Abstract
Although aerobic glycolysis (the Warburg effect) is a hallmark of cancer, key questions, including when, how, and why cancer cells become highly glycolytic, remain less clear. For a largely unknown regulatory mechanism, a rate-limiting glycolytic enzyme pyruvate kinase M2 (PKM2) isoform is exclusively expressed in embryonic, proliferating, and tumor cells, and plays an essential role in tumor metabolism and growth. Because the receptor tyrosine kinase/PI3K/AKT/mammalian target of rapamycin (RTK/PI3K/AKT/mTOR) signaling cascade is a frequently altered pathway in cancer, we explored its potential role in cancer metabolism. We identified mTOR as a central activator of the Warburg effect by inducing PKM2 and other glycolytic enzymes under normoxic conditions. PKM2 level was augmented in mouse kidney tumors due to deficiency of tuberous sclerosis complex 2 and consequent mTOR activation, and was reduced in human cancer cells by mTOR suppression. mTOR up-regulation of PKM2 expression was through hypoxia-inducible factor 1α (HIF1α)-mediated transcription activation, and c-Myc–heterogeneous nuclear ribonucleoproteins (hnRNPs)-dependent regulation of PKM2 gene splicing. Disruption of PKM2 suppressed oncogenic mTOR-mediated tumorigenesis. Unlike normal cells, mTOR hyperactive cells were more sensitive to inhibition of mTOR or glycolysis. Dual suppression of mTOR and glycolysis synergistically blunted the proliferation and tumor development of mTOR hyperactive cells. Even though aerobic glycolysis is not required for breach of senescence for immortalization and transformation, the frequently deregulated mTOR signaling during multistep oncogenic processes could contribute to the development of the Warburg effect in many cancers. Components of the mTOR/HIF1α/Myc–hnRNPs/PKM2 glycolysis signaling network could be targeted for the treatment of cancer caused by an aberrant RTK/PI3K/AKT/mTOR signaling pathway.
Keywords: PTEN, tuberous sclerosis 1, hexokinase II, lactate dehydrogenase-B, glyceraldehyde 3-phosphate dehydrogenase
Unlike in normal cells, glycolysis is induced by hypoxia, and cancer cells preferentially metabolize glucose by glycolysis, even in an aerobic environment (1–3). Increased glucose consumption and an elevated rate of lactate production by cancer cells are characteristics of glycolysis, first described by Otto Warburg in the 1920s and thereafter known as the Warburg effect (4). Because this altered metabolism can occur even in the presence of oxygen, glycolysis presumably confers a selective advantage for the survival and proliferation of cancer cells. This catabolic process is, however, inefficient for energy production in that it generates only 2 mol of ATP, instead of an additional 36 mol through the tricarboxylic acid (TCA) cycle, in the presence of oxygen by using 1 mol of glucose (2, 3, 5).
Although the Warburg effect is a well-recognized hallmark of cancer metabolism, its regulatory mechanism is still largely obscure. Critical issues, including how and when cancer cells acquire this highly glycolytic phenotype, and its causal relationship with cancer progression, remain to be addressed. In addition, a cellular adaptation model could not explain the constitutively high rate of glycolysis in cancer cells in tissue culture conditions with 20% oxygen in vitro or in pseudohypoxic tumors in vivo. Nevertheless, changes of rate-limiting glycolytic enzymes are observed during tumor formation. Among these enzymes is pyruvate kinase, which catalyzes the dephosphorylation of phosphoenolpyruvate to pyruvate and yields one molecule of ATP independent of oxygen supply during glycolysis (6). There are four pyruvate kinase isoenzymes: PKL and PKR are encoded by the PKLR gene but under the control of different promoters; and PKM1 and PKM2 are two different splicing forms of the same mRNA transcribed from the PKM gene. The ratio of PKM1 to PKM2 is dictated by heterogeneous nuclear ribonucleoproteins (hnRNPs) (7, 8). PKL, PKR, and PKM1 are tissue-specific isoenzymes, whereas PKM2 is considered an embryonic and cancer cell-specific isoform. By still-unknown regulatory mechanisms, PKM2 is gradually replaced by the respective tissue-specific isoenzyme during embryogenesis. By contrast, tissue-specific isoenzymes are switched to PKM2 during tumorigenesis, and PKM2 plays a critical role in tumor metabolism and growth (6, 9). Because PKM2 is an inactive form of pyruvate kinase for the last step of glycolysis, the buildup of phosphoenolpyruvate is then shunted to an alternative glycolytic pathway for anabolic synthesis and cell growth (10).
The receptor tyrosine kinase/PI3K/AKT/mammalian target of rapamycin (RTK/PI3K/AKT/mTOR) signaling pathway plays a crucial role in regulating cell growth, survival, and metabolism (11, 12). Various alterations of the proto-oncogenes and tumor suppressors along this pathway mark this network as one of the most frequently dysregulated signaling cascades in cancers (13–16). A major activated effector of this pathway is mTOR, a serine/threonine protein kinase. mTOR integrates a broad spectrum of input, ranging from growth factor signaling to cellular nutrient status to energy supply, for regulation of protein synthesis and cell growth (14, 17). Even though the downstream events of oncogenic mTOR leading to cancer development are still largely unknown, among numerous mTOR effectors, the proto-oncogene Myc family and hypoxia-inducible factors (HIFs) are often activated in various cancers and have been considered an “axis of evil” in cancer development (5, 18). Although the function of normal c-Myc is inhibited by physiological HIF1α signaling, oncogenic Myc and HIFs collaborate with each other (though the molecular detail of this interaction is less certain) to confer metabolic advantages to cancer cells by induction of the Warburg effect through transcriptional activation of glycolytic enzymes (19).
We initially noticed prominent glycolysis in cells with constitutively active mTOR while characterizing primary and immortalized tuberous sclerosis 1 (Tsc1)- or tuberous sclerosis 2 (Tsc2)-null MEFs. Subsequently we found that mTOR was a major positive regulator of the Warburg effect, not only in cancer cells but also, surprisingly, in benign tumor cells and even in premature senescent primary cells with activated mTOR signaling, under normoxic conditions. We then identified PKM2 as a critical glycolytic enzyme in oncogenic mTOR-induced Warburg effect. Next, we determined that HIF1α and c-Myc–hnRNPs cascades were the transducers of mTOR regulation of PKM2. Moreover, disruption of mTOR signaling, PKM2, and/or glycolysis suppressed the cell proliferation and tumorigenesis caused by oncogenic mTOR signaling. We suggest that PKM2-stimulated glycolysis contributes to the development of tumors caused by hyperactive mTOR, and therefore this cascade may be targeted for the treatment of these cancers.
Results
Hyperactive mTOR Promotes Aerobic Glycolysis.
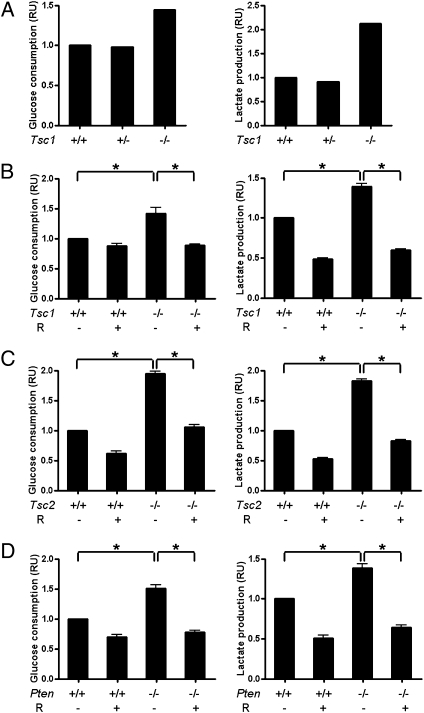
The protein complex of TSC1 and TSC2 tumor suppressors is a key negative regulator of mTOR signaling, and the loss of its function causes tuberous sclerosis complex (TSC) and lymphangioleiomyomatosis (LAM) with features of benign tumors (20–26). Deletion of Tsc1 or Tsc2 is mouse embryonic lethal. In comparison with WT, Tsc1+/−, or Tsc2+/− mouse embryonic fibroblasts (MEF), Tsc1−/− or Tsc2−/− MEFs derived from E10–10.5 embryo cultures underwent premature senescence. Immortalized MEF lines were established by multiple passages of MEFs in culture or in the p53-null background (20, 27). Initially, we noticed the cultured media of Tsc1 or Tsc2 null MEF turned to yellow much faster than the cultured media of WT MEF cells did in both cultured primary cells and immortalized cell lines. Suspicious of lactic acidosis in these media, we performed metabolic analysis of these conditioned media for glucose consumption and lactate production. We first checked the culture media from the primary MEFs of a Tsc1−/− embryo and its Tsc1+/+ and Tsc1+/− littermates from a Tsc1+/− interbreeding. Both glucose consumption and lactate production were increased in Tsc1 knockout MEFs compared with their WT or heterozygous control cells under normoxic conditions (Fig. 1A). This metabolic change is a typical feature of aerobic glycolysis (4). Based on this preliminary finding, we then statistically quantified the culture media with or without the treatment of mTOR inhibitor rapamycin from the immortalized Tsc1−/– or Tsc2−/– MEF lines vs. WT MEF lines. This Warburg effect was prominent in Tsc1- or Tsc2-deficient cells and was reversed by rapamcyin (Fig. 1 B and C). The inactive mutations of Pten tumor suppressor, another major negative regulator of mTOR signaling through down-regulation of AKT activity, can lead to aberrant activation of mTOR in many cancers (15, 16, 28, 29). As expected, Pten−/− MEFs also exhibited enhanced aerobic glycolysis that was sensitive to rapamycin interference (Fig. 1D). Therefore, our data demonstrates that mTOR is a positive regulator of the Warburg effect.
Fig. 1.
Hyperactive mTOR promotes aerobic glycolysis. (A) Cultures of primary Tsc1+/+, Tsc1+/−, and Tsc1−/− MEF isolated from a Tsc1+/− interbreeding. Tsc1−/− (B), Tsc2−/− (C), or Pten−/− (D) and WT MEFs were treated with or without 5 nM rapamycin (R) for 48 h. The cultured media were collected for the measurement of glucose and lactate. Data represent mean ± SEM. *P < 0.05. RU, relative unit.
mTOR Is a Positive Regulator of PKM2 Expression.
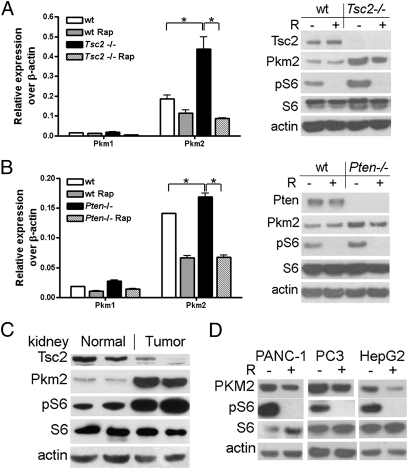
To elucidate the molecular mechanism of mTOR signaling pathway in the activation of aerobic glycolysis, we examined the abundance of PKM2, a key glycolytic enzyme often present in cancer cells. We found that both the elevated Pkm2 mRNA and protein in either Tsc2−/− or Pten−/− MEF cell lines were reduced by rapamycin treatment (Fig. 2 A and B). Pkm2 protein was increased in cells with mTOR activation, caused by lack of Tsc1 (Fig. S1A), Tsc2, or Pten, and was then reduced by rapamycin treatment in all of these cell lines, suggesting that the regulation of Pkm2 is in mTOR dependent (Fig. 2 A and B and Fig. S1A). In contrast, Pkm2 expression was not influenced by the treatment of U0126, an inhibitor of MEK1/2 in the MAPK signaling pathway (Fig. S1B). Pkm1 mRNA was detected at a much lower level (about 10- to 20-fold) than that of Pkm2 mRNA, and the PKLR mRNA value was undetectable in WT, Tsc2−/−, and Pten−/− MEF cell lines (Fig. 2 A and B). Nonetheless, immunoblotting analysis indicated that Pkm1 and PKLR proteins were up-regulated in a mTOR-dependent fashion in Tsc2−/− or Pten−/− MEF cell lines (Fig. S2 A and B). Furthermore, glycolytic enzymes hexokinase II (HKII), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and lactate dehydrogenase-B (LDHB) proteins were also increased in Tsc2−/− and Pten−/− MEF cell lines. mTOR suppression by rapamycin then decreased the expression of these proteins (Fig. S2 A and B). These data indicate that all of the glycolytic enzymes examined here were positively regulated by mTOR.
Fig. 2.
mTOR is a positive regulator of Pkm2 expression. Total RNA and protein lysates were extracted from WT, Tsc2−/− (A), or Pten−/− (B) MEFs treated with or without 10 nM rapamycin (R) for 24 h for RT-qPCR and immunoblotting, respectively. *P < 0.05. (C) Age-matched kidneys from two normal mice and kidney tumors from two Tsc2del3/+ mice were immunoblotted. (D) Human pancreatic (PANC-1), prostate (PC3), and liver (HepG2) cancer cell lines were treated with or without 10 nM rapamycin for 24 h and then subjected to immunoblotting.
Unlike other glycolytic enzymes, PKM2 mainly presents in rapidly proliferating cells and tumor cells. To investigate whether mTOR regulation of PKM2 exists in tumor samples, we checked mouse kidney tumors driven by up-regulated mTOR in a TSC mouse model (Tsc2del3/+) (30). As expected, these kidney tumor samples exhibited robust elevation of Pkm2 (Fig. 2C). Consistent with the mTOR regulation of Pkm2 in mouse cells, PKM2 expression was antagonized by rapamycin treatment in human cancer cell lines as well (Fig. 2D). Because rapamycin treatment did not completely abolish the expression of PKM2, other factors might also regulate PKM2 expression.
Pkm2 Is Critical for Aerobic Glycolysis of the Cells with Active mTOR Signaling.
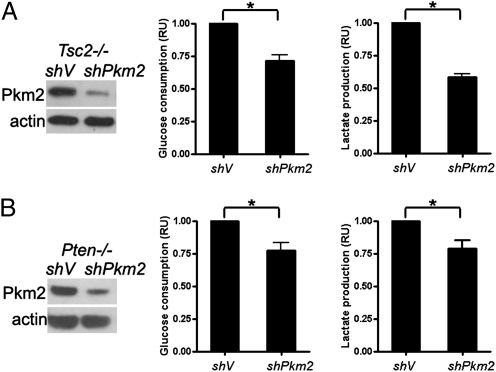
Because both Pkm2 and mTOR promote aerobic glycolysis, we postulated that mTOR regulated aerobic glycolysis at least partially through Pkm2. To test this hypothesis, we first knocked down Pkm2 expression in Tsc2−/− and Pten−/− MEFs and then measured the glucose consumption and lactate production of these cells. Reduction of PKM2 expression led to decreased glucose consumption and lactate production in these cells (Fig. 3 A and B). Likewise, knockdown of HKII or LDHB also led to decreased glycolysis in Tsc2−/− MEFs (Fig. S3 A and B). Therefore, mTOR indeed regulates aerobic glycolysis via up-regulation of Pkm2 and other glycolytic enzymes.
Fig. 3.
Pkm2 is critical for aerobic glycolysis of cells with activated mTOR. Lysates of Tsc2−/− (A) or Pten−/− (B) MEFs stably expressing the shRNA for Pkm2 were subjected to immunoblotting, and the conditioned media from the cultures of these MEFs were examined for glucose and lactate. Data represent mean ± SEM. *P < 0.05. RU, relative unit.
mTOR Stimulates Pkm2 Expression Through Induction of HIF1α Expression.
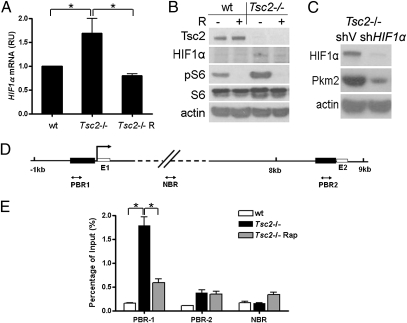
HIF1α, a downstream target of mTOR, is a major transcription factor controlling cellular adaptation to hypoxia, and it promotes glycolytic metabolism (31–34). To dissect the mechanism by which mTOR activation leads to aerobic glycolysis mediated by overexpression of Pkm2, we explored the possibility that HIF1α might be a link between mTOR activation and Pkm2 expression. Both the mRNA and the protein of HIF1α were increased in the cells with mTOR hyperactivation due to Tsc2 deficiency, and were repressed after rapamycin treatment (Fig. 4 A and B and Table S1). In addition, knockdown of HIF1α led to drastic reduction of Pkm2 expression in Tsc2−/− and Pten−/− MEFs (Fig. 4C and Fig. S4), indicating that mTOR activates Pkm2 expression through up-regulation of HIF1α expression under normoxic conditions. We thereby tested whether HIF1α might stimulate PKM expression as a transcriptional activator (35). Five potential binding sites were identified by the computer software program Genomatix at two predicted HIF1α binding regions on mouse PKM gene (Fig. 4D). Real-time PCR analysis of ChIP DNA revealed that HIF1α binding to a DNA region immediately upstream of the exon 1 of the PKM gene was significantly higher in Tsc2−/− cells than in WT cells. The interaction between HIF1α and the PKM2 promoter was disrupted by rapamycin treatment (Fig. 4E). Nevertheless, HIF1α-mediated transcription activation in the promoter region of the PKM gene could not fully account for the drastic elevation of PKM2, an alternative splicing product.
Fig. 4.
mTOR stimulates Pkm2 expression through induction of HIF1α. RT-qPCR (A) and immunoblotting (B) analysis for WT and Tsc2−/− MEFs treated with or without 10 nM rapamycin (R) for 24 h. *P < 0.05. RU, relative unit. (C) Tsc2−/− MEFs were transfected with shHIF1α or scramble shRNA (shV) in pSilencer2.1 and then subjected to immunoblotting. (D) Schematic representation of the promoter regions of mouse PKM gene. E1 and E2 indicate the location of PKM exons 1 and 2. Dark rectangles indicate predicted HIF1α binding regions; two-way arrows indicate fragment amplified in ChIP real-time PCR analysis. The transcription start site is indicated by an arrow above the gene. PBR, predicted binding region; NBR, nonspecific binding region. (E) Tsc2−/− MEFs treated with or without 10 nM rapamycin (R) for 24 h. HIF1α antibody-immunoprecipitated DNA was PCR amplified for regions indicated in D. The data are plotted as the ratio of immunoprecipitated DNA subtracting nonspecific binding to IgG vs. total input DNA. Representative data from two independent experiments are shown. Data represent mean ± SEM of replicate real-time PCR. *P < 0.05.
mTOR-Dependent c-Myc Up-Regulation of hnRNPs Enhances Pkm2 Production.
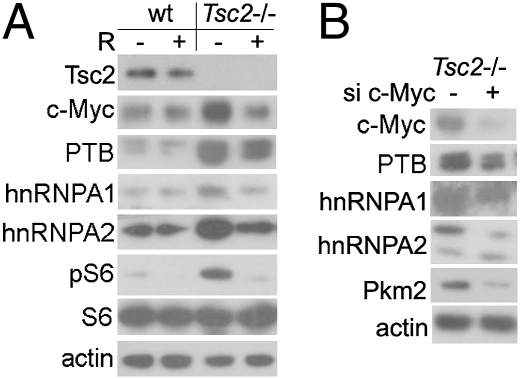
PKM1 and PKM2 are generated from mutually exclusive alternative splicing of the PKM pre-mRNA. PKM1 contains exon 9, whereas PKM2 has exon 10 (6). Recently, three hnRNPs, polypyrimidine tract binding protein (PTB), hnRNPA1, and hnRNPA2, were identified as alternative splicing repressors for PKM1 (7, 8). Because c-Myc is a transcriptional activator of these hnRNPs and a downstream effector of mTOR, we speculated that the c-Myc–hnRNPs signaling cascade might play an important role in the overproduction of Pkm2 in mTOR active cells. c-Myc, PTB, hnRNPA1, and hnRNPA2 were all increased in Tsc2−/− cells and suppressed by rapamycin treatment (Fig. 5A). Moreover, knockdown of c-Myc led to reduction of PTB, hnRNPA1, and hnRNPA2, as well as Pkm2, in Tsc2−/− cells (Fig. 5B). We thus conclude that mTOR is a positive regulator of c-Myc–mediated expression of hnRNPs. In conjunction with HIF1α, the c-Myc–hnRNPs axis promotes PKM2 expression.
Fig. 5.
mTOR up-regulates Pkm2 expression through the c-Myc–hnRNPs axis. (A) WT and Tsc2−/− MEFs treated with or without 10 nM rapamycin for 24 h were subjected to immunoblotting. (B) Tsc2−/− MEFs were transfected with siRNA of c-Myc or scramble siRNA and then subjected to immunoblotting.
Inhibition of mTOR, PKM2, and Glycolysis Suppresses Cell Proliferation and Tumorigenesis Mediated by Oncogenic mTOR Signaling.
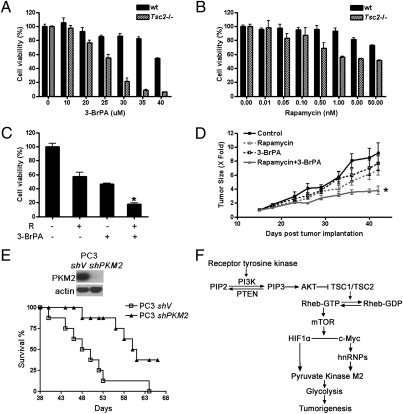
Because a higher rate of glycolysis occurred in the cells with augmented mTOR signaling, and these cells could therefore be more vulnerable to the suppression of either glycolysis or mTOR, we measured the viability of these cells in the presence of either mTOR inhibitor rapamcyin or glycolytic inhibitor 3-bromopyruvate (3-BrPA) (36). Tsc2−/− MEFs were more sensitive not only to rapamcyin treatment but also to 3-BrPA intervention in comparison with WT MEFs (Fig. 6 A and B). These data suggest that the proliferation of these mTOR-activated cells might be more dependent on mTOR/PKM2 glycolysis regulation, and this feature therefore could be exploited for the treatment of tumors caused by deregulated mTOR signaling. Furthermore, the combination of rapamycin and 3-BrPA exerted synergistic suppression on the proliferation of Tsc2−/− MEFs in vitro (Fig. 6C and Fig. S5). This finding prompted us to test the efficacy of this combinatory strategy for the treatment of cancer in a preclinical animal model. PC3 cells, a human prostate cancer cell line with PTEN deficiency and mTOR hyperactivation, were injected s.c. into nude mice to establish a xenografic tumor model. Peritoneal injection of low-dosage rapamycin and 3-BrPA significantly retarded the development of these tumors, in comparison with the application of a single drug (Fig. 6D). Considering that PKM2 is critical for cell proliferation (6) and is more abundant in the cells with hyperactive mTOR signaling, we reasoned the enhanced Pkm2 expression was critical for RTK/PI3K/AKT/mTOR-mediated tumorigenesis. Hence, we first stably knocked down PKM2 in PC3 cells, and then evaluated its significance for tumorigenesis in a nude mouse xenograft tumor model. Reduction of PKM2 significantly extended the survival of these tumor-bearing mice (Fig. 6E).
Fig. 6.
Inhibition of mTOR, glycolysis, and PKM2 suppresses cell proliferation and tumorigenesis. WT and Tsc2−/− MEF cells were treated with 3-BrPA (A) and rapamycin (B) for 48 h at the indicated concentration. Cell viability was determined with MTT assay. P < 0.05. (C) Cells were treated with or without 0.5 nM rapamycin (R) for 18 h before the addition of 25 μM 3-BrPA or solvent control for 48 h. Cell viability was detected with MTT assay. Combination index < 1; *P < 0.05. (D) Nude mice bearing xenografted s.c. tumor from PC3 cells were treated with a single drug or the combination of 3-BrPA and rapamycin. The relative tumor sizes were plotted. Data represent mean ± SEM. *P < 0.05. (E) PC3 cells were transduced with the shPKM2 (shPKM2) or scramble shRNA lentiviruses (shV) and then inoculated s.c. into nude mice. (Upper) Immunoblotting. (Lower) Kaplan–Meier survival analysis of the mice with xenografting tumors. P < 0.05. (F) Schematic illustration of the RTK/PI3K/AKT/mTOR pathway regulated glycolysis and tumorigenesis through the HIF1α/c-Myc–hnRNPs/PKM2 network. Active mTOR switches on PKM2 production through up-regulation of HIF1α-mediated transcriptional activation, and the c-Myc–hnRNPs regulated alternative splicing. The consequent activation of glycolysis promotes tumorigenesis.
Discussion
By studying cells with hyperactive mTOR signaling resulting from deletion of a Tsc1, Tsc2, or Pten tumor suppressor, we have identified the PI3K/AKT/mTOR signaling pathway as a major positive regulator of the Warburg effect, a hallmark of cancer metabolism. Rate-limiting glycolytic enzyme PKM2 is a major effector of mTOR signaling, downstream of HIF1α and c-Myc–hnRNPs, and the mTOR/HIF1α/Myc–hnRNPs/PKM2 signaling cascade is critical for oncogenic mTOR-mediated tumorigenesis (Fig. 6F).
The RTK/PI3K/AKT/mTOR signaling pathway plays an important role in the regulation of cell growth, proliferation, and differentiation (37). Though aberrant activation of mTOR is often present in both benign and malignant tumors, the mechanism of oncogenic mTOR-mediated tumor development remains poorly understood. Here we present that mTOR suppressor Tsc1-, Tsc2-, and Pten-deficient MEFs exhibited pronounced glycolysis under normal oxygen conditions. This aerobic glycolysis is dependent on mTOR signaling, because rapamycin blunted the occurrence of glycolysis in those cells. Our data implicates mTOR as a critical driver of aerobic glycolysis, and this observation is consistent with findings from several recent reports (38–40). Akt activity is elevated in Pten-null cells, but reduced in Tsc1- or Tsc2- deficient cells, suggesting that the oncogenic AKT-stimulated aerobic glycolysis occurs mainly through the action of mTOR signaling (41, 42).
Even though aerobic glycolysis is widely recognized as a hallmark of malignant cancers, whether it is a consequence or cause of cancer is still debatable (2). Because we have observed the presence of augmented aerobic glycolysis in malignant tumorigenic Pten−/− MEF cell lines (37), benign tumorigenic Tsc1- and Tsc2-null MEF cell lines (43), and even senescent primary Tsc1−/− or Tsc2−/− MEFs (20, 27) (Fig. 1), we argue that aerobic glycolysis is not required for breach of senescence for immortalization and transformation. We speculate that the Warburg effect is neither necessarily an intrinsic property of cancer cells nor a consequence of carcinogenesis; instead, it maybe a manifestation of hyperactive mTOR signaling under certain circumstances.
PKM2 is an embryonic M2 isoform of the glycolytic enzyme pyruvate kinase, which is expressed mainly in proliferating cells such as cancer cells. PKM2 expression is necessary for the switch from regular cell metabolism to aerobic glycolysis, and this metabolic phenotype provides a selective growth advantage for tumor cells (6, 10, 44). In searching for the molecular link between mTOR activation and enhanced aerobic glycolysis, we have identified PKM2 as a major mediator of glycolysis downstream of mTOR in cell lines and tumor tissues. Furthermore, PKM2 is elevated, and consequently contributes to, the glycolytic phenotype of mTOR-activated cells (Figs. 2 and 3).
HIF1α and glycolytic enzymes are both overexpressed in many tumor cells. HIF1α can regulate expression of a set of genes involved in reaction to hypoxia, including several glycolytic enzymes, such as aldolase A, phosphoglycerate kinase 1, and pyruvate kinase M, though the precise mechanism of this regulation is obscure (44–46). The concomitant induction of angiogenesis and enhancement of glycolysis are mediated partially by activating HIF1α (46). Deficiency of Tsc1 or Tsc2 gene indeed causes enhanced angiogenesis and augmented glycolysis (Fig. 1) (38, 47, 48). Although HIF1α levels are usually increased by hypoxia through stabilization of HIF1α protein in adaptation to the changes of tissue oxygenation, HIF1α may be induced transcriptionally by mTOR even under normoxic environments through 4EBP1 and Stat3 (32, 38, 47, 49–51). We have now established mTOR regulation of PKM2 expression partially through HIF1α-mediated transcriptional activation (Fig. 4). mTOR-mediated HIF1α induction mimics the effect of hypoxia and then leads to glycolysis. Therefore, mTOR regulation of PKM2 through induction of HIF1α in part explains why cancer cells undergo glycolysis even under normoxic conditions.
PKM2 is an alternative splicing product of PKM pre-mRNA. Recently, PKM2 expression was reported to be regulated by splicing regulators, hnRNP proteins, which are transcriptionally activated by c-Myc (7, 8). The proto-oncogene Myc family is frequently overactivated in cancers and plays an important collaborative role with HIF in cancer metabolism by enhancing expression of many glycolytic enzymes (5, 18, 19). In addition, both HIF and c-Myc can be up-regulated by mTOR signaling. Here we present evidence that enhanced expression of c-Myc, PTB, hnRNPA1, and hnRNPA2 in mTOR-activated cells is dependent on mTOR activity (Fig. 5A), and that this mTOR-mediated c-Myc–hnRNPs cascade is essential for PKM2 overexpression in mTOR hyperactive cells (Fig. 5B).
In comparison with aerobic oxidation of glucose, aerobic glycolysis is a very low economic process in terms of energy production. The potential benefit provided by glycolysis for cancer cells is unclear. The preference for aerobic glycolysis by cancer cells is now believed to provide building blocks for their accelerated growth and proliferation, even though this metabolic alteration might not directly and by itself induce malignancy (3, 10, 52, 53). Because mTOR is a major regulator of cell growth, proliferation, and differentiation, cell metabolism and proliferation now share a common regulatory pathway (54). Thus, mTOR-mediated aerobic glycolysis may play an essential role in tumorigenesis. In support of this notion, disruption of PKM2 expression in mTOR-activated cells blunted aerobic glycolysis in vitro (Fig. 3) and suppressed tumor formation in nude mouse xenografts (Fig. 6).
Enhanced aerobic glycolysis renders cells with oncogenic AKT and mTOR more susceptible than control cells to death after glucose deprivation (41, 55). Similarly, the addiction to aerobic glycolysis by hyperactive mTOR cells may be exploited for the treatment of tumors caused by dysregulated mTOR signaling. Tsc2−/− cells were indeed hypersensitive not only to mTOR inhibition by rapamycin but also to glycolytic blockade by 3-BrPA (Fig. 6). Notably, combinatorial suppression of mTOR and glycolysis synergistically retarded the proliferation of these cells in vitro and blocked tumor development of human PTEN-deficient cancer cells in nude mice (Fig. 6 C and D). This result demonstrates a proof of concept that combination therapy may provide a better opportunity for the management of cancers caused by the oncogenic RTK/PI3K/AKT/mTOR signaling transduction cascade.
In summary, mTOR activation simulates the hypoxia effect by inducing HIF1α expression, and HIF1α in turn enhances PKM2 expression through collaboration with c-Myc–hnRNPs splicing regulators. Subsequently, along with other glycolytic enzymes, PKM2 stimulates aerobic glycolysis, a metabolic shift often observed in pseudohypoxic tumors. This newly identified mTOR/HIF1α/Myc–hnRNPs/PKM2 glycolysis cascade is essential for cell proliferation and tumor growth. We speculate that the frequent hyperactivation of mTOR signaling during the course of the multistep oncogenesis could contribute to the development of the Warburg effect present in many human cancers. This study may provide us a mechanistic insight into novel targets controlled by mTOR signaling, and the components in the mTOR/HIF1α/Myc–hnRNPs/PKM2 glycolysis process may thus be targeted for the treatment of cancer caused by the abnormal RTK/PI3K/AKT/mTOR signaling propagation.
Materials and Methods
For descriptions of reagents, cell culture, cell proliferation assay, immunoblotting, measurements of glucose and lactate, siRNA gene knockdown, generation of stable gene knockdown cell lines, mRNA expression profiling analysis, RT-qPCR, real-time PCR analysis of ChIP DNA, mouse kidney tumor assessment, xenografting tumorigenesis and treatment, and statistics, see SI Materials and Methods.
Supplementary Material
Acknowledgments
This study was supported by National Natural Science Foundation of China Grant 30788004, the National Basic Research Program of China 973 Program Grants 2009CB522202 and 2009CB522203, and Ministry of Education of China 111 Project B08007.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014769108/-/DCSupplemental.
References
- 1.Pan JG, Mak TW. Metabolic targeting as an anticancer strategy: Dawn of a new era? Sci STKE. 2007;2007:pe14. doi: 10.1126/stke.3812007pe14. [DOI] [PubMed] [Google Scholar]
- 2.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 3.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 5.Yoo YG, Hayashi M, Christensen J, Huang LE. An essential role of the HIF-1alpha-c-Myc axis in malignant progression. Ann N Y Acad Sci. 2009;1177:198–204. doi: 10.1111/j.1749-6632.2009.05043.x. [DOI] [PubMed] [Google Scholar]
- 6.Christofk HR, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 7.David CJ, Chen M, Assanah M, Canoll P, Manley JL. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature. 2010;463:364–368. doi: 10.1038/nature08697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clower CV, et al. The alternative splicing repressors hnRNP A1/A2 and PTB influence pyruvate kinase isoform expression and cell metabolism. Proc Natl Acad Sci USA. 2010;107:1894–1899. doi: 10.1073/pnas.0914845107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spoden GA, et al. Pyruvate kinase isoenzyme M2 is a glycolytic sensor differentially regulating cell proliferation, cell size and apoptotic cell death dependent on glucose supply. Exp Cell Res. 2009;315:2765–2774. doi: 10.1016/j.yexcr.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 10.Vander Heiden MG, et al. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010;329:1492–1499. doi: 10.1126/science.1188015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moritz A, et al. Akt-RSK-S6 kinase signaling networks activated by oncogenic receptor tyrosine kinases. Sci Signal. 2010;3:ra64. doi: 10.1126/scisignal.2000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Govindarajan B, et al. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J Clin Invest. 2007;117:719–729. doi: 10.1172/JCI30102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knobbe CB, Lapin V, Suzuki A, Mak TW. The roles of PTEN in development, physiology and tumorigenesis in mouse models: A tissue-by-tissue survey. Oncogene. 2008;27:5398–5415. doi: 10.1038/onc.2008.238. [DOI] [PubMed] [Google Scholar]
- 14.Efeyan A, Sabatini DM. mTOR and cancer: Many loops in one pathway. Curr Opin Cell Biol. 2010;22:169–176. doi: 10.1016/j.ceb.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–414. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Hill R, et al. PTEN loss accelerates KrasG12D-induced pancreatic cancer development. Cancer Res. 2010;70:7114–7124. doi: 10.1158/0008-5472.CAN-10-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoki K, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 18.Podar K, Anderson KC. A therapeutic role for targeting c-Myc/Hif-1-dependent signaling pathways. Cell Cycle. 2010;9:1722–1728. doi: 10.4161/cc.9.9.11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dang CV, Kim JW, Gao P, Yustein J. The interplay between MYC and HIF in cancer. Nat Rev Cancer. 2008;8:51–56. doi: 10.1038/nrc2274. [DOI] [PubMed] [Google Scholar]
- 20.Kwiatkowski DJ, et al. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet. 2002;11:525–534. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- 21.Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4:658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- 22.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 23.El-Hashemite N, Zhang H, Henske EP, Kwiatkowski DJ. Mutation in TSC2 and activation of mammalian target of rapamycin signalling pathway in renal angiomyolipoma. Lancet. 2003;361:1348–1349. doi: 10.1016/S0140-6736(03)13044-9. [DOI] [PubMed] [Google Scholar]
- 24.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 25.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 26.Bissler JJ, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, et al. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest. 2003;112:1223–1233. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keniry M, Parsons R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene. 2008;27:5477–5485. doi: 10.1038/onc.2008.248. [DOI] [PubMed] [Google Scholar]
- 29.Hill R, et al. PTEN loss accelerates KrasG12D-induced pancreatic cancer development. Cancer Res. 2010;70:7114–7124. doi: 10.1158/0008-5472.CAN-10-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollizzi K, et al. A hypomorphic allele of Tsc2 highlights the role of TSC1/TSC2 in signaling to AKT and models mild human TSC2 alleles. Hum Mol Genet. 2009;18:2378–2387. doi: 10.1093/hmg/ddp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semenza GL. HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J Bioenerg Biomembr. 2007;39:231–234. doi: 10.1007/s10863-007-9081-2. [DOI] [PubMed] [Google Scholar]
- 32.Land SC, Tee AR. Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J Biol Chem. 2007;282:20534–20543. doi: 10.1074/jbc.M611782200. [DOI] [PubMed] [Google Scholar]
- 33.Dekanty A, Lavista-Llanos S, Irisarri M, Oldham S, Wappner P. The insulin-PI3K/TOR pathway induces a HIF-dependent transcriptional response in Drosophila by promoting nuclear localization of HIF-alpha/Sima. J Cell Sci. 2005;118:5431–5441. doi: 10.1242/jcs.02648. [DOI] [PubMed] [Google Scholar]
- 34.Brugarolas J, Kaelin WG., Jr. Dysregulation of HIF and VEGF is a unifying feature of the familial hamartoma syndromes. Cancer Cell. 2004;6:7–10. doi: 10.1016/j.ccr.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 35.Semenza GL. HIF-1: Upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20:51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yun J, et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325:1555–1559. doi: 10.1126/science.1174229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma J, et al. Mammalian target of rapamycin regulates murine and human cell differentiation through STAT3/p63/Jagged/Notch cascade. J Clin Invest. 2010;120:103–114. doi: 10.1172/JCI37964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Düvel K, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toschi A, et al. Phospholipase D-mTOR requirement for the Warburg effect in human cancer cells. Cancer Lett. 2010;299:72–79. doi: 10.1016/j.canlet.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blouin MJ, et al. Loss of function of PTEN alters the relationship between glucose concentration and cell proliferation, increases glycolysis, and sensitizes cells to 2-deoxyglucose. Cancer Lett. 2010;289:246–253. doi: 10.1016/j.canlet.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 41.Elstrom RL, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 42.Khatri S, Yepiskoposyan H, Gallo CA, Tandon P, Plas DR. FOXO3a regulates glycolysis via transcriptional control of tumor suppressor TSC1. J Biol Chem. 2010;285:15960–15965. doi: 10.1074/jbc.M110.121871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H, et al. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Invest. 2007;117:730–738. doi: 10.1172/JCI28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazurek S. Pyruvate kinase type M2: A key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol. 2010 doi: 10.1016/j.biocel.2010.02.005. 10.1016/j.biocel.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 46.Robey IF, Lien AD, Welsh SJ, Baggett BK, Gillies RJ. Hypoxia-inducible factor-1alpha and the glycolytic phenotype in tumors. Neoplasia. 2005;7:324–330. doi: 10.1593/neo.04430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brugarolas JB, Vazquez F, Reddy A, Sellers WR, Kaelin WG., Jr. TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell. 2003;4:147–158. doi: 10.1016/s1535-6108(03)00187-9. [DOI] [PubMed] [Google Scholar]
- 48.El-Hashemite N, Walker V, Zhang H, Kwiatkowski DJ. Loss of Tsc1 or Tsc2 induces vascular endothelial growth factor production through mammalian target of rapamycin. Cancer Res. 2003;63:5173–5177. [PubMed] [Google Scholar]
- 49.Toschi A, Lee E, Gadir N, Ohh M, Foster DA. Differential dependence of hypoxia-inducible factors 1 alpha and 2 alpha on mTORC1 and mTORC2. J Biol Chem. 2008;283:34495–34499. doi: 10.1074/jbc.C800170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jung JE, et al. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J. 2005;19:1296–1298. doi: 10.1096/fj.04-3099fje. [DOI] [PubMed] [Google Scholar]
- 51.Xu Q, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24:5552–5560. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- 52.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: Metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laplante M, Sabatini DM. An emerging role of mTOR in lipid biosynthesis. Curr Biol. 2009;19:R1046–R1052. doi: 10.1016/j.cub.2009.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choo AY, et al. Glucose addiction of TSC null cells is caused by failed mTORC1-dependent balancing of metabolic demand with supply. Mol Cell. 2010;38:487–499. doi: 10.1016/j.molcel.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.