Abstract
The extinction of a species is inevitably preceded by the extirpation of a series of local populations. Ecological theory predicts that vulnerability to extirpation varies between populations and is ultimately linked to environmental heterogeneity. If populations of a species are present in multiple regions separated by abrupt changes in environmental conditions (e.g., biomes), spatial variation in vulnerability to extirpation may be closely linked to the distribution of these regions. In the absence of abrupt shifts in environmental conditions, populations at the edge of a species’ range should have low growth rates and be more vulnerable to extirpation, whereas populations located in the core of the species’ range should be exposed to more favorable environmental conditions, have higher growth rates, and be less vulnerable. Here, we ask whether the distribution of biomes or range position better reflects spatial variation in vulnerability for 43 mammal species distributed through four continents. We control for the distribution of human threats and quantify the importance of protected areas in population persistence. We conclude that the distribution of biomes is a better predictor of vulnerability than position in the geographic range. We also find that core populations are less vulnerable than edge populations (after controlling for threats levels and protected areas). Protected areas are important for the persistence of most species we studied. By providing a measure of vulnerability linked directly to the distribution of threats, our results offer insights for scaling up from species vulnerability to extinction risk.
The geographic ranges of many species are composed of multiple interacting local populations (1). Range loss occurs as local populations are progressively extirpated (2).Focusing on range loss, and the extirpation of these local populations, provides insight into the process of extinction, and can help inform conservation action and management. For example, studies of range collapse can draw attention to declining species before they reach levels requiring intensive interventions (2, 3) and this focus can uncover the drivers of range loss, providing important insights for management (3, 4). Even if a species is still globally common, studying range loss can also identify where ecosystems are losing local populations of a given species (5).
Although it is clear that various forms of human modification of the environment are the major extrinsic drivers of extirpations (2, 6), there is ongoing debate about the role that other factors, including intraspecific variation in vulnerability play in determining patterns of range loss. A number of authors have theorized that the spatial distribution of suitable environmental conditions (including biotic interactions; e.g., ref. 7) leads to variation in the size and demography of local populations and that this variation influences these populations’ ability to tolerate anthropogenic disturbance (threats; refs. 6 and 8). Consider two populations of the same species with similar birth rates but different adult mortality and imagine that some pollutant that lowers birth rates is introduced into both populations. For some level of pollutant, the population with lower adult mortality should persist, whereas the population with higher adult mortality is extirpated. In other words, one population is more vulnerable. If vulnerability differs greatly between local populations, this underlying natural heterogeneity could have consequences for range-wide priority setting, reintroductions, and other forms of conservation planning and management (9, 10). For example, accounting for variation between populations in their vulnerability may help us to better evaluate species level extinction risk. Furthermore, if intraspecific variation in vulnerability is related to climatic variables, understanding these relationships may allow us to predict where local populations are capable of persisting and/or colonizing under predicted scenarios of climate change and land use change.
The range structure and dynamics that result from the interaction of individuals of a species and the distributions of relevant environmental conditions within a species’ range are complex and difficult to understand without some degree of simplification (11). One way that ecologists have made sense of this complexity is through the concept of a species ecological niche. A species ecological niche can be defined as the set of conditions under which a species has a growth rate greater or equal to zero (12). The growth rates of many species are influenced by interactions with humans (including interactions with environmental changes associated with humans such land use change or hunting), and we argue that a species’ response to interactions with humans should be thought of as a part of a species’ niche. Although the growth rates of some species are increased by human actions, facilitating range expansion (e.g., ref. 13), we focus here on species that have been adversely affected and define the inability to tolerate interactions with humans as a species’ vulnerability.
A species’ niche also includes interactions between multiple environmental factors. For example, populations of a species might fail to grow under hot and dry conditions or cool and wet conditions, but have a positive growth rate at high temperatures under humid conditions. If we consider the ability to tolerate the consequences of human activities as an aspect of a species’ niche, then we might expect similar interactions between the intensity of human activities and other aspects of the species’ niche. For example, a species may be able to tolerate a given level of hunting in a wetter habitat, but have a negative growth rate for the same hunting intensity in an arid habitat. When a species persists throughout a large region and there is an abrupt shift in an important environmental condition within that region, we might expect populations on either side of the transition zone to vary substantially in their vulnerability. If dispersal links local populations across the transition zone, then natural selection favoring adaptations to the local environment in the more sparsely inhabited region may be inhibited, further magnifying the differences in demographic rates between the two populations (14).
Ecological theory recognizes environmental heterogeneity at various spatial scales. Coarse environmental heterogeneity in terrestrial ecosystems is represented by the fourteen biome classification (15). Each biome is a biogeographic region characterized by a range of environmental conditions, in particular precipitation and temperature, and is distinguished primarily by its distinct vegetation type (e.g., temperate conifer forests or tundra). For species with wide geographic ranges, the distribution of biomes may represent, to a first-order approximation, the distribution of relevant environmental conditions.
An alternative hypothesis, supported by a large body of ecological theory, posits that optimal environmental conditions occur in one or a few areas near the core of most species’ ranges, and habitat suitability declines toward the edges of the range (8, 16). As a consequence, densities and/or growth rates are greater in the core, and vulnerability is lower. For those species that generally comply with this generalization, declining densities at the edges are often the result of both lower frequency of suitable habitat and lower habitat quality within these areas (17). These smaller and more isolated edge populations are expected to be more susceptible to demographic stochasticity. Furthermore, because range edges often occur where species are close to some environmental limit, these edge populations may be more prone to extinction driven by environmental stochasticity than core populations (16, 18, 19). Lastly, local populations at the periphery of a species’ range may be poorly adapted to local environmental conditions because of asymmetric gene flow from the core of a species range (20–22). The hypothesis that species are more abundant, and thus less vulnerable, in the center of their ranges may be most appropriate in systems where biological, rather than physical, processes constrain dispersal and where environmental gradients are fairly smooth (18, 23).
To our knowledge, the hypothesis that vulnerability varies between biomes in large mammals has never been tested; however, researchers have discussed the differential impacts of gradual environmental gradients (ramps) versus abrupt changes (steps) on densities and growth rates across a species’ range (e.g., ref. 24). The hypothesis that core populations are less vulnerable has been tested and, contrary to initial expectations, researchers have found that core populations have a greater probability of extirpation than edge ones (e.g., refs. 6 and 25). However, these studies did not control for the distribution of human threats and thus could not disentangle intraspecific variation in exposure to threats from variation in vulnerability. Controlling for threat levels is critical to understanding spatial variation in range loss because humans preferentially settle areas that are also the best habitat for many species (26, 27).
Using data from 43 species of large mammals distributed across four continents and four taxonomic orders, we quantify the relative importance of human activities and vulnerability in explaining modern range loss. We focus on large mammals because human threats for this group have been identified and mapped (5, 28–31). For each species, we compare two models of range collapse that make different assumptions about the drivers of spatial variation in vulnerability while controlling for human activities (Fig. 1). The first model assumes that vulnerability varies between biomes. In other words, we ask whether a given intensity of human modification has a greater impact on population persistence in one biome than in another. The second model uses distance to the centroid of the historic range, a core–edge-based variable, as the index of vulnerability, and test whether population persistence at a given intensity of human modification is greater near the core or the edge of a species’ range. We use these models to quantify the relative importance of natural and anthropogenic factors in driving modern range collapse. Lastly, we examine how results from our study of within-species variation in vulnerability are linked to the study of between species variation in extinction risk.
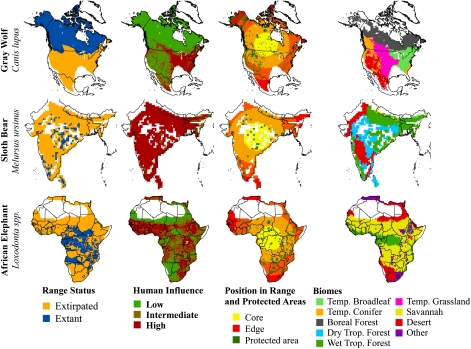
Fig. 1.
For 43 species, we analyzed the distribution of range loss and persistence (column 1) as a function of human influence (column 2), protected areas (column 3), and either the distance to centroid of the historic range (column 3) or the distribution of biomes (column 4). Here, we illustrate three examples: Canis lupus (first row), Melursus ursinus (second row), and Loxodonta spp. (third row).
Results
The distribution of biomes was a better proxy for vulnerability than the distance to the centroid of range. The distribution of 37 of the 43 species included in the analyses spanned more than one biome. For 26 of these 37 species, the biome model was a better fit to the data than the distance to centroid model based on deviance information criterion values (Fig. S1 and Tables S1 and S2). Species varied greatly in their mean vulnerability to human impacts and in the relative differences in vulnerability between biomes (Fig. 2); however, there were some consistent patterns across species. For example, temperate species (mostly North American) appeared to be less vulnerable in coniferous forest and more vulnerable in grasslands, with deciduous forests falling somewhere in the middle (Table S2). Tropical species (mostly African), on the other hand, were less vulnerable in savannah/grassland biomes, and more vulnerable in more arid desert regions and in wetter forests.
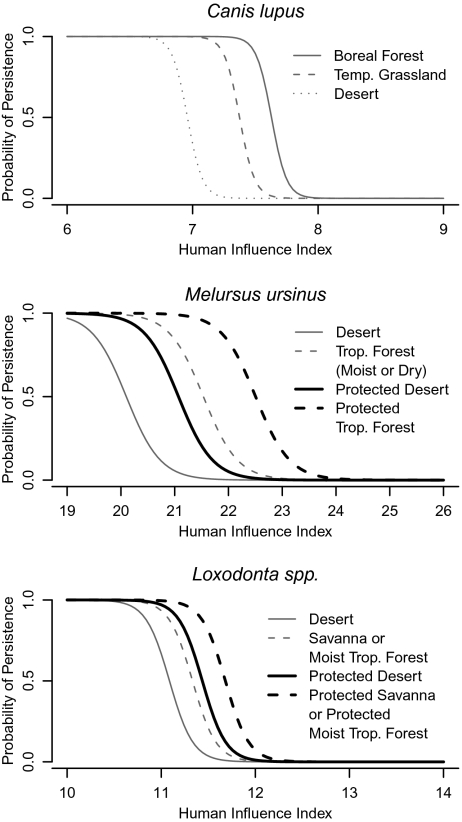
Fig. 2.
The relationship between the human influence index and the predicted probability of persistence varies as a function of biome and protection status. Curves are for the same three species in Fig. 1 and represent the most common biomes in the species’ historic ranges. Protected areas were not included for Canis lupus because they relationship with persistence was not significant in our models (Table S2).
Contrary to the conclusions of previous studies, we found consistent and broad support for the prediction that edge populations are more vulnerable than core populations (Table S1). Based on 95% credible intervals, 27 of 43 species supported this hypothesis, and no species contradicted it. Using less conservative 50% credible intervals, 37 species supported this hypothesis and only 2 contradicted it. Support for the distance-to-centroid predictor was relatively constant across taxonomic orders and continents.
The hypotheses that greater human impacts decrease population persistence and that protected areas increase persistence were also supported. Both the protected areas variable (14 of 37 species) and the human influence index (18 of 37 species) were significant and had expected signs across a similar percentage of species in the biome model as in the distance-to-centroid model (Table S1 and S2). Our proxy for threats was significant in 60% of species classified as threatened by the International Union for Conservation of Nature (IUCN; 9 of 15) as opposed to 38% of species that were not classified as threatened (10 of 28). Similarly, the presence of protected areas significantly increased persistence for 67% of threatened species (10 of 15) versus 46% of nonthreatened species (13 of 28). For 68% of species (25 of 37), the impact of protected areas was greater than the difference in vulnerability between biomes (Table S2 and Fig. 2). As we consider progressively more threatened species in the most well represented family in our dataset, the family Felidae, the impact of protected areas becomes more positive and the effect of the human influence index on persistence generally decreases (Fig. 3).
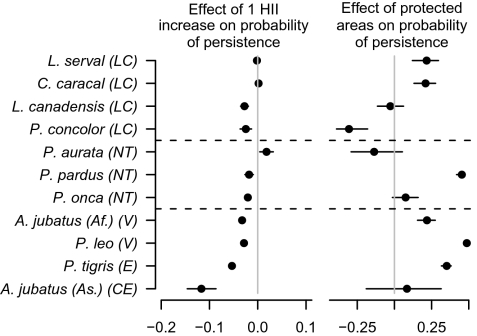
Fig. 3.
Among the 11 cat species in our study the estimated effect sizes of increasing human impacts (predicted to be negative) and protected areas (predicted to be positive) were greater in species of greatest conservation concern. Codes in parentheses are abbreviations for their status (least concern, LC; near threatened, NT; vulnerable, V; endangered, E; critically endangered, CR), with the horizontal dashed lines showing the divisions between threatened, near threatened, and least concern species. Bars around estimates represent 2 SDs. Values were calculated as the change in the predicted probability of persistence based on the addition of either parks or a one unit increase in the human influence index to a range cell with a predicted probability of persistence of 50%.
The focus of our study was intraspecific variation in vulnerability, but our results also offer insights about interspecific variation in extinction risk. Specifically, we can use our models to distinguish the roles that mean vulnerability (i.e., the predicted vulnerability of an average population outside a protected area subjected to a low intensity of human activities) and aggregate exposure to threats (i.e., proportion of range exposed to a low intensity of threats) play in determining the conservation status of a species (Fig.4). This type of analyses allows us not only to explain current conservation status but also to predict which species are most likely to become more threatened as the extent of human influence (i.e., exposure to threats) increases within their range. For instance, Panthera onca (Po), Hyaena brunnea (Hb), and Ursus arctos (Uar) are currently listed as being of least concern or near threatened; however, they appear predisposed to significant range collapse if a greater portion of their ranges became exposed to higher levels of human modification.
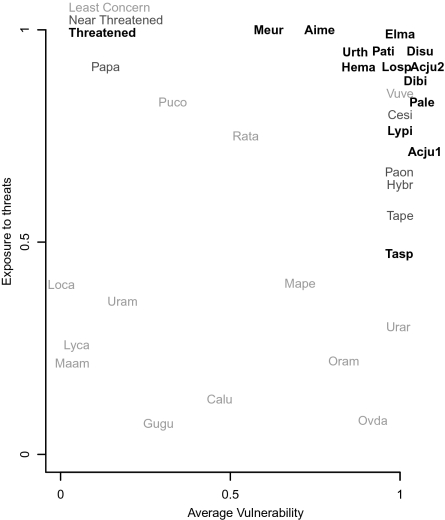
Fig. 4.
Conservation status of species is related to both their exposure to threats and their vulnerability (where vulnerability is defined as a species ability to tolerate human modification of their environment). For each species in this analysis, we defined a species’ average vulnerability as the predicted probability of persistence based on the parameter estimates of each species in the distance to centroid model (Table S1) and following values for the covariates: human influence index equal to 5 (the human influence index ranges from 0 to 50, with most values between 4 and 20; a value of 5 corresponds to an untransformed area within 2–15 km of a road with a population density of 1 human per km2); distance to the centroid equal to half of the maximum distance; spatial random effects equal to 0 and outside of a protected area. Exposure to threats was defined as the percentage of the grid cells in the historic range where the median human influence is ≤ 5. Letters indicate codes for species derived from the first two letters of their genus and species and their shade of gray indicates whether the species are least concern, near threatened or threatened according to the IUCN red list (69). Five of the species (Disu, Dibi, Acju2, Acju1, Pale) with vulnerability just barely below 1 were shifted over one for the purposes of clarity; however, all values were <1.
Discussion
We found broad support for the hypothesis that intraspecific variation in vulnerability has helped shape patterns of range loss during modern times. For those species for which we compared the biome and distance to centroid models, the biome model was favored in 27 of 37 species. The results from the biome model suggest some trends in the factors that determine vulnerability across large mammals. Among temperate species, vulnerability was highest in grasslands and lower in both deciduous and coniferous forests. Among tropical species, vulnerability was higher in deserts and moist forests. However, further study is required to determine whether these patterns apply to other continents (they are based mostly on North American and African species) and to a larger suite of species. We suspect that vulnerability in many species will be associated with habitat productivity within their ranges. Gaining a better understanding of these relationships and their generality across species is important for understanding past range loss and for predicting how ongoing climate change and concomitant shifts in the distribution and productivity of biomes may alter the future distribution of intraspecific variation in vulnerability.
In contrast to results from previous studies, we found strong support for greater vulnerability of edge populations relative to those located near the core of the range. Our analysis differed in three important ways from past studies that have found that edge populations are more likely to persist than core populations (6, 25). Unlike past studies, we controlled for threat levels, protected areas, and spatial autocorrelation, which should lead to more robust inference. On the other hand, we restricted our analysis to large mammals that tend to have large geographic ranges, whereas previous studies examined a more diverse sample of species. The choice to focus on large mammals was motivated by the belief that the human influence index would be a reasonable proxy for threats in these species, an assumption that is generally supported by our results. Our methods could be extended to other taxonomic groups as long as the appropriate threats can be identified and mapped. Lastly, we defined position in range based on the distance from centroid, whereas past studies divided a species range into two categories (core and edge) based on distance from the edge. We preferred our measure because it is continuous; however, these two measures were highly correlated for all species we examined.
The degree to which persistence in grid cells responds to the environment (including changes in the intensity of anthropogenic threats) is a function of a species’ niche, but is also contingent on patterns of dispersal and the resulting spatial population structure (32).We have represented vulnerability in the distance to centroid model as declining linearly, under the assumptions that growth rate is linked to environmental conditions and environmental conditions vary gradually. In the biome model, we assumed environmental conditions step up abruptly between biomes and result in an immediate change in growth rate. Clearly, neither of these caricatures is true, because heterogeneity in environmental conditions is more subtle and dispersal from neighboring areas also influences species demographic rates (31, 32). If the persistence of populations in the periphery of a species range is linked to metapopulation dynamics, the degree of range loss may vary depending on initial colonization and extinction rates and on the influence of anthropogenic on extinction rates, colonization rates, and habitat availability (11). Furthermore, if competition or predator–prey dynamics plays an important role in determining a species’ historic range limit, changes in the regional demographic rates of strongly interacting species may lead to unexpected responses to local environmental change (including increasing anthropogenic influences; refs. 7 and 33). Although the complexities of spatial population structure and ecological interactions can hinder our ability to detect the impacts of environmental heterogeneity, threats, and protected areas on patterns of range loss, it should not introduce a systematic bias in our results.
Explicitly linking range loss and population loss is difficult. Defining a local population and determining its spatial extent is often arbitrary, even though populations play a central role in ecological theory (34–36). The difficulties in linking ranges and populations and defining the area of a population arise from the fact that populations are a theoretical construct, whereas ranges (and area) are empirical constructs (37). The link between range loss and population loss is only an approximation. In this study, we chose our grid cell size after considering average home range sizes and persistence in protected areas for large mammals (38, 39). We also wanted to avoid confusing individual mortality or migration away from areas of human conflict with population loss, so we further assumed that it was preferable to err on the side of overestimating the spatial extent of local populations. So although persistence in our grid cells is only approximately related to population loss, it is a defensible approximation.
Including protected areas and the human influence index in our models allowed us to control for coarse spatial variation in the distribution of threats. Both the human influence index and the presence of protected areas explained patterns of range collapse in species from four taxonomic orders across four continents. Both variables were more likely to be included in the models of species that are considered to be most threatened by the IUCN (Fig. 3). For many species, the effect of protection status was of a similar or greater magnitude than the differences between biomes in vulnerability (Fig. 2 and Table S2). It is reassuring that protected areas and the human influence index were significant in so many species given the complications introduced by spatial population structure (see above), range map limitations (40), and the coarseness of both proxies. Despite these robust patterns, two important caveats to our conclusions bear discussion. First, historical threats have played a role in determining current ranges. For example, Puma concolor was extirpated from many parts of eastern North America before the creation of protected areas in this region, and this may explain why this species has a negative coefficient for protected areas. Second, the human influence index combines the impacts of multiple factors (e.g., land use change, human population density, roads) that negatively impact large mammals. This index, however, cannot control for variability between species in their relative vulnerability to different threats (e.g., one species might be more vulnerable to hunting than land use change, whereas another is more vulnerable to land use), nor can it account for nonlocal threats such climate change.
We have attempted to link our study of range collapse and intraspecific variation in vulnerability to the well developed literature addressing interspecific variation in extinction risk. Our results suggest that an estimate of species-level average vulnerability, coupled with information on the extent of human impacts, can provide a reasonable prediction of a species extinction risk. Extended to a larger suite of species, this approach could complement current studies linking species traits (e.g., body size and space requirements) to their IUCN conservation status (e.g., refs. 41–44). In particular, such an analysis could help shed light on the degree to which variation between species in extinction risk is related to the distribution of threats versus differences in life history strategies, traits, or taxonomic affiliation (45). Furthermore, studying how these same traits diverge within species and how these patterns are related to intraspecific variation in vulnerability may offer unique insights into understanding how traits are causally linked to extinction risk. For example, intraspecific variation in home range size in some carnivore species is substantial (e.g., 80–1,800 km2 in Canis lupus) and can be comparable to variation between species (46, 47). Studying intraspecific variation in vulnerability could also determine whether the required size of a protected area for species persistence varies at different locations within a species range, just as it varies between different species.
We conclude that intraspecific variation in vulnerability plays an important role in explaining patterns of range loss in large mammals. The degree of persistence at a given intensity of human modification varies between biomes and is greater in the core of a species range. Because multiple factors influence patterns of range loss, the importance of intraspecific variation in vulnerability must be understood in the context of the distribution of anthropogenic drivers of extirpation and intrinsic predictors of between species variation in extinction risk. Given the important biological differences between populations of large mammals in different biomes, the often unique roles these species play in ecosystems, the large uncertainties introduced by unstable governments and environmental variability, and the potential role of rapid evolution to ongoing environmental change, we suggest that effective and sustainable conservation strategies requires spreading efforts across the entirety of a species’ range with the recognition that requirements for persistence may vary between different sites.
Methods
The 43 mammal species included in this study were chosen based on two criteria: (i) availability of reasonably accurate historic and current maps (28, 48–61); (ii) range loss >20% of historical extent, but with current range not smaller than 25,000 km2. In other words, we chose mammals that have experienced range loss but still have substantive range remaining. Although we carefully examined historic and current maps, range maps often contain errors (40), which may introduce noise, but should not have systematically biased our analyses. For most species, the historic range was one continuous polygon; however, for those species that had discontinuous historic ranges (multiple discrete polygons), we only included those natural populations (discrete polygons) in which there had been some but not total range loss. Removal of these polygons (typically small islands) was necessary for the spatial models to converge. Parameter estimates from nonspatial models with and without these islands were similar, so we are confident that the removal of these islands did not bias our results.
For each species, we divided the historic ranges into grids with 50 × 50-km cells and determined whether any part of the current range overlapped with each cell. Human activities were included in the models in two ways. The human influence index, which is based on human population densities and land-use change (62), was included as a metric of threats. We chose the human influence index over the human footprint because the former has a consistent interpretation across all biomes, whereas the latter was modified based on the minimum and maximum human influences within each biogeographical region and thus must be interpreted within the context of each biome. The distribution of protected areas, obtained from the world database of protected areas (63), was also included in the models because these management structures can be instrumental in population persistence (64). Although not a comprehensive metric of wildlife management structures, geographically explicit data for protected areas are readily available. At finer spatial resolutions it was clear that the current range data and the World Commission on Protected Areas data were misaligned, however this misalignment was greatly lessened once we resampled at the 50 × 50-km resolution. To determine whether vulnerability varied between biomes, we included biome as a random effect in our models. For the biome model we focused only on the 37 species with at least 15 cells (37,500 km2) of current range and 15 or more cells of lost range in two or more biomes. Biome type for each cell was derived from the Terrestrial Ecosystems of the World Database (15). To determine the effects of position in the range on extinction in all 43 species, we first determined the centroid of each polygon in the historic geographic range and then calculated the distance from the center of each grid cell to the centroid of the polygon that contained it. The response variable was the current occupancy state of each cell (i.e., whether a cell did or did not overlap with a portion of the current range), given the cells in the historic range of a species. We modeled error using a Gaussian conditional autoregressive hierarchical Bayesian model with binary errors (65, 66). Parameters were estimated using WinBugs 1.4.3 with weak or noninformative priors. We compared the distance to centroid and biome models using deviance information criterion (67, 68).
To account for spatial autocorrelation, the original 50 × 50-km cells were grouped into larger 100 × 100-km blocks, and random effects for each block were conditioned on the model predictions for neighboring blocks. One consequence of this hierarchical design is that blocks centered on the border of the current range, where some, but not all, cells were occupied, were distinguishable from blocks where all cells shared the same value (i.e., whether the whole block was part of the current range or absent from the current range). The effect of this structure is to focus more attention on patterns of range loss along edges of the current range. We chose the spatial scales of the cells and blocks based on accepted accuracies of our coarsest range maps, examination of residuals, and past analyses that determined how much area was needed for different species of carnivores to have a >50% probability of persistence (38, 39).
Supplementary Material
Acknowledgments
We thank J. Ginsberg, S. Naeem, J. Nichols, and W. Jetz for critical discussions and reading of earlier versions of this manuscript. We also thank the editor and two anonymous reviewers for their helpful comments.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015097108/-/DCSupplemental.
References
- 1.Andrewartha HG, Birch LC. The ecological web: More on the distribution and abundance of animals. Chicago: University of Chicago Press; 1984. [Google Scholar]
- 2.Caughley G, Gunn A. Conservation biology in theory and practice. Cambridge, MA: Blackwell Science; 1996. [Google Scholar]
- 3.Caughley G. Directions in conservation biology. J Anim Ecol. 1994;63:215–244. [Google Scholar]
- 4.Soule ME. What do we really know about extinctions? In: Schonewald-Cox SM, Chambers SM, MacBryde B, Thomas WL, editors. Genetics and Conservation: A Reference for Managing Wild Animals and Plant Populations. Menlo Park, CA: Benjamin/Cummings Publishing Company; 1983. pp. 111–124. [Google Scholar]
- 5.Ceballos G, Ehrlich PR. Mammal population losses and the extinction crisis. Science. 2002;296:904–907. doi: 10.1126/science.1069349. [DOI] [PubMed] [Google Scholar]
- 6.Channell R, Lomolino MV. Dynamic biogeography and conservation of endangered species. Nature. 2000;403:84–86. doi: 10.1038/47487. [DOI] [PubMed] [Google Scholar]
- 7.Holt RD, Barfield M. Trophic interactions and range limits: The diverse roles of predation. Proc Royal Soc B. 2009;276:1435–1442. doi: 10.1098/rspb.2008.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawton JH. Range, population abundance and conservation. Trends Ecol Evol. 1993;8:409–413. doi: 10.1016/0169-5347(93)90043-O. [DOI] [PubMed] [Google Scholar]
- 9.Lomolino MV, Channell R. Range collapse, re-introductions, and biogeographic guidelines for conservation. Conserv Biol. 1998;12:481–484. [Google Scholar]
- 10.Wolf CM, Griffith B, Reed C, Temple SA. Avian and mammalian translocations: Update and reanalysis of 1987 survey data. Conserv Biol. 1996;10:1142–1154. [Google Scholar]
- 11.Holt RD, Keitt TH, Lewis MA, Maurer BA, Taper ML. Theoretical models of species' borders: single species approaches. Oikos. 2005;108:18–27. [Google Scholar]
- 12.Hutchinson GE. An Introduction to Population Ecology. New Haven, CT: Yale University Press; 1978. [Google Scholar]
- 13.Kanda LL, Fuller TK, Sievert PR, Kellogg RL. Seasonal source-sink dynamics at the edge of a species’ range. Ecology. 2009;90:1574–1585. doi: 10.1890/08-1263.1. [DOI] [PubMed] [Google Scholar]
- 14.Antonovics J. The Nature of Limits to Natural Selection. Ann Mo Bot Gard. 1976;63:224–247. [Google Scholar]
- 15.Olson DM, et al. Terrestrial ecoregions of the world: A New map of life on earth. Bioscience. 2001;51:933–938. [Google Scholar]
- 16.Brown JH, Mehlman DW, Stevens GC. Spatial variation in abundance. Ecology. 1995;76:2028–2043. [Google Scholar]
- 17.Gaston KJ. The structure and dynamics of geographic ranges. New York: Oxford University Press; 2003. p. 266. [Google Scholar]
- 18.Brown JH. On the relationship between Abundance and distribution of species. Am Nat. 1984;124:255–279. [Google Scholar]
- 19.Gaston KJ. Patterns in the geographical ranges of species. Biol Rev Camb Philos Soc. 1990;65:105–129. [Google Scholar]
- 20.Kirkpatrick M, Barton NH. Evolution of a species’ range. Am Nat. 1997;150:1–23. doi: 10.1086/286054. [DOI] [PubMed] [Google Scholar]
- 21.Bridle JR, Vines TH. Limits to evolution at range margins: when and why does adaptation fail? Trends Ecol Evol. 2007;22:140–147. doi: 10.1016/j.tree.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Sexton JP, McIntyre PJ, Angert AL, Rice KJ. Evolution and ecology of species range limits. Annu Rev Ecol Evol Syst. 2009;40:415–436. [Google Scholar]
- 23.Sagarin RD, Gaines SD, Gaylord B. Moving beyond assumptions to understand abundance distributions across the ranges of species. Trends Ecol Evol. 2006;21:524–530. doi: 10.1016/j.tree.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Caughley G, Grice D, Barker R, Brown B. The edge of the range. J Anim Ecol. 1988;57:771–785. [Google Scholar]
- 25.Channell R, Lomolino MV. Trajectories to extinction: Spatial dynamics of the contraction of geographical ranges. J Biogeogr. 2000;27:169–179. [Google Scholar]
- 26.Cincotta RP, Wisnewski J, Engelman R. Human population in the biodiversity hotspots. Nature. 2000;404:990–992. doi: 10.1038/35010105. [DOI] [PubMed] [Google Scholar]
- 27.Pautasso M. Scale dependence of the correlation between human population presence and vertebrate and plant species richness. Ecol Lett. 2007;10:16–24. doi: 10.1111/j.1461-0248.2006.00993.x. [DOI] [PubMed] [Google Scholar]
- 28.Laliberte AS, Ripple WJ. Range contractions of North American carnivores and ungulates. Bioscience. 2004;54:123–138. [Google Scholar]
- 29.Woodroffe R. Predators and people: using human densities to interpret declines of large carnivores. Anim Conserv. 2000;3:165–173. [Google Scholar]
- 30.Brashares JS, Arcese P, Sam MK. Human demography and reserve size predict wildlife extinction in West Africa. Proc Biol Sci. 2001;268:2473–2478. doi: 10.1098/rspb.2001.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrison JC, Sechrest W, Dinerstein E, Wilcove DS, Lamoreux JF. Persistence of large mammal faunas as indicators of global human impacts. J Mammal. 2007;88:1363–1380. [Google Scholar]
- 32.Brown JH, Lomolino MV. Biogeography. 2nd Ed. Sunderland, MA: Sinauer Associates; 1998. [Google Scholar]
- 33.Case TJ, Holt RD, McPeek MA, Keitt TH. The community context of species’ borders: ecological and evolutionary perspectives. Oikos. 2005;108:28–46. [Google Scholar]
- 34.Millstein RL. Populations as individuals. Biol Theory. 2009;4:267–273. [Google Scholar]
- 35.Wells JV, Richmond ME. Populations, metapopulations, and species populations: what are they and who should care? Wildl Soc Bull. 1995;23:458–462. [Google Scholar]
- 36.Schaefer JA. Towards maturation of the population concept. Oikos. 2006;112:236–240. [Google Scholar]
- 37.Maurer BA. Predicting distribution and abundance: Thinking within and between scales. In: Scott JM, et al., editors. Predicting Species Occurrences: Issues of Accuracy and Scale. Washington: Island Press; 2002. [Google Scholar]
- 38.Woodroffe R, Ginsberg JR. Edge effects and the extinction of populations inside protected areas. Science. 1998;280:2126–2128. doi: 10.1126/science.280.5372.2126. [DOI] [PubMed] [Google Scholar]
- 39.Woodroffe R, Ginsberg JR. Ranging behavior and vulnerability to extinction in carnivores. In: Sutherland WJ, Gosling LM, editors. Behaviour and Conservation. Cambridge: Cambridge University Press; 2000. pp. 125–140. [Google Scholar]
- 40.Boakes EH, et al. Distorted views of biodiversity: spatial and temporal bias in species occurrence data. PLoS Biol. 2010;8:e1000385. doi: 10.1371/journal.pbio.1000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purvis A, Gittleman JL, Cowlishaw G, Mace GM. Predicting extinction risk in declining species. Proc Biol Sci. 2000;267:1947–1952. doi: 10.1098/rspb.2000.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cardillo M, et al. Human population density and extinction risk in the world's carnivores. PLoS Biol. 2004;2:E197. doi: 10.1371/journal.pbio.0020197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cardillo M, et al. The predictability of extinction: biological and external correlates of decline in mammals. Proc Biol Sci. 2008;275:1441–1448. doi: 10.1098/rspb.2008.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davidson AD, Hamilton MJ, Boyer AG, Brown JH, Ceballos G. Multiple ecological pathways to extinction in mammals. Proc Natl Acad Sci USA. 2009;106:10702–10705. doi: 10.1073/pnas.0901956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davies TJ, et al. Colloquium paper: phylogenetic trees and the future of mammalian biodiversity. Proc Natl Acad Sci USA. 2008;105(Suppl 1):11556–11563. doi: 10.1073/pnas.0801917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moorcroft P, Lewis MA. Mechanistic Home Range Analysis. Princeton: Princeton University Press; 2006. [Google Scholar]
- 47.Karanth KU, Nichols JD, Kumar NS, Link WA, Hines JE. Tigers and their prey: Predicting carnivore densities from prey abundance. Proc Natl Acad Sci USA. 2004;101:4854–4858. doi: 10.1073/pnas.0306210101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taber A, et al. El Destino de los Arquitectos de los Bosques Neotropicales: Evaluación de la Distribución y el Estado de Conservación de los Pecaríes Labiados y los Tapires de Tierras Bajas (Grupo Especialista de la CSE/UICN en Cerdos, Pecaríes & Hipopótamos; Grupo & Tapires. New York: Wildlife Conservation Society & Wildlife Trust; 2008. [Google Scholar]
- 49.Seymour KL. Panthera onca. Mamm Species. 1989;340:1–9. [Google Scholar]
- 50.Blanc JJ, et al. African Elephant Specialist Group African Elephant Database. Gland, Switzerland: IUCN/SSC/African Specialist Group; 1998. [Google Scholar]
- 51.Ray J, Hunter L, Zigouris J. WCS Working Paper No. 24. New York: Wildlife Conservation Society; 2005. Setting conservation and research priorities for larger African carnivores. [Google Scholar]
- 52.International Rhino Foundation . Distribution Maps for White and Black Rhinos. Yulee, FL: International Rhino Foundation; 2006. [Google Scholar]
- 53.Laursen L, Beckoff M. Loxodonta africana. Mamm Species. 1978;92:1–8. [Google Scholar]
- 54.WCS . Range Wide Assessment for Mongolian Gazelles. Ulaanbaatar, Mongolia: Wildlife Conservation Society; 2004. [Google Scholar]
- 55.Sanderson EW, et al. Setting Priorities for the Conservation and Recovery of Wild Tigers: 2005-2015. The Technical Assessment. New York: WCS, WWF, Smithsonian, and NTWF-STF; 2006. [Google Scholar]
- 56.IUCN Bear Specialist Group. International Bear Association. WCS . Range-Wide Priority Settings for Asian Bears. Bronx, NY: Wildlife Conservation Society; 2006. [Google Scholar]
- 57.Sechrest W. Charlottesville: Univ of Virginia; 2003. Global diversity, endemism, and conservation of mammals. PhD dissertation. [Google Scholar]
- 58.Nowell K, Jackson P. Wild Cats Status Survey and Conservation. Gland, Switzerland: IUCN; 1996. [Google Scholar]
- 59.Santiapillai C, Jackson P. The Asian Elephant: An Action Plan for its Conservation. Gland, Switzerland: IUCN; 1990. [Google Scholar]
- 60.Sanderson EW, et al. Planning to save a species: the jaguar as a model. Conserv Biol. 2002;16:58–72. doi: 10.1046/j.1523-1739.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 61.Patterson BD, et al. Digital Distribution Maps of the Mammals of the Western Hemisphere, version 1.0. Arlington, VA: NatureServe; 2003. [Google Scholar]
- 62.Sanderson EW, et al. The human footprint and the last of the wild. Bioscience. 2002;52:891–904. [Google Scholar]
- 63.WCPA World database on protected areas. 2006. Available at http://www.unep-wcmc.org/wdpa. Accessed March 18, 2007.
- 64.Linnell J, Swenson J, Andersen R. Predators and people: conservation of large carnivores is possible at high human densities if management policy is favourable. Anim Conserv. 2001;4:345–349. [Google Scholar]
- 65.Latimer AM, Wu S, Gelfand AE, Silander JA., Jr Building statistical models to analyze species distributions. Ecol Appl. 2006;16:33–50. doi: 10.1890/04-0609. [DOI] [PubMed] [Google Scholar]
- 66.Besag J. Spatial Interaction and the Statistical Analysis of Lattice Systems. J R Stat Soc, B. 1974;36:192–236. [Google Scholar]
- 67.Spiegelhalter DJ, Best NG, Carlin BR, van der Linde A. Bayesian measures of model complexity and fit. London: Blackwell; 2002. pp. 583–616. [Google Scholar]
- 68.Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. New York: Cambridge Univ Press; 2007. [Google Scholar]
- 69.IUCN Red list of threatened species. 2010. Version 2010.3. Available at http://www.iucnredlist.org. Accessed September 2, 2010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.