Abstract
Histone deacetylase inhibitors (HDACi) have been successfully used as monotherapies for the treatment of hematological malignancies; however, the single agent effects of HDACi against solid tumors are less robust. Using preclinical models of lymphoma, we have recently demonstrated that HDACi induce tumor cell-specific apoptosis and that this is essential for the therapeutic effects of these agents. Herein, we demonstrate that HDACi can be combined with immune-activating antibodies designed to promote the function of antigen-presenting cells (APCs) and enhance proliferation and survival of cytotoxic T cells (CTL) to stimulate a host antitumor immune response resulting in eradication of established solid tumors. This unique combination therapy was dependent on tumor cell apoptosis mediated by HDACi that stimulated the uptake of dead tumor cells by APCs. Tumor eradication was mediated by CD8+ CTL that used perforin as the key immune effector molecule. This combination therapy was well tolerated and induced long-term immunological antitumor memory capable of mediating spontaneous tumor eradication upon rechallenge. These studies indicate that the ability of HDACi to mediate subtherapeutic levels of tumor cell apoptosis can be exploited by combining with antibodies that augment host antitumor immune responses to mediate robust and prolonged eradication of solid tumors.
Keywords: anticancer therapy, epigenetic regulatory agent, immunogenic cell death
Chemotherapy and radiation can efficiently kill tumor cells (1) and recent studies indicate that certain chemotherapeutics (e.g., anthracyclines) and radiotherapy can induce a form of “immunogenic cell death” that results in concomitant antitumor immune responses (2–5). The demonstrated protective role of the host immune system against tumor onset and progression in experimental mouse models of cancer, together with accumulating clinical data, provides strong evidence that cancer immunosurveillance functions as an effective extrinsic tumor suppressor mechanism (6–8). Accordingly, harnessing and enhancing patient antitumor immune responses for the control of tumor growth concomitant with directly killing tumor cells is an appealing therapeutic strategy.
Previous studies from our laboratory have shown that combining tumor cell apoptosis induced by an agonistic anti–TNF-related apoptosis-inducing ligand (TRAIL) receptor mAb (MD5-1) with immunomodulatory anti-CD40 and anti-CD137 mAbs that stimulate antigen-presenting cells (APCs) and costimulate cytotoxic T cells (CTLs), respectively (termed trimAb), potently eradicated established solid tumors (9). That study provided compelling evidence that combining tumor cell apoptosis with immune modulation was therapeutically viable (9). Although trimAb therapy was effective against a range of TRAIL-sensitive solid tumors, the requirement for inherent TRAIL sensitivity of tumor targets for therapeutic success limits the broader application of this therapy. The use of alternate apoptotic stimuli that circumvent TRAIL sensitivity may expand the application of this immunotherapeutic strategy. This is of particular importance with the identification of an increasing number of TRAIL-resistant malignancies (10). Based on our previous studies demonstrating that inactivation of the TRAIL death pathway had no effect on the ability of histone deacetylase inhibitors (HDACi) to mediate tumor cell apoptosis (11, 12), we hypothesized that these agents would be suitable for testing in combination with immune-stimulating antibodies.
HDACis are a promising class of anticancer agents that can selectively kill tumor cells, block cell cycle progression, and induce cellular differentiation (13, 14). Using genetic mouse models of cancer, we and others have demonstrated a direct link between HDACi-mediated apoptosis and therapeutic efficacy (11, 12, 15). In addition to intrinsic effects on tumor cells, HDACi may affect neoplastic growth by regulating host immune responses and tumor vasculature (13). Indeed, enhanced tumor cell immunogenicity through HDACi-mediated up-regulation of MHC, costimulatory, and adhesion molecules (16–19) has been shown to both activate IFNγ-secreting T cells (19) and increase susceptibility of tumor cells to CTL-mediated destruction (18). Moreover, the ability of HDACi to stimulate the differentiation of CD8+ effector T cells to functional memory cells provides further evidence that combining HDACi with immunostimulatory therapeutics may prove effective (20, 21).
Using syngeneic murine tumor models of mammary, renal, prostate, and colorectal carcinomas, we demonstrated herein that combining the HDACis vorinostat and panobinostat with agonistic mAbs that target CD40 and CD137 (termed V/bimAb and P/bimAb, respectively) was highly efficacious for the treatment of established solid tumors within a therapeutic window that was safe and well tolerated. Furthermore, this unique combination strategy demonstrated that combining an HDACi with targeted stimulation of APC function and T cell survival and expansion can generate a CTL response important for the eradication of solid tumors. Subsequent tumor rechallenge experiments indicated the strong possibility that long-term immunological antitumor memory has been induced. HDACi treatment did not appear to alter the immunogenicity of tumor targets; however, HDACi-induced tumor cell apoptosis was critical for uptake of tumor cells by APCs and therapeutic efficacy. We identified a critical role for CD8+ and natural killer (NK) cells but not CD4+ cells for therapeutic efficacy. Moreover, we showed that host IFNγ and perforin but not TRAIL were essential for tumor eradication and that V/bimAb and P/bimAb therapy was effective for the treatment of TRAIL-resistant malignancies.
Results
V/bimAb Therapy Potently Eradicates Established Solid Tumors of Diverse Tissue Origins.
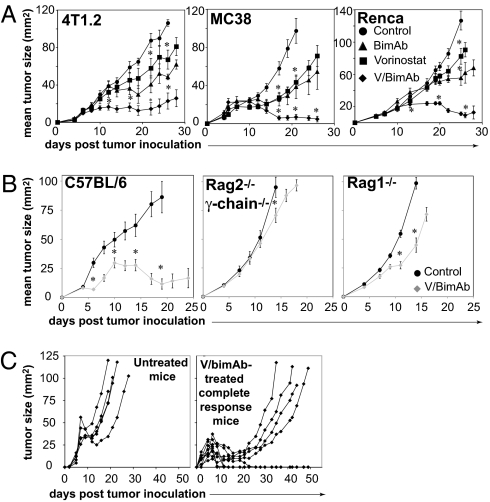
We have previously shown that vorinostat induces dose-dependent apoptosis of murine 4T1.2 (mammary), Renca (renal), and MC38 (colon) carcinoma cells in vitro (22). We investigated whether combining vorinostat with bimAb (V/bimAb) would prove therapeutically beneficial against established solid tumors. Treatment with vorinostat alone slightly delayed growth of established tumors; however, it did not induce any complete tumor regressions (Fig. 1A). BimAb treatment slightly suppressed the growth of all tumors tested (Fig. 1A); however, tumor regression was only observed in a small percentage (17%) of mice bearing MC38 tumors. In contrast, V/bimAb treatment significantly delayed the outgrowth of all three tested carcinomas such that tumors completely regressed below palpable detection in mice bearing 4T1.2, MC38, and Renca carcinomas (25, 56, and 25%, respectively) (Fig. 1A).
Fig. 1.
V/bimAb therapy induces the regression of established s.c. carcinoma of diverse tissue origins. (A) Cohorts of mice with established (>9 mm2) s.c. carcinoma (4T1.2, MC38, or Renca) were treated i.p. with 150 mg/kg vorinostat daily for 7 d. The dose was then decreased to 100 mg/kg for the following 8 d. BimAb (100 μg anti-CD137, 25 μg anti-CD40) was administered every 4 d for 4 doses. Mice were treated with either vorinostat and bimAb (V/bimAb, n = 8), bimAb or vorinostat alone (n = 6), or vehicle with isotype mAb (control, n = 6). Tumor growth was assessed every 2–3 d; mean tumor size ± SEM are shown. (B) Therapeutic efficacy of V/bimAb was also assessed in Rag2−/−c-γ-chain−/− and Rag1/J mice with established MC38 carcinomas. (C) Mice that achieved complete tumor regressions (MC38, from A) when treated with V/bimAb were rechallenged on the opposite hind flank with 1 × 106 MC38 and tumor growth assessed compared with naïve C57BL/6 mice. Tumors spontaneously regressed in 5/9 rechallenge mice, demonstrating V/bimAb therapy facilitates the generation of long-term immunological memory. Data shown is representative of at least two independent experiments. Enhancement of therapeutic efficacy comparing bimAb alone and in combination with vorinostat and single agent activity compared with control was assessed using the Mann-Whitney test; *P < 0.05.
Complete eradication of tumors from mice bearing MC38 and Renca tumors following V/bimAb treatment was maintained (>100 d) after the completion of V/bimAb therapy. Similar antitumor responses were observed when a different HDACi, panobinostat, was combined with bimAb for the treatment mice with established RM1 (prostate), CT26 (colon), and 4T1.2 carcinomas (Fig. S1A). TrimAb therapy was more efficacious than P/bimAb therapy for the treatment of established TRAIL pathway-sensitive tumors CT26 and 4T1.2 (80% and 100% complete tumor regressions, respectively, using TrimAb). However, P/bimAb therapy was more efficacious for the treatment of established TRAIL-resistant RM1 tumors (no complete tumor regressions recorded using trimAb; Fig. S1A).
No overt signs of toxicity were observed in the control, vorinostat alone, or bimAb alone treatment groups, with all mice gaining weight during the experiment. V/bimAb-treated, tumor-bearing mice initially decreased in weight upon commencement of therapy but stabilized while undergoing therapy and demonstrated an overall weight gain over the course of the experiment. We have therefore demonstrated using syngeneic s.c. tumor transplant models that V/bimAb and P/bimAb are highly efficacious and safe for the treatment of carcinomas of diverse tissue origins.
Because previous studies have demonstrated some toxic side effects may arise following administration of CD40 agonists (23–25), we substituted the agonistic anti-CD40 mAb with the NKT cell glycolipid α-c-galactosylceramide (α-c-GC). α-c-GC presentation to invariant NKT cells by dendritic cells (DCs) stimulates production of key cytokines, including IFNγ and IL-4, the downstream effects of which include activation of CD8+ T cells, NK cells, DCs, and B cells (26). Mice bearing established s.c. CT26 tumors were treated with panobinostat combined with α-c-GC and anti-CD137 mAb (P/α-c-GC/anti-CD137). The combination of all three therapeutic reagents demonstrated potent antitumor activity inducing the regression of 5/5 tumors (Fig. S1B). These data are consistent with those previously published by our group, demonstrating α-c-GC can substitute for anti-CD40 mAb in trimAb therapy (27).
V/bimAb-Induced Tumor Regressions Are Immune Mediated.
To determine if an adaptive immune response was important in V/bimAb-mediated rejection of MC38 tumors, we assessed the therapeutic efficacy of V/bimAb in Rag2 common-γ chain knockout mice (Rag2−/−c-γ-chain−/−) and Rag1 knockout mice (Rag1−/−) (Fig. 1B). V/bimAb was effective against MC38 tumors grown in C57BL/6 mice with complete tumor regressions observed in 50% of treated mice (Fig. 1B). However, in Rag2−/−c-γ-chain−/− and Rag1−/− mice, V/bimAb was ineffective against MC38 tumors; no tumor regressions were recorded and little if any delay in tumor growth was observed (Fig. 1B). We next assessed the development of a long-term, tumor-specific immune memory response in mice with primary tumor eradication following treatment with V/bimAb. Mice bearing MC38 tumors that had achieved complete tumor regressions following V/bimAb therapy were rechallenged with MC38 cells on the opposite hind flank >100 d after the end of the first experiment. As expected, MC38 tumors progressed rapidly in naïve untreated C57BL/6 mice (Fig. 1C). In contrast, when mice that had effectively cleared MC38 tumors following initial treatment with V/bimAb were subsequently rechallenged, only four of nine secondary challenge tumors grew, with delayed kinetics compared with naïve C57BL/6 mice. Importantly, the remaining five mice spontaneously rejected the growth of secondary MC38 tumors (Fig. 1C). Similar spontaneous tumor rejection was also observed in C57BL/6 mice originally engrafted with CT26 tumors and cured with P/α-c-GC/anti-CD137 mAb (i.e., the mice from Fig. S1B). These findings suggest that treatment with V/bimAb and P/α-c-GC/anti-CD137 mAb results in the generation of long-term protective adaptive immunity capable of the rejection of secondary tumor challenge.
Vorinostat Does Not Increase Expression of Immune-Regulatory Molecules.
We hypothesized that direct killing of target tumor cells by vorinostat and/or the ability of vorinostat to up-regulate cell surface expression of immune-modulating molecules may be important for the observed synergy with bimAb. We assessed the expression of key immune molecules on the cell surface of MC38 tumors following treatment with vorinostat (5 μM) for 16 h (Fig. S2A). Minimal to no changes in the expression of MHC Class I (Kb and Db) and Class II (IAb), CD40 and CD137, or the NK cell ligand family (Rae) were observed in MC38 cells following treatment with vorinostat (Fig. S2A). Similarly, the adhesion molecule ICAM-1, CD70 (the ligand for CD27, a receptor that supports T-cell expansion), and the costimulatory ligands CD80 and CD86 were largely unchanged following treatment with vorinostat (Fig. S2A). We therefore conclude that in contrast to other studies using different systems (16–19, 28, 29), vorinostat does not substantially induce expression of immune regulatory molecules on the surface of tumor cells.
Role of Vorinostat-Induced Cell Death in Mediating Synergy with Anti-CD40 and Anti-CD137 mAbs.
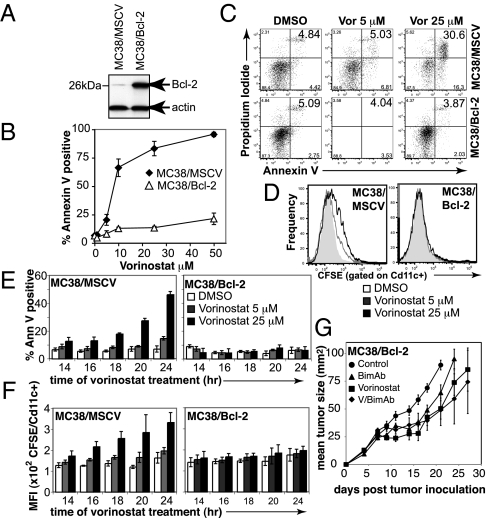
We next determined the link between vorinostat-induced tumor cell apoptosis and uptake by APCs. We had previously demonstrated overexpression of prosurvival Bcl-2 family proteins inhibited HDACi-induced apoptosis (11, 12, 22, 30). MC38 cells were therefore retrovirally transduced to overexpress Bcl-2 (Fig. 2A) and, in comparison with MC38 cells transduced with the empty MSCV vector, were resistant to vorinostat-induced apoptosis (Fig. 2 B and C).
Fig. 2.
Vorinostat-induced apoptosis is required for therapeutic efficacy of V/bimAb. (A) MC38 carcinomas retrovirally transduced to overexpress murine Bcl-2 (MC38/Bcl-2) were assessed for overexpression of exogenous protein by Western blotting. (B) Empty vector control cells (MC38/MSCV) and MC38/Bcl-2 cells were assessed for in vitro sensitivity to vorinostat following culture for 48 h. Data shown are the mean ± SEM of three independent experiments. (C) Tumor cell apoptosis following treatment of MC38/MSCV and MC38/Bcl-2 cells with 5 μM and 25 μM vorinostat for 24 h was assessed using annexinV/propidium iodide staining and flow cytometry. Representative flow cytometry profiles are shown. (D) Engulfment of CFSE-labeled vorinostat-treated (24 h) MC38/MSCV and MC38/Bcl-2 cells by bone marrow-derived CD11c+ APCs was assessed by flow cytometry. Bcl-2 overexpression blocked uptake of MC38 carcinomas following treatment with vorinostat. (E and F) To confirm apoptosis correlated with engulfment by APCs, a time course was performed. Annexin V-positive staining of vorinostat-treated MC38/MSCV and MC38/Bcl-2 cells are shown in E, with the corresponding median fluorescence intensity in FL-1 (i.e., CFSE) following co-culture of tumor cells with APCs, gated on CD11c positive cells shown in F. Data shown are the mean ± SEM of three independent experiments. (G) Efficacy of V/bimAb therapy was then assessed in mice bearing established MC38/Bcl-2 carcinomas. Cohorts of mice with established (>9 mm2) s.c. MC38/Bcl-2 carcinoma were treated with vehicle (n = 6), vorinostat (n = 6), bimAb (n = 6), or V/bimAb (n = 8) using the therapeutic schedule described in Fig. 1. Tumor growth was assessed every 2–3 d; mean tumor size ± SEM are shown. No complete tumor regressions were observed in mice in any of the treatment groups. Data shown are representative of two independent experiments.
Phagocytosis of vorinostat-treated MC38/MSCV and MC38/Bcl-2 cells by bone marrow-derived CD11c+ APCs was then investigated via co-culture experiments. Treatment of MC38/MSCV but not MC38/Bcl-2 cells with vorinostat for 24 h induced significant levels of annexin V staining (Fig. 2C). CFSE-labeled MC38/MSCV tumor cells were phagocytosed by CD11c+ APCs following treatment for 24 h with 25 μM vorinostat (and to a lesser extent 5 μM vorinostat), whereas CFSE-labeled MC38/Bcl-2 cells were not phagocytosed following treatment with vorinostat at either dose (Fig. 2D). To confirm that engulfment of MC38 tumor targets correlated with apoptosis, a time course was performed (Fig. 2 E and F). The phagocytosis of vorinostat-treated MC38/MSCV cells by APCs correlated with the degree of apoptosis over the time course, whereas MC38/Bcl-2 cells that were refractory to vorinostat-induced apoptosis were also refractory to phagocytosis by APCs (Fig. 2 E and F). This demonstrates vorinostat-mediated tumor cell apoptosis is required for engulfment by APCs.
To determine if the induction of tumor cell apoptosis was required for the eradication of established MC38 tumors following V/bimAb therapy, C57BL/6 mice bearing MC38/Bcl-2 tumors were treated with vorinostat alone, bimAb alone, or V/bimAb (Fig. 2G). Mice bearing MC38/Bcl-2 tumors were refractory to V/bimAb therapy, with only minor delays in tumor growth profiles observed and no tumor regressions achieved (Fig. 2G). These findings demonstrate that vorinostat-induced apoptosis is critical for the successful eradication of MC38 tumors following V/bimAb therapy.
V/bimAb Is Efficacious Against TRAIL-Resistant Tumors.
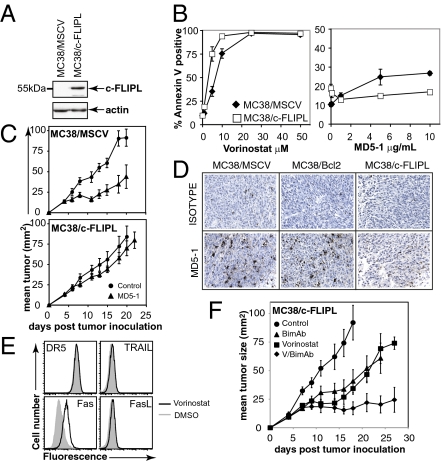
One limitation of trimAb therapy is the requirement for the tumor target cells to be TRAIL sensitive (9), and substituting MD5-1 with an alternative apoptotic stimulus such as an HDACi may circumvent TRAIL resistance. We already demonstrated that P/bimAb treatment of mice bearing TRAIL-insensitive RM1 tumors resulted in tumor eradication, whereas trimAb treatment did not eradicate these tumors (Fig. S1A). To extend these studies, we generated MC38 tumors that were refractory to apoptosis via the TRAIL-receptor. MC38 tumors were retrovirally transduced to overexpress c-FLIPL (Fig. 3A) and these cells were as sensitive to vorinostat-induced apoptosis in vitro as control MC38/MSCV cells (Fig. 3B) but were resistant to MD5-1-induced apoptosis in vitro (Fig. 3B) and in vivo (Fig. 3 C and D). Although up-regulation of death receptors (Fas, DR5, DR4) and their cognate ligands (FasL, TRAIL) has been proposed to play an important role in HDACi-mediated death of certain cell types (13), we saw little change in expression of TRAIL, TRAIL receptor (DR5), or FasL in vorinostat-treated MC38 cells, with only Fas showing a substantial change in expression (Fig. 3E). These data, coupled with our functional data showing that overexpression of Bcl-2 but not c-FLIPL inhibits vorinostat-mediated apoptosis, demonstrates that activation of the death receptor pathway plays little or no role in vorinostat-mediated death of MC38 cells.
Fig. 3.
V/bimAb therapy is efficacious against established TRAIL-resistant carcinomas. (A) MC38 carcinomas retrovirally transduced to overexpress murine c-FLIPL were assessed for overexpression of exogenous protein by Western blotting. (B) MC38/MSCV and MC38/c-FLIPL cells were treated with increasing concentrations of vorinostat for 48 h or plate-bound MD5-1 for 24 h and apoptosis was assessed using annexin V staining and flow cytometry. Data shown are the mean ± SEM of three independent experiments; statistical significance was assessed using the Student's t test. *P < 0.05. (C) Mice with established MC38/MSCV or MC38/c-FLIPL carcinomas were treated with isotype control or MD5-1 mAb (4 doses, every 4 d, 50 μg per dose, n = 6 per treatment group). Tumor growth was assessed every 2–3 d; mean tumor size ± SEM are shown. (D) TRAIL pathway sensitivity was also assessed in situ via TUNEL staining of established tumors taken from MD5-1 and control-treated mice (tissue harvested 8 h post-mAb (50 μg) treatment). (E) The expression of cell surface DR5, TRAIL, Fas, and FasL was assessed on untreated and vorinostat-treated MC38 cells by flow cytometry. (F) Efficacy of V/bimAb therapy was assessed in mice bearing established MC38/c-FLIPL carcinomas. Cohorts of mice with established (>9 mm2) s.c. MC38/c-FLIPL carcinoma were treated with vehicle (n = 6), vorinostat (n = 6), bimAb (n = 6), or V/bimAb (n = 8) using the therapeutic schedule described in Fig. 1. Tumor growth was assessed every 2–3 d; mean tumor size ± SEM are shown and are representative of two independent experiments. Efficacy of MD5-1, V/bimAb, and the single agent activity compared with control treatment was assessed using the Mann-Whitney test. *P < 0.05.
The efficacy of V/bimAb combination therapy was then assessed in C57BL/6 mice bearing established TRAIL-resistant MC38/c-FLIPL tumors (Fig. 3F). V/bimAb treatment significantly retarded tumor growth compared with the effects of vehicle, vorinostat, or bimAb treatment (Fig. 3F). These data coupled with the data shown in Fig. S1A demonstrated that HDACi in combination with bimAb was effective for the treatment of TRAIL-resistant malignancies.
V/bimAb Therapy Requires CD8+ T Cells and NK Cells for Antitumor Activity.
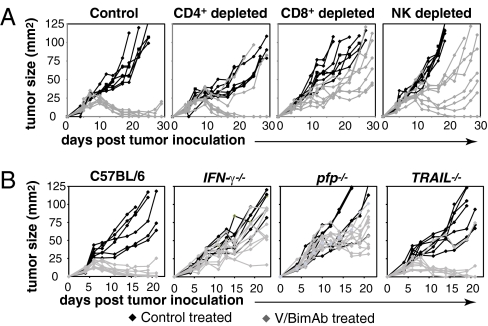
To identify the immune cells necessary for the in vivo effects of V/bimAb, mice deficient in functional CD8+, CD4+, or NK cells were used. Tumor eradication following V/bimAb therapy was not dependent on CD4+ cells (Fig. 4A); however, importantly, therapeutic efficacy was completely lost in mice lacking functional CD8+ cells, with no complete tumor regressions observed (Fig. 4A). This is supported by the finding that IFNγ was also required for therapeutic efficacy of V/bimAb (Fig. 4B) and P/bimAb (Fig. S3) and clearly demonstrates a critical role for CD8+ cells following treatment with HDACi and bimAb. These findings suggest CD8+ CTLs are the primary effector cell important for tumor eradication following V/bimAb treatment. NK cell depletion resulted in the partial loss of V/bimAb therapeutic efficacy with very few complete tumor rejections observed in these mice (Fig. 4A), indicating NK cells play a relatively minor role in mediating the therapeutic effects of V/bimAb. Similar results were observed using P/bimAb and RM1 prostate cancer cells, with therapeutic efficacy lost in RAG1−/−-, IFNγ−/−-, and CD8-depeleted mice (Fig. S3).
Fig. 4.
Role of immune-specific effector cells and regulatory proteins in mediating V/bimAb therapy. (A) Cohorts of mice with established (>9 mm2) s.c. MC38 tumors were treated with anti-CD4 (GK1.5), anti-CD8 (53-6.7), or anti-asialo GM1 to deplete and/or inactivate CD4+, CD8+, and NK cells, respectively. Cell lineage-deficient tumor-bearing mice were then treated with either vehicle (n = 6) or V/bimAb (n = 8) using the therapeutic schedule described in Fig. 1. Tumor growth was assessed every 2–3 d and tumor size in individual mice (mm2) is shown. Data shown are representative of two independent experiments. (B) Cohorts of knockout mice deficient in IFNγ (IFN-γ−/−), TRAIL (TRAIL−/−), or perforin (pfp−/−) bearing established MC38 carcinomas (>9 mm2) were treated with either vehicle (n = 6) or V/bimAb (n = 8) using the therapeutic schedule described in Fig. 1. Tumor growth was assessed every 2–3 d and tumor size in individual mice (mm2) is shown. Data shown are representative of two independent experiments.
V/bimAb Therapy Requires Perforin and Not TRAIL for Therapeutic Efficacy.
CTL-mediated death of transformed cells can occur via two well-defined effector mechanisms: (i) the membrane-disrupting protein perforin and secretion of cytotoxic serine proteases (granzymes); and (ii) the direct activation of target cell death receptors by ligands such as TRAIL (31). Given the important role of the adaptive immune system in mediating effective V/bimAb therapy (Figs. 1B and 4A and Fig. S3), we investigated which of these key CTL effector mechanisms contributed to therapeutic efficacy using gene-targeted TRAIL- or perforin-deficient mice (Fig. 4B). Tumor growth suppression and complete tumor regression responses following V/bimAb therapy in TRAIL−/− mice were similar to those observed in wild-type C57BL/6 mice with eradication of MC38 tumors by V/bimAb recorded in 56% of TRAIL−/− mice (Fig. 4B). In contrast, no V/bimAb-mediated tumor regressions were observed in perforin−/− mice (Fig. 4B). These experiments demonstrate a critical requirement for host perforin, but not TRAIL, in mediating the therapeutic efficacy of V/bimAb combination treatment of MC38 tumors. The importance of perforin was also demonstrated for P/bimAb (Fig. S3).
Discussion
HDACi are effective in the clinic as single agents against certain hematological malignancies but have more limited activity against solid tumors (13, 32). Given that HDACi can be safely administered to patients with manageable side effects (32) and can synergize with a diverse array of anticancer agents (33), it is likely that future therapeutic regimens using HDACi will be in combination with other agents (34). The concept of combining chemo- and immunotherapies (i.e., trimAb) to provide a more potent antitumor response has emerged based on promising preclinical studies (9, 35, 36). A limitation of the trimAb system is that the target tumor cells need to be TRAIL-sensitive for the therapy to be effective (9). We have demonstrated that HDACi can kill tumor cells that have inactive death receptor pathways (11, 12, 37, 38) and hypothesized that HDACi could be used in place of MD5-1 and combined with immunomodulatory agents (such as anti-CD40 and anti-CD137 mAbs) for the treatment of solid tumors, including those insensitive to TRAIL-mediated apoptosis.
Herein, we demonstrated that two HDACi, vorinostat and panobinostat, could be combined with anti-CD40 and anti-CD137 mAbs in therapeutic regimens we termed V/bimAb and P/bimAb, respectively. Both V/bimAb and P/bimAb induced complete regression of tumors of diverse tissue origin. Anti-CD40 mAb could be replaced with α-c-GC in the P/bimAb regimen, indicating that the immune-modulatory component of combination chemo-/immunotherapies involving HDACi could be manipulated without affecting efficacy. Taken together, these data clearly demonstrated that combining HDAC inhibition with immunostimulation that promotes DC activation and CTL proliferation and survival, was highly effective against established carcinomas and that these combination regimens were well tolerated.
The failure of V/bimAb therapy against MC38 tumors in Rag2−/−c-γ-chain−/− mice that lack B, T, and NK cells and in mice with Ab-mediated depletion of CD8+ cells demonstrated that effector CTLs played an essential role in mediating the primary antitumor response of V/bimAb. In addition to the primary antitumor effects mediated by tumor-specific CTLs, an appealing characteristic of immunotherapeutic strategies is that they may enhance the generation of immunological memory capable of protection from both tumor recurrence and metastases (39). We demonstrated in this study that mice that achieved complete tumor responses following V/bimAb or P/α-c-GC/anti-CD137 therapy rejected tumors upon secondary challenge, suggesting this combination therapy may generate immunological memory. These findings are supported by two recent studies that demonstrate HDAC inhibition during T cell activation facilitates the differentiation of CD8+ T cells into functional memory cells that exhibit enhanced survival in vivo, via increased proinflammatory cytokine secretion, and the ability to exert rapid and robust effector functions, mediated by constitutively higher levels of perforin and granzyme B (20, 21). Therefore, it is postulated that increased effector cell function both in response to the initial primary antitumor effect and as a part of the secondary memory response may be one mechanism by which vorinostat treatment enhanced the antitumor response of bimAb (Fig. S4).
Although other groups have shown that HDACi can enhance the immunogenicity of target tumor cells by altering the expression of MHC class I and II, MHC-related molecules MICA/B (Rae ligand family in mice), the adhesion molecule ICAM1, and costimulatory molecules CD40, CD80, and CD86 (16–19), we saw no evidence that these molecules were significantly altered in our HDACi-treated tumor cells. Whether expression of other, as-yet-unidentified cell surface molecules on the tumor cell surface following HDACi treatment plays an important role in initiating a host antitumor immune response remains to be determined. However, we did demonstrate that tumor cells treated with vorinostat that subsequently underwent apoptosis were efficiently phagocytosed by APCs and inhibition of apoptosis by overexpression of Bcl-2 inhibited tumor cell engulfment by the APCs. We have preliminary data demonstrating that vorinostat-treated, apoptotic tumor cells express cell surface calreticulin and release HMGB1 and the role these candidate immunogenic molecules play in V/bimAb therapeutic efficacy remains to be determined.
In our combination chemo-/immunotherapeutic regimen, the anti-CD137 Ab acted to enhance CTL activity (40). However, it is possible that HDACis can intrinsically enhance tumor-specific CTL generation, activity, and specificity. For example, HDACi have been shown to stimulate both the activation of CTLs and their ability to recognize tumor targets (19, 41). Sensitized tumor cells to effector CTL-mediated TRAIL/Fas-induced apoptosis (42) and down-regulated Tregs mediated by HDACi treatment removed immunosuppressive immune regulation and potentiated effector T cell function (43). The role of vorinostat or panobinostat in mediating such effects in our system has yet to be clarified, although the stimulation of CTL activity using anti-CD137 is essential, because combination treatment of tumor-bearing mice with anti-CD40 and vorinostat did not result in tumor regressions. Using gene knockout mice, we demonstrated a critical role for IFNγ and the CTL effector molecule perforin but not TRAIL in mediating tumor regressions following V/bimAb treatment. These findings were supported by the key role described for these molecules in tumor immunosurveillance (31) and the eradication of tumor targets following CTL-stimulating immunotherapies such as trimAb (9).
In summary, we demonstrated that combining an HDACi with targeted stimulation of APC function and T cell survival and expansion can generate a CTL response capable of the eradication of solid tumors. HDACi-induced apoptosis was critical for therapeutic efficacy with host perforin key to tumor eradication. Our data has demonstrated that when the apoptotic sensitivities of a given tumor are exploited and combined with therapies that target key steps involved in the generation of a tumor-specific cytotoxic lymphocyte response, complete tumor eradication can be achieved. Tumor rechallenge experiments supported our postulate that a concomitant, long-lasting antitumor immune response was induced by V/bimAb treatment. Our preclinical studies provide the basis for clinical evaluation of this combination anticancer approach.
Materials and Methods
In Vivo Experiments.
4T1.2 (7 × 104), Renca (2 × 105), MC38 (1 × 106), CT26 (2 × 105), and RM1 (5 × 105) cells were injected s.c. into the right-hand hind flank of mice. Therapy commenced when s.c. tumors reached ∼9 mm2. Tumor size was measured every 2–3 d and data are represented as the mean ± SEM of at least six mice in each group.
In Vitro Cytotoxicity Assays.
Tumor cells (2.5 × 104) were cultured in 24-well plates in complete media with increasing concentrations of vorinostat for 24 or 48 h. Apoptosis mediated by MD5-1 (0.1–10 μg/mL) and vorinostat (0.5–50 μM) was assessed as previously described (22).
In Vitro Phagocytosis Assays.
Phagocytosis of vorinostat-treated MC38/MSCV and MC38/Bcl-2 cells by bone marrow-derived CD11c-positive APCs was investigated via co-culture experiments. Briefly, bone marrow cells were harvested from the femurs of C57BL/6 mice and cultured in complete RPMI containing GM-CSF for 7 d. Target tumors were labeled with CFSE and were treated with vorinostat (5 or 25 μM) for the time periods stated. These treated cells were then co-cultured with bone marrow-derived APCs labeled with fluorescently tagged CD11c. Two and one-half hours following co-culture, cells were harvested, and flow cytometry was performed to determine the shift of CD11c-positive cells in FL-1 (i.e., the degree of CFSE/tumor phagocytosed by these APCs). Median fluorescence intensity for the FL-1 channel on the LSR II is shown. The degree of tumor cell apoptosis following vorinostat treatment was also assessed via annexin V/PI staining (as described above) in samples run concurrently with the phagocytosis assay samples.
In Situ Apoptosis.
C57BL/6 mice with established s.c. MC38/MSCV, MC38/Bcl-2, or MC38/c-FLIPL carcinomas (∼9 mm2) were treated i.p. with control (50 μg isotype antibody) or MD5-1 (50 μg). Treated tumors were resected 16 h after treatment, fixed in 4% buffered formalin, and paraffin embedded, and sections were cut. In situ apoptosis was detected using the Apoptag Peroxidase in situ Apoptosis Detection kit (Chemicon International) per the manufacturer's instructions.
Supplementary Material
Acknowledgments
We thank Merck & Co and Novartis for supply of vorinostat and panobinostat, respectively. R.W.J. is a Principal Research Fellow and M.J.S. is an Australian Fellow of the National Health and Medical Research Council of Australia (NHMRC). This work is supported by NHMRC program and project grants, and project grants from the Susan G. Komen for the Cure Foundation, the Victorian Breast Cancer Research Consortium, and the Prostate Cancer Foundation of Australia. A.J.C. is supported by a postdoctoral fellowship from the Cancer Council Victoria. R.W.J. is the recipient of collaborative grants from Merck and Novartis.
Footnotes
Conflict of interest statement: The R.W.J. laboratory has collaborative research grants from Merck & Co and Novartis for studies involving vorinostat and panobinostat, respectively.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011037108/-/DCSupplemental.
References
- 1.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 2.Zitvogel L, et al. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghiringhelli F, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 4.Aymeric L, et al. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. 2010;70:855–858. doi: 10.1158/0008-5472.CAN-09-3566. [DOI] [PubMed] [Google Scholar]
- 5.Ma Y, et al. Chemotherapy and radiotherapy: Cryptic anticancer vaccines. Semin Immunol. 2010;22:113–124. doi: 10.1016/j.smim.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Stagg J, Johnstone RW, Smyth MJ. From cancer immunosurveillance to cancer immunotherapy. Immunol Rev. 2007;220:82–101. doi: 10.1111/j.1600-065X.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 7.Pagès F, et al. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–1102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 8.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uno T, et al. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med. 2006;12:693–698. doi: 10.1038/nm1405. [DOI] [PubMed] [Google Scholar]
- 10.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 11.Lindemann RK, et al. Analysis of the apoptotic and therapeutic activities of histone deacetylase inhibitors by using a mouse model of B cell lymphoma. Proc Natl Acad Sci USA. 2007;104:8071–8076. doi: 10.1073/pnas.0702294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis L, et al. The histone deacetylase inhibitors LAQ824 and LBH589 do not require death receptor signaling or a functional apoptosome to mediate tumor cell death or therapeutic efficacy. Blood. 2009;114:380–393. doi: 10.1182/blood-2008-10-182758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 14.Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1:287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 15.Insinga A, et al. Inhibitors of histone deacetylases induce tumor-selective apoptosis through activation of the death receptor pathway. Nat Med. 2005;11:71–76. doi: 10.1038/nm1160. [DOI] [PubMed] [Google Scholar]
- 16.Maeda T, Towatari M, Kosugi H, Saito H. Up-regulation of costimulatory/adhesion molecules by histone deacetylase inhibitors in acute myeloid leukemia cells. Blood. 2000;96:3847–3856. [PubMed] [Google Scholar]
- 17.Magner WJ, et al. Activation of MHC class I, II, and CD40 gene expression by histone deacetylase inhibitors. J Immunol. 2000;165:7017–7024. doi: 10.4049/jimmunol.165.12.7017. [DOI] [PubMed] [Google Scholar]
- 18.Manning J, et al. Induction of MHC class I molecule cell surface expression and epigenetic activation of antigen-processing machinery components in a murine model for human papilloma virus 16-associated tumours. Immunology. 2008;123:218–227. doi: 10.1111/j.1365-2567.2007.02689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan AN, Gregorie CJ, Tomasi TB. Histone deacetylase inhibitors induce TAP, LMP, Tapasin genes and MHC class I antigen presentation by melanoma cells. Cancer Immunol Immunother. 2008;57:647–654. doi: 10.1007/s00262-007-0402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Araki Y, Fann M, Wersto R, Weng NP. Histone acetylation facilitates rapid and robust memory CD8 T cell response through differential expression of effector molecules (eomesodermin and its targets: perforin and granzyme B) J Immunol. 2008;180:8102–8108. doi: 10.4049/jimmunol.180.12.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Northrop JK, Wells AD, Shen H. Cutting edge: chromatin remodeling as a molecular basis for the enhanced functionality of memory CD8 T cells. J Immunol. 2008;181:865–868. doi: 10.4049/jimmunol.181.2.865. [DOI] [PubMed] [Google Scholar]
- 22.Frew AJ, et al. Combination therapy of established cancer using a histone deacetylase inhibitor and a TRAIL receptor agonist. Proc Natl Acad Sci USA. 2008;105:11317–11322. doi: 10.1073/pnas.0801868105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hixon JA, Blazar BR, Anver MR, Wiltrout RH, Murphy WJ. Antibodies to CD40 induce a lethal cytokine cascade after syngeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2001;7:136–143. doi: 10.1053/bbmt.2001.v7.pm11302547. [DOI] [PubMed] [Google Scholar]
- 24.Hixon JA, et al. Administration of either anti-CD40 or interleukin-12 following lethal total body irradiation induces acute lethal toxicity affecting the gut. Biol Blood Marrow Transplant. 2002;8:316–325. [PubMed] [Google Scholar]
- 25.Vonderheide RH, et al. Phase I study of recombinant human CD40 ligand in cancer patients. J Clin Oncol. 2001;19:3280–3287. doi: 10.1200/JCO.2001.19.13.3280. [DOI] [PubMed] [Google Scholar]
- 26.Hayakawa Y, Godfrey DI, Smyth MJ. Alpha-galactosylceramide: potential immunomodulatory activity and future application. Curr Med Chem. 2004;11:241–252. doi: 10.2174/0929867043456115. [DOI] [PubMed] [Google Scholar]
- 27.Teng MW, et al. Combined natural killer T-cell based immunotherapy eradicates established tumors in mice. Cancer Res. 2007;67:7495–7504. doi: 10.1158/0008-5472.CAN-07-0941. [DOI] [PubMed] [Google Scholar]
- 28.Armeanu S, et al. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res. 2005;65:6321–6329. doi: 10.1158/0008-5472.CAN-04-4252. [DOI] [PubMed] [Google Scholar]
- 29.Skov S, et al. Cancer cells become susceptible to natural killer cell killing after exposure to histone deacetylase inhibitors due to glycogen synthase kinase-3-dependent expression of MHC class I-related chain A and B. Cancer Res. 2005;65:11136–11145. doi: 10.1158/0008-5472.CAN-05-0599. [DOI] [PubMed] [Google Scholar]
- 30.Whitecross KF, et al. Defining the target specificity of ABT-737 and synergistic antitumor activities in combination with histone deacetylase inhibitors. Blood. 2009;113:1982–1991. doi: 10.1182/blood-2008-05-156851. [DOI] [PubMed] [Google Scholar]
- 31.Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol. 2006;6:940–952. doi: 10.1038/nri1983. [DOI] [PubMed] [Google Scholar]
- 32.Rasheed W, Bishton M, Johnstone RW, Prince HM. Histone deacetylase inhibitors in lymphoma and solid malignancies. Expert Rev Anticancer Ther. 2008;8:413–432. doi: 10.1586/14737140.8.3.413. [DOI] [PubMed] [Google Scholar]
- 33.Bots M, Johnstone RW. Rational combinations using HDAC inhibitors. Clin Cancer Res. 2009;15:3970–3977. doi: 10.1158/1078-0432.CCR-08-2786. [DOI] [PubMed] [Google Scholar]
- 34.Frew AJ, Johnstone RW, Bolden JE. Enhancing the apoptotic and therapeutic effects of HDAC inhibitors. Cancer Lett. 2009;280:125–133. doi: 10.1016/j.canlet.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 35.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 36.Teng MW, Sharkey J, McLaughlin NM, Exley MA, Smyth MJ. CD1d-based combination therapy eradicates established tumors in mice. J Immunol. 2009;183:1911–1920. doi: 10.4049/jimmunol.0900796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruefli AA, et al. The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc Natl Acad Sci USA. 2001;98:10833–10838. doi: 10.1073/pnas.191208598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peart MJ, et al. Novel mechanisms of apoptosis induced by histone deacetylase inhibitors. Cancer Res. 2003;63:4460–4471. [PubMed] [Google Scholar]
- 39.Lake RA, Robinson BW. Immunotherapy and chemotherapy—a practical partnership. Nat Rev Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 40.Melero I, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 41.Murakami T, et al. Transcriptional modulation using HDACi depsipeptide promotes immune cell-mediated tumor destruction of murine B16 melanoma. J Invest Dermatol. 2008;128:1506–1516. doi: 10.1038/sj.jid.5701216. [DOI] [PubMed] [Google Scholar]
- 42.Lundqvist A, et al. Bortezomib and depsipeptide sensitize tumors to tumor necrosis factor-related apoptosis-inducing ligand: a novel method to potentiate natural killer cell tumor cytotoxicity. Cancer Res. 2006;66:7317–7325. doi: 10.1158/0008-5472.CAN-06-0680. [DOI] [PubMed] [Google Scholar]
- 43.Kato N, et al. Regulation of the expression of MHC class I-related chain A, B (MICA, MICB) via chromatin remodeling and its impact on the susceptibility of leukemic cells to the cytotoxicity of NKG2D-expressing cells. Leukemia. 2007;21:2103–2108. doi: 10.1038/sj.leu.2404862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.