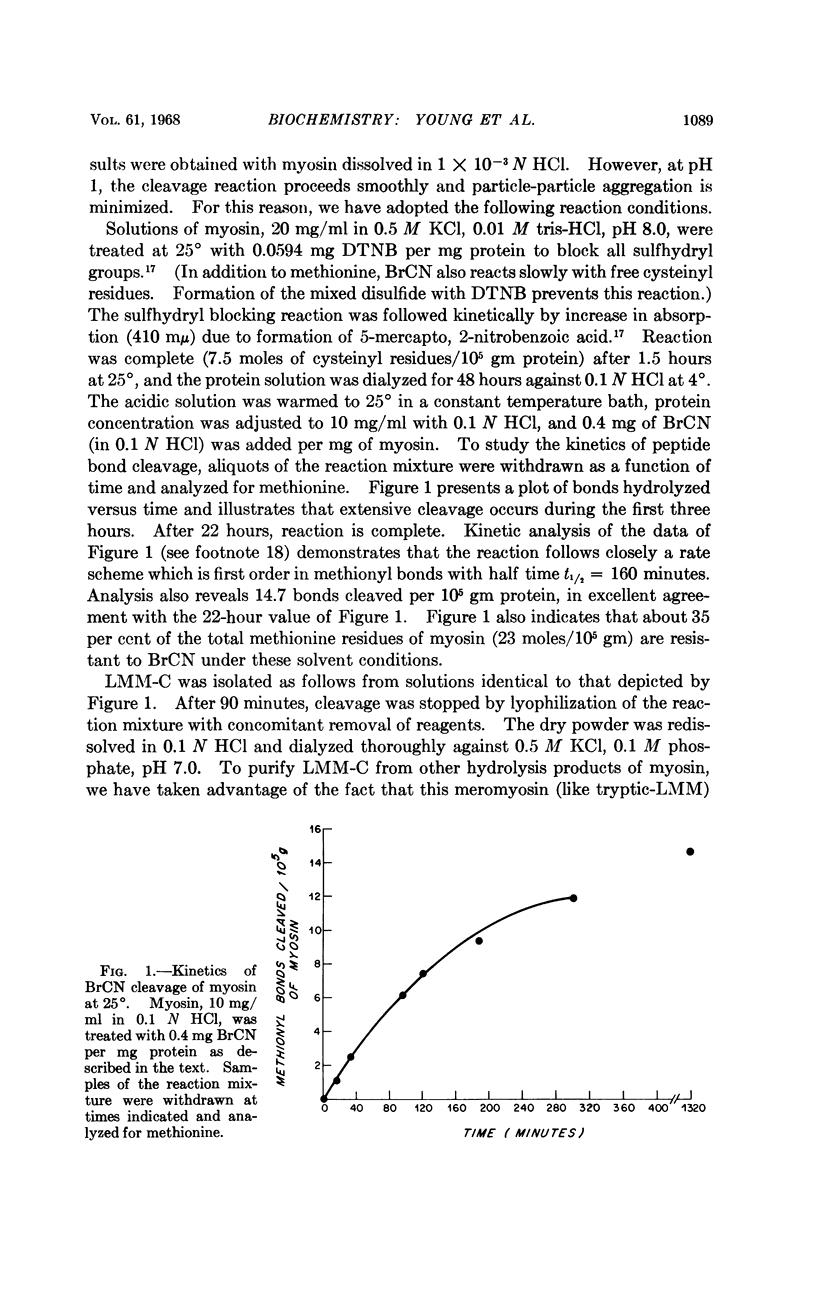
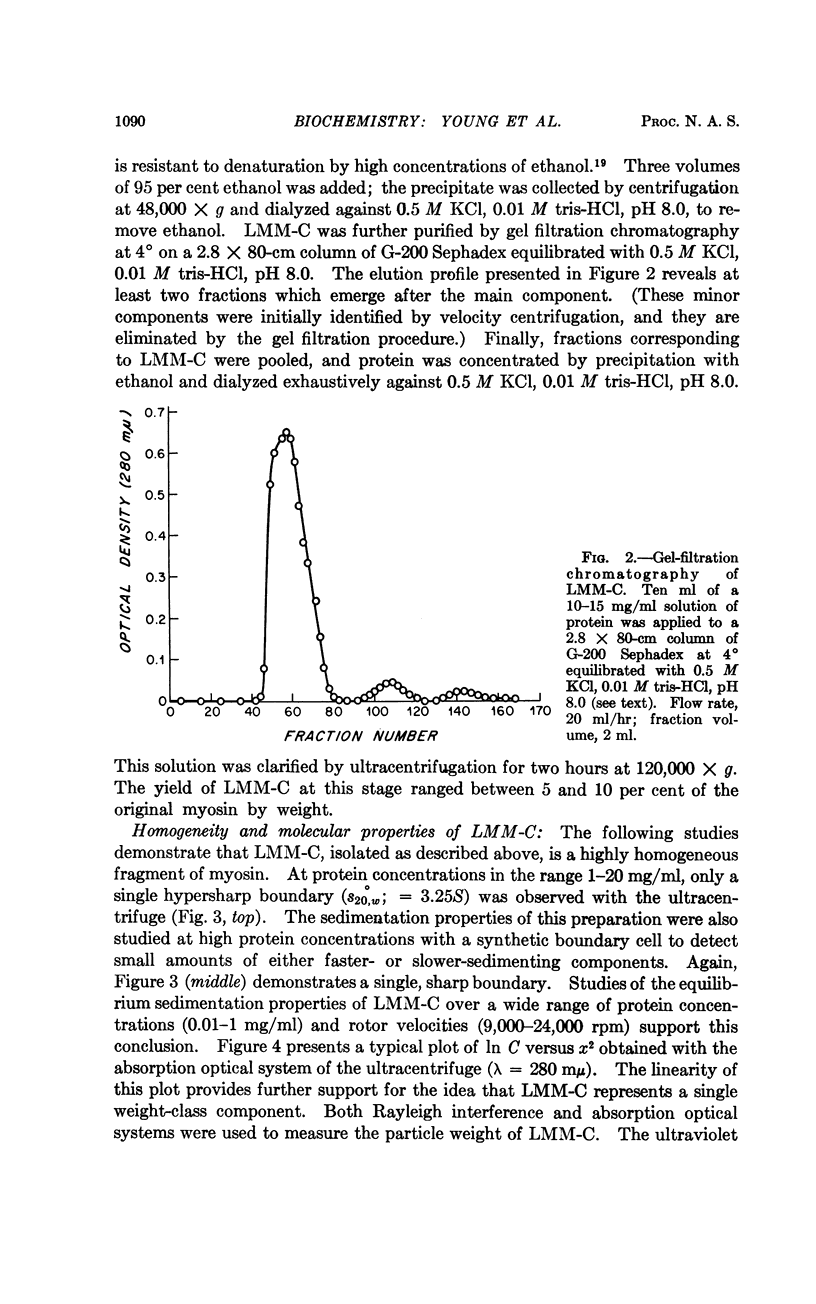
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAHNMANN H. J., ARNON R., SELA M. ACTIVE FRAGMENTS OBTAINED BY CLEAVAGE OF RABBIT ANTIBODY WITH CYANOGEN BROMIDE. J Biol Chem. 1965 Jun;240:2762–2764. [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- GERGELY J. Studies on myosin-adenosinetriphosphatase. J Biol Chem. 1953 Feb;200(2):543–550. [PubMed] [Google Scholar]
- GROSS E., WITKOP B. Nonenzymatic cleavage of peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1856–1860. [PubMed] [Google Scholar]
- KIELLEY W. W., BRADLEY L. B. The relationship between sulfhydryl groups and the activation of myosin adenosinetriphosphatase. J Biol Chem. 1956 Feb;218(2):653–659. [PubMed] [Google Scholar]
- KIELLEY W. W., HARRINGTON W. F. A model for the myosin molecule. Biochim Biophys Acta. 1960 Jul 15;41:401–421. doi: 10.1016/0006-3002(60)90037-8. [DOI] [PubMed] [Google Scholar]
- LOWEY S., COHEN C. Studies on the structure of myosin. J Mol Biol. 1962 Apr;4:293–308. doi: 10.1016/s0022-2836(62)80007-2. [DOI] [PubMed] [Google Scholar]
- MIHALYI E., SZENT-GYORGYI A. G. Trypsin digestion of muscle proteins. I. Ultracentrifugal analysis of the process. J Biol Chem. 1953 Mar;201(1):189–196. [PubMed] [Google Scholar]
- Rees M. K., Young M. Studies on the isolation and molecular properties of homogeneous globular actin. Evidence for a single polypeptide chain structure. J Biol Chem. 1967 Oct 10;242(19):4449–4458. [PubMed] [Google Scholar]
- SZENT-GYORGYI A. G., BORBIRO M. Depolymerization of light meromyosin by urea. Arch Biochem Biophys. 1956 Jan;60(1):180–197. doi: 10.1016/0003-9861(56)90410-6. [DOI] [PubMed] [Google Scholar]
- Segal D. M., Himmelfarb S., Harrington W. F. Composition and mass of peptides released during tryptic and chymotryptic hydrolysis of myosin. J Biol Chem. 1967 Mar 25;242(6):1241–1252. [PubMed] [Google Scholar]
- YOUNG D. M., HIMMELFARB S., HARRINGTON W. F. THE RELATIONSHIP OF THE MEROMYOSINS TO THE MOLECULAR STRUCTURE OF MYOSIN. J Biol Chem. 1964 Sep;239:2822–2829. [PubMed] [Google Scholar]
- Young M. On the interaction of adenosine diphosphate with myosin and its enzymically active fragments. Evidence for three identical catalytic sites per myosin molecule. J Biol Chem. 1967 Jun 10;242(11):2790–2792. [PubMed] [Google Scholar]