Abstract
Pertussis is a highly contagious respiratory disease that is especially dangerous for infants and children. Despite mass vaccination, reported pertussis cases have increased in the United States and other parts of the world, probably because of increased awareness, improved diagnostic means, and waning vaccine-induced immunity among adolescents and adults. Licensed vaccines do not kill the organism directly; the addition of a component inducing bactericidal antibodies would improve vaccine efficacy. We investigated Bordetella pertussis and Bordetella bronchiseptica LPS-derived core oligosaccharide (OS) protein conjugates for their immunogenicity in mice. B. pertussis and B. bronchiseptica core OS were bound to aminooxylated BSA via their terminal Kdo residues. The two conjugates induced similar anti-B. pertussis LPS IgG levels in mice. B. bronchiseptica was investigated because it is easier to grow than B. pertussis. Using B. bronchiseptica genetically modified strains deficient in the O-specific polysaccharide, we isolated fractions of core OS with one to five repeats of the terminal trisaccharide, having at the nonreducing end a GlcNAc or GalNAc, and bound them to BSA at different densities. The highest antibody levels in mice were elicited by conjugates containing an average of 8–17 OS chains per protein and with one repeat of the terminal trisaccharide. Conjugate-induced antisera were bactericidal against B. pertussis, and the titers correlated with ELISA-measured antibody levels (r = 0.74). Such conjugates are easy to prepare and standardize; added to a recombinant pertussis toxoid, they may induce antibacterial and antitoxin immunity.
Keywords: hydroxylamine, Gram-negative
The genus Bordetella comprises Gram-negative bacteria pathogenic for mammals and birds. The most common Bordetella species are the respiratory pathogens Bordetella pertussis, Bordetella parapertussis, and Bordetella bronchiseptica. B. pertussis is an exclusively human pathogen causing paroxysmal coughing in infants (whooping cough) and persistent respiratory infections in adults (1). Killed whole-cell pertussis vaccines were used to immunize infants and children worldwide for many years but are too reactogenic for adults (2). Acellular pertussis vaccines containing pertussis toxoid and other proteins, such as filamentous hemagglutinin, pertactin, and fimbriae, have recently largely replaced whole-cell vaccines in most developed countries (3).
Despite a high rate of vaccination, B. pertussis is responsible for an estimated 260,000 deaths annually, and a resurgence of pertussis in the United States and Europe has initiated calls for an improved vaccine (4, 5). However, there is no agreement among experts or regulatory agencies about how to achieve this goal. There is, however, agreement that pertussis toxoid is an essential component of the acellular vaccines; a postimmunization level of ≥100 ELISA units (EU) to pertussis toxin (PT) is the only reliable method for its serologic diagnosis, and low levels of anti-PT are related to infection with this pathogen (6). The confusion is mainly due to the nature of pertussis vaccines’ complex mode of action. The primary action of pertussis vaccines is serum IgG antitoxin immunity that blocks the inactivating action of PT on phagocytic cells, thus allowing them to opsonize the B. pertussis; i.e., the antibodies elicited by acellular or whole-cell vaccines do not directly kill the pathogen. There is also the “herd” immunity effect of pertussis vaccines that results in decreased transmission of B. pertussis in the susceptible population (2, 6, 7). Similar to the effect induced by widespread immunization with diphtheria toxoid, this indirect effect of antitoxin accounts for the incomplete immunity of both vaccines on an individual basis (~80%; ref. 6).
Because IgG antibodies to the lipopolysaccharide (LPS) of noncapsulated Gram-negative bacteria were protective in humans (8, 9), we studied the oligosaccharide (OS) of B. pertussis LPS as a potential vaccine component. Several articles indicate that serum IgG anti-OS antibodies can confer complement mediated killing effect upon B. pertussis (10, 11). B. pertussis LPS is comprised of Lipid A and a branched dodecasaccharide core, composed of unusual sugars with free amino and carboxylic acid groups but with no O-specific polysaccharide (O-SP; ref. 12). SDS/PAGE analysis of B. pertussis LPS reveals two bands, band A and band B. Band B is composed of Lipid A and a branched nonasaccharide core, and band A consists of band B further substituted by a trisaccharide unit. B. bronchiseptica LPS was reported to have an identical core structure to that of B. pertussis but substituted by an O-SP, composed of a linear polymer of 1,4-linked 2,3-diacetamido-2,3-dideoxy-α-l-galacturonic acid (GalNAc3NAcA), connected to the core by a five-sugar linker (13–16).
B. pertussis and B. bronchiseptica core OS have low molecular weights and require covalent binding to a protein to be immunogenic. The conjugate-induced antibody levels are a function of the saccharide chain length, their loading on the protein, and the structure of the nonreducing terminal monosaccharide (17, 18). To evaluate these variables, we prepared conjugates of B. pertussis and B. bronchiseptica OS isolated from native and genetically modified strains (B. bronchiseptica RBA2b OS differed from that of B. pertussis in the OS chain length by producing multiples of band A terminal trisaccharide, and B. bronchiseptica RBB1a OS had ~50% of GlcNAc replaced by GalNAc at the nonreducing end) and studied their chemical, serological, and immunological properties as an addition to pertussis vaccine.
Results
Chemical Characterization of the LPS.
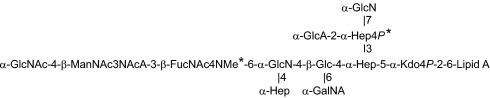
B. bronchiseptica RB50 LPS core OS structure is similar to that of B. pertussis Tohama I and Tax 113, with an additional component, an O-SP. The dodecasaccharide core of B. bronchiseptica RB50 without its O-SP (commonly referred to as “band A”) was separated on a Bio-Gel P-4 column. The first fraction, eluted in the void volume, contained band A substituted with O-SP (~80% by weight). The second fraction contained nonsubstituted band A, which was used in this study (~20% by weight). Electrospray ionization (ESI)-MS and NMR analysis confirmed the published LPS structure (13, 14). Mass spectra showed minor differences between band A of B. pertussis and of B. bronchiseptica (Fig. 1). In B. pertussis, phosphate (P) was not detected, and methylation (Me) of the N-4 of FucNAc4N was complete (a peak of 2,294 Da; the Kdo was present only in the anhydro-Kdo form), whereas in B. bronchiseptica RB50, ~50% of N-4 of FucNAc4N were methylated and ~30% of O-4 of the Hep substituted with GlcA was phosphorylated (peaks detected were as follows: 2,374 Da, band A with P and Me; 2,360 Da, band A without Me; 2,294 Da, band A without P; and 2,280 Da, band A without both P and Me).
Fig. 1.
Structure of B. pertussis LPS and B. bronchiseptica RB50 core-Lipid A fraction (band A). * indicates differences in methylation and phosphorylation between the strains (Results).
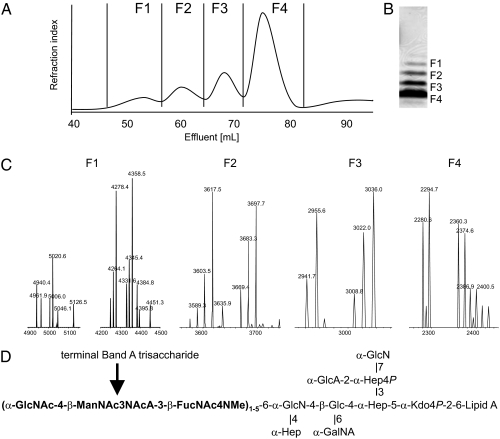
Three mutants deficient in O-SP, derived from B. bronchiseptica RB50, were used. The first was RBwbmΔ, which lacks the complete wbm locus and does not express O-SP, but produces core OS structurally almost identical to that of B. pertussis, differing from it only by the degree of phosphorylation and methylation, and identical to its parental strain RB50. RBB1a and RBA2b produced LPS containing several repeats of the band A trisaccharide. RBA2b produced oligomers of up to five repeats of the band A trisaccharide, visible by SDS/PAGE and mass spectra. OS with different numbers of repeats were separated on a Bio-Gel P-10 column from the acetic acid RBA2b LPS hydrolysate (Fig. 2). ESI-MS spectra confirmed that the OS in each fraction differed by one additional band A trisaccharide repeat (661 Da). NMR spectra of the RBA2b structures with repeats of terminal trisaccharide of various lengths were identical except for a new spin system of α-GlcNAc linking band A trisaccharide repeats together, compared with the core fraction of one band A trisaccharide only. LPS of the RBB1a strain contained mostly core with only small amounts of the band A trisaccharide repeats, but ~50% of the terminal GlcNAc was replaced by GalNAc.
Fig. 2.
Characterization of B. bronchiseptica RBA2b LPS hydrolyzate. (A) Bio-Gel P-10 gel filtration of B. bronchiseptica RBA2b OS with different number of band A nonreducing end trisaccharide repeats. (B) SDS/PAGE of B. bronchiseptica RBA2b LPS. (C and D) ESI-MS spectrum of the separated fractions (C) and their chemical structures (D). Each fraction differs by 661 Da (one repeat). Each OS is represented by several peaks due to incomplete methylation of FucNAc4N (14 Da) and/or phosphate (80 Da). F1, four and five repeats; F2, three repeats; F2, two repeats; F4, one repeat.
Serological Characterization of the LPS.
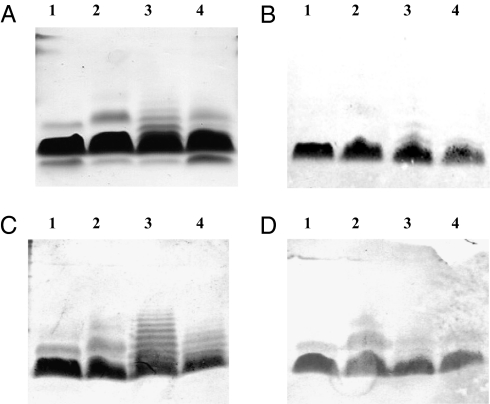
Western blot analysis showed binding of antiserum to B. pertussis Tohama I to the homologous and to the three B. bronchiseptica mutant LPS, confirming the similarity of these structures (Fig. 3). However, this serum bound only weakly to B. bronchiseptica RBA2b LPS containing several repeats of the band A trisaccharide. The same was observed for a serum induced by a BSA/RBA2b-F4 conjugate prepared with only one repeat of trisaccharide (conjugate 10; Table 1; structure the same as that of B. pertussis band A). Antisera induced by BSA/RBA2b-F3, -F2, and -F1 conjugates prepared with two, three, or four trisaccharide repeats (conjugates 11, 12, and 13; Table 1) bound to the LPS with one trisaccharide repeat, as well as to LPS with several repeats of the trisaccharide.
Fig. 3.
SDS/PAGE and Western blots of B. pertussis Tohama I (lane 1), B. bronchiseptica RBwbmΔ (lane 2), B. bronchiseptica RBA2b (lane 3), and B. bronchiseptica RBB1a (lane 4) LPS. (A) SDS/PAGE. (B –D) Western blots with anti-B. pertussis Tohama I bacteria (one band A trisaccharide repeat; B) with anti-BSA/RBA2b-F1 (four repeats of band A trisaccharide; C; similar picture was obtained with anti-BSA/RBA2b-F2 and BSA/RBA2b-F3 conjugates with three and two repeats of band A trisaccharide); and with anti-BSA/ RBA2b-F4 (one repeat of band A trisaccharide; D).
Table 1.
Composition and geometric mean of mouse IgG of anti-B. pertussis Tohama I LPS induced by conjugates of B. pertussis and B. bronchiseptica core fractions bound to BSA
| Conj. no. | OS in conjugate | Conj. Mm, kDa | Saccharide Mm, kDa | S/P ratio | No. of chains per BSA | No. of band A trisaccharide repeats | Terminal saccharide | IgG (25–75%), EU |
| 1 | B.p. Tohama I | 94 | 19 | 0.3 | 8 | 1 | GlcNAc | 4.2 (2.0–7.4) |
| 2 | B.p. Tax113 | 132 | 57 | 0.9 | 25 | 1 | GlcNAc | 1.2 (0.5–1.9) |
| 3 | B.p. Tax113 | 115 | 40 | 0.6 | 17 | 1 | GlcNAc | 5.6 (3.2–9.0) |
| 4 | B.p. Tax113 | 100 | 25 | 0.4 | 11 | 1 | GlcNAc | 3.3 (1.6–6.5) |
| 5 | B.b. RB50 core | 97 | 21 | 0.3 | 9 | 1 | GlcNAc | 4.6 (4.3–7.2) |
| 6 | B.b. RBwbmΔ | 120 | 45 | 0.7 | 19 | 1 | GlcNAc | 2.6 (2.1–4.5) |
| 7 | B.b. RBwbmΔ | 102 | 27 | 0.4 | 11 | 1 | GlcNAc | 2.8 (0.9–7.1) |
| 8 | B.b. RBB1a | 121 | 46 | 0.7 | 20 | 1 | GlcNAc/GalNAc | 0.4 (0.1–0.9) |
| 9 | B.b. RBB1a | 104 | 29 | 0.4 | 12 | 1 | GlcNAc/GalNAc | 8.7 (5.3–14.3) |
| 10 | B.b. RBA2b, F4 | 98 | 25 | 0.4 | 8 | 1 | GlcNAc | 5.3 (2.1–16.3) |
| 11 | B.b. RBA2b, F3 | 109 | 34 | 0.5 | 9 | 2 | GlcNAc | 0.3 (0.1–4.1) |
| 12 | B.b. RBA2b, F2 | 107 | 32 | 0.5 | 11 | 3 | GlcNAc | 0.1 (0.1–0.3) |
| 13 | B.b. RBA2b, F1 | 111 | 36 | 0.4 | 9 | 4–5 | GlcNAc | 0.3 (0.1–2.5) |
| — | PBS | — | — | — | — | — | — | <0.01 |
Mice (10 per group) were injected with 2.5 μg of saccharide as a conjugate per mouse, s.c., three times 2 weeks apart and bled 1 week after last injection. All conjugates induced statistically higher antibody levels than PBS group. Conjugate 10 vs. 11, 12, and 13, P < 0.005; conjugate 9 vs. 8, P = 0.03. Conj., conjugate; B.p., B. pertussis; B.b., B. bronchiseptica; Mm, molecular mass; S/P, saccharide/protein; –, not applied.
Chemical and Serological Characterization of Conjugates.
Schemes for preparing conjugates by derivatization of BSA with O-(3-thiopropyl)hydroxylamine followed by binding to a carbonyl group of acetic acid-hydrolyzed LPS to form an oxime linkage are shown in Fig. 4. This procedure yielded high-molecular-mass conjugates, detected by MALDI mass spectrometry (Table 1). The reactivity of the aminooxy group of the linker with anhydro-Kdo of the OS was established in another experiment: ESI-MS spectra showed complete incorporation of the linker into the OS molecules (Fig. S1). The number of OS chains per protein was calculated from the masses of the aminooxylated BSA, the OS, and the conjugate. For example, the average mass of the aminooxylated BSA was 72 kDa; the mass of the OS from B. pertussis Tohama I was 2,294 Da, and that of the conjugate was 94 kDa (94 − 72 kDa/2.294 kDa), leading to an estimate of eight chains per one BSA (conjugate 1).
Fig. 4.
Scheme of conjugation of B. pertussis and B. bronchiseptica core OS.
An identity line formed between all conjugates and the anti-B. pertussis Tohama I and anti-BSA sera by immunodiffusion. An example of an SDS/PAGE profile of conjugates prepared with RBA2b OS with different numbers of band A trisaccharide repeats and an immunodiffusion assay between these conjugates and anti-B. pertussis Tohama I and anti-BSA sera is shown in Fig. S2. All conjugates had <5 EU/μg of conjugate measured by Limulus amebocyte lysate assay.
Antibodies to B. pertussis LPS and Their Bactericidal Activity.
The conjugates varied in their chain lengths (number of band A trisaccharide repeats), nonreducing terminal saccharide (GlcNAc or a mixture of GlcNAc/GalNAc), and the density of OS chains per BSA (8–25 chains; Table 1). All conjugates, injected s.c. at 2.5 μg of saccharide per mouse, induced significantly higher antibody levels than the controls (Table 1). The antibody response elicited by the conjugates depended on the density of OS chains per protein. For B. pertussis Tax 113, the highest antibody levels were elicited by a conjugate with 17 chains per BSA (conjugate 3 vs. 2 and 4; Table 1), and for B. bronchiseptica RBB1a, 12 chains were better that 20 chains per protein (conjugate 8 vs. 9; P = 0.03; Table 1). Conjugate 9 of OS with mixed GlcNAc/GalNAc at the nonreducing terminal (~1:1 ratio) induced antibody levels similar to those induced by conjugates containing only GlcNAc termini (conjugates 1, 3, 5, and 10). Conjugates with two to five repeats of band A trisaccharide induced significantly lower anti-B. pertussis LPS levels than the conjugate with only one repeat (conjugate 10 vs. 11, 12, and 13; P < 0.005).
Bactericidal activity and IgM antibody levels were measured in sera representing various IgG antibody levels (Table 2). The correlation coefficients between the reciprocal bactericidal titers of these sera and their ELISA-measured antibody levels were as follows: IgG, r = 0.66; IgM, r = 0.73; and IgG plus IgM, r = 0.74.
Table 2.
Bactericidal activity, IgG, and IgM anti-B. pertussis Tohama I LPS (ELISA) elicited by different conjugates
| Conj no. | OS in a conjugate | No. of chains per BSA | Individual serum | IgG, EU | IgM, EU | Reciprocal bactericidal titers |
| 1 | B.p. Tohama I | 8 | 1786A | 3.6 | 3.2 | 20 |
| 2 | B.p. Tax113 | 25 | 2058H | 22.6 | 1.4 | 50 |
| 3 | B.p. Tax113 | 17 | 2059A | 9.4 | 4.2 | 100 |
| 3 | B.p. Tax113 | 17 | 2059E | 1.1 | 0.8 | 10 |
| 3 | B.p. Tax113 | 17 | 2059I | 13.8 | 9.5 | 750 |
| 4 | B.p. Tax113 | 11 | 2060A | 6.2 | 0.2 | 100 |
| 5 | B.b. RB50 core | 9 | 1787C | 9.6 | 1.7 | 500 |
| 5 | B.b. RB50 core | 9 | 1787G | 64.9 | 2.8 | 750 |
| 6 | B.b. RBwbmΔ | 19 | 2064A | 2.4 | 3.1 | 20 |
| 7 | B.b. RBwbmΔ | 11 | 2063A | 0.06 | 0.3 | <10 |
| 7 | B.b. RBwbmΔ | 11 | 2063C | 0.04 | 0.07 | <10 |
| 9 | B.b. RBB1a | 12 | 2093H | 38.4 | 13.9 | 750 |
| 9 | B.b. RBB1a | 12 | 2093J | 14.0 | 1.6 | 10 |
| 10 | B.b. RBA2b, F1 | 8 | 2152B | 5.3 | 0.6 | <10 |
| 11 | B.b. RBA2b, F2 | 9 | 2153D | 4.2 | 0.7 | 10 |
| 12 | B.b. RBA2b, F3 | 11 | 2154H | 4.1 | 0.2 | 10 |
| 13 | B.b. RBA2b, F4 | 9 | 2155D | 39.3 | 0.1 | 50 |
| 13 | B.b. RBA2b, F4 | 9 | 2155F | 2.1 | 3.8 | <10 |
| 13 | B.b. RBA2b, F4 | 9 | 2155H | 0.8 | 0.5 | <10 |
| PBS | — | 2156A | <0.01 | <0.01 | <10 |
Correlation coefficients between the reciprocal bactericidal titer and IgG, r = 0.66; IgM, r = 0.73; and IgG plus IgM, r = 0.74. *B.p., B. pertussis; B.b., B. bronchiseptica; –, not applied.
Discussion
Conjugates of B. pertussis core OS injected s.c. in PBS, at a fraction of an estimated human dose, induced serum bactericidal antibodies in young outbred mice. A dodecasaccharide was obtained from acid-hydrolyzed B. pertussis and B. bronchiseptica LPS, and each was bound to BSA by a single-point attachment at its reducing end. Conjugation was performed between the carbonyl group of acid-released anhydro-Kdo (19–21) and an aminooxy group of a bifunctional thio-aminooxy linker bound to BSA. This efficient reaction was used for preparing conjugates of Bordetella and Shigella O-SP-core fragments (22–25). The method allows the binding of saccharide components of different lengths and at different densities per protein molecule to select the most immunogenic construct. The final product is easy to standardize, and the methodology is suitable for clinical use.
Another type of B. pertussis LPS-based conjugate was immunogenic in rabbits, inducing antibodies that bound to B. pertussis cells as assayed by immunofluorescence (26). In that study, the conjugate was composed of the nonreducing end pentasaccharide (band A trisaccharide, GlcN, Hep) with the reducing end GlcN modified to anhydro-mannose used for conjugation. The authors (26) suggested that the epitope was located predominantly at the band A distal trisaccharide.
These results, together with the fact that the immunodominant fragment of B. bronchiseptica LPS was its nonreducing O-SP end saccharides (22), prompted us to prepare conjugates containing several repeats of the band A nonreducing end trisaccharide produced by recombinant strains of B. bronchiseptica to examine whether conjugates of longer trisaccharide chains would induce higher antibody levels. We isolated OS with one to five repeats of the trisaccharide from the B. bronchiseptica RBA2b strain and bound them separately to BSA. The LPS with more than one repeat did not bind to the hyperimmune B. pertussis antiserum or to antiserum raised against conjugates prepared with OS containing only one trisaccharide. Our interpretation is that the epitope of B. pertussis LPS includes not only the terminal trisaccharide but also some core sugars, possibly the side chain Hep of the terminal pentasaccharide, as observed in saturation transfer difference NMR experiments (26). This suggestion could explain why conjugates containing two to five repeats induced lower antibody levels to B. pertussis LPS than the conjugate containing one repeat only. The ELISA-measured antibody levels were in accordance with the bactericidal data; sera induced by the conjugates of several band A trisaccharide repeats had no or very low bactericidal activity. On the bases of these experiments, we suggest a dodecasaccharide with only one trisaccharide repeat as a vaccine candidate.
Other variables reported to influence the immunogenicity of conjugates were the number of OS chains per protein molecule and the identity of the nonreducing end molecule (17, 18, 22, 27, 28). In the present study, the range of optimal chain density was wide, with an average number of chains between 8 and 17. For the evaluation of the nonreducing end molecule we used a B. bronchiseptica mutant RBB1a LPS, which expressed band A with a terminal GlcNAc or GalNAc in an ~1:1 ratio. Conjugates prepared with this OS at the optimal densities were as immunogenic as conjugates with terminal GlcNAc only. We were unable to obtain OS with terminal GalNAc only; therefore, the role of the end group cannot be definitively established.
To summarize, we present an easy method of preparing B. pertussis OS/protein conjugates using either B. pertussis or B. bronchiseptica strains. The benefits of using B. bronchiseptica instead of B. pertussis are its ability to grow on simple media, in a short time, and with higher bacterial mass yield. We plan to conjugate the band A of RBwbmΔ LPS—the same as that of B. pertussis—to a clinically useful protein for clinical evaluation. The “herd” immunity induced by our current acellular pertussis vaccines has been successful in reducing significantly the incidence of pertussis but leaves many individuals who are not immune. Induction of antibacterial immunity by OS conjugates could further reduce the circulation of B. pertussis and decrease outbreaks of this disease. We believe that our OS-based conjugate, added to a recombinant pertussis toxoid, could provide antibacterial immunity to antitoxin immunity.
Materials and Methods
Bacteria and Cultivation.
B. pertussis Tohama I (ATCC BAA-589) was obtained from the Culture Collection of Göteborg. Construction of genetically inactivated B. pertussis strain Tax 113 was done as described, except that in that strain, the codon for the glutamic acid residue at position 129 of the mature S1 subunit was changed to an alanine (GCC; ref. 29). This strain produces pertussis holotoxin with R9K and E129A mutations in the S1 subunit of the toxin, which abolishes the enzymatic activity of the toxin. B. bronchiseptica RB50 (ATCC BAA-588) was obtained from ATCC. Genetically modified strains were as follows: RBwbmΔ is a mutant of B. bronchiseptica RB50 in which a large chromosomal XbaI fragment encompassing wbmA-wbmZ is replaced with a kanamycin-resistance cassette; RBA2b and RBB1a are B. bronchiseptica RB50 mutants in which wbmA (RBA2b) or wbmB (RBB1a) coding sequences are disrupted by insertion of a tetracycline resistance cassette.
B. pertussis and B. bronchiseptica strains were cultivated on Bordet–Gengou agar plates. B. bronchiseptica was then transferred to Stainer–Scholte liquid medium containing 50 mM MgSO4 (30). After 16–24 h of cultivation at 37 °C with shaking in baffled flasks, bacteria were harvested by centrifugation, killed by boiling for 1 h, and stored at −20 °C for LPS extraction.
LPS and OS Preparation.
LPS was isolated by hot phenol-water extraction and purified by enzyme treatment and ultracentrifugation as described (31). LPS was heated in 1% acetic acid for 90 min at 100 °C and ultracentrifuged at 142,000 × g for 5 h at 4 °C, and the carbohydrate-containing supernatant was passed through a 1× 100-cm column of Bio-Gel P-4—or, in the case of B. bronchiseptica RBA2b, Bio-Gel P-10—in pyridine/acetic acid/water buffer (4/8/988 mL, pH 5.7) monitored with a Knauer differential refractometer. Fractions were collected based on the refractometer profile and freeze-dried twice to remove traces of the buffer components.
Conjugation.
BSA (Sigma) was converted to aminooxylated derivatives in a two-step procedure, similar to that described for B. bronchiseptica and B. parapertussis (22): (i) BSA was treated with succinimidyl 3-(bromoacetamido)propionate (Pierce) to introduce thiol-reactive bromoacetamido groups (BSA-Br); and (ii) BSA-Br was coupled to O-(3-thiopropyl)hydroxylamine, a heterobifunctional linker, to form the aminooxylated protein through stable thioether linkages (BSA–ONH2) as described (32). For conjugation, BSA-ONH2 (5 mg) was reacted with OS isolated from different B. pertussis or B. bronchiseptica stains in quantities of 2.5 mg (conjugates 1, 4, 5, 7, and 9–13; see Table 1), 5 mg (conjugates 3, 6, and 8), and 7.5 mg (conjugate 2) of OS in 1.5 mL of buffer A (PBS/0.1% glycerol/5 mM EDTA) at pH 5.7 for 15 h. The ratio of BSA to OS was adjusted to achieve a range of OS chains incorporated per BSA. Next, the reaction mixture was passed through a 1× 100-cm Sephadex G-75 column in 0.2 M NaCl as eluent, and the void volume fraction was characterized by protein assay, immunodiffusion, SDS/PAGE, and MALDI-TOF spectroscopy.
Reactivity of B. pertussis OS with O-(3-thiopropyl)hydroxylamine.
B. pertussis Tax 113 OS (5 mg) was reacted with 1 mg of O-(3-thiopropyl)hydroxylamine in 1.5 mL buffer A (PBS/0.1% glycerol/5 mM EDTA) at pH 5.7 for 15 h. Next, the reaction mixture was passed through a 1× 100-cm Bio-Gel P-4 column in pyridine/acetic acid/water buffer (4/8/988 mL, pH 5.7) monitored with a Knauer differential refractometer, and then fractions were collected and freeze-dried. ESI-MS spectra of B. pertussis OS before and after reaction were recorded.
Immunization.
For immunization, 5- to 6-week-old female NIH Swiss Webster mice were injected s.c. three times at 2-week intervals with 2.5 μg of saccharide as a conjugate in 0.1 mL of PBS. Groups of 10 mice were exsanguinated 7 d after the second or third injection (33). Controls received PBS. Hyperimmune antiserum against B. pertussis Tohama I was prepared with heat-killed bacteria as described (34).
Analytic.
Protein concentration was measured by the method of Lowry (35). Double immunodiffusion was performed in 1% agarose gel in PBS. SDS/PAGE gel and immunoblotting used 16% Tris·glycine gels and PVDF membranes and were performed according to the manufacturer's instructions (Invitrogen). Endotoxin activity was measured by the limulus amebocyte lysate assay as described by the manufacturer (Cambrex,).
Spectroscopy.
MALDI-TOF mass spectra of the derivatized carrier proteins and of the conjugates were obtained with an OmniFlex MALDI-TOF instrument (Bruker Daltonics) operated in the linear mode. Samples for analysis were desalted, and 1 μL was mixed with 20 μL of sinnapinic acid matrix made in 30% CH3CN and 0.1% trifluoroacetic acid. Next, 1 μL of mixture was dried on the sample stage and placed in the mass spectrometer. NMR spectra were recorded at 30 °C in D2O on a Varian UNITY INOVA 600 instrument using acetone as reference for both proton (2.225 ppm) and carbon (31.5 ppm) spectra. Varian standard programs COSY, NOESY (mixing time of 400 ms), TOCSY (spinlock time 120 ms), HSQC, and gHMBC (long-range transfer delay 70 ms) were used (14).
Antibodies.
Serum IgG and IgM antibodies were measured by ELISA (36). Nunc Maxisorb plates were coated with B. pertussis Tohama I LPS at 10 μg/mL in PBS; 1% human serum albumin in PBS was used in a blocking step. The optimal concentration of the coating antigen was determined by checkerboard titration. A PA MRX Dynatech reader was used. Antibody levels were calculated relative to the hyperimmune standard antiserum diluted 1:5,000 for IgG and 1:3,000 for IgM, and a value of 100 EU was assigned for each. Results were computed with an ELISA data processing program provided by the Biostatistics and Information Management Branch of the Centers for Disease Control and Prevention (37).
Bactericidal Assay.
The bactericidal activity of sera was tested in vitro (12). Briefly, antisera were inactivated at 56 °C for 15 min and diluted 10-fold in PBS with 0.15 mM CaCl2, 0.5 mM MgCl2; and 0.1% BSA (buffer B, pH 7.4). B. pertussis Tohama I was diluted to ~300–500 bacteria per 25 μL of buffer B. A precolostral calf serum (PCS) was used as a source of complement. Then, 45 μL of antisera was mixed with 25 μL of bacteria and 15 μL of undiluted PCS. Finally, 140 μL of buffer B was added (final volume of reagents, 225 μL). Mixtures of bacteria in buffer B without PCS or without serum were used as controls. After incubation for 60 min at 37 °C, 100 μL of each mixture was plated onto Bordet–Gengou agar and incubated for 3–5 d, and then colonies were counted. The bactericidal titer of the antiserum was defined as the highest dilution giving killing of 50% of the inoculum. The lowest positive titer was a dilution of 1:10.
Supplementary Material
Acknowledgments
We thank Chris Mocca and Chunyan Guo (National Institute of Child Health and Human Development) for technical assistance. This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100782108/-/DCSupplemental.
References
- 1.Edwards KM, Decker MD, Mortimer EA. Pertussis vaccine. In: Plotkin S, Orenstein WA, editors. Vaccines. 3rd Ed. Philadelphia: Saunders; 1999. pp. 293–342. [Google Scholar]
- 2.Linnemann CC, Jr, Ramundo N, Perlstein PH, Minton SD, Englender GS. Use of pertussis vaccine in an epidemic involving hospital staff. Lancet. 1975;2:540–543. doi: 10.1016/s0140-6736(75)90907-1. [DOI] [PubMed] [Google Scholar]
- 3.Robbins JB, et al. Pertussis vaccine: A critique. Pediatr Infect Dis J. 2009;28:237–241. doi: 10.1097/INF.0b013e31818a8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celentano LP, Massari M, Paramatti D, Salmaso S, Tozzi AE. EUVAC-NET Group Resurgence of pertussis in Europe. Pediatr Infect Dis J. 2005;24:761–765. doi: 10.1097/01.inf.0000177282.53500.77. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Summary of notifiable diseases—United States, 2007. MMWR. 2009;56:1–94. [Google Scholar]
- 6.Schneerson R, Robbins JB, Taranger J, Lagergård T, Trollfors B. A toxoid vaccine for pertussis as well as diphtheria? Lessons to be relearned. Lancet. 1996;348:1289–1292. doi: 10.1016/S0140-6736(96)05243-9. [DOI] [PubMed] [Google Scholar]
- 7.Higginbotham TW, Cleveland KW. Decreasing childhood pertussis infection through vaccination of the elderly. Consult Pharm. 2008;23 doi: 10.4140/tcp.n.2008.976. 976, 979–981. [DOI] [PubMed] [Google Scholar]
- 8.Cohen D, et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet. 1997;349:155–159. doi: 10.1016/S0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- 9.Passwell JH, et al. Israeli Shigella Study Group Age-related efficacy of Shigella O-specific polysaccharide conjugates in 1-4-year-old Israeli children. Vaccine. 2010;28:2231–2235. doi: 10.1016/j.vaccine.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trollfors B, et al. Serum immunoglobulin G antibody responses to Bordetella pertussis lipooligosaccharide and B. parapertussis lipopolysaccharide in children with pertussis and parapertussis. Clin Diagn Lab Immunol. 2001;8:1015–1017. doi: 10.1128/CDLI.8.5.1015-1017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Archambault D, Rondeau P, Martin D, Broudeur BR. Characterization and comparative bactericidal activity of monoclonal antibodies to Bordetella pertussis lipo-oligosaccharide A. J Gen Micro. 1991;137:905–911. doi: 10.1099/00221287-137-4-905. [DOI] [PubMed] [Google Scholar]
- 12.Mountzouros KT, Kimura A, Cowell JL. A bactericidal monoclonal antibody specific for the lipooligosaccharide of Bordetella pertussis reduces colonization of the respiratory tract of mice after aerosol infection with B. pertussis. Infect Immun. 1992;60:5316–5318. doi: 10.1128/iai.60.12.5316-5318.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caroff ML, et al. Structural variability and originality of the Bordetella endotoxins. J Endotoxin Res. 2001;7:63–68. [PubMed] [Google Scholar]
- 14.Vinogradov E. The structure of the carbohydrate backbone of the lipopolysaccharides from Bordetella hinzii and Bordetella bronchiseptica. Eur J Biochem. 2000;267:4577–4582. doi: 10.1046/j.1432-1327.2000.01512.x. [DOI] [PubMed] [Google Scholar]
- 15.Di Fabio JL, Caroff M, Karibian D, Richards JC, Perry MB. Characterization of the common antigenic lipopolysaccharide O-chains produced by Bordetella bronchiseptica and Bordetella parapertussis. FEMS Microbiol Lett. 1992;76:275–281. doi: 10.1016/0378-1097(92)90348-r. [DOI] [PubMed] [Google Scholar]
- 16.Preston A, et al. Complete structures of Bordetella bronchiseptica and Bordetella parapertussis lipopolysaccharides. J Biol Chem. 2006;281:18135–18144. doi: 10.1074/jbc.M513904200. [DOI] [PubMed] [Google Scholar]
- 17.Pozsgay V, et al. Protein conjugates of synthetic saccharides elicit higher levels of serum IgG lipopolysaccharide antibodies in mice than do those of the O-specific polysaccharide from Shigella dysenteriae type 1. Proc Natl Acad Sci USA. 1999;96:5194–5197. doi: 10.1073/pnas.96.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pozsgay V, Kubler-Kielb J, Schneerson R, Robbins JB. Effect of the nonreducing end of Shigella dysenteriae type 1 O-specific oligosaccharides on their immunogenicity as conjugates in mice. Proc Natl Acad Sci USA. 2007;104:14478–14482. doi: 10.1073/pnas.0706969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bock K, Vinogradov EV, Holst O, Brade H. Isolation and structural analysis of oligosaccharide phosphates containing the complete carbohydrate chain of the lipopolysaccharide from Vibrio cholerae strain H11 (non-O1) Eur J Biochem. 1994;225:1029–1039. doi: 10.1111/j.1432-1033.1994.1029b.x. [DOI] [PubMed] [Google Scholar]
- 20.Volk WA, Salomonsky NL, Hunt D. Xanthomonas sinensis cell wall lipopolysaccharide. I. Isolation of 4,7-anhydro- and 4,8-anhydro-3-deoxy-octulosonic acid following acid hydrolysis of Xanthomonas sinensis lipopolysaccharide. J Biol Chem. 1972;247:3881–3887. [PubMed] [Google Scholar]
- 21.Auzanneau FI, Charon D, Szabo L. Phosphorylated sugars. Part 27. Synthesis and reactions, in acid medium, of 5-O-substituted methyl 3-deoxy-α-D-manno-oct-2-ulopyranosidonic acid 4-phosphates. J Chem So Perkin Trans. 1991;1:509–517. [Google Scholar]
- 22.Kubler-Kielb J, et al. Saccharide/protein conjugate vaccines for Bordetella species: preparation of saccharide, development of new conjugation procedures, and physico-chemical and immunological characterization of the conjugates. Vaccine. 2008;26:3587–3593. doi: 10.1016/j.vaccine.2008.04.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lees A, Sen G, LopezAcosta A. Versatile and efficient synthesis of protein-polysaccharide conjugate vaccines using aminooxy reagents and oxime chemistry. Vaccine. 2006;24:716–729. doi: 10.1016/j.vaccine.2005.08.096. [DOI] [PubMed] [Google Scholar]
- 24.Robbins JB, et al. Synthesis, characterization, and immunogenicity in mice of Shigella sonnei O-specific oligosaccharide-core-protein conjugates. Proc Natl Acad Sci USA. 2009;106:7974–7978. doi: 10.1073/pnas.0900891106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubler-Kielb J, et al. Immunochemical studies of Shigella flexneri 2a and 6, and Shigella dysenteriae type 1 O-specific polysaccharide-core fragments and their protein conjugates as vaccine candidates. Carbohydr Res. 2010;345:1600–1608. doi: 10.1016/j.carres.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niedziela T, et al. Epitope of the vaccine-type Bordetella pertussis strain 186 lipooligosaccharide and antiendotoxin activity of antibodies directed against the terminal pentasaccharide-tetanus toxoid conjugate. Infect Immun. 2005;73:7381–7389. doi: 10.1128/IAI.73.11.7381-7389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, et al. On the antigenic determinants of the lipopolysaccharides of Vibrio cholerae O:1, serotypes Ogawa and Inaba. J Biol Chem. 1998;273:2777–2783. doi: 10.1074/jbc.273.5.2777. [DOI] [PubMed] [Google Scholar]
- 28.Kubler-Kielb J, et al. A bicomponent Plasmodium falciparum investigational vaccine composed of protein-peptide conjugates. Proc Natl Acad Sci USA. 2010;107:1172–1177. doi: 10.1073/pnas.0913374107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown DR, Keith JM, Sato H, Sato Y. Construction and characterization of genetically inactivated pertussis toxin. In: Karger S, editor. Developments in Biological Standardization. Basel: International Association of Biological Standardization; 1991. pp. 63–73. [PubMed] [Google Scholar]
- 30.Stainer DW, Scholte MJ. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970;63:211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- 31.Westphal O, Jann K. Extraction with phenol-water and further application of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 32.Kubler-Kielb J, Pozsgay V. A new method for conjugation of carbohydrates to proteins using an aminooxy-thiol heterobifunctional linker. J Org Chem. 2005;70:6987–6990. doi: 10.1021/jo050934b. [DOI] [PubMed] [Google Scholar]
- 33.Schneerson R, Barrera O, Sutton A, Robbins JB. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980;152:361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu CY, et al. Preparation, characterization, and immunogenicity of conjugates composed of the O-specific polysaccharide of Shigella dysenteriae type 1 (Shiga's bacillus) bound to tetanus toxoid. Infect Immun. 1991;59:4450–4458. doi: 10.1128/iai.59.12.4450-4458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 36.Taylor DN, et al. Synthesis, characterization, and clinical evaluation of conjugate vaccines composed of the O-specific polysaccharides of Shigella dysenteriae type 1, Shigella flexneri type 2a, and Shigella sonnei (Plesiomonas shigelloides) bound to bacterial toxoids. Infect Immun. 1993;61:3678–3687. doi: 10.1128/iai.61.9.3678-3687.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plikaytis BD, Carlone GM. Program ELISA for Windows User's Manual. Atlanta: Centers for Disease Control and Prevention; 2005. Version 2.22. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.