigenvalues of 0.104 (axis 1), 0.087 (axis 2), 0.079 (axis 3), 0.069 (axis 4), 0.067 (axis 5), and 0.052 (axis 6), each explaining 22.67%, 19.02%, 17.31%, 15.02%, 14.7%, and 11.28% of the variation, respectively. The analysis showed gene flow among the Mariager Fjord samples but also separation, especially along axis 1, in accordance with the weak but significant FST values of samples 3 and 4 versus the other samples (Fig. 2).
Fig. 2.
FCA of the genetic distances based on a plot using FST values of the individual strains belonging to the seven groups of strains established from resting stages in discrete sediment layers accumulated in Mariager Fjord from before 1921 to 2006 (samples 1–7 with approximate years when resting stages accumulated given in parentheses). Axes 1, 2, and 3 explain 59% of the variance in distribution of the individuals from the discrete sediment layers.
All genotyped individuals isolated from the Mariager Fjord and the Kattegat were genetically distinct (100%). Tests for differences in genetic diversity between the Mariager Fjord and the Kattegat populations showed significantly reduced allelic richness (P < 0.05), inbreeding coefficient (FIS), and gene diversity and significantly higher corrected relatedness within the Mariager Fjord population as compared with the open-sea samples (Table 3). The observed heterozygosity (HO) and relatedness were not significantly different between the two groups. Genetic diversity and the degree of clonality among the groups of strains sampled in the Mariager Fjord indicated less diversity within sample 4, established from S. marinoi resting stages that settled in the sediment in 1980. This population had significantly lower allelic richness (P < 0.05), and two significantly (P < 0.05 after Bonferroni adjustment) linked loci were detected (S.mar1 and S.mar6).
Table 3.
Test for significant difference of genetic diversity between Mariager Fjord (n = 158) and Kattegat (n = 87) populations
| Diversity index | Differences | P value |
| Allelic richness | Mariager < Kattegat | 0.023 |
| FIS | Mariager < Kattegat | 0.029 |
| HS | Mariager < Kattegat | 0.004 |
| HO | Mariager = Kattegat | NS |
| Relatedness | Mariager = Kattegat | NS |
| Corrected relatedness | Mariager > Kattegat | 0.042 |
Discussion
We investigated the genetic structure of S. marinoi throughout a sediment archive spanning from recent times to more than 100 y ago, equivalent to ∼40,000 diatom mitotic generations. By germinating the resting stages from discrete layers of an anoxic sediment core and subsequently applying powerful molecular markers, we have revealed the population structure within the fjord. The Mariager Fjord maintains an endemic population and a reduced level of gene flow with adjacent populations from the Kattegat despite continuous water exchange. Throughout the investigated time period, the genetic structure within the fjord was homogenous, although weak genetic differentiation also was recorded within the fjord.
The viability of the diatom resting stages reported here is at least twice as long as the previously documented period of 65 y (1). The age of the upper layers of the sediment core is most certain, whereas the 210Pb dating is uncertain in the oldest two layers (layers 1 and 2, accumulated before 1921). Thus, our results suggest we have encountered and successfully cultured viable cells from sediment as old as 150 y or more (based on extrapolation of the 210Pb chronology). Survival rate of the strains from the germinated resting stages was high, and most strains were able to resume growth when placed in an illuminated nutrient-enriched medium. The highest proportion of strain survival was recorded in the 28-y-old sediment accumulation, whereas the strains germinated from the older layers displayed less viability, in agreement with previous studies of other aquatic organisms (21, 22).
S. marinoi reproduces mainly asexually; however high levels of genetic diversity characterizing the populations in this study imply occasional sexual reproduction. Sexual reproduction acts to diversify diatom populations. The frequency of sexual reproduction probably varies among different species and populations (23), and therefore the contribution made by the reproductive modes is difficult to estimate. The reproduction strategy has a significant effect on the allele and genotype frequencies, but the level of heterozygosity is influenced only when the proportion of asexually reproducing individuals is very high (24). In populations with mainly asexual propagation, large population sizes, high growth rates, and short generation time, genotypic diversity is maintained even if the proportion of sexually derived individuals is very low (25). The populations analyzed here all displayed heterozygote deficiency, possibly because of the mode of reproduction and nonrandom mating but also, especially in some loci, because of the presence of null alleles.
Our results indicate strong genetic differentiation between the S. marinoi populations in the Mariager Fjord and the Kattegat, despite weak or no physical dispersal barriers. Genetic differentiations of similar magnitudes between populations of seemingly well-connected habitats have been confirmed previously for S. marinoi (22), for a different diatom (26), and for a few other marine protists (10, 27). However, the most remarkable outcome is that diatom populations can be extremely long lived, and patterns of genetic structure are well conserved. Groups of strains established from revived resting stages from layers 1, 2, 5, 6, and 7, corresponding to sediment accumulated over a century, could not be distinguished. Thus, in the Mariager Fjord, despite constant water exchange and the influx of new potential colonizers from the Kattegat, the populations do not mix and most likely have not done so for thousands of generations. Investigations of the secondary structure of the ITS sequence predict meaningful intercrossing ability between the fjord and the open-sea populations, with a constant inflow of Kattegat individuals obtaining sufficient proximity for interbreeding. The paradox of reduced gene flow despite high dispersal capacities in aquatic organisms has been recorded for multicellular animals (cladocerans, rotifers, bryozoans) and macrophytes in aquatic habitats (28, 29). High genetic differentiation between well-connected habitats can be explained by rapid population growth after an historical founder event, enhanced by a large propagule bank buffering against new immigrants, and the rapid adaptation of the resident population to local conditions. These characteristics effectively increase the persistence of the founder event, and initial colonizers will strongly dominate the genetic structure through priority effects that preclude subsequent colonization (30). Because S. marinoi reproduces mainly asexually, dividing approximately once per day, selection for individual clonal lineages can be swift, resulting in rapid population differentiation. In addition, a rich seed bank with continuously germinating propagules will enhance the effect of genetic differentiation. Thus, the genetic stability of the Mariager Fjord population over thousands of generations suggests that the original population has adapted to the local environment, and the large population sizes in the plankton and benthos will effectively outcompete later invaders. An additional and complementary explanation for the complete separation between the two neighboring populations could be attributed to the means of propagation. During the diatom lifecycle, the cell size is reduced because of the way it divides. Some species can restore their cell size through vegetative cell enlargement, but for the majority sexual reproduction is a required stage to avoid critical cell sizes and subsequent death (31). Models of population growth and the timing of cell size-dependant sexual reproduction suggest that a small proportion of the parental population (0.05%) must reproduce at least every 4 y to avoid local extinction (32). We therefore hypothesize that differential selection pressure coupled with founder effects, infrequent sexual reproduction, and perhaps different periods of sexual induction prevent the open-sea cells from reproducing and colonizing the fjord.
Genetic diversity among the individuals from the Mariager Fjord was reduced significantly compared with the open-sea population. Ecologically marginal environments accommodate populations that experience extreme selection regimes and contain individuals that are genetically impoverished (33). The occurrence of eutrophication processes is known to be associated with reduced diversity (34) and serves as an explanation here, because Mariager Fjord is hypertrophic. Increased frequency of asexual reproduction reduces genetic variation, and among facultative asexual species, populations living in marginal environments tend to reproduce asexually to a large extent (35, 36). Thus, the reduced frequency of sexual reproduction within the Mariager Fjord as a consequence of the perturbed environment may explain the reduced genetic diversity compared with the open-sea population.
Some groups of strains isolated from the discrete sediment layers of the Mariager core were weakly but significantly differentiated (Table 2). Strains originating from the 1980 sediment accumulation (sample 4) were significantly differentiated from all other groups of strains except those isolated from samples 2 and 3. Four groups of strains (1, 2, 6, and 7) could not be distinguished. The reason for the similarities between strains isolated from the two most recent sediment layers can be attributed to the short time span between them, with the possibility that they overlap in time. The other similarity, that of samples 1, 2, 6, and 7, lacks the strong coupling in time but is the more interesting and challenging. All the other revived strains are decades younger than those isolated from sample 1. What the planktonic cells of strains 1, 2, 6, and 7 may have in common are the environmental conditions at the time they formed resting stages and sank to the bottom of the fjord. Nutrient loading had not reached an extreme when resting stages of samples 1 and 2 accumulated (19). During the accumulation of resting stages in sample 6 (2000) and 7 (2006), the nutrient loadings, especially P, had started to decrease drastically because of successful efforts to reduce nutrient concentrations by the end of the 1980s. Because of uncertain dating in pre-1921 sediment layers, no environmental data can be extrapolated from other analyzed sediment cores from the Mariager Fjord. The lower germination and culturing success from the older sediment layers also adds to the speculative nature of any conclusions. However, it is possible that similar environmental factors occurring >100 y apart in the same geographic area select from a pool of propagules in which all clones are represented, and specific genotypes shared by samples 1, 2, 6, and 7 are advantageous in an environment without a nutrient excess. The distribution of individuals along the FCA axis one (Fig. 2) may be an effect of the environmental conditions in the fjord. Individuals originating from samples 3 (1921) and 4 (1980) are positioned on the extreme right of axis one and probably are a remnant of the corresponding planktonic population that was influenced by eutrophication. Increasingly, during this period, the N and P load was reported as extreme (19). The reduced genetic diversity among the strains isolated from sample 4 relative to the other group of strains further indicates that the S. marinoi population lived in an extreme environment during this time. Information in coding loci would have been advantageous for establishing our hypothesis of differential selection in sample 4; however, we did not have any coding loci to compare with the noncoding loci. Neutral microsatellite loci will not be affected initially by divergent selection. However, given enough time in divergent environments, especially if more extensive asexual reproduction is present and at larger population sizes, neutral microsatellites also will become differentiated (37).
We have investigated the genetic structure of S. marinoi spanning more than a century and found that the population in a Danish fjord is genetically conserved. Dispersal potential via water exchange had no impact on the genetic structuring of the populations investigated. Instead, environmental conditions, such as the extreme eutrophication of the Mariager Fjord, are perhaps more important. With the exception of a period when the fjord was highly eutrophic and a weakly differentiated population displaying reduced genetic diversity was dominant, the S. marinoi population of the Mariager Fjord is genetically homogenous.
Materials and Methods
The sampling sites were located in Mariager Fjord on the east coast of Jutland, Denmark, and in the open sea of the Kattegat (Fig. S1). The narrow sill-fjord (42 km long) is connected to the Kattegat and has high primary production and a permanently anoxic basin (19). Tidal movement controls the exchange of saline water between the fjord and the Kattegat. Salinity is generally stable [20 practical salinity units (PSU)] in the deeper, inner part of the Mariager Fjord. The eastern part of the fjord is shallow (<6 m), and the maximum depth is 30 m. Thus, the shape of the fjord allows little water exchange, and the deep water in the inner part of the fjord is renewed on average once every 17 mo (17). Because the deeper part of the inner fjord is anoxic, there is no infauna and therefore no bioturbation. The Mariager Fjord has a sparse mesozooplankton community and periodically intense blooms of S. marinoi (38).
Sediment cores were collected on January 21, 2008, in Mariager Fjord (56°40.696′ N, 10°01.411′ E) at 30-m depth using a modified HAPS corer (KC-Denmark). The cores were kept in the dark at 4 °C. On February 2, 2008, a single core (MF08/II) was sliced into 1-cm samples from the bottom up to avoid smearing from younger to older layers. The outer few millimeters of each slice were removed. The remains were transferred to reclosable plastic bags and stored in the dark at 4 °C to prevent resting cells from germinating. Subsamples of every 1-cm layer (1- to 29-cm core depth) were analyzed for 210Pb, 226Ra, and 137Cs activity via gamma-spectrometry at the Gamma Dating Centre, Department of Geography, University of Copenhagen. The measurements were carried out on an ultra-low-background germanium detector (Canberra). 210Pb was measured by its gamma-peak at 46.5 keV, 226Ra by the granddaughter 214Pb peaks at 295 and 352 keV, and 137Cs by its peak at 661 keV. Content of unsupported 210Pb decreased quickly with depth in the sediment core to levels below the detection limit at depths deeper than 10 cm. The calculated flux of unsupported 210Pb was low, 18 Bq·m−2·y−1, indicating that the site might have experienced temporary erosion. The content of 137C was high in the top of the core and decreased quickly with depth. The profile showed similarities to that of the unsupported 210Pb, also indicating that the deposition profiles might be influenced to some extent by downward transport of surface material or mobility of 137C. The chronology of the core was established using a slightly modified constant rate of supply (CRS) model (39). The activity below 10 cm is calculated on the basis of a regression of unsupported 210Pb versus accumulated dry mass. The chronology is shown in Fig. S2. Monoclonal cultures of S. marinoi were established from seven discrete sediment layers from the Mariager Fjord core (Table 1). The layers for germination were selected to represent the periods before, during, and after nutrient excess was discharged to the Mariager Fjord. Recent open-sea sediment from the Kattegat (Anholt, depth 53 m, 56°40.00′ N, 12°07.00′ E, and Vinga, depth 77 m, 57°33.00′ N, 11°31.50′ E) was collected once at each location (Fig. S1). A box corer was used to collect undisturbed sediment samples. The top (<1 cm) of the sediment cores was scraped off and kept at 4 °C in the dark.
To establish monoclonal strains of S. marinoi, ∼1 g of sediment from each sediment sample was distributed in smaller aliquots and inoculated into 24-well plates (Nunc). The wells were filled with f/2 medium (40), 26 PSU. The sediment slurries were kept at 10 °C on a 12:12-h light:dark cycle at an irradiance of 60 μmol photons·m−2·s−1. Slurries were examined daily for germination and vegetative growth of S. marinoi using an Axiovert 135 inverted microscope (Zeiss). One cell chain from each well was isolated by micropipetting and then was transferred to a Petri dish (50-mm diameter) with 5 mL of f/2 medium and incubated under the same conditions as above. When growth was confirmed, the cells were transferred to 50-mL Nunc flasks with 10 mL of f/2 medium. After further propagation, another 40 mL of medium was added. Monoclonal cultures in the exponential phase were filtered onto Versapor-3000 filters with 25-mm diameter and 3-μm pore size (Pall Corporation) and were kept at −80 °C.
Genomic DNA was extracted from the monoclonal cultures following a phenol-chloroform–based protocol described in ref. 41. DNA was resuspended in 50 μL of sterile Milli-Q (Millipore) water. Eight microsatellite loci (S.mar 1–8) were amplified (42). PCR was run in total volumes of 24 μL consisting of ∼2 ng template DNA, Perkin–Elmer-PCR buffer, 0.33 μM of each primer, 250 μM of dNTP, 0.04 U of Taq polymerase (AmpliTaq Gold, Applied Biosystems), and sterile Milli-Q water. Amplification conditions and allele size assignment were as specified in ref. 22.
The internal transcriber spacer 1 (ITS1), 5.8S rRNA gene, and the ITS2 were sequenced from four isolates from the Mariager Fjord (strains originating from samples 2, 3, 4, and 6), four isolates from Anholt, and four isolates from Vinga. The DNA fragment from the 5′ end of the 5.8S ending in the conserved motif of helix III of the ITS2 has been proposed for bar coding of diatoms (43), and the identity of a 30-nt motif in the highly conserved region of the 5′ side of the ITS2 helix III predicts meaningful intercrossing ability among protists (44). The nuclear-encoded ITS region was amplified and sequenced according to the protocol in ref. 14. A consensus sequence for each clone was produced and aligned for comparison using Sequencher 4.1.2 (Gene Codes Corporation). The secondary structure of the ITS was analyzed as described in ref. 45. Blast searches (http://www.ncbi.nlm.nih.gov/BLAST) were performed on all obtained DNA sequences to compare their identity with S. marinoi sequences in the database. The sequences have been deposited in GenBank (accession numbers HM582852–63).
Deviations from the Hardy–Weinberg equilibrium (HWE) at each locus in each sample (10,000 Markov chain dememorizations, 20 batches, and 5,000 iterations per batch), genotypic linkage disequilibrium (LD) between pairs of loci in each sample, and genotypic LD between pairs of loci for all samples (10,000 dememorizations, 100 batches, and 5,000 iterations per batch) were estimated using Genepop v. 4.0.7 (46). The microsatellite dataset was examined for integer or inconsistent modulus, null alleles, and stuttering using MicroChecker 1.0 (47) (95% confidence interval and 1,000 randomizations). To investigate whether null alleles (20) could explain deviation from the HWE, the relationship between the null allele frequencies and the P values from the HWE tests was examined using χ2 tests.
Genetic differentiation between pairs of populations was determined by calculating pair-wise FST using Genetix version 4.05 (48) with 1,000 permutations and P values corrected using the Bonferroni test. An UPGMA cluster of the Mariager Fjord and the Kattegat populations was constructed based on Nei's genetic distance (49), using Tools for Population Genetic Analyses (TFPGA) v. 1.3 (50). Bootstrap values were based on 1,000 permutations. Bayesian analysis, as implemented in Structure version 2.2 (51), was used to gain further insight into the gene flow between different populations. We assessed the number of potential clusters (K) among the nine S. marinoi samples from five different runs at each K, ranging from 1 to 9, and calculated the estimated posterior log probability of the data, L(K), and the stability of assignment patterns across runs. For each run, a burn-in period of 100,000 generations and 100,000 Markov chain Monte Carlo replications was applied. We used the model of no-admixture ancestry and the assumption of correlated allele frequencies as suggested in ref. 52. An ad hoc statistic, ΔK, was calculated on the basis of the rates of change in the log likelihood of data between consecutive K values. ΔK assists in determining the uppermost hierarchical level of structure (53). An FCA that was carried out using genotypic data displays the structural relationships among the Mariager Fjord populations and individuals using Genetix 4.05 (48).
Microsatellite Tools for Excel (54) was used to search for identical genotypes. Tests for differences in genetic diversity between the Mariager Fjord and the Kattegat populations were examined using Fstat [allelic richness, observed heterozygosity (HO), FIS, gene diversity (HS), relatedness, and corrected relatedness, 1,000 permutations]. The extent of genetic diversity within the Mariager Fjord samples was assessed by allelic richness corrected for sample size, HO, HS, and FIS and was estimated for each sample using Fstat (55). Significant differences (P < 0.05) in genetic diversity between the Mariager Fjord groups of strains were estimated with pair-sampled t tests performed in SPSS version 16.0 for Mac (SPSS Inc.).
Supplementary Material
Acknowledgments
We thank Lovisa Jansson, Jenny Egardt, and Dr. Adil Yusuf Al-Handal for assistance in the laboratory, Prof. Carl André for help with statistics, and Dr. Tatiana Rynearson and two anonymous reviewers for constructive comments on the manuscript. The fragment analysis was performed at the Competence Centre for Clinical Research, Malmö University Hospital. ITS sequencing was done at the Genomics Core Facility, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, by Dr. Elham Rekabdar. This project was supported by Sida Grant SWE-2004-129, Swedish Research Council Formas Grant 2006-1892, Danish Research Council Grant 2111-04-0011, European Community-RI Action ASSEMBLE Grant 227799, and by SYNTHESYS Danish Taxonomic Facility, Kapten Carl Stenholms Donationsfond, Stiftelsen Lars Hiertas Minne, The Royal Society of Arts and Sciences in Gothenburg, Magnus Bergvalls Stiftelse, and Stiftelsen Oscar and Lilli Lamms Minne.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. HM582852–HM582863).
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013528108/-/DCSupplemental.
References
- 1.McQuoid MR, Godhe A, Nordberg K. Viability of phytoplankton resting stages in the sediments of a coastal Swedish fjord. Eur J Phycol. 2002;37:191–201. [Google Scholar]
- 2.Hairston NG, Kearns CM, Ellner SP. Phenotypic variation in a zooplankton egg bank. Ecology. 1996;77:2282–2392. [Google Scholar]
- 3.Dale B, Thorsen TA, Fjellså A. Dinoflagellate cysts as indicators of cultural eutrophication in the Oslofjord, Norway. Estuar Coast Shelf Sci. 1999;48:371–382. [Google Scholar]
- 4.Karlsen AW, et al. Historical trends in Chesapeake Bay dissolved oxygen based on benthic foraminifera from sediment cores. Estuaries. 2000;23:488–508. [Google Scholar]
- 5.Hairston NG, Vanbrunt RA, Kerrns CM, Engstrom DR. Age and survivorship of diapausing eggs in a sediment egg bank. Ecology. 1995;76:1706–1711. [Google Scholar]
- 6.Brede N, et al. The impact of human-made ecological changes on the genetic architecture of Daphnia species. Proc Natl Acad Sci USA. 2009;106:4758–4763. doi: 10.1073/pnas.0807187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finlay BJ. Global dispersal of free-living microbial eukaryote species. Science. 2002;296:1061–1063. doi: 10.1126/science.1070710. [DOI] [PubMed] [Google Scholar]
- 8.Rynearson TA, Armbrust EV. Maintenance of clonal diversity during a spring bloom of the centric diatom Ditylum brightwellii. Mol Ecol. 2005;14:1631–1640. doi: 10.1111/j.1365-294X.2005.02526.x. [DOI] [PubMed] [Google Scholar]
- 9.Evans KM, et al. Highly differentiated populations of the freshwater diatom Sellaphora capitata suggest limited dispersal and opportunities for allopatric speciation. Protist. 2009;160:386–396. doi: 10.1016/j.protis.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Nagai S, et al. Genetic structuring and transfer of marine dinoflagellate Cochlodinium polykrikoides in Japanese and Korean coastal waters revealed by microsatellites. Mol Ecol. 2009;18:2337–2352. doi: 10.1111/j.1365-294X.2009.04193.x. [DOI] [PubMed] [Google Scholar]
- 11.Kooistra WHCF, et al. Global diversity and biogeography of Skeletonema species (bacillariophyta) Protist. 2008;159:177–193. doi: 10.1016/j.protis.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Migita S. Sexual reproduction of centric diatom Skeletonema costatum. Bull Jap Soc Sci Fish. 1967;33:392–398. [Google Scholar]
- 13.Taylor R, Abrahamsson K, Godhe A, Wängberg S-Å. Seasonal variability in polyunsaturated aldehyde production potential between strains of Skeletonema marinoi (Bacillariophyceae) J Phycol. 2009;45:46–53. doi: 10.1111/j.1529-8817.2008.00625.x. [DOI] [PubMed] [Google Scholar]
- 14.Godhe A, McQouid MR, Karunasagar I, Karunasagar I, Rehnstam-Holm A-S. Comparison of three common molecular tools for distinguishing among geographically separated clones of the diatom Skeletonema marinoi Sarno et Zingone (Bacillariophyceae) J Phycol. 2006;42:280–291. [Google Scholar]
- 15.Clarke AL, et al. Long-term trends in eutrophication and nutrients in the coastal zone. Limnol Oceanogr. 2006;51:385–397. [Google Scholar]
- 16.Diaz RJ, Rosenberg R. Spreading dead zones and consequences for marine ecosystems. Science. 2008;321:926–929. doi: 10.1126/science.1156401. [DOI] [PubMed] [Google Scholar]
- 17.Fallesen G, Andersen F, Larsen B. Life, death and revival of the hypertrophic Mariager Fjord, Denmark. J Mar Syst. 2000;25:313–321. [Google Scholar]
- 18.Hansen PJ. Effect of high pH on the growth and survival of marine phytoplankton: Implications for species succession. Aquat Microb Ecol. 2002;28:279–288. [Google Scholar]
- 19.Ellegaard M, et al. Multi-proxy evidence of long-term changes in ecosystem structure in a Danish marine estuary, linked to increase nutrient loading. Estuar Coast Shelf Sci. 2006;68:567–578. [Google Scholar]
- 20.Brookfield JFY. A simple new method for estimating null allele frequency from heterozygote deficiency. Mol Ecol. 1996;5:453–455. doi: 10.1111/j.1365-294x.1996.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 21.Brendonck L, De Meester L. Egg banks in freshwater zooplankton: Evolutionary and ecological archives in the sediment. Hydrobiologia. 2003;491:65–84. [Google Scholar]
- 22.Godhe A, Härnström K. Linking the planktonic and benthic habitat: Genetic structure of the marine diatom Skeletonema marinoi. Mol Ecol. 2010;19:4478–4490. doi: 10.1111/j.1365-294X.2010.04841.x. [DOI] [PubMed] [Google Scholar]
- 23.Mann DG. Patterns of sexual reproduction in diatoms. Hydrobiologia. 1993;269/270:11–20. [Google Scholar]
- 24.Balloux F, Lehmann L, de Meeûs T. The population genetics of clonal and partially clonal diploids. Genetics. 2003;164:1635–1644. doi: 10.1093/genetics/164.4.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bengtsson BO. Genetic variation in organisms with sexual and asexual reproduction. J Evol Biol. 2003;16:189–199. doi: 10.1046/j.1420-9101.2003.00523.x. [DOI] [PubMed] [Google Scholar]
- 26.Rynearson TA, Newton JA, Armbrust EV. Spring bloom development, genetic variation, and population succession in the planktonic diatom Ditylum brightwellii. Limnol Oceanogr. 2006;51:1249–1261. [Google Scholar]
- 27.Alpermann TJ, Beszteri B, John U, Tillmann U, Cembella AD. Implications of life-history transitions on the population genetic structure of the toxigenic marine dinoflagellate Alexandrium tamarense. Mol Ecol. 2009;18:2122–2133. doi: 10.1111/j.1365-294X.2009.04165.x. [DOI] [PubMed] [Google Scholar]
- 28.Pálsson S. Microsatellite variation in Daphnia pulex from both sides of the Baltic Sea. Mol Ecol. 2000;9:1075–1088. doi: 10.1046/j.1365-294x.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 29.Campillo S, García-Roger EM, Carmona MJ, Gómez A, Serra M. Selection on life-history traits and genetic population divergence in rotifers. J Evol Biol. 2009;22:2542–2553. doi: 10.1111/j.1420-9101.2009.01871.x. [DOI] [PubMed] [Google Scholar]
- 30.De Meester L, Goméz A, Okamura B, Schwenk K. The monopolization hypothesis and the dispersal–gene flow paradox in aquatic organisms. Acta Oecol. 2002;23:121–135. [Google Scholar]
- 31.Round FE, Crawford R, Mann DG. The Diatoms. Cambridge: Cambridge Univ Press; 1990. [Google Scholar]
- 32.D'Alelio D, et al. The time for sex: A biennial life cycle in a marine planktonic diatom. Limnol Oceanogr. 2010;55:106–114. [Google Scholar]
- 33.Johannesson K, André C. Life on the margin: Genetic isolation and diversity loss in a peripheral marine ecosystem, the Baltic Sea. Mol Ecol. 2006;15:2013–2029. doi: 10.1111/j.1365-294X.2006.02919.x. [DOI] [PubMed] [Google Scholar]
- 34.Kerfoot WC, Weider LJ. Experimental paleoecology (resurrection ecology): Chasing Van Valen's Red Queen hypothesis. Limnol Oceanogr. 2004;49:1300–1316. [Google Scholar]
- 35.Eckert CG. The loss of sex in clonal plants. Evol Ecol. 2002;15:501–520. [Google Scholar]
- 36.Tatarenkov A, et al. Intriguing asexual life in marginal populations of the brown seaweed Fucus vesiculosus. Mol Ecol. 2005;14:647–651. doi: 10.1111/j.1365-294X.2005.02425.x. [DOI] [PubMed] [Google Scholar]
- 37.Thibert-Plante X, Hendry AP. When can ecological speciation be detected with neutral loci? Mol Ecol. 2010;19:2301–2314. doi: 10.1111/j.1365-294X.2010.04641.x. [DOI] [PubMed] [Google Scholar]
- 38.Tiselius P, et al. High reproduction, but low biomass: Mortality estimates of the copepod Acartia tonsa in a hyper-eutrophic estuary. Aquat Biol. 2008;2:93–103. [Google Scholar]
- 39.Appelby P. In: Tracking Environmental Change Using Lake Sediments. Volume 1: Basin Analysis, Coring and Chronological Techniques. Last W, Smol JP, editors. Dordrecht, The Netherlands: Kluwer; 2001. pp. 171–203. [Google Scholar]
- 40.Guillard RRL. In: Culture of Marine Invertebrate Animals. Smith W, Chanley M, editors. New York: Plenum; 1975. pp. 29–60. [Google Scholar]
- 41.Godhe A, et al. Quantification of diatom and dinoflagellate biomasses in coastal marine seawater samples by real-time PCR. Appl Environ Microbiol. 2008;74:7174–7182. doi: 10.1128/AEM.01298-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almany GR, De Arruda MP, Arthofer W, Atallah ZK, Beissinger SR, et al. Permanent genetic resources added to Molecular Ecology Resources Database 1 May 2009-31 July. Mol Ecol Resour. 2009;9:1460–1466. doi: 10.1111/j.1755-0998.2009.02759.x. [DOI] [PubMed] [Google Scholar]
- 43.Moniz MBJ, Kaczmarska I. Barcoding of diatoms: Nuclear encoded ITS revisited. Protist. 2010;161:7–34. doi: 10.1016/j.protis.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Coleman AW. Is there a molecular key to the level of “biological species” in eukaryotes? A DNA guide. Mol Phylogenet Evol. 2009;50:197–203. doi: 10.1016/j.ympev.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Mai JC, Coleman AW. The internal transcribed spacer 2 exhibits a common secondary structure in green algae and flowering plants. J Mol Evol. 1997;44:258–271. doi: 10.1007/pl00006143. [DOI] [PubMed] [Google Scholar]
- 46.Raymond M, Rousset F. GENEPOP (version 1.2): Population genetics software for exact tests and ecumenicism. J Hered. 1995;86:248–249. [Google Scholar]
- 47.Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P. MICRO-CHECKER: Software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes. 2004;4:535–538. [Google Scholar]
- 48.Belkhir K. GENETIX 4.05, logiciel sous Windows pour la génétique des populations. Montpellier, France: Laboratoire Génome, Populations, Interactions, CNRS UMR 5000, Université de Montpellier II; 2004. [Google Scholar]
- 49.Nei M. Genetic distance between populations. Am Nat. 1972;106:283–292. [Google Scholar]
- 50.Miller M. Tools for Population Genetic Analyses (TFPGA) version 1.3. A Windows Program for the Analysis of Allozyme and Molecular Population Genetic Data. Flagstaff, AZ: Department of Biological Sciences, Northern Arizona Univ; 1997. [Google Scholar]
- 51.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Falush D, Stephens M, Pritchard JK. Inference of population genetic structure: Extensions to linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol Ecol. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- 54.Park S. Trypanotolerance in West African Cattle and the Population Genetic Effects of Selection. Dublin: Univ of Dublin; 2001. [Google Scholar]
- 55.Goudet J. Fstat, a Program to Estimate and Test Gene Diversities and Fixation Indices (Version 2.9.3.2) 2001. Available at http://www2.unil.ch/popgen/softwares/fstat.htm. Accessed on June 1, 2010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.