Abstract
Claudins (Clds) are crucial constituents of tight-junction strands in epithelial cells and have a central role in barrier functions. We show that Cld4 is unexpectedly expressed in normal thymic lymphocytes independently of tight junctions. The Cld4 expression was mostly confined to a portion of the CD4/CD8 double-positive (DP) cells. The proportion of Cld4+ DP cells was markedly increased in MHC-I−/− II−/− mice but decreased in Rorγ−/− mice, and Cld4+ DP cells contained higher levels of the rearranged Tcra transcripts involving the most distal Va and Ja segments than Cld4– DP cells. The Cld4 expression levels were reduced in E47-deficient mice in a gene dose-dependent manner, and ChIP analysis indicated that E2A and HEB were bound to the E-box sites of the putative Cldn4 promoter region. Functionally, Cld4 showed a potent T-cell receptor costimulatory activity by coligation with CD3. The Cld4 was distributed diffusely on the cell surface and associated with CD4/lck independently of CD3 in the resting thymocytes. However, Cld4 was strongly recruited to the immunological synapse on specific T-cell receptor engagement through antigen-presenting cells. In the fetal thymic organ culture, knockdown of Cldn4 resulted in the reduced generation of CD4/CD8 single-positive cells from the DP cells. These results suggest that Cld4 is induced by E-protein activity in the later stages of DP cells to increase the efficiency of positive selection, uncovering a hitherto unrecognized function of a Cld family protein.
Keywords: thymus, repertoire, costimulation
In most epithelial tissues, epithelial cells form cellular sheets through junctional complexes, of which tight junction (TJ) is the most apical component and functions as a fluid barrier between the distinct body compartments (1). Claudins (Clds), a family of small (20–23 kDa) tetraspan membrane proteins, are central structural and functional constituents of the epithelial TJ barrier, forming continuous polymerized rows called TJ strands (2, 3). Among epithelial tissues, thymus is rather exceptional in that the thymic epithelial cells (TECs) form a meshwork rather than a sheet structure (4). We previously reported that a subset of the medullary TECs expressing an autoimmune regulator (Aire) expressed Cld3 and Cld4 (5). Nonetheless, these TECs showed no evidence of TJ formation (5), and the significance and function of such Clds independent of TJs remained elusive.
Although the expression of Clds is thought to be specific for epithelial and endothelial cells, we have unexpectedly discovered that Cld4 is expressed in the normal thymocytes at specific developmental stages, particularly at the CD4/CD8 double-positive (DP) stage. The DP thymocytes are the first to express αβ–T-cell receptors (TCRs) during T-cell development, which are tested for self-MHC specificity. The vast majority of DP cells die, because they are not signaled or are signaled too strongly by their TCRs, whereas only those signaled weakly by their TCRs are rescued from cell death, a process called positive selection (6). The E-protein family of transcriptional factors, including E2A and HEB, has a central role in controlling the fate of DP cells (7–9). E-protein activity sustains the unique gene expression pattern in DP cells and regulates their survival, Tcra rearrangements, and possibly, other activities to maximize the positive selection and differentiation of thymocytes with a functional TCR repertoire (9).
We show that Cld4 expression is induced by E-protein activity in the later stages of DP thymocytes before positive selection and suggest that it increases the efficiency of positive selection through unique TCR costimulatory activity.
Results
Cld4 Is Expressed in the Later Stages of DP Thymocytes Before Positive Selection.
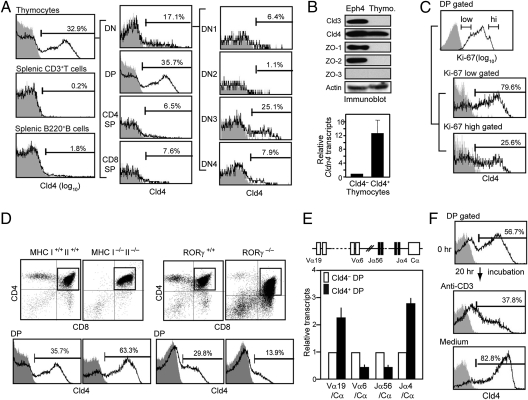
We found that a significant proportion of the thymic but not splenic lymphocytes expressed Cld4 on the cell surface with the use of a newly developed monoclonal antibody specific for the extracellular region of Cld4 (Fig. 1A and Fig. S1). The Cld4+ cells were mostly confined to the DP and double-negative 3 (DN3) stages of adult thymocytes (Fig. 1A). The expression of Cld4 was confirmed at the levels of both protein and transcripts, although zonula occludens (ZO) proteins that are essential for TJ formation in epithelial cells were undetectable (Fig. 1B). The Cld4 was a predominant Cld expressed in the normal thymocytes among 24 family members (Fig. S2 and Table S1). The proportions of Cld4+ DP cells were the highest in newborn stage (more than 90%) but decreased thereafter, and around one-third of the thymocytes continued to express Cld4 in the adults at least until 20 wk old (Fig. S3A). The proportions of CD69+ and TCRβ/CD3high cells as well as CD5 intensity were indistinguishable between Cld4+ and Cld4– DP populations (Fig. S3B). However, the Cld4+ DP cells tended to be enriched in the Ki67low fraction and showed slower BrdU incorporation rate in vivo than Cld4– DP cells (Fig. 1C and Fig. S3C). The proportion of Cld4+ DP cells was markedly increased in MHC-I−/− II−/− compared with MHC-I+/+ II+/+ age-matched control mice, whereas it was significantly diminished in Rorγ−/− mice, which showed shorter lifespan of the DP cells (10, 11) (Fig. 1D). In addition, the Cld4+ DP cells showed higher levels of the rearranged Tcra transcripts involving the most distally located Va and Ja segments than Cld4– DP cells (Fig. 1E). However, the levels of Cld4 and the transcripts were drastically reduced in CD4high CD8int DP cells and became negligible in CD4high CD8– cells (Fig. S4A). Furthermore, stimulation of the thymocytes with anti-CD3 antibody caused significant reduction of Cld4 and its transcripts in the DP population in vitro within 20 h, whereas the incubation in medium alone resulted in rather enhanced expression (Fig. 1F and Fig. S4B). These results collectively suggest that Cld4 expression is increased in the later stages of DP cells and extinguished after positive selection.
Fig. 1.
Cld4 is expressed in the later stages of DP thymocytes. (A) The thymocytes and splenocytes from 8-wk-old B6 mice were multicolor analyzed with antibodies for Cld4 (HKH-189), CD4, CD8, CD44, and CD25 and antibodies for Cld4, CD3, and B220, respectively. Proportions of Cld4+ cells in the indicated cell gates are shown. Data are representative of at least three experiments. (B) Eph4 epithelial cells and thymocytes were lysed and immunoblotted with the indicated antibodies (Upper). RNA was extracted from Cld4– and Cld4+ thymocytes, and relative Cldn4 transcripts were assessed by quantitative RT-PCR (Lower). The means and SDs of duplicate analyses are indicated. (C) The thymocytes were multicolor stained with antibodies for CD4, CD8, Cld4, and Ki-67. Proportions of Cld4+ cells in the Ki-67high and Ki-67lo DP cell gates are shown. Data are representative of two experiments. (D) The thymocytes from 8-wk-old MHC I−/− II−/−, Rorγ−/−, and respective age-matched control mice were three-color analyzed with the indicated antibodies, and the proportions of Cld4+ cells in the DP cell gates are shown. Data are representative of three experiments. (E) RNA was extracted from Cld4– and Cld4+ DP cells from B6 thymocytes, and the rearranged Tcra transcripts were assessed by quantitative RT-PCR using the indicated primer combinations. The means and SDs of duplicate analyses are indicated. (F) The B6 thymocytes were incubated in the presence or absence of solid-phase anti-CD3 antibody for 20 h, and the proportions of Cld4+ DP cells were examined before and after the incubation. Data are representative of four experiments.
Expression of Cld4 Is Regulated by E-Protein Activity.
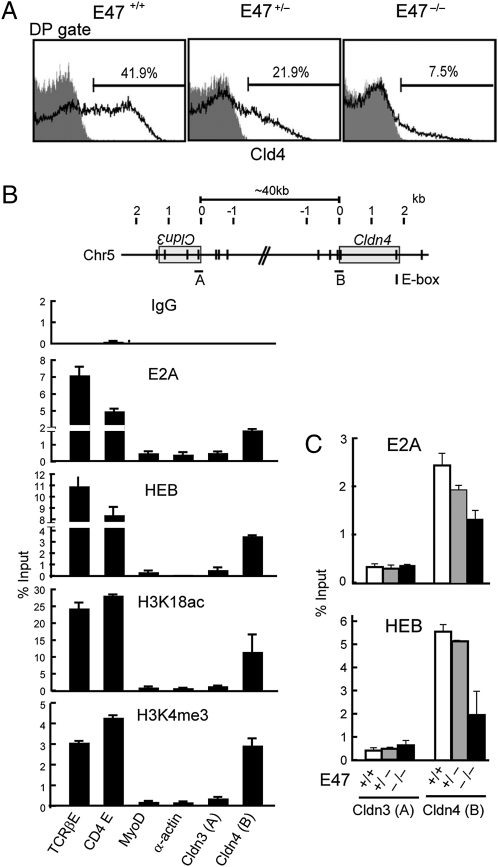
The characteristic expression profile of Cld4 during thymocyte development was reminiscent of E-protein activity (12), and indeed, we found that Cld4 expression in the DP cells was reduced in E47-deficient mice in a gene dose-dependent manner (Fig. 2A). There are a number of consensus E-protein binding sites (E-boxes) in and around the Cldn4, and ChIP analysis indicated the specific binding of endogenous E2A and HEB at the putative Cldn4 promoter region containing two E-box sites (Fig. 2B). In addition, increased H3K18 acetylation and H3K4 methylation of the corresponding region were also detected (Fig. 2B). In contrast, the binding of E2A/HEB or the modification of H3 was hardly detectable at the corresponding promoter region of Cldn3 (Fig. 2B). As anticipated, the E2A binding to the Cldn4 promoter was reduced in the thymocytes derived from E47-deficient mice in a gene dose-dependent manner (Fig. 2C). The residual E2A binding in E47−/− thymocytes might be attributable to the known cross-reactivity of anti-E2A antibody to HEB homodimer, which is detected by HEB ChIP in E47−/− thymocytes. These results suggest that Cld4 expression in DP cells is under the transcriptional regulation by E-protein activity.
Fig. 2.
Cld4 expression is regulated by E-protein activity. (A) The thymocytes from 6-wk-old littermate E47−/−, E47+/−, and E47+/+ mice were three-color analyzed with anti-CD4, CD8, and Cld4 antibodies, and the proportions of Cld4+ cells in the DP cell gates are shown. Data are representative of at least three independent experiments. (B and C) Schematic representation of the E-box consensus sequences around Cldn3 and Cldn4 loci (Upper). A and B in B Upper indicate the putative promoter regions containing E-box sites of Cldn3 and Cldn4, respectively. Chromatin prepared from normal B6 thymocytes (B) or those from 4-wk-old littermate E47−/−, E47+/−, and E47+/+ mice (C) were immunoprecipitated with the indicated antibodies, and ChIP analysis was performed for the indicated gene segments. The mean bound to input ratios (in percentages) of duplicate analyses are indicated with ranges. Data are representative of three independent experiments.
Cld4 Shows Negligible Homotypic Cell Adhesion Activity in the Thymocytes.
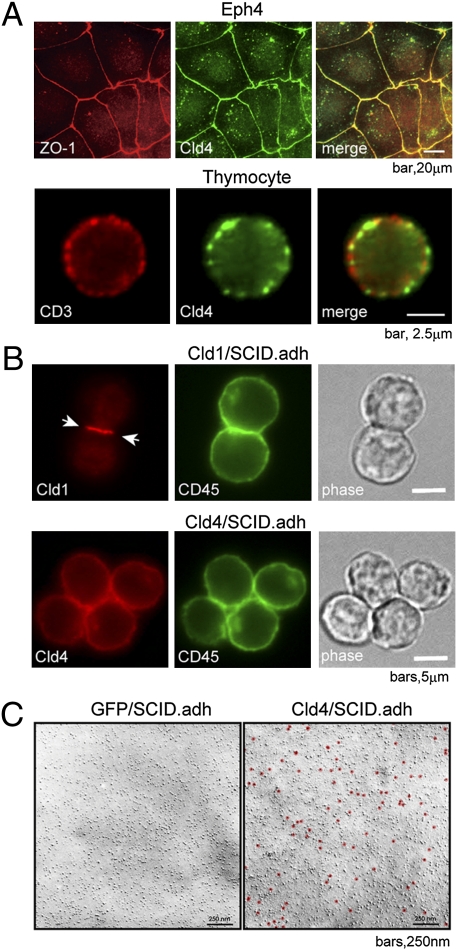
In an epithelial cell line (Eph4) with typical TJs, the Cld4 was sharply concentrated at the cell–cell contact sites colocalizing with ZO-1 (Fig. 3A Upper). However, Cld4 was expressed in a patchy pattern diffusely on the cell surface of thymocytes, and it showed little colocalization with CD3 (Fig. 3A Lower). We then examined the intrinsic homotypic adhesion activity of Cld4 compared with prototypic Cld1. It is reported that Cld1 is capable of reconstituting TJs in a ZO-1+ fibroblastic L-cell line (2, 3), and as expected, Cld1 expressed in L cells (Cld1L) was sharply localized at cell adhesion sites along with ZO-1 and conferred a homotypic cell adhesion activity. In contrast, Cld4 was distributed on the apical surface of Cld4L cells and failed to induce the homotypic cell adhesion (Fig. S5). Moreover, although Cld1 expressed in a Cld− thymocyte cell line (SCID.adh) was localized at the cell–cell contact sites, Cld4 was distributed diffusely around the cell surface of SCID.adh cells (Fig. 3B). Although there seemed to be some enrichment of Cld4 at the contact sites, the pattern was essentially identical to that of CD45, which has no homotypic adhesion activity, and thus, this was considered to be because of a cell overlapping effect. We further confirmed that Cld4 molecules showed no evidence for TJ strand formation or any clustering in SCID.adh cells by the immunoelectron microscopic analysis (Fig. 3C). These results suggest that Cld4 may not be involved in homotypic cell–cell adhesion in thymocytes.
Fig. 3.
Cld4 shows negligible homotypic cell adhesion activity. (A) Cultured Eph4 epithelial cell lines and freshly isolated newborn thymocytes were fixed and two-color stained with the indicated antibodies. (B) Cld− thymocyte cell lines (SCID.adh) transfected with Cldn1 or Cldn4 were stained with the corresponding and anti-CD45 antibodies. Phase-contrast images are also shown. Arrows indicate the accumulation of Cld1 at the cell contact sites. (C) The Pt/C replicas of SCID.adh cells transduced with Cldn4 or control vector (GFP) were stained with anti-Cld4 antibody followed by colloidal gold-conjugated protein A and examined with EM. Gold labels were indicated as red dots. Data are representative of two independent experiments.
Cld4 Shows a Potent TCR Costimulatory Effect by Coligation with CD3.
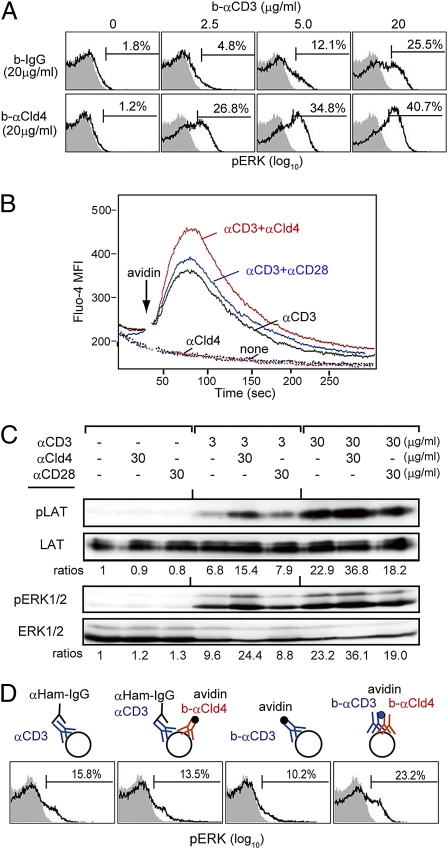
Given that Cld4 is expressed in the DP cells before positive selection, we then investigated the possible effects of Cld4 on TCR signaling. To this end, we incubated the normal thymocytes with biotinylated (b-) anti-CD3 in the presence or absence of b–anti-Cld4 antibody followed by cross-linking with avidin. Whereas the ligation of CD3 alone induced ERK activation in a dose-dependent manner, ligation of Cld4 failed to do so even at the highest antibody concentration (Fig. 4A). However, the coligation of CD3 and Cld4 resulted in the significantly augmented ERK phosphorylation compared with the CD3 ligation alone, the effect being more prominent at lower concentrations of anti-CD3 antibody (Fig. 4A). The kinetics of ERK activation were not affected (Fig. S6). The coligation also caused a markedly enhanced Ca2+ influx (Fig. 4B). Both ERK activation and Ca2+ influx depend on linker for activation of T cells (LAT) phosphorylation, and we confirmed that the Cld4/CD3 coligation induced a significant enhancement of LAT phosphorylation as well (Fig. 4C). However, the CD28/CD3 coligation induced little enhancement of LAT phosphorylation and accordingly, no significant costimulatory effects on ERK activation and Ca2+ influx (Fig. 4 B and C). When CD3 and Cld4 were ligated simultaneously but only homologously, however, no costimulatory effect was detectable (Fig. 4D), suggesting that the effect required the physical juxtaposition of Cld4 to CD3.
Fig. 4.
Cld4 shows a potent TCR costimulatory effect by coligation with CD3. (A) Newborn B6 thymocytes were incubated with the indicated concentrations of b–anti-CD3 together with 20 μg/mL rat IgG or b–anti-Cld4 antibody for 20 min followed by cross-linking with 25 μg/mL avidin. Two minutes later, the cells were fixed and analyzed with anti-phospho-Erk (pERK) antibody. Shaded areas indicate the isotype control. The proportions of pERK+ cells are shown. (B) Newborn B6 thymocytes loaded with 5 μM Fluo-4 AM were incubated with the combinations of b–anti-CD3 (3 μg/mL), b–anti-Cld4 (30 μg/mL), and b–anti-CD28 (30 μg/mL) antibodies as indicated followed by cross-linking with avidin (25 μg/mL). Mean fluorescence intensities (MFIs) of Fluo-4 were scanned for 5 min. (C) Newborn B6 thymocytes were incubated with the indicated concentrations of b–anti-CD3 in the absence or presence of b–anti-Cld4 or b–anti-CD28 antibody followed by cross-linking with avidin. Two minutes later, the cells were lysed and immunoblotted with the indicated antibodies. Relative activation ratios are shown. (D) Newborn B6 thymocytes were incubated with 3 μg/mL anti-CD3 plus 30 μg/mL b-IgG or b–anti-Cld4 antibody followed by cross-linking with 10 μg/mL anti-hamster IgG and 25 μg/mL avidin (left two columns) or 3 μg/mL b–anti-CD3 plus 30 μg/mL b-IgG or b–anti-Cld4 antibody followed by cross-linking with 25 μg/mL avidin (right two columns). The cells were analyzed with anti-pERK antibody 2 min later. Data are representative of at least three experiments.
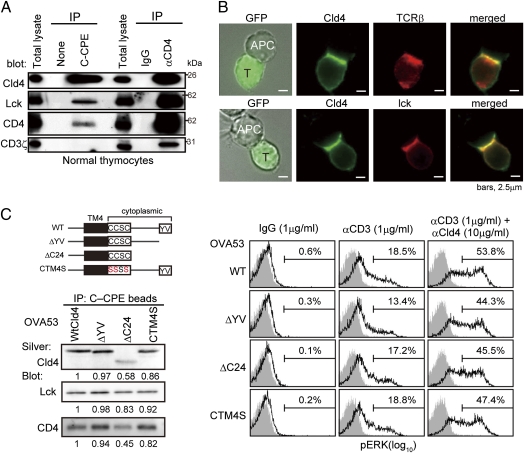
Cld4 Is Associated with CD4/Lymphocyte Kinase (Lck) and Recruited to Immunological Synapse.
We found that CD4 and lck, but not CD3ζ, were coimmunoprecipitated with Cld4 from the thymocyte lysate by a C-terminal fragment of Clostridium perfringens enterotoxin (C-CPE) that is bound to the extracellular region of Cld4 with high affinity (13) (Fig. 5A). The effect was specific for Cld4, because no lck was coimmunoprecipitated by C-CPE in the Cld4– thymocytes cell line (Fig. S7A). In the reciprocal experiments, Cld4 was coimmnoprecipitated by anti-CD4 antibody together with lck and CD3ζ (Fig. 5A). The results suggest that Cld4 is constitutively associated with a portion of the CD4/lck pool independently of CD3. We next investigated whether the juxtaposition of Cld4 to CD3 could occur during the physiological TCR engagement with the use of a DP cell line (OVA53) derived from ovalbumin (OVA)-specific TCR transgenic mice. When the OVA53 cells transduced with Cldn4 were incubated with OVA-loaded antigen-presenting cells (APCs), Cld4 was strongly recruited to the immunological synapse, colocalizing with TCR and lck (Fig. 5B). To examine the requirement of the cytoplasmic region of Cld4 for the effects, we generated OVA53 cells expressing Cldn4 mutant deleted of a C-terminal postsynaptic density-95/discs large/zonula occludens-1 (PDZ)-binding motif required for ZO protein binding (ΔYV) or the entire cytoplasmic region (ΔC24) except for the membrane proximal four residues (CCSC). Because additional deletion of CCSC, a potential palmitoylation site, resulted in the failure of cell surface expression, we also generated the cells expressing a mutant of the palmitoylation motif (CTM4S). The ΔC24 mutant showed reduced immunoprecipitation efficiency; nonetheless, all of the Cld4 mutants coimmunoprecipitated lck and CD4 with comparable efficiency to WT Cld4 (Fig. 5C Left). Moreover, all of the OVA53 cells expressing the mutants showed the TCR costimulatory effect by coligation with CD3 comparable with WT OVA53 cells (Fig. 5C Right), and also, ΔC24 mutant was recruited to the immunological synapse similarly to WT Cld4 (Fig. S7B). These results strongly suggest that the cytoplasmic region of Cld4 is dispensable for the association with CD4/lck as well as the TCR costimulatory effect.
Fig. 5.
Cld4 is associated with CD4/lck and recruited to immunologic synapse. (A) Newborn B6 thymocytes were lysed and immunoprecipitated with b–C-CPE or b–anti-CD4 and avidin beads followed by immunoblotting with the indicated antibodies. Data are representative of five experiments. (B) OVA53/Cld4 cells were incubated for 6 h with APCs (A20 cells) preloaded overnight with 1 μM OVA, fixed, and immunostained with the indicated antibodies. OVA53/Cld4 cells were identified with GFP. Data are representative of three experiments. (C) OVA53 cells were retrovirally transduced with the mutant Cldn4 as illustrated (Upper Left), and the cell lysates were immunoprecipitated with b–C-CPE and avidin beads followed by immunoblotting with the indicated antibodies. Aliquotes of the immunoprecipitates were electrophoresed and silver-stained (Lower Left). Relative intensities of the signals are indicated. Data are representative of five experiments. OVA53 cells expressing WT or mutant Cld4 were incubated with 1 μg/mL b-hamster IgG, 1 μg/mL b–anti-CD3, or 1 μg/mL b–anti-CD3 plus 10 μg/mL b–anti-Cld4 antibodies followed by cross-linking with 25 μg/mL avidin; 2 min later, ERK phosphorylation was assessed. Data are representative of four experiments (Right).
Knockdown of Cldn4 Results in the Reduced Generation of Single Positive (SP) Cells in Fetal Thymus Organ Culture (FTOC).
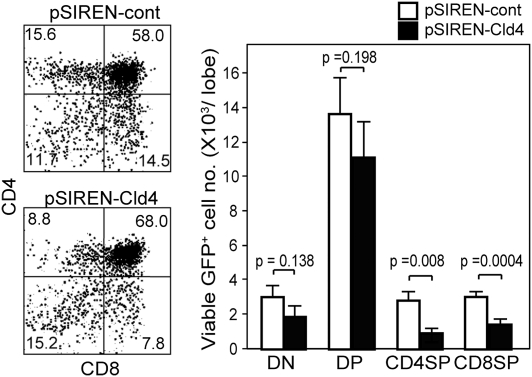
Finally, we investigated the physiologic role of Cld4 in thymic T-cell development. The thymocytes from E15 embryos, which were mostly at the DN stage, were infected with pSIREN retrovirus vector containing shCldn4 (pSIREN/Cld4) or scrambled oligomer (pSIREN/cont) and cultured together with the E15 thymic lobes that had been treated with deoxyguanosine (dGuo). After 10 d in the FTOC, 25% of the control GFP+ thymocyte population was Cld4+, whereas only 6.5% of the pSIREN/Cld4-infected cell population expressed Cld4 at lower levels (Fig. S8). The generation of both CD4+ and CD8+ SP cells in the pSIREN/Cld4-infected (GFP+) population was significantly reduced compared with that in the control GFP+ population, whereas the numbers of DP and DN cells were affected insignificantly (Fig. 6). These results suggest that Cld4 expression may contribute to the efficiency of thymic positive selection.
Fig. 6.
Knockdown of Cldn4 results in the reduced generation of SP cells in FTOC. The E15 thymocytes were infected with pSIREN retrovirus containing Cldn4 shRNA (pSIREN-Cld4) or scrambled oligonucleotides (pSIREN-cont) and cultured with the dGuo-treated E15 thymic lobes. Ten days later, the thymocytes were stained with antibodies for CD4, CD8, and CD3, and the representative profiles in the GFP+ gate are indicated (Left). The mean cell numbers and SEs of DN, DP, CD3high CD4, and CD3high CD8 SP cells per lobe in five independent experiments are shown (Right).
Discussion
We have shown that Cld4 is expressed in a minor population of DP cells in the adult thymus. The DP cells consist of thymocytes at the heterogeneous stages of development, and several lines of evidence indicate that Cld4 expression is induced in the later stages of DP cells before positive selection. First, the Cld4+ DP cells tend to be less proliferative than Cld4– DP cells and are markedly increased in MHC I−/− II−/− mice that lack the TCR ligands for positive selection. Second, Rorγ−/− mice, in which DP cells have shortened lifespan because of premature apoptosis (10, 11), show a diminished proportion of Cld4+ DP cells. Third, Cld4+ DP cells in normal mice contain higher levels of the rearranged Tcra transcripts involving the most distally located Va and Ja segments than Cld4– DP cells, suggesting the increased secondary rearrangements. Finally, Cld4 expression is almost completely repressed in SP cells. Because Cld4 in DP cells was rapidly down-regulated by the stimulation with anti-CD3 antibody in vitro, it is suggested that the Cld4 expression is extinguished after positive selection.
We have shown that such a characteristic expression of Cld4 is regulated by E-protein activity. The E proteins, including E2A and HEB, have a crucial role in coordinating the DP cell fate by regulating the expression of a large set of genes at the DP stage (9, 14). HEB is essential for the secondary Tcra rearrangements to increase the chance for generation of TCRs suitable for positive selection (9). A crucial factor allowing the progression of secondary Tcra rearrangements is the lifespan of DP cells, which is controlled by Rorγ (15), and HEB may also mediate the Rorγ activation (9). Although Cldn4 was listed in an array of DP stage-specific genes up-regulated by E proteins (9, 14), the significance has remained unknown. We found that artificial coligation of Cld4 with CD3 strongly enhanced the TCR signaling in the DP thymocytes in vitro, in which the juxtaposition of Cld4 to CD3 seemed to be essential. Indeed, it was shown that Cld4 was efficiently recruited to the immunological synapse formed with the antigen-bearing MHC-II+ APCs in a physiological setting. However, Cld4 was associated with a portion of the CD4/lck pool independently of CD3 in the resting thymocytes. Therefore, it may be possible that Cld4 promotes the recruitment of free CD4/lck to the TCR proximity on antigen engagement directly or indirectly, although the mechanisms remain to be investigated.
Considering the static nature of Clds in epithelial TJs, such a dynamic movement of Cld4 along the plasma membrane was rather unanticipated. Whereas prototypic Cld1 expressed alone in fibroblastic or thymocyte cell lines was localized at the cell adhesion sites, such a localization was hardly detectable in Cld4, suggesting that Cld4 has much lower homotypic adhesion activity than Cld1. Actually, the cytoplasmic region of Cld4, which has important roles in the formation and function of TJs (16), was dispensable for the TCR costimulatory activity. A group of tetraspan proteins called tetraspanins are known to associate laterally with the partner proteins through the extracellular regions and facilitate their lateral positioning in the plasma membrane (17). For instance, CD81 is laterally associated with CD21/CD19 linked to lyn and shows B cell receptor (BCR) costimulatory activity (18). Although Clds are not usually classified as tetraspanins (19), the TCR costimulatory activity of Cld4 seems to resemble the function of tetraspanins.
We found that the knockdown of Cldn4 in the fetal DN thymocytes resulted in a significant, if not complete, reduction of both CD4+ and CD8+ SP cell development, with only marginal effect on the generation of DP cells in the FTOC. The results suggest that endogenous Cld4 expression promotes the positive selection efficiency of at least a portion of DP cells. Inasmuch as Cld4 expression is increased at the later stages of preselected DP cells, which may include the cells bearing TCRs with suboptimal affinity for the self-MHC ligands, it is conceivable that the Cld4 expression increases the chance for such DP cells with borderline TCR affinity to be positively selected. Such an effect of Cld4 may constitute a unique part of the E protein-mediated function in maximizing overall generation of functional SP thymocytes in the thymus (20).
Current results provide one instance for the expression of a Cld family member, which is believed to be specific in epithelial cells, in normal lymphocytes. Recently, a number of unique interactions have been described between lymphocytes and epithelial cells in epithelial tissues (21) including the thymus, the only epithelial organ in the immune system. We previously reported that a subset of medullary TECs also expressed Cld4, although they apparently lacked TJs (5). It seems unlikely that Cld4 mediates the cellular interaction between thymocytes and TECs through homotypic interaction because of their distinct localization in the thymus. However, it may be still possible that Cld4 mediates unique thymocyte–TEC interactions through heterotypic adhesion activity with other Cld members in TJ-independent manners, and this possibility remains to be investigated.
In conclusion, we have shown that Cld4 is a marker of the later stages of preselected DP thymocytes, and we discovered a functional aspect of Clds as a signaling modifier in lymphoid cells. Our current results may provide insights into the understanding of T-cell development and selection in the thymus.
Materials and Methods
SI Materials and Methods has details on the materials and methods used in this study.
Mice.
The C57BL/6 (B6) and Rorγ −/− (11) as well as E47−/− and MHC I−/− II−/− mice provided by C. Murre (University of California, San Diego, CA) and Y. Takahama (University of Tokushima, Tokushima, Japan), respectively, were maintained in specific pathogen-free conditions at Kyoto University's Laboratory Animal Center in accordance with university guidelines.
Flow Cytometry.
Multicolor flow cytometric analysis and cell sorting were performed with FACSCalibur and FACSAria (BD Biosciences). Ca2+ influx was analyzed using the thymocytes loaded with 5 μM Fluo-4 AM (DOJINDO Laboratories) in Tyrode's buffer.
Immunoelectron Microscopy.
The freeze–fracture replicas of cell pellets prepared at −120 °C were treated with 2.5% SDS/PBS and incubated with anti-Cld4 antibody followed by protein A conjugated with colloidal gold. The specimens were observed with a JEOL 1400EX electron microscope operated at 100 kV.
Gene Transduction.
The cDNAs and shRNA of Cldns were subcloned into the pMCs-Ires-EGFP retroviral vector provided by Kitamura (University of Tokyo, Tokyo, Japan) and pSIREN-RetroQ vector (Clontech) containing Ires-EGFP, respectively. Recombinant retrovirus was produced in Plat-E packaging cells. The shRNA primer sequences are indicated in SI Materials and Methods.
Fetal Thymus Organ Culture.
E15 fetal thymocytes were cultured with E15 thymic lobes that had been treated with 1.35 mM dGuo (Nacalai Tesque) at 103 cells/lobe in a gas mixture of 5% CO2, 70% O2, and 25% N2.
ChIP Assay.
Soluble chromatin prepared from 1% formaldehyde-fixed thymocytes was immunoprecipitated with various antibodies, and DNA purified from the immunoprecipitants was subjected to quantitative PCR using QuantiTect SYBR Green PCR mix (Qiagen) on a LightCycler Real-Time PCR System (Roche). The primer sets are described in SI Materials and Methods.
Statistical Analysis.
Statistical analysis was performed using the Student t test.
Supplementary Material
Acknowledgments
We thank Drs. Y. Tanaka, Y. Kato, and M. Hayashi for the generation of the anti-Cld4 monoclonal antibody, Mr. K. Wakae for the ChIP assay, Dr. M. Itoh for the anti–ZO-1 antibody, Dr. Y. Horiguchi for a C-CPE plasmid, Dr. K. Moriwaki for Cldn plasmids, Dr. T. Kitamura for the retroviral vector, Dr. D. L. Wiest for the Scid.adh cell line, and Dr. C. Murre for the E47−/− mice. This work was supported by grants from the Ministry of Education, Culture, Science, Sports, and Technology of the Japanese Government (to Y.H. and N.M.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014178108/-/DCSupplemental.
References
- 1.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 2.Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Ewijk W, et al. Thymic microenvironments, 3-D versus 2-D? Semin Immunol. 1999;11:57–64. doi: 10.1006/smim.1998.0158. [DOI] [PubMed] [Google Scholar]
- 5.Hamazaki Y, et al. Medullary thymic epithelial cells expressing Aire represent a unique lineage derived from cells expressing claudin. Nat Immunol. 2007;8:304–311. doi: 10.1038/ni1438. [DOI] [PubMed] [Google Scholar]
- 6.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 7.Bain G, Quong MW, Soloff RS, Hedrick SM, Murre C. Thymocyte maturation is regulated by the activity of the helix-loop-helix protein, E47. J Exp Med. 1999;190:1605–1616. doi: 10.1084/jem.190.11.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engel I, Johns C, Bain G, Rivera RR, Murre C. Early thymocyte development is regulated by modulation of E2A protein activity. J Exp Med. 2001;194:733–745. doi: 10.1084/jem.194.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Cruz LM, Knell J, Fujimoto JK, Goldrath AW. An essential role for the transcription factor HEB in thymocyte survival, Tcra rearrangement and the development of natural killer T cells. Nat Immunol. 2010;11:240–249. doi: 10.1038/ni.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Z, et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 11.Kurebayashi S, et al. Retinoid-related orphan receptor gamma (RORgamma) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc Natl Acad Sci USA. 2000;97:10132–10137. doi: 10.1073/pnas.97.18.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murre C. Helix-loop-helix proteins and lymphocyte development. Nat Immunol. 2005;6:1079–1086. doi: 10.1038/ni1260. [DOI] [PubMed] [Google Scholar]
- 13.Sonoda N, et al. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: Evidence for direct involvement of claudins in tight junction barrier. J Cell Biol. 1999;147:195–204. doi: 10.1083/jcb.147.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz R, Engel I, Fallahi-Sichani M, Petrie HT, Murre C. Gene expression patterns define novel roles for E47 in cell cycle progression, cytokine-mediated signaling, and T lineage development. Proc Natl Acad Sci USA. 2006;103:9976–9981. doi: 10.1073/pnas.0603728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo J, et al. Regulation of the TCRalpha repertoire by the survival window of CD4(+)CD8(+) thymocytes. Nat Immunol. 2002;3:469–476. doi: 10.1038/ni791. [DOI] [PubMed] [Google Scholar]
- 16.Umeda K, et al. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 17.Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol. 2005;5:136–148. doi: 10.1038/nri1548. [DOI] [PubMed] [Google Scholar]
- 18.Witherden DA, Boismenu R, Havran WL. CD81 and CD28 costimulate T cells through distinct pathways. J Immunol. 2000;165:1902–1909. doi: 10.4049/jimmunol.165.4.1902. [DOI] [PubMed] [Google Scholar]
- 19.Hemler ME. Specific tetraspanin functions. J Cell Biol. 2001;155:1103–1107. doi: 10.1083/jcb.200108061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones ME, Zhuang Y. Stage-specific functions of E-proteins at the β-selection and T-cell receptor checkpoints during thymocyte development. Immunol Res. 2010 doi: 10.1007/s12026-010-8182-x. in press. [DOI] [PubMed] [Google Scholar]
- 21.Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: In search of the ‘epimmunome.’. Nat Immunol. 2010;11:656–665. doi: 10.1038/ni.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.