Abstract
Signaling through N-methyl-d-aspartate–type glutamate receptors (NMDARs) is essential for the development of behavioral sensitization to psychostimulants such as amphetamine (AMPH). However, the cell type and brain region in which NMDAR signaling is required for AMPH sensitization remain unresolved. Here we use selective inactivation of Grin1, the gene encoding the essential NR1 subunit of NMDARs, in dopamine neurons or their medium spiny neuron (MSN) targets, to address this issue. We show that NMDAR signaling in dopamine neurons is not required for behavioral sensitization to AMPH. Conversely, removing NMDARs from MSNs that express the dopamine D1 receptor (D1R) significantly attenuated AMPH sensitization, and conditional, virus-mediated restoration of NR1 in D1R neurons in the nucleus accumbens (NAc) of these animals rescued sensitization. Interestingly, sensitization could also be restored by virus-mediated inactivation of NR1 in all remaining neurons in the NAc of animals lacking NMDARs on D1R neurons, or by removing NMDARs from all MSNs. Taken together, these data indicate that unbalanced loss of NMDAR signaling in D1R MSNs alone prevents AMPH sensitization, whereas a balanced loss of NMDARs from both D1R and dopamine D2 receptor-expressing (D2R) MSNs is permissive for sensitization.
In rodents, repeated amphetamine (AMPH) treatment results in locomotor sensitization, which persists even after long periods of withdrawal. Although sensitization is not a measure of addiction, it is a stable alteration of behavior that has been associated with addictive behaviors (1). The mechanism by which repeated psychostimulant treatment leads to a sensitized locomotor response remains incompletely understood. However, it has been proposed that stable alterations in the nucleus accumbens (NAc) are involved (1). Consistent with this hypothesis, a large number of adaptations are observed in the NAc after repeated psychostimulant treatment. These changes include increased dopamine and glutamate release into the NAc upon subsequent stimulant treatment (2, 3), increased dopamine D1 receptor (D1R) sensitivity (4), and changes in synaptic strength onto NAc medium spiny neurons (MSNs) (5, 6). In addition to the changes that occur in the NAc, there is an N-methyl-d-aspartate–type glutamate receptor (NMDAR)-dependent potentiation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor currents in dopamine neurons in response to psychostimulants (7).
Glutamate signaling through NMDARs is required for the development of AMPH sensitization. Systemic NMDAR antagonism before AMPH treatment prevents sensitization (8). NMDAR antagonists delivered directly to the ventral tegmental area (VTA) block a sensitized response to subsequent AMPH challenge, whereas NMDAR antagonism in the NAc has no effect on AMPH sensitization (9).
Genetic inactivation of functional NMDARs in specific neuronal populations in mice by Cre-mediated recombination of the gene encoding the essential NR1 subunit of the NMDAR, Grin1, is another approach to studying the role of these receptors in sensitization. This approach was used to study the role of NMDARs on dopamine neurons in cocaine sensitization (10, 11), and revealed that NMDAR signaling in dopamine neurons was not necessary for sensitization. In contrast, NMDAR antagonists delivered to the VTA prevent cocaine sensitization (12). These disparate results have been recently reconciled by the observation that an NMDAR antagonist injected into the VTA of mice lacking NMDARs on dopamine neurons still blocks cocaine sensitization (13). Thus, NMDARs on nondopamine neurons of the VTA appear to be the critical substrate for cocaine sensitization in this brain region.
Although, to date, no genetic models lacking NMDARs in MSNs have been tested for their ability to sensitize to psychostimulants, it appears that activity of D1R- and D2R-expressing neurons may have opposing effects in mediating cocaine-induced locomotion and sensitization (14–16). These studies are generally consistent with the model that activity of direct-pathway MSNs (D1R-expressing) stimulates locomotion, whereas activity of indirect pathway MSNs (D2R-expressing) inhibits locomotion (17). In addition to evidence that D1R and D2R neurons have antagonistic effects in psychostimulant sensitization, a growing body of work suggests that convergence of NMDAR and D1R signaling are required to mediate changes associated with repeated psychostimulant treatment (4, 18–20). These data led us to hypothesize that inactivating NMDARs specifically on D1R neurons, while leaving those on D2R neurons intact, might reveal a role for NMDAR signaling in the NAc in AMPH sensitization. We report that inactivation of NMDAR signaling on D1R-expressing MSNs prevents sensitization, whereas inactivation of NMDARs in both MSN populations is permissive for AMPH sensitization.
Results
NMDAR Signaling in Dopamine Neurons Is Not Required for Behavioral Sensitization to AMPH.
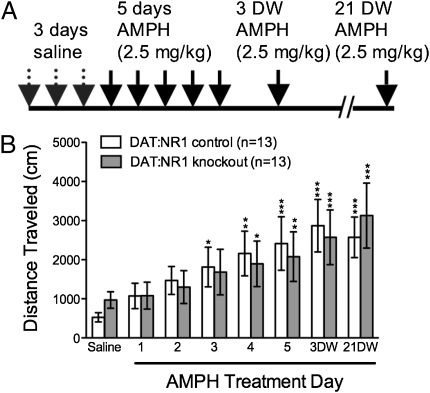
It has been reported that NMDARs in the VTA are required for AMPH sensitization (9), but the cell types involved are unknown. To determine whether NMDARs on dopamine neurons are required for AMPH sensitization, we used animals that lack NR1 specifically in these cells. Mice with a floxed Grin1 locus (Grin1lox/lox) were crossed to animals in which Cre recombinase expression is driven by the endogenous dopamine transporter locus (Slc6a3Cre/+;Grin1Δ/+) to generate knockout mice with the genotype Slc6a3Cre/+;Grin1Δ/lox (DAT:NR1 knockouts) and controls with the genotype Slc6a3Cre/+;Grin1lox/+ (DAT:NR1 controls) (10). We tested the development of AMPH sensitization in these animals by monitoring their locomotion in activity chambers after AMPH (2.5 mg/kg) administration for 5 consecutive days. This dose is in the range used in AMPH sensitization paradigms in mice (21–23), and was chosen because it resulted in a robust acute locomotor response (4.4-fold increase compared with saline) across all control groups, as well as profound locomotor sensitization (4.8-fold increase in locomotion on AMPH treatment day 5 versus day 1) among all control groups. The long-term maintenance of a sensitized response was tested by challenge injections at 3 d of withdrawal (3 DW) and 21 DW (Fig. 1A). DAT:NR1 knockouts and DAT:NR1 controls developed comparable locomotor sensitization to AMPH, which was maintained at both withdrawal time points (Fig. 1B; all statistics are reported in figure legends). This finding suggests that NMDAR signaling in dopamine neurons is not required for AMPH sensitization.
Fig. 1.
NMDARs on dopamine neurons are not required for AMPH sensitization. (A) AMPH sensitization paradigm. On all days, animals were injected i.p. after 90-min habituation in locomotion chambers, and locomotion was measured for 90 min after injection. (B) Cumulative 90-min locomotor response to AMPH in DAT:NR1 control (n = 13) and DAT:NR1 knockout (n = 13) mice across days. DW, days of withdrawal. Two-way, repeated-measures ANOVA: genotype effect, F(1, 24) = 0.01, P = 0.90; day effect, F(6, 144) = 14.41, P = 0.01; genotype × day effect, F(6, 144) = 0.79, P = 0.58.
Inactivating NMDAR Signaling Specifically in D1R-Expressing Neurons Impairs AMPH Sensitization and Conditioned Place Preference.
Due to evidence that convergent D1R and NMDAR signaling are required for long-term cellular and behavioral adaptations to repeated AMPH (4, 18–20), and studies supporting opposing roles for activity in D1R- and D2R-expressing MSNs in AMPH sensitization (14–16), we hypothesized that NMDARs on D1R neurons are required for AMPH sensitization. To test this hypothesis, we selectively removed NR1 from D1R-expressing neurons. Mice with a floxed Grin1 locus (Grin1lox/lox) were crossed to animals with Cre recombinase knocked into the D1R locus (Drd1aCre/+) to generate knockout mice with the genotype Drd1aCre/+;Grin1Δ/lox (D1R:NR1 knockout mice) and controls with the genotype Drd1aCre/+;Grin1lox/+ (D1R:NR1 control mice).
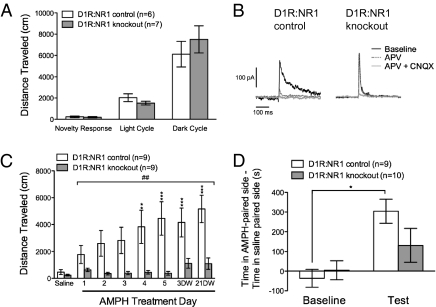
As reported previously, D1R-Cre is expressed in the dorsal striatum and NAc, which are the predominant sites of D1R expression in adult animals (24). Consistent with another model lacking NMDAR signaling in striatal D1R-expressing neurons (19), these knockout mice exhibited normal 24-h locomotion (Fig. 2A). To confirm a lack of functional NMDAR signaling in D1R Cre-positive striatal neurons of D1R:NR1 knockout mice, control and knockout animals carrying a conditional, Cre-activated TdTomato reporter (mouse line Ai14) were generated (25). Whole-cell, voltage-clamp recordings from red-fluorescing cells in knockout and control mice were obtained from brain slices through the ventral striatum. Application of the NMDAR antagonist D-(-)-2-amino-5-phosphonopentanoic acid (APV) significantly attenuated excitatory postsynaptic currents (EPSCs) in control mice relative to knockout mice (inhibition in controls: 66.9% ± 6.3% vs. knockout: 15.2% ± 9.4%; P < 0.01 by unpaired t test; Fig. 2B). Six of seven knockout cells were completely unresponsive to APV, but one neuron had an intact APV-sensitive component of the EPSC.
Fig. 2.
Removing NMDARs from D1R-expressing neurons impairs AMPH sensitization. (A) Baseline locomotion in D1R:NR1 control and knockout mice; novelty response (distance traveled in 60 min in a novel environment by D1R:NR1 control mice: 230 ± 80 cm vs. D1R:NR1 knockout mice: 200 ± 60 cm), P = 0.74; light cycle, P = 0.22; dark cycle, P = 0.45 by unpaired t tests. (B) Representative traces of NMDAR and AMPAR EPSCs from TdTomato-expressing neurons in the ventral striatum of D1R:NR1 control and knockout mice. (C) Cumulative 90-min locomotor response to AMPH in D1R:NR1 control (n = 9) and knockout (n = 9) mice across days; two-way, repeated-measures ANOVA: genotype effect, F(1, 16) = 9.51, P = 0.007; day effect, F(6, 96) = 6.80, P < 0.0001; genotype × day effect, F(6, 96) = 3.96, P = 0.0014 (##P < 0.01); **P < 0.01; ***P < 0.001 compared with AMPH day 1 within genotype. (D) Difference between time spent in AMPH-paired and saline-paired compartments at baseline and after 1 d of AMPH pairing in D1R:NR1 control (n = 9) and knockout (n = 10) mice. Two-way, repeated-measures ANOVA: genotype effect, F(1, 17) = 0.78, P = 0.39; day effect, F(1, 17) = 22.56, P = 0.0002; genotype × day effect, F(1, 17) = 4.76, P = 0.04. *P < 0.05 vs. baseline.
The acute AMPH response in D1R:NR1 knockout mice was intact (fold increase in locomotion on AMPH relative to saline in controls: 4.9 ± 1.8 vs. knockout: 3.9 ± 1.1; P = 0.60 by unpaired t test). However, the ability of D1R:NR1 knockout mice to undergo AMPH sensitization was impaired compared with controls (Fig. 2C). To explore whether the failure to sensitize observed in D1R:NR1 knockout mice was due to an inability to achieve locomotion comparable to control animals, we treated both groups of animals with the NMDAR antagonist MK-801, a drug known to induce locomotion via a dopamine-independent mechanism (26). D1R:NR1 knockout mice showed a robust locomotor response to MK-801 [distance traveled in 90 min after 0.5 mg/kg MK-801 by D1R:NR1 control mice: 8,647 ± 961.6 cm (n = 11); knockout mice: 16,344 ± 3,884 cm (n = 11); P = 0.07 by unpaired t test]. Thus, the failure to manifest sensitization as robustly as control animals is not due to impaired locomotor ability. Moreover, the locomotor response to MK-801 does not depend upon NMDAR antagonism of cells expressing D1R. Finally, we measured conditioned place preference (CPP), another conditioned behavioral response to AMPH. After 1 d of AMPH pairing (1.5 mg/kg), D1R:NR1 control animals showed a significant preference for the AMPH-paired context, whereas D1R:NR1 knockout mice failed to do so (Fig. 2D).
Restoration of NMDAR Signaling to D1R-Expressing Neurons in the NAc Is Sufficient to Rescue Sensitization.
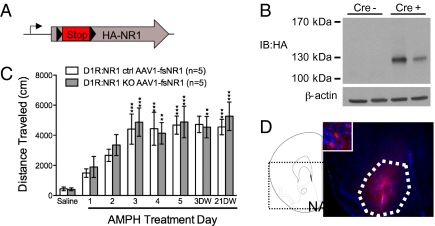
D1R:NR1 knockout mice lack NMDARs in several brain regions; therefore, the anatomical location of the NMDARs on D1R-expressing cells required for AMPH sensitization is not discernable. Because a variety of long-lasting changes associated with sensitization are observed in the NAc (6, 27, 28), we hypothesized that restoring NMDAR signaling to this brain region in D1R:NR1 knockout mice might be sufficient to rescue AMPH sensitization in these animals. To address this hypothesis, we used a conditional virus-mediated restoration approach. An adeno-associated virus containing a cDNA cassette of rat NR1 with a floxed-stop cassette followed by an HA tag inserted after amino acid 31 (AAV1-fsNR1) was generated (Fig. 3A). A Western blot against HA confirmed that full-length NR1 was expressed in the NAc of virus-injected animals in a Cre-dependent manner (Fig. 3B).
Fig. 3.
Restoring NMDAR signaling to the NAc of D1R:NR1 knockouts rescues AMPH sensitization. (A) Schematic diagram of AAV1-fsNR1 virus construct. (B) Western blot of striatal homogenates from two D1R-Cre (−) and two D1R-Cre (+) mice that had received intra-NAc injections of AAV1-fsNR1, probed with anti-HA antibody showing Cre-dependent expression of full-length viral NR1. (C) Cumulative 90-min locomotor response to AMPH from AAV1-fsNR1–injected D1R:NR1 control (n = 5) and knockout (n = 5) mice across days; two-way, repeated-measures ANOVA: genotype effect, F(1, 8) = 0.1246, P = 0.73; day effect, F(6, 48) = 9.10, P < 0.0001; genotype × day effect, F(6, 48) = 0.25, P = 0.96. **P < 0.01, ***P < 0.001 compared with AMPH day 1 within genotype. (D) Immunohistochemistry with anti-HA antibody showing NAc-specific expression of viral NR1. NAc is outlined in white. A DAPI counterstain was used.
To test whether restoration of NMDAR signaling to the NAc would be sufficient to rescue sensitization in D1R:NR1 knockout mice, AAV1-fsNR1 was bilaterally injected into the NAc of naive D1R:NR1 knockout and control mice. Subsequently, both groups of animals were subjected to our AMPH sensitization protocol. AAV1-fsNR1 injection into the NAc completely restored AMPH sensitization to D1R:NR1 knockout mice (Fig. 3C).
Following completion of behavioral analysis, NR1 reexpression and proper viral targeting were assessed by HA immunostaining (Fig. 3D). These data indicate that NMDAR signaling in D1R-expressing cells in the NAc is sufficient for the development of AMPH sensitization.
Removing NMDAR Signaling Throughout the Striatum Allows AMPH Sensitization.
D1R:NR1 knockout mice lack a major excitatory neurotransmitter receptor selectively in D1R neurons, potentially favoring neuronal activity in D2R cells, a situation that impairs psychostimulant-induced locomotion (14–16). Hence, the unopposed activity of D2R-expressing MSNs might be responsible for the lack of AMPH sensitization, rather than there being a strict requirement for NMDAR signaling by D1R-expressing MSNs. To examine the possibility that AMPH sensitization might require balanced NMDAR signaling in the two populations of MSN, we performed three experiments.
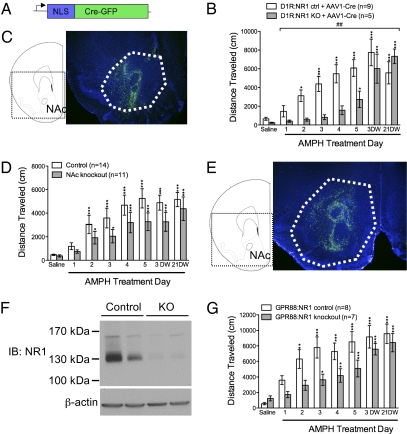
First, we removed NMDAR signaling from the remaining NAc neurons in D1R:NR1 knockout mice using a viral vector carrying a Cre-GFP fusion gene (AAV1-Cre-GFP) (Fig. 4A), the function of which has been validated in a number of studies (10, 29). AAV1-Cre-GFP was injected into the NAc of D1R:NR1 knockout and control mice. After recovering from surgery, both groups of mice underwent the AMPH sensitization protocol. Both D1R:NR1 control and knockout mice injected with AAV1-Cre-GFP showed comparable AMPH sensitization, although the knockout mice became sensitized more slowly than controls, and achieved comparable locomotion on AMPH only at the withdrawal time points (Fig. 4B). Following AMPH sensitization, viral targeting to the NAc was verified by visualizing GFP in the NAc of these animals (Fig. 4C). These data indicate that unbalanced NMDAR signaling through D1R- and non–D1R-expressing neurons prevents AMPH sensitization.
Fig. 4.
Removing NMDAR signaling from both classes of MSNs is permissive for AMPH sensitization. (A) Schematic diagram of AAV1-Cre-GFP virus construct. (B) Cumulative 90-min locomotor response to AMPH from AAV1-Cre-GFP–injected D1R:NR1 control (n = 9) and knockout (n = 5) mice across days; two-way, repeated-measures ANOVA: genotype effect, F(1, 12) = 3.343, P = 0.09; day effect, F(6, 72) = 22.08, P < 0.0001; genotype × day effect, F(6, 72) = 4.23, P = 0.01 (##P < 0.01). *P < 0.05, ***P < 0.001 compared with AMPH day 1 within genotype. (C) GFP fluorescence showing NAc-specific injection of AAV1-Cre-GFP into D1R:NR1 knockout mice. NAc is outlined in white. A DAPI counterstain was used. (D) Cumulative 90-min locomotor response to AMPH from control mice (n = 14) and mice in which NR1 is inactivated in the NAc (n = 12) across days; two-way, repeated-measures ANOVA: genotype effect, F(1, 23) = 2.00, P = 0.17; day effect, F(6, 138) = 26.59; P < 0.0001; genotype × day effect, F(6, 138) = 1.07, P = 0.39. *P < 0.05, ***P < 0.001 compared with AMPH day 1 within genotype. (E) GFP fluorescence showing NAc-specific injection of AAV1-Cre-GFP into Grin1lox/lox mice. NAc is outlined in white. A DAPI counterstain was used. (F) Western blot of striatal homogenates from two GPR88:NR1 control and two GPR88:NR1 knockout animals probed with anti-NR1 antibody. (G) Cumulative 90-min locomotor response to AMPH from GPR88:NR1 control (n = 8) and knockout (n = 7) mice across days; two-way, repeated-measures ANOVA: genotype effect, F(1, 13) = 4.163, P = 0.06; day effect, F(6, 78) = 26.96, P < 0.0001; genotype × day effect, F(6, 78) = 1.81, P = 0.11. *P < 0.05, **P < 0.01, ***P < 0.001 compared with AMPH day 1 within genotype.
The previous findings strongly suggests that genetically inactivating NMDAR signaling throughout the NAc is permissive for sensitization. However, because NMDAR signaling is inactivated in D1R-expressing cells early in development in the D1R:NR1 knockout mice, it remains possible that the results obtained in the previous experiment are due to a developmental alteration in striatal circuitry. We addressed this issue in our second experiment, in which we nonselectively inactivated NMDAR signaling in the NAc of adult mice using the AAV1-Cre-GFP approach. AAV1-Cre-GFP was injected into the NAc of Grin1lox/lox animals to generate NAc-specific Grin1 knockout mice, and into Grin1lox/+ control animals. Two weeks after surgery, both groups of mice underwent the AMPH sensitization protocol. There was no significant difference between NAc knockout and control mice in our AMPH sensitization paradigm (Fig. 4D). Following behavioral analysis, NAc-specific viral targeting was confirmed by visualization of GFP-positive neurons in that region (Fig. 4E). This result suggests that NMDARs in the NAc as a whole are not necessary for the development of AMPH sensitization.
Histological analysis revealed that viral injection only inactivates Grin1 in a fraction of cells in the NAc. Therefore, it is possible that our failure to see a phenotype in Grin1lox/lox animals injected with AAV1-Cre-GFP is due to an insufficient number of cells transduced by virus. In addition, Grin1 inactivation by AAV1-Cre-GFP affects all neuronal types in the NAc; thus, it is also possible that any phenotype observed in either the Grin1lox/lox animals or the D1R:NR1 knockout mice may be due to disrupted NMDAR signaling in striatal interneurons. These issues are addressed in our third experiment, in which we generated mice that lack NMDAR signaling selectively all MSNs. Grin1lox/lox mice were crossed to mice with Cre recombinase targeted to the striatum-specific Gpr88 locus (Gpr88Cre/+), a gene that is expressed in both populations of MSNs throughout the dorsal and ventral striatum but not in striatal interneurons (30). Striatal dissections were performed on the resulting Gpr88Cre/+;Grin1Δ/lox (GPR88:NR1 knockout) and Gpr88Cre/+;Grin1lox/+ (GPR88:NR1 control) animals. A Western blot confirmed that GPR88:NR1 knockout mice lack nearly all NR1 throughout the dorsal and ventral striatum (Fig. 4F). We tested the ability of GPR88:NR1 control and knockout mice to undergo AMPH sensitization, and found that both groups were able to sensitize (Fig. 4G). The sensitized response in GPR88:NR1 knockout mice appeared to develop more slowly than in control mice, similar to what we observed in D1R:NR1 knockout mice injected with AAV1-Cre-GFP into the NAc. Taken together, these results indicate that although loss of NMDARs in D1R neurons impairs sensitization, NMDARs in the NAc are not strictly required for AMPH sensitization. Rather, balanced NMDAR-mediated activity in the two antagonistic populations of MSNs is essential for behavioral sensitization to occur.
Discussion
We have shown that NMDARs on dopamine neurons are not required for AMPH sensitization. Furthermore, we demonstrated that disrupting NMDAR signaling in D1R-expressing cells prevents sensitization, but that a balanced loss of NMDARs in both classes of MSNs is permissive for sensitization. The VTA has been thought of as critical for the induction of psychostimulant sensitization, whereas the NAc is required for its expression (9, 31, 32). Because the genetic models used in this study lack NMDAR signaling throughout the behavioral paradigm, it is not possible for us to draw conclusions regarding the phase of sensitization at which these receptors are important, only whether a lack of NMDAR signaling in particular cell types and brain regions affects the behavioral output of the animal. It would be possible to determine the role of NMDARs in induction versus expression of AMPH sensitization by viral injection between the induction phase and withdrawal time points of this paradigm.
The finding that NMDARs in dopamine neurons are not required for the development of AMPH sensitization is somewhat surprising given previous pharmacological experiments exploring the role of NMDARs in psychostimulant sensitization (9). However, we and others have shown that DAT:NR1 knockout mice also develop normal cocaine sensitization, similar to what we describe here (10, 11, 13). There are multiple possible explanations for this discrepancy. First, antagonists affect all cell types in a region, whereas the genetic approach allows only a particular cell type to be affected. Recent evidence strongly suggests that nondopaminergic cells in the VTA are the critical substrate in this region for mediating sensitization (13). Alternatively, it has been shown that not all dopamine neurons express DAT; therefore, Cre-mediated removal of Grin1 in DAT:NR1 knockout mice may be incomplete (33). It has been shown that application of NMDAR antagonists to the VTA of animals lacking NMDARs on dopamine neurons was able to block cocaine sensitization, suggesting that NMDARs on nondopaminergic cells in this region are important in cocaine sensitization, or that the NMDAR antagonist used has off-target effects (13). Finally, it is possible that DAT:NR1 knockout mice are able to sensitize to psychostimulants due to a compensatory mechanism. However, we believe this is also unlikely, because inactivating Grin1 in the dopamine neurons of adult mice has been shown to leave cocaine CPP intact (11).
The findings that mice lacking NMDAR signaling in D1R neurons are unable to sensitize, and that restoring NR1 to D1R neurons in the NAc of these animals rescues AMPH sensitization, support other studies showing that cocaine-induced changes in plasticity are both NMDAR and D1R dependent (34). In addition, a number of adaptations in the NAc elicited by chronic psychostimulant treatment, such as increased dendritic spine density (27), are maintained selectively in D1R-expressing cells of the NAc (35). Furthermore, there is a growing body of literature implicating the convergence of NMDAR and D1R signaling in a number of forms of behavioral and cellular adaptation, including AMPH sensitization (18–20, 36). Our work provides a direct demonstration of the importance of convergent NMDAR and D1R signaling within the NAc for behavioral sensitization to AMPH. Remarkably, although only 30–40% of NAc cells in the rescue experiment expressed the viral NR1 subunit (corresponding to roughly 60–80% of D1 neurons in the region), the behavioral rescue was complete. Therefore, it is possible that only a fraction of D1R-expressing MSNs in the NAc is required for AMPH sensitization. Alternatively, overexpression of the viral NR1 in injected control and knockout mice might account for the complete behavioral rescue. Furthermore, it is impossible to determine from these data alone whether NMDARs in D1R-expressing MSNs in the dorsal striatum are involved in AMPH sensitization in a wild-type mouse.
Surprisingly, removing NMDARs from the remaining neurons in the NAc of D1R:NR1 knockout mice also restores sensitization, and inactivating NMDARs in all MSNs preserves AMPH sensitization. We believe these observations are significant for a number of reasons. First, they provide direct support for several recent studies suggesting that activity of D1R MSNs is crucial for the development of psychostimulant-driven behaviors, whereas activity of D2 MSNs may play an inhibitory role in the formation of these behaviors (14–16, 37, 38). In addition, these findings shed light on previously seemingly contradictory findings that NMDAR antagonism in the NAc does not affect AMPH sensitization (9), but that a large number of NMDAR-dependent changes occur in the NAc in association with sensitization (4, 36).
The finding that D1R and D2R neurons have opposing roles in mediating AMPH sensitization can be explained using a classical model of D1R and D2R MSN function (17, 39). Briefly, activity of D1R MSNs (direct pathway) promotes locomotion, whereas activity of D2R MSNs (indirect pathway) inhibits locomotion. Dopamine, released by AMPH or relevant natural stimuli, positively modulates D1R MSNs and negatively modulates D2R MSN activity (40), thus promoting locomotion by its action on both pathways. Consistent with this general model, both D1R antagonists (41) and D2R antagonists (42) have been shown to impair AMPH sensitization, possibly by preventing AMPH-mediated dopamine release from up-regulating D1R MSN activity and down-regulating D2R MSN activity, respectively. Removing NMDARs, a major class of glutamate receptors, from D1R MSNs in the D1R:NR1 knockout mice may cause a severe imbalance of activity between the two classes of MSNs, favoring D2R MSNs, and in this way impair AMPH-mediated locomotor sensitization. By contrast, a balanced loss of NMDAR signaling in both classes of MSNs, as is present in the GPR88:NR1 knockout mice, may dampen MSN activity but allow the opposite effects of AMPH-induced dopamine efflux on the two classes of MSNs to remain intact. This disruption in NMDAR signaling is therefore permissive of AMPH sensitization. Interestingly, all groups of animals in which NR1 was inactivated in both classes of MSNs appeared to sensitize more slowly than controls. This delay in sensitization was significant in the AAV1-Cre–injected D1R:NR1 knockout mice, the animals in which NMDAR signaling was most skewed in favor of D2R neurons. These animals only exhibit sensitization comparable to that seen in controls after a period of withdrawal, an interesting phenomenon that may merit further study. It is not clear what leads to the slower onset of a sensitized response in these animals. One possibility is that the neuronal changes that underlie AMPH sensitization, which may normally be induced by NMDAR signaling in the two classes of MSNs, are now mediated by other neurotransmitter receptors; the residual glutamate receptors are prime candidates.
Interestingly, baseline locomotion in all knockout animals studied here was normal. Therefore, it appears that altered NMDAR signaling throughout the striatum, specifically in D1R-expressing MSNs, disrupts hyperlocomotion evoked by hyperdopaminergia without affecting basal ambulatory activity. Basal ambulatory activity is likely to be more dependent on D2R signaling because D2Rs have a higher affinity for dopamine than D1Rs; hence, they may be preferentially occupied under normal conditions (43).
Taken together, our results indicate that D1R- and D2R-expressing neurons have opposing roles in mediating sensitization to AMPH. Specifically, D1R neuron activity promotes whereas D2R neuron activity inhibits AMPH sensitization. Unbalancing the contribution of the direct and indirect pathways, by in our case compromising the glutamatergic activation of the D1R-expressing MSNs, prevents sensitization. This conclusion has cautionary implications regarding the interpretation of the role of dopamine in other behavioral experiments in which only D1R or D2R signaling were manipulated by either genetic or pharmacological means. Furthermore, our findings suggest that differentially modulating the activity of the two classes of MSNs to favor D2R neuron activity might have potential therapeutic benefit in combating addiction.
Materials and Methods
Mice.
All animal protocols were approved by the University of Washington Institutional Animal Care and Use Committee. The generation and maintenance of all mouse lines are described in SI Materials and Methods.
AMPH Sensitization.
On days 1–3, animals were habituated to activity chambers (Columbus Instruments). On days 4–8, day 12 [3-d withdrawal (DW)], and day 30 (21 DW), animals received i.p. AMPH (2.5 mg/kg; Sigma), and their locomotion was monitored for an additional 90 min. A more detailed protocol can be found in SI Materials and Methods.
AMPH Conditioned Place Preference.
Baseline compartment preference was measured, and mice were paired using an unbiased paradigm (22). Animals received 1 d of AMPH (1.5 mg/kg) and saline pairing. A more detailed paradigm description can be found in SI Materials and Methods.
MK-801–Induced Locomotion.
Mice received i.p. MK-801 (0.5 mg/kg; Sigma) in activity chambers, and their locomotion was monitored for 90 min. For a more detailed description, see SI Materials and Methods.
Overnight Locomotion.
Animals were placed in locomotion chambers with ad libitum access to food and water for 48 h. Distance traveled in the first hour is reported as a novelty response. Distance traveled during the second light and dark cycles are reported.
Viral Injections.
All viruses were injected bilaterally at the coordinates (x = ±1.35 mm; y = 1.70 mm; z = −3.75 mm) from bregma. Details of viral constructs and preparation can be found in SI Materials and Methods.
Immunohistochemistry.
After behavioral experiments, all stereotactically injected animals were euthanized and perfused as described (44). Immunostaining with an anti-HA antibody and visualization of GFP were performed as described in SI Materials and Methods.
Electrophysiology.
Mice were anesthetized with pentobarbital (200 mg/kg i.p.), and coronal brain slices (300 μm) were prepared. Whole-cell patch-clamp recordings in voltage clamp mode were obtained from MSNs. d-APV (100 μM) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10 μM) were used to block NMDA and AMPA receptor currents, respectively. Detailed methods can be found in SI Materials and Methods.
Western Blots.
Western blots for HA, NR1, and β-actin were performed as described elsewhere (45) and in SI Materials and Methods.
Statistics.
Sensitization and CPP data were analyzed using two-way, repeated-measures ANOVA. Fisher LSD post hoc tests were performed. Acute MK-801 and APV responses were compared using unpaired t tests.
Supplementary Material
Acknowledgments
We thank Glenda Froelick for help with histology and Kiara Eldred for assistance with genotyping. This work was supported by National Institute of General Medical Sciences Grant T32 GM07735 and the Achievement Rewards for College Scientists (ARCS) Foundation. A.Q. and E.S. are recipients of Ministerio de Ciencia e Innovacion (MCINN) postdoctoral mobility program fellowships. Work by M.J.W. and N.S.B. was supported by National Institutes of Health Grants F32-DA026273 NS052536, NS060803, and HD02274.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1101424108/-/DCSupplemental.
References
- 1.Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 2.Robinson TE, Jurson PA, Bennett JA, Bentgen KM. Persistent sensitization of dopamine neurotransmission in ventral striatum (nucleus accumbens) produced by prior experience with (+)-amphetamine: A microdialysis study in freely moving rats. Brain Res. 1988;462:211–222. doi: 10.1016/0006-8993(88)90549-5. [DOI] [PubMed] [Google Scholar]
- 3.Pierce RC, Kalivas PW. Repeated cocaine modifies the mechanism by which amphetamine releases dopamine. J Neurosci. 1997;17:3254–3261. doi: 10.1523/JNEUROSCI.17-09-03254.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf ME, White FJ, Hu XT. MK-801 prevents alterations in the mesoaccumbens dopamine system associated with behavioral sensitization to amphetamine. J Neurosci. 1994;14:1735–1745. doi: 10.1523/JNEUROSCI.14-03-01735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: A neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- 6.Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- 8.Wolf ME, Jeziorski M. Coadministration of MK-801 with amphetamine, cocaine or morphine prevents rather than transiently masks the development of behavioral sensitization. Brain Res. 1993;613:291–294. doi: 10.1016/0006-8993(93)90913-8. [DOI] [PubMed] [Google Scholar]
- 9.Vezina P, Queen AL. Induction of locomotor sensitization by amphetamine requires the activation of NMDA receptors in the rat ventral tegmental area. Psychopharmacology (Berl) 2000;151:184–191. doi: 10.1007/s002130000463. [DOI] [PubMed] [Google Scholar]
- 10.Zweifel LS, Argilli E, Bonci A, Palmiter RD. Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors. Neuron. 2008;59:486–496. doi: 10.1016/j.neuron.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engblom D, et al. Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron. 2008;59:497–508. doi: 10.1016/j.neuron.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Kalivas PW, Alesdatter JE. Involvement of N-methyl-d-aspartate receptor stimulation in the ventral tegmental area and amygdala in behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1993;267:486–495. [PubMed] [Google Scholar]
- 13.Luo Y, et al. NMDA receptors on non-dopaminergic neurons in the VTA support cocaine sensitization. PLoS ONE. 2010;5:e12141. doi: 10.1371/journal.pone.0012141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Bateup HS, et al. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc Natl Acad Sci USA. 2010;107:14845–14850. doi: 10.1073/pnas.1009874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson SM, et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerfen CR. The neostriatal mosaic: Multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- 18.Snyder GL, Fienberg AA, Huganir RL, Greengard P. A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J Neurosci. 1998;18:10297–10303. doi: 10.1523/JNEUROSCI.18-24-10297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heusner CL, Palmiter RD. Expression of mutant NMDA receptors in dopamine D1 receptor-containing cells prevents cocaine sensitization and decreases cocaine preference. J Neurosci. 2005;25:6651–6657. doi: 10.1523/JNEUROSCI.1474-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valjent E, et al. Mechanisms of locomotor sensitization to drugs of abuse in a two-injection protocol. Neuropsychopharmacology. 2010;35:401–415. doi: 10.1038/npp.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bedingfield JB, Calder LD, Thai DK, Karler R. The role of the striatum in the mouse in behavioral sensitization to amphetamine. Pharmacol Biochem Behav. 1997;56:305–310. doi: 10.1016/s0091-3057(96)00331-0. [DOI] [PubMed] [Google Scholar]
- 22.Ramsey AJ, et al. Genetic NMDA receptor deficiency disrupts acute and chronic effects of cocaine but not amphetamine. Neuropsychopharmacology. 2008;33:2701–2714. doi: 10.1038/sj.npp.1301663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon J, et al. A subpopulation of neuronal M4 muscarinic acetylcholine receptors plays a critical role in modulating dopamine-dependent behaviors. J Neurosci. 2010;30:2396–2405. doi: 10.1523/JNEUROSCI.3843-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heusner CL, Beutler LR, Houser CR, Palmiter RD. Deletion of GAD67 in dopamine receptor-1 expressing cells causes specific motor deficits. Genesis. 2008;46:357–367. doi: 10.1002/dvg.20405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chartoff EH, Heusner CL, Palmiter RD. Dopamine is not required for the hyperlocomotor response to NMDA receptor antagonists. Neuropsychopharmacology. 2005;30:1324–1333. doi: 10.1038/sj.npp.1300678. [DOI] [PubMed] [Google Scholar]
- 27.Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fadok JP, Dickerson TMK, Palmiter RD. Dopamine is necessary for cue-dependent fear conditioning. J Neurosci. 2009;29:11089–11097. doi: 10.1523/JNEUROSCI.1616-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massart R, Guilloux JP, Mignon V, Sokoloff P, Diaz J. Striatal GPR88 expression is confined to the whole projection neuron population and is regulated by dopaminergic and glutamatergic afferents. Eur J Neurosci. 2009;30:397–414. doi: 10.1111/j.1460-9568.2009.06842.x. [DOI] [PubMed] [Google Scholar]
- 31.Paulson PE, Robinson TE. Sensitization to systemic amphetamine produces an enhanced locomotor response to a subsequent intra-accumbens amphetamine challenge in rats. Psychopharmacology (Berl) 1991;104:140–141. doi: 10.1007/BF02244569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perugini M, Vezina P. Amphetamine administered to the ventral tegmental area sensitizes rats to the locomotor effects of nucleus accumbens amphetamine. J Pharmacol Exp Ther. 1994;270:690–696. [PubMed] [Google Scholar]
- 33.Lammel S, et al. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 34.Fourgeaud L, et al. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24:6939–6945. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee KW, et al. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci USA. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valjent E, et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durieux PF, et al. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci. 2009;12:393–395. doi: 10.1038/nn.2286. [DOI] [PubMed] [Google Scholar]
- 38.Lobo MK, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kravitz AV, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Vezina P. D1 dopamine receptor activation is necessary for the induction of sensitization by amphetamine in the ventral tegmental area. J Neurosci. 1996;16:2411–2420. doi: 10.1523/JNEUROSCI.16-07-02411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuribara H. Modification of cocaine sensitization by dopamine D1 and D2 receptor antagonists in terms of ambulation in mice. Pharmacol Biochem Behav. 1995;51:799–805. doi: 10.1016/0091-3057(95)00037-w. [DOI] [PubMed] [Google Scholar]
- 43.Richfield EK, Penney JB, Young AB. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience. 1989;30:767–777. doi: 10.1016/0306-4522(89)90168-1. [DOI] [PubMed] [Google Scholar]
- 44.Hnasko TS, et al. Cre recombinase-mediated restoration of nigrostriatal dopamine in dopamine-deficient mice reverses hypophagia and bradykinesia. Proc Natl Acad Sci USA. 2006;103:8858–8863. doi: 10.1073/pnas.0603081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanz E, et al. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc Natl Acad Sci USA. 2009;106:13939–13944. doi: 10.1073/pnas.0907143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.