Abstract
It has been suggested that the frontal operculum (fO) is a key node in a network for exerting control over cognitive processes. How it exerts this influence, however, has been unclear. Here, using the complementary approaches of functional MRI and transcranial magnetic stimulation, we have shown that the fO regulates increases and decreases of activity in multiple occipitotemporal cortical areas when task performance depended on directing attention to different classes of stimuli held in memory. Only one region, the fO, was significantly more active when subjects selectively attended to a single stimulus so that it determined task performance. The stimuli that guided task performance could belong to three categories—houses, body parts, and faces—associated with three occipitotemporal regions. On each trial, the pattern of functional correlation between the fO and the three occipitotemporal regions became either positive or negative, depending on which stimulus was to be attended and which ignored. Activation of the fO preceded both activity increases and decreases in the occipitotemporal cortex. The causal dependency of the distributed occipitotemporal pattern of activity increases and decreases on the fO was demonstrated by showing that transcranial magnetic stimulation–mediated interference of the fO diminished top-down selective attentional modulation in the occipitotemporal cortex, but it did not alter bottom-up activation of the same areas to the same stimuli when they were presented in isolation. The fO's prominence in cognitive control may stem from a role in regulating the level of activity of representations in posterior brain areas that are relevant or irrelevant, respectively, for response selection.
Keywords: top-down control, prefrontal cortex, bottom-up signal, category-selective regions, inhibition
It has been suggested that an inferior frontal region, the frontal operculum (fO), is a critical node in a network controlling activity in other brain areas to perform the wide array of cognitive tasks of which humans are capable (1–3). Activity in the fO, among other areas, predicts how well subjects will perform a task even many seconds into the future (4). The fO is also important when it is suddenly necessary to cease engagement in a task (5, 6).
Given that most frontal cortical areas have a unique pattern of anatomical connections it is likely that they are also functionally different (7). However, despite several demonstrations of the fO's activation in correlation with complex tasks requiring cognitive control, its frequent coactivation with dorsomedial and other frontal regions has made it difficult to determine the fO's specific contribution. Finally, showing that task-contingent changes in activity in other brain areas diminish when the fO is compromised is necessary to establish whether the fO exerts any causal influence over these coactivated brain regions.
In the current report, we examine whether the fO's contribution to cognitive control could partly be attributable to its exerting a changing, trial-by-trial, physiological influence over multiple higher-order sensory association areas as the type of stimulus that should determine task performance changes. We carried out two experiments in which human subjects performed a task (Fig. 1A and SI Methods) requiring match/nonmatch decisions about faces, houses, and body parts while functional MRI (fMRI) data were recorded. The stimulus type that determined performance changed from trial to trial. Each trial began with presentation of two stimuli drawn from two of these three categories. Four seconds later, on selective trials, a cue instructed subjects to focus on making a match/nonmatch decision about just one of the classes of stimuli (face, house, or body part) they had seen, which also meant that subjects could ignore the other stimulus class they had seen. After the offset of this cue plus a 1.5- to 2.5-s variable delay, an array of three stimuli, including one stimulus in every class, was presented, and the match/nonmatch decision was made. In a second type of trial, nonselective trials, the same sequence of events occurred with the exception that the cue now instructed subjects to attempt to make the same match/nonmatch judgments about both of the stimuli they had seen (subjects were informed that there would be a match for either one or none of the stimuli seen at the start of the trial). In experiment 1, we looked for evidence that a specific brain region was engaged in the process of selective attention. In experiment 2, we applied transcranial magnetic stimulation (TMS) over the fO, the region identified by the first experiment, in an attempt to disrupt fO function and test whether it determined the trial-by-trial changes in the distributed pattern of activity increases and decreases in the occipitotemporal cortex also seen in the experiment 1. The occipitotemporal pattern of facilitation and inhibition depended on which stimulus classes were attended and which were ignored. Initial localizer scans in both experiments made it possible to identify the occipitotemporal brain regions—the parahippocampal place area (PPA), fusiform face area (FFA), and extrastriate body area (EBA)—specialized for processing house, face, and body stimuli, respectively (8).
Fig. 1.
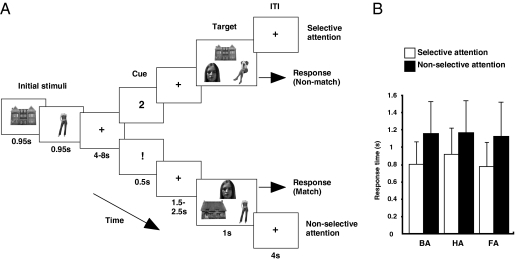
Schematic overview of experimental design and behavioral performance during scanning. (A) Subjects were initially presented with two stimuli drawn from two of three possible categories: body (whole body without head), house, and face. After a variable delay, a selective cue, indicated by a number (1 or 2), or a nonselective cue, indicated by an exclamation mark (!), instructed subjects to focus on the first or second stimulus they had seen or not to focus on only one stimulus but to try and hold both in mind. On presentation of the final target array containing one stimulus of all three types, subjects decided whether any of the three images matched the image (selective condition) or images (nonselective condition) they had been instructed to hold in mind. (B) Median response time in six conditions in which subjects indicated that body (BA), face (FA), and house (HA) stimuli were present under selective and nonselective conditions.
Results
In experiment 1, subjects responded significantly faster (F1, 19 = 66.543, P < 0.001; Fig. 1B) and more accurately on selective than nonselective trials (F1, 19 = 21.524, P < 0.001; Fig. S1). There was an interaction between selection and category of stimulus (response time: F2, 38 = 4.429, P = 0.0201; accuracy: F2, 38 = 14.684, P < 0.001). Nevertheless, it was clear that response times for selective trials were faster than for nonselective trials, when each stimulus category was considered in isolation (all t > 6.482, all P < 0.001). Furthermore, body- and face-matching judgments were both clearly more accurate on selective trials (both t > 6.281, both P < 0.002).
To identify regions associated with the selective guidance of task performance by a single stimulus class, we contrasted blood oxygen level–dependent (BOLD) signal changes to selective and nonselective trial cues. Although a number of brain regions were more active in the more difficult nonselective condition with higher demands on memory (Fig. S2 and Table S1), only a single bilateral region, the fO [Z = 3.89, Montreal Neurological Institute (MNI) coordinates x = −30, y = 22, z = −10, Z = 2.80, MNI x = 34, y = 18, z = −10], was more active in response to selective cues (Fig. 2A). There was no evidence that the fO signal was related to the match/nonmatch decision; even when using a low threshold, a supplementary analysis found no evidence (P > 0.1, uncorrected) of different fO activity on these two trial types. Moreover, fO activity correlated with the behavioral performance increases observed for the selective condition. Individual differences in the regression coefficients relating the fO BOLD response on selective trials were correlated with individual differences in faster response times on selective trials (left fO: r = 0.739, n = 20, P < 0.001; right fO: r = 0.566, n = 20, P = 0.001; Fig. 2B).
Fig. 2.
(A) Coronal (Left) and axial (Right) views of fO cue-locked activity, which were greater for the selective than the nonselective condition at a cluster-corrected threshold of P < 0.05, Z > 2.3 shown on the group average MRI scan. (B) Correlation between selective-attention effect size in left (Upper) and right (Lower) fO and decrement in median response time (RT) in the selective condition compared with the nonselective condition [(selective RT − nonselective RT)/nonselective RT].
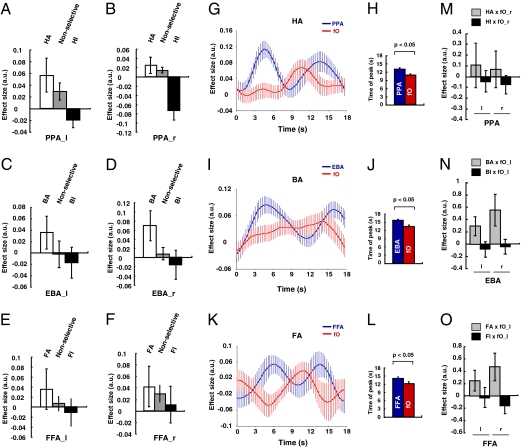
Next, we examined what changes occurred in the occipitotemporal areas—the PPA, FFA, and EBA—concerned with specific categories of stimuli. We were interested in whether activity in these areas was elevated in the selective-attention case, when stimuli handled by the area in question were the focus of attention or whether activity was diminished when stimuli handled by the area in question were no longer the focus of attention in the selective condition. We therefore compared activity in the PPA (Fig. 3 A and B) when subjects attended to houses (HA) or ignored houses (HI) in the selective condition with activity on nonselective trials that involved similar house stimuli (H!). Analogous analyses were performed on EBA BOLD signals (Fig. 3 C and D) on body attention (BA), body ignore (BI), and nonselective body trials (B!) and on FFA BOLD signals (Fig. 3 E and F) in face attend (FA), face ignore (FI), and nonselective face trials (F!). The BOLD signal in all three areas in both hemispheres was significantly modulated by whether subjects were selectively attending, nonselectively attending, or selectively ignoring stimuli of the category for which the area was specialized (F2, 12 = 5.385, P = 0.021). By focusing just on the selective-attention and nonselective conditions (Fig. 3 A–F, first and second bars), it was possible to show that selective attention resulted in an increase in activity in all three occipitotemporal regions (F1, 6 = 10.365, P = 0.018). Similarly, by focusing just on the trials in which subjects selectively ignored stimuli belonging to the category for which the area was specialized and by comparing the BOLD signal with the signal recorded in nonselective trials (Fig. 3 A–F, second and third bars), it was possible to assess whether ignoring a stimulus led to a decrement in activity in all three occipitotemporal regions. Although there initially appeared to be no decrement in activity (P > 0.05), it was partly because of the small size and variability of the decrement in the FI condition in the right and left FFA. A clear decrement was, however, apparent when the data from all three areas were considered but when the BOLD effects were time-locked to the presentation of the target array that followed the cue rather than to the cue itself (main effect of selective ignoring: F1, 9 = 7.634, P = 0.022; interaction of selective ignoring and area: F2, 18 = 6.474, P = 0.014; and interaction of selective ignoring and hemisphere: F1, 9 = 6.068, P = 0.036 reflected the weak decrement in the left FFA, but a decrease was clearly manifested in the right FFA: F1, 14 = 7.825, P = 0.014). It might be argued that the interpretation of such decrement effects is made difficult by not knowing against which baseline they should be compared. What can be said, however, is that BOLD effects, in all three occipitotemporal areas, associated with ignoring the preferred stimulus types were major in that they were no different than those associated with trials in which the preferred stimulus was never even presented (no main effect or interaction of trial type—selective ignoring versus stimulus never presented—with area or hemisphere: F2, 12 < 1.7, P > 0.1). In other words, occipitotemporal activity was in a similar state if a stimulus was ignored or if it was never seen in the first place. In summary, facilitation and, at a very slightly later time, a decrement of the BOLD signal were apparent in all three occipitotemporal areas, depending on whether the stimuli for which the areas were specialized were attended or ignored.
Fig. 3.
Selective-attention effects in the three occipitotemporal regions. (A−F) The mean cue-locked BOLD effect sizes were extracted for conditions in which subjects were selectively attending to or ignoring stimuli from the category for which each occipitotemporal region is specialized and for the nonselective condition in which subjects had seen a stimulus from the same category earlier in the trial: left PPA (PPA_l) (−29, −50, −12) (A), right PPA (PPA_r) (29, −47, −13) (B), left EBA (EBA_l) (−46, −79, −5) (C), right EBA (EBA_r) (49, −74, −4) (D), left FFA (FFA_l) (−37, −54, −22) (E), and right FFA (FFA_r) (41, −51, −24) (F), where the numbers in parentheses indicate mean MNI coordinates of the center of each individual's region of interest. (G–L) fO activation preceded activations in the three regions during selective attention. Time courses for the regressor coefficients relating the fO and PPA BOLD signal to the HA condition (G), the fO and EBA BOLD signal to the BA condition (I), and the fO and FFA BOLD signal to the FA condition (K) throughout the duration of the trial, respectively. There are two peaks in effect size in each occipitotemporal area at the time of initial stimulus presentation and at the time of presentation of the match array. The second peak is preceded by an fO peak in the HA (H), BA (J), and FA (L) conditions. (M−O) The fO engaged both in facilitatory and inhibitory interactions with the three occipitotemporal regions, the EBA, PPA, and FFA, during HA and HI (M), BA and BI (N), and FA and FI (O) conditions, respectively. Note interactions with the PPA were stronger with the right fO (M). All data are mean ± SEM.
To understand whether the fO was in a position to modulate activity in the occipitotemporal areas, we examined the time course of the regression coefficients relating the BOLD signal in each of the areas to the selective-attention manipulation. In Fig. 3 G–L, we focus on the HA, FA, and BA conditions. In the PPA (Fig. 3 G and H), the HA-related regression coefficient had two peaks on HA trials. The first peak occurred ∼4.8 s after trial onset, and the second peak was ∼6.6 s after the selective cue was presented. By contrast, the HA-related regression coefficient in the fO peaked only once ∼4.2 s after the selective cue. In summary, the PPA was first activated, in a “bottom-up” manner, by the isolated presentation of a house stimulus in the absence of any distracting information and in the absence of fO activation. Later in the trial, when subjects were focusing on the house stimulus within the context of distracting memories of other stimuli, there was “top-down” activity in the PPA, which was preceded by activity in the fO. An analogous pattern of activity was seen in the EBA and FFA on BA and FA trials, respectively (Fig. 3 I–L). A comparison of the timing of the fO peak regression coefficient and the second regression coefficient peak in the EBA, PPA, and FFA during BA, HA, and FA conditions revealed that the fO peak occurred significantly earlier than the occipitotemporal peaks (main effect of area: F1, 10 = 12.182, P = 0.005; no interaction of task and area: F2, 20 = 0.356, P > 0.05). A similar analysis was used to compare the fO regression coefficient peak time and the regression trough times in the EBA, PPA, and FFA during the inhibition conditions BI, HI, and FI, respectively (Fig. S3 A–C). Again, it revealed that the fO peak occurred significantly earlier than the inhibition of each occipitotemporal area (main effect of area: F1, 10 = 34.215, P < 0.001; no interaction of task and area: F2, 20 = 2.688, P > 0.05). In summary, fO signal modulation occurred before either facilitation or suppression of activity in all three occipitotemporal areas but only when the presence of multiple stimuli, rather than a single stimulus, meant that there was a need for selective attention.
To further examine whether the fO was in a position to both up- and down-regulate task-relevant and task-irrelevant representations in the occipitotemporal areas, we performed a psychophysiological interaction (PPI) analysis (9) (Fig. 3 M–O). To examine the role that fO exerted both when subjects were attending to houses and when they were ignoring houses, we carried out a PPI analysis that looked at the influence of fO activity in the context of the HA and HI conditions. Formally, these effects correspond to the HA psychological condition × fO physiological activity interaction term and HI psychological condition × fO physiological interaction term in a PPI analysis that also included main effects of fO activity, HA and HI. Note that independent effects of HA and HI can be estimated because the absence of HA does not imply HI. This possibility follows from using three different stimulus types—houses, faces, and body parts—in six combinations on selective trials. We then performed a repeated-measures ANOVA of the regression coefficients relating the BOLD signal to the HA × fO PPI interaction term and the HI × fO PPI interaction term in the three occipitotemporal areas in both hemispheres.
The regression coefficients representing the degree of interaction between each of the occipitotemporal brain regions and the fO differed between areas in a manner that depended on task condition (interaction of PPI type, HA × fO or HI × fO, and brain area: F1, 12 = 5.747, P = 0.034; Fig. 3M). Further investigation showed that the two PPIs had a different effect on the regression coefficients in the PPA (main effect of attention: F1, 16 = 4.981, P = 0.04) but not in the FFA (F1, 13 = 0.969, P > 0.1) or the EBA (F1, 19 = 1.726, P > 0.1). In other words, the influence of the fO on PPA activity, but on neither EBA nor FFA activity, was modulated by whether subjects were attending to or ignoring houses.
The same type of PPI analyses were conducted to examine the influence of fO activity in the context of, first, the BA and BI conditions and, second, the FA and FI conditions, with analogous results (Fig. 3 N and O). The fO entered into distinct patterns of functional interaction with the EBA, but not with the PPA or FFA, depending on whether subjects were attending to or ignoring body parts. There was a significant interaction of PPI type, BA × fO or BI × fO, and brain area (F1, 13 = 6.686, P = 0.023; Fig. 3N). Further investigation showed that the two PPIs had a different effect on the regression coefficients in the EBA (main effect of attention: F1, 19 = 6.076, P = 0.023) but not the FFA (F1, 13 = 2.748, P > 0.1) or EBA (F1, 17 = 0.266, P > 0.1). The fO entered into distinct patterns of functional interaction with the FFA, but not with the PPA or EBA, depending on whether subjects were attending to or ignoring faces. There was a significant interaction of PPI type, FA × fO or FI × fO, and brain area (F1, 13 = 4.744, P = 0.048; Fig. 3O). Further investigation showed that the two PPIs had a different effect on the regression coefficients in the FFA (main effect of attention: F1, 13 = 11.082, P = 0.005) but not the PPA (F1, 17 = 0.175, P = 0.681) or EBA (F1, 19 = 0.942, P = 0.344).
An alternative way to summarize the impact of fO activity on occipitotemporal activity is to test for differences between the consequences of attending to or ignoring the stimulus type associated with each occipitotemporal area in the PPI analyses. In other words, whether the HA, BA, and FA conditions had different consequences than the HI, BI, and FI conditions for the BOLD signal effects recorded in the PPA, EBA, and FFA, respectively, was tested (which corresponds to testing the differences between the gray and black bars in Fig. 3 M–O). There was a significant effect of the attentional manipulation (F1, 11 = 10.621, P = 0.008) that did not change across the occipitotemporal area (F2, 22 = 0.351, P > 0.1), and planned comparisons testing whether fO activity, on average, was positively correlated with activity in the occipitotemporal areas when the areas’ associated stimulus type was to be attended and negatively correlated with activity in the occipitotemporal areas when the areas’ associated stimulus type was to be ignored revealed that both effects were present (t11 = 2.745, P = 0.010; t11 = −1.968, P = 0.038).
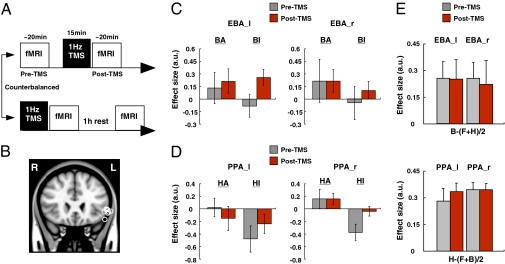
The relative timing of fO and occipitotemporal activity suggest that the fO could determine selective processing in the occipitotemporal cortex. To directly test this hypothesis, 1-Hz TMS was used to temporarily interfere with fO activity, and its effect on modulation of the BOLD signal in the occipitotemporal cortex was examined (Fig. 4). Despite being located in a sulcus, fO activity can be seen, even after thresholding, extending toward the brain surface and is therefore likely to be amenable to investigation with TMS (compare Figs. 2A and 4B). We have previously shown that 15 min of 1-Hz TMS leads to measurable changes in task-specific BOLD signal changes in brain regions interconnected with the site of stimulation for at least a further 15 min (10). Because such effects are likely to be short-lived (10), we adjusted the task so that it was optimized for collection of the maximum amount of fMRI data pertaining to the question of whether modulation of occipitotemporal activity by selective attention proceeds in the normal manner. Therefore, experiment 2 used just the selective-attention condition so that it would, for example, be possible to compare the EBA BOLD data in BA and BI conditions. The key prediction is that the BOLD signal-change difference between BA and BI conditions in EBA should be smaller after fO repetitive TMS if the fO determines occipitotemporal attentional modulation.
Fig. 4.
TMS-mediated interference of fO activity diminished the selective attentional modulation in the occipitotemporal regions. (A) TMS/fMRI design in experiment 2. Two fMRI sessions were performed by each subject (18–20 min). One session was preceded by a 1-Hz TMS application to the left fO (15 min). The order of scan sessions was counterbalanced across subjects. (B) TMS stimulation sites. Each circle represents MNI coordinates of an individual subject's fO TMS site (n = 9). (C and D) Effects of the fO TMS on left EBA (EBA_l) (−44, −79, −6), right EBA (EBA_r) (49, −75, −5), left PPA (PPA_l) (−26, −45, −17), and right PPA (PPA_r) (30, −44, −13) activity. (E) Effects of the fO TMS on EBA (Upper) and PPA (Lower) activity in response to the initial presentation of stimuli. All results are mean ± SEM.
Nine subjects completed two fMRI sessions before (pre-TMS) and after (post-TMS) the application of TMS to the left fO (because the left fO had been more activated than the right fO in the first experiment). Four subjects participated in the post-TMS session before participating in the pre-TMS session; scan order and task practice were therefore appropriately counterbalanced with respect to repetitive TMS application (Fig. 4 A and B). fO TMS diminished the difference in activation associated with top-down attend and ignore conditions (Fig. 4 C and D). Once again, localizer scans were used to localize the EBA, FFA, and PPA. The analysis focused on the PPA and EBA because the FFAs were only identified bilaterally in five of the subjects, and the analysis ideally required data from both hemispheres for each area. A four-way ANOVA was used to test whether application of TMS to the fO affected top-down modulation of activity in the EBA and PPA. The first three factors were area (EBA or PPA), hemisphere (left or right), and TMS (pre-TMS or post-TMS session). The final factor was attention; for EBA data, this factor compared BA trials and BI trials, whereas, for PPA data, this factor compared HA and HI trials. A two-way interaction between TMS and attention (F1, 5 = 30.115, P = 0.003) indicated fO TMS indeed influenced attentional modulation in the occipitotemporal cortex. The lack of higher-order interactions suggested a similar effect occurred regardless of area or hemisphere, which was confirmed by further analyses that demonstrated two-way TMS × attention interactions in the left EBA (F1, 7 = 390.376, P < 0.001), right EBA (F1, 5 = 11.955, P = 0.009), and right PPA (F1, 7 = 11.329, P = 0.012), although the interaction did not reach significance in the left PPA (F1, 7 = 2.572, P = 0.153).
We carried out two control analyses to assess the specificity of the fO TMS effects. In the first, we examined whether the impact of fO TMS on occipitotemporal modulation could be explained by any general distracting effect. Such a distracting effect is unlikely given that TMS ceased several minutes before the start of the occipitotemporal BOLD data collection. However, should any such distracting effect occur, it is likely to be proportional to the intensity of the TMS because this intensity will partly determine the degree of peripheral muscle and nerve activation. TMS intensity had been adjusted in relation to each subject's individual threshold for motor-evoked responses (individual differences in threshold probably reflect individual differences in scalp–cortex distance; ref. 11), but these differences in intensity failed to correlate with the modulatory effects seen in the EBA or PPA in left and right hemispheres (−0.310 < r < 0.614, P > 0.1, 8 < n < 9; Fig. S4).
A separate control analysis was conducted to test whether fO TMS effects were specific to the top-down modulation of occipitotemporal activity (Fig. 4E). In addition to the modulation of occipitotemporal activity that occurred ∼6.6 s after presentation of selective cues (Fig. 3 A–L and Fig. 4 C and D), there was also an earlier increase in occipitotemporal activity ∼4.8 s after the presentation of the initial stimuli (Figs. 1A and 3 G–L). Because subjects could have no prior expectations about the identity of these stimuli and because each stimulus was presented in isolation and in the absence of others competing for attention, any activity increases in the PPA, EBA, or FFA that occurred in response to houses, body parts, or faces, respectively was likely to be bottom-up and stimulus-driven in nature. Unlike the second peak in the occipitotemporal BOLD signal, such effects were not preceded by prior activation of the fO (Fig. 3 G–L). The analysis of stimulus-driven, bottom-up effects was conducted in a similar manner to the one used to investigate top-down effects. There were four factors and, as before, the first three were area (EBA or PPA), hemisphere (left or right), and TMS (pre-TMS or post-TMS session). Now, however, the final factor was stimulus type; for the EBA data, this factor compared trials in which subjects saw body parts (B) and trials in which they saw either houses or faces (HF), whereas for PPA data, this factor compared trials in which subjects saw houses (H) and trials in which they saw either body parts or faces (BF). There was no two-way interaction of TMS and stimulus type (F1, 7 = 0.008, P = 0.930) nor was there any higher-order interaction involving these two factors (all F1, 7 < 1, P > 0.1). In conclusion, although the fO TMS diminished top-down modulation of occipitotemporal activity at the end of each selective trial, it had no impact on bottom-up, stimulus-driven modulation at the beginning of the same trials several seconds earlier.
Discussion
Although prefrontal activity is known to occur in situations that require switching of attention between different categories of information and, therefore, activation of different posterior brain regions (12–14) this study is a unique demonstration that activity in a particular prefrontal region determines multiple patterns of task-dependent activity change in a network of specific posterior brain regions. As such, it complements and extends earlier demonstrations that large prefrontal lesions disrupt attentional modulation of scalp-recorded event-related potentials (15) and that frontal eye field TMS modulates event-related potentials during task switching (16). It has been suggested that the frontal eye field and ventrolateral prefrontal cortex have complementary roles in selecting information that should guide behavior (17). Unlike in previous experiments, it was the latter system that was investigated in the current experiment.
Lateral and dorsomedial frontal cortical activity is augmented when people are engaged in difficult cognitive tasks (18). Although a number of brain regions were activated in the more difficult nonselective condition in the present experiment, in which subjects had to retain information about a greater number of stimuli (Fig. S2 and Table S1), only the fO was more active, bilaterally, in the easier selective-attention condition. In the easier, selective condition, subjects were in a position to exert greater control over the manner in which they performed the task. Consistent with reports that populations of prefrontal neurons encode both match and nonmatch decisions, there was no difference in activation on selective trials that resulted in correct match or nonmatch decisions (19).
It is widely held that the prefrontal cortex exerts a top-down influence over other brain areas. Implicit in this view is that influence is not restricted to a single area or circuit but rather encompasses a collection of posterior brain regions. Such a widespread pattern of influence would be different from the more specific influence exerted by, for example, one sensory area over another (20). Although there is evidence that some prefrontal regions influence particular posterior brain circuits (13, 21), the evidence that a single prefrontal area influences processing in several posterior brain regions has been weak. Even if a single prefrontal area is shown to be active as a person switches from one of two tasks to another and back again, it is possible that its activation reflects engagement and disengagement of just one of the component tasks rather than independent modulatory influences specific to each task. By using three different selective-attention conditions, when faces, houses, and body part information were determining task performance and the FFA, PPA, and EBA were predominantly active, it was possible to show that control by the fO was not just restricted to one posterior brain region.
It is difficult to be certain of the precise identity of the fO region and its correspondence with the frontal cortical regions studied in macaques. One team that has made careful comparisons of ventral prefrontal cortex in macaques and humans (22) has labeled activity in an almost identical fO location, found as subjects switched task sets in a version of the Wisconsin Card Sorting Task, as belonging to area 47/12 (23). Activity related to switches of attention has been reported in this region by others (24). Such an assignment would make sense because 47/12 is distinguished by strong interconnections with a number of sensory association areas (25), which may underlie its role in retrieving information from posterior association cortex (26). In the macaque, neurons in this region encode transitions between learned task events (27), and lesions to this region disrupt learning of learned arbitrary task relationships (28). This disruptive effect, however, is partly a consequence of a failure of identification of the environmental features that should guide task performance (29).
A region that includes the pars opercularis of the inferior frontal gyrus and the tissue posterior to the inferior limb of the precentral sulcus is active when people suppress motor responses (5) and, when stimulated, causes changes in motor cortical activity (30, 31). Despite the similarity in their names, this region is more closely associated with the premotor cortex and it is distinct to the fO; it has strong connections to anterior inferior parietal cortex but is not so densely connected with the occipitotemporal cortex (32, 33). This area is 34.8 mm from the stimulation site in the present experiment, and so it is unlikely to have mediated the effects that we saw.
The nature of the modulatory influence exerted by the prefrontal cortex has been unclear. Particular emphasis has been given to the possibility that some inferior frontal regions exert an inhibitory influence over activity in other brain regions, and there is now evidence that they influence activity in the motor cortex when subjects try to stop themselves from making a response (30, 34). Whether such regions also exert facilitatory influences over other brain regions has been unclear. The patterns of functional connectivity observed between the fO and the three occipitotemporal regions investigated (Fig. 3 M–O), however, suggest that, even if this region does exert an inhibitory influence over other brain regions, it is also able to exert control by facilitating representations in posterior brain areas.
If anything it is more questionable whether it is correct to refer to the decrements in occipitotemporal activity observed in the present study as inhibitory effects. That trial-by-trial variation in occipitotemporal decrements were associated with trial-by-trial-by-trial variation in the fO signal increases (Fig. 3 M–O) suggests that, whether the decrements are best described as inhibition or as a removal of facilitation, they appear to reflect an active rather than a passive process. More direct evidence for an inhibitory process that depends on the inferior frontal cortex comes from a study in which activity in this area was found when words that were to be ignored were presented; activity levels predicted the subsequent proactive interference that the words exerted (17).
The prominence of the fO in studies of task control (1–4) may reflect a dual role that the region has in using arbitrary rules to guide response selection, such as the use of match and nonmatch rules (28), and in retrieving information from posterior cortical regions to do so (26). As task performance demands change, then so do the modulatory influences of this region on posterior brain areas.
Methods
Twenty-one (eight females, mean age = 21.8 y, range = 19–29 y) and nine (two females, mean age = 25.1 y, range = 19–41 y) right-handed, healthy subjects participated in the fMRI and TMS/fMRI experiments, respectively. MRI data were acquired on a 3T Siemens MRI scanner with maximum gradient strength 40 mT·m−1 at the Oxford Centre for Clinical Magnetic Resonance Imaging. fMRI analysis was carried out with tools from the software library of the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB). A general linear model included explanatory variables for all phases of a trial, which were convolved with the hemodynamic response function (HRF). The onsets for the following events were entered into the model. For the main attentional task, 17 regressors were created: BA cues, HA cues, FA cues, BI cues, HI cues, FI cues, nonselective cues, initial body stimuli, initial house stimuli, initial face stimuli, target array, and six motion regressors produced during realignment. For localizer tasks, three regressors, presentation of body stimuli, house stimuli, and face stimuli, were included. In the PPI analyses, time courses were deconvolved to remove the effects of the HRF, multiplied with the psychological regressor of interest, and reconvolved with the canonical HRF. The PPI regressors, together with the fO time series and psychological regressor, were then entered into the general linear model as confound regressors. The parameter estimates were extracted for each subject and region of interest. Further details of fMRI and TMS procedures and localizer tasks are presented in SI Methods.
Supplementary Material
Acknowledgments
We thank MaryAnn Noonan, Vanessa Johnen, Steven Knight, and Jan Scholz. This work was supported by the Human Frontier Science Program (T.H.) and the Medical Research Council (M.F.S.R. and R.B.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013361108/-/DCSupplemental.
References
- 1.Dosenbach NU, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fair DA, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dosenbach NU, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eichele T, et al. Prediction of human errors by maladaptive changes in event-related brain networks. Proc Natl Acad Sci USA. 2008;105:6173–6178. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: Inhibition and attentional control. Neuroimage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Passingham RE, Stephan KE, Kötter R. The anatomical basis of functional localization in the cortex. Nat Rev Neurosci. 2002;3:606–616. doi: 10.1038/nrn893. [DOI] [PubMed] [Google Scholar]
- 8.Reddy L, Kanwisher N. Coding of visual objects in the ventral stream. Curr Opin Neurobiol. 2006;16:408–414. doi: 10.1016/j.conb.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Friston KJ, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 10.O'Shea J, Johansen-Berg H, Trief D, Göbel S, Rushworth MF. Functionally specific reorganization in human premotor cortex. Neuron. 2007;54:479–490. doi: 10.1016/j.neuron.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 11.Stokes MG, et al. Simple metric for scaling motor threshold based on scalp-cortex distance: Application to studies using transcranial magnetic stimulation. J Neurophysiol. 2005;94:4520–4527. doi: 10.1152/jn.00067.2005. [DOI] [PubMed] [Google Scholar]
- 12.Lepsien J, Nobre AC. Attentional modulation of object representations in working memory. Cereb Cortex. 2007;17:2072–2083. doi: 10.1093/cercor/bhl116. [DOI] [PubMed] [Google Scholar]
- 13.Gazzaley A, et al. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb Cortex. 2007;17(Suppl 1):i125–i135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- 15.Knight RT. Attention regulation and human prefrontal cortex. In: Thierry A-M, Glowinski J, Goldman-Rakic PS, Christen Y, editors. Motor and Cognitive Functions of the Prefrontal Cortex. Berlin: Springer; 1994. pp. 161–173. [Google Scholar]
- 16.Morishima Y, et al. Task-specific signal transmission from prefrontal cortex in visual selective attention. Nat Neurosci. 2009;12:85–91. doi: 10.1038/nn.2237. [DOI] [PubMed] [Google Scholar]
- 17.Nee DE, Jonides J. Common and distinct neural correlates of perceptual and memorial selection. Neuroimage. 2009;45:963–975. doi: 10.1016/j.neuroimage.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 19.Kusunoki M, Sigala N, Nili H, Gaffan D, Duncan J. Target detection by opponent coding in monkey prefrontal cortex. J Cogn Neurosci. 2010;22:751–760. doi: 10.1162/jocn.2009.21216. [DOI] [PubMed] [Google Scholar]
- 20.Hupé JM, et al. Cortical feedback improves discrimination between figure and background by V1, V2 and V3 neurons. Nature. 1998;394:784–787. doi: 10.1038/29537. [DOI] [PubMed] [Google Scholar]
- 21.Sakai K, Rowe JB, Passingham RE. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nat Neurosci. 2002;5:479–484. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- 22.Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- 23.Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: Distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cereb Cortex. 2006;16:1679–1689. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- 25.Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1995;363:642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- 26.Petrides M. Specialized systems for the processing of mnemonic information within the primate frontal cortex. Philos Trans R Soc Lond. 1996;351:1455–1462. doi: 10.1098/rstb.1996.0130. [DOI] [PubMed] [Google Scholar]
- 27.Sigala N, Kusunoki M, Nimmo-Smith I, Gaffan D, Duncan J. Hierarchical coding for sequential task events in the monkey prefrontal cortex. Proc Natl Acad Sci USA. 2008;105:11969–11974. doi: 10.1073/pnas.0802569105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wise SP, Murray EA. Arbitrary associations between antecedents and actions. Trends Neurosci. 2000;23:271–276. doi: 10.1016/s0166-2236(00)01570-8. [DOI] [PubMed] [Google Scholar]
- 29.Rushworth MF, et al. Attentional selection and action selection in the ventral and orbital prefrontal cortex. J Neurosci. 2005;25:11628–11636. doi: 10.1523/JNEUROSCI.2765-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neubert FX, Mars RB, Buch ER, Olivier E, Rushworth MF. Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proc Natl Acad Sci USA. 2010;107:13240–13245. doi: 10.1073/pnas.1000674107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buch ER, Mars RB, Boorman ED, Rushworth MF. A network centered on ventral premotor cortex exerts both facilitatory and inhibitory control over primary motor cortex during action reprogramming. J Neurosci. 2010;30:1395–1401. doi: 10.1523/JNEUROSCI.4882-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Croxson PL, et al. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. J Neurosci. 2005;25:8854–8866. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrides M, Pandya DN. Distinct parietal and temporal pathways to the homologues of Broca's area in the monkey. PLoS Biol. 2009;7:e1000170. doi: 10.1371/journal.pbio.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swann N, et al. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J Neurosci. 2009;29:12675–12685. doi: 10.1523/JNEUROSCI.3359-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.