Abstract
The brain's histaminergic system has been implicated in hippocampal synaptic plasticity, learning, and memory, as well as brain reward and reinforcement. Our past pharmacological and lesion studies indicated that the brain's histamine system exerts inhibitory effects on the brain's reinforcement respective reward system reciprocal to mesolimbic dopamine systems, thereby modulating learning and memory performance. Given the close functional relationship between brain reinforcement and memory processes, the total disruption of brain histamine synthesis via genetic disruption of its synthesizing enzyme, histidine decarboxylase (HDC), in the mouse might have differential effects on learning dependent on the task-inherent reinforcement contingencies. Here, we investigated the effects of an HDC gene disruption in the mouse in a nonreinforced object exploration task and a negatively reinforced water-maze task as well as on neo- and ventro-striatal dopamine systems known to be involved in brain reward and reinforcement. Histidine decarboxylase knockout (HDC-KO) mice had higher dihydrophenylacetic acid concentrations and a higher dihydrophenylacetic acid/dopamine ratio in the neostriatum. In the ventral striatum, dihydrophenylacetic acid/dopamine and 3-methoxytyramine/dopamine ratios were higher in HDC-KO mice. Furthermore, the HDC-KO mice showed improved water-maze performance during both hidden and cued platform tasks, but deficient object discrimination based on temporal relationships. Our data imply that disruption of brain histamine synthesis can have both memory promoting and suppressive effects via distinct and independent mechanisms and further indicate that these opposed effects are related to the task-inherent reinforcement contingencies.
Neuronal histamine has been implicated in a variety of physiological, pathophysiological, and behavioral processes (Huston et al. 1997; Brown et al. 2001; Haas and Panula 2003). Neuronal histamine is exclusively derived from the nucleus tuberomammillaris (TM) of the posterior hypothalamus, which receives major inputs from limbic areas, and from where diffuse projections to wide parts of the brain arise, including the hippocampal formation (Wada et al. 1991). Histamine synthesis is executed by histidine-decarboxylase (HDC) converting histidine to histamine. Two postsynaptic (H1 and H2) and one presynaptic receptor (H3), with auto- and heteroreceptor functions, were identified (Hill et al. 1997). Histamine facilitated (Kamei et al. 1993) and suppressed active avoidance conditioning (Alvarez and Banzan 1996). The HDC-blocker α-FMH both improved (Sakai et al. 1998) and impaired spatial memory in a radial-maze task (Chen et al. 1999). Furthermore, H1 receptor antagonism improved water-maze (Hasenöhrl et al. 1999) and impaired radial-maze performance (Taga et al. 2001), whereas learning and memory in H1 knockout mice were unaffected (Yanai et al. 1998a,b). Contradictory results were also found with agents acting at H2 (Flood et al. 1998; Onodera et al. 1994) and H3 receptors (Blandina et al. 1996; Rubio et al. 2002). Finally, lesions and temporary inactivation of the TM region improved habituation learning, inhibitory avoidance, discrimination, and water-maze learning in adult and aged rats (Frisch et al. 1998, 1999). A selective, significant, and lasting disruption of brain histamine synthesis through the HDC-blocker α-fluoromethyl histidine (α-FMH) or the simultaneous inhibition of all histamine receptors has failed (Watanabe et al. 1990). Systemic injections of high doses of α-FMH did not reduce hippocampal histamine levels significantly (Onodera et al. 1992). Most histaminergic agents also show activity at nonhistamine, for example, cholinergic receptors (Hill et al. 1997). Furthermore, lesions of the TM may not only lead to neuronal histamine depletion but also to the depletion of the transmitter systems colocalized in the TM or even coreleased by histaminegic neurons (Köhler et al. 1985; Yamatodani et al. 1991). These shortcomings might have contributed to some extent to the fact that the functions of brain histamine in learning and memory are still controversial. Alternatively, modulation of central histaminergic transmission might, indeed, have both memory promoting and suppressive effects possibly via distinct and independent mechanisms. One mechanism might act directly on the brain's memory substrate via the modulation of hippocampal synaptic plasticity as recently reviewed in Haas and Panula (2003), whereas the other might have an indirect effect on memory inscription via modulation of the brain's reinforcement system (for review, see Huston et al. 1997). Our results indicate that the brain's histamine system exerts inhibitory effects on the brain's reinforcement respective reward system reciprocal to mesolimbic dopamine systems (Wise 1996; Huston et al. 1997). We thus evaluated whether neo- and ventro-striatal dopamine concentrations and metabolism were affected by the histidine decarboxylase knockout (HDC-KO), because these brain structures were implicated in brain reward and reinforcement (Fibinger and Phillips 1988; Wise 1996). Given the close functional relationship between brain reinforcement and memory processes (Huston et al. 1997; Huston and Oitzl 1989), the HDC-KO might have differential effects on learning dependent on the task-inherent reinforcement contingencies. We expected to find unaffected performance of HDC-KO mice in a nonreinforced object exploration task, where possible disinhibitory effects of the HDC-KO on the brain's reinforcement system should play a minor role. On the contrary, improved performance might be evident in water-maze tasks that closely depend on negative reinforcement, as indicated by our previous work (Frisch et al. 1998, 1999; Hasenöhrl et al. 1999).
RESULTS
Habituation to Object Stimuli
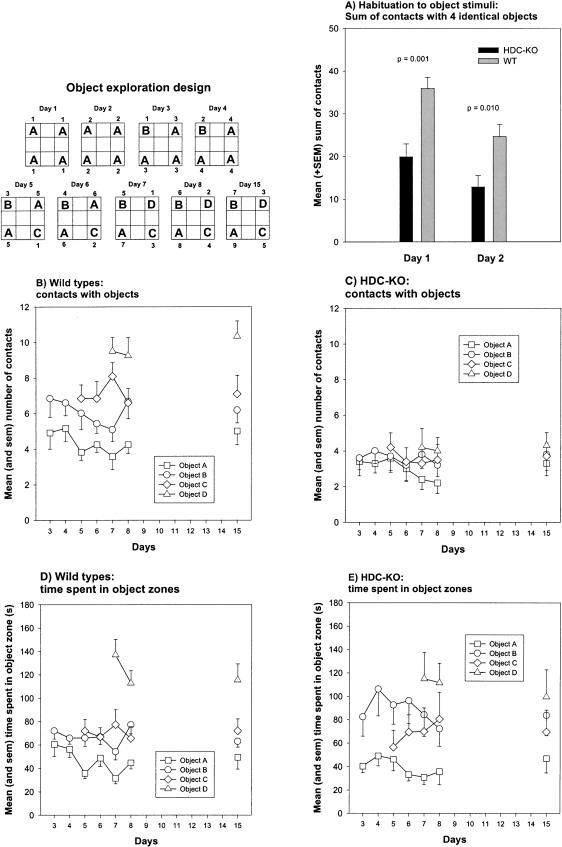
During the 2 d of object exploration with four equivalent objects (A), the HDC-KO mice showed a fewer number of total contacts than the controls (F(1,20) = 12.430, p = 0.002; repeated measures ANOVA; Fig. 1A). Post hoc t-tests revealed that the HDC-KO mice contacted the objects on day 1 (p = 0.001; t-test for independent samples) and day 2 (p = 0.010) less frequently than the wild-type (WT) mice. However, both groups showed a reduced number of contacts on day 2 relative to day 1 (HDC-KO: p = 0.001; WT: p < 0.001). These findings indicate that although HDC-KO mice show reduced contacts with objects, they nevertheless habituate to object stimuli.
Figure 1.
Effects of the HDC gene disruption on nonreinforced object habituation (A) and episodic object memory based on temporal relationships (B-E). (Inserttop left) Scheme of the object exploration design. (A) Mean and sem total contacts for all identical objects on indicated days. (B,C) Mean and sem number of contacts with different objects on indicated days for HDC-KO and wild-type mice. (D,E) Mean and sem time spent in the four object quadrants on indicated days for HDC-KO and wild-type mice. P = HDC-KO versus WT, t-test for independent samples.
Nonreinforced Relational Object Memory
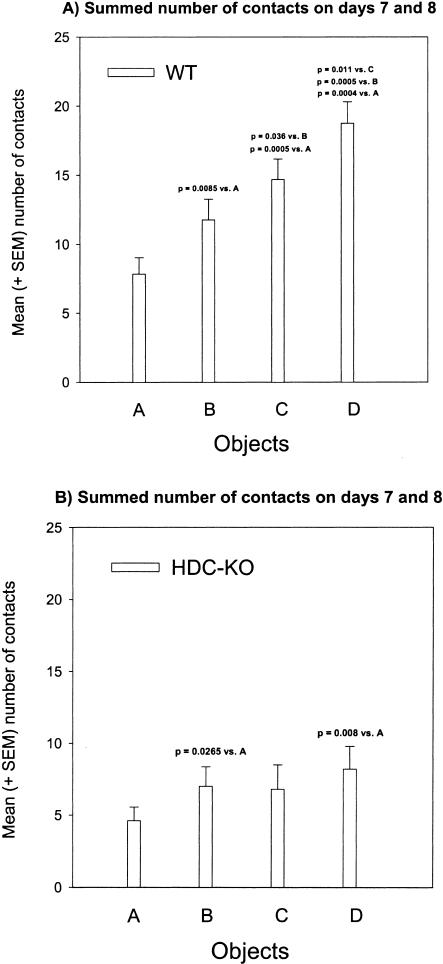
From days 3 to 8, the wild-type mice and HDC-KO mice showed a similar low number of contacts with the most familiar Object A (Object A: F(1,20) = 3.356, p = 0.082; repeated measures ANOVA; Fig. 1B,C). The number of contacts with Objects B, C, and D, however, was higher for wild-type mice relative to HDC-KO mice (Object B: F(1,20) = 9.100, p = 0.007; Object C: F(1,20) = 9.309, p = 0.006; Object D: F(1,20) = 20.515, p < 0.001). Within-group comparisons of contacts with pairs of objects (A vs. B, A vs. C, A vs. D, B vs. C, B vs. D, and C vs. D) on corresponding days, revealed that wild-type mice generally contacted the less familiar objects more frequently than the more familiar ones (Fig. 1B; see also p-values in Table 1 for comparisons of object pairs). These findings indicate that the control mice were able to establish temporal relationships between discrete object stimuli. We additionally computed for each animal the sum of contacts with Objects A, B, C, and D for days 7 and 8 (Fig. 2A,B) and performed within-group comparisons for different contact numbers. As shown in Figure 2A, the wild-type mice showed the expected rank order A < B < C < D. On the contrary, the HDC-KO mice were unable to discriminate between objects in dependence of the number of previous encounters with those objects (Figs. 1C and 2B; see also p-values in Table 1 for within-group comparisons of object pairs), indicating that relational object memory based on temporal discrimination is disrupted in HDC-KO mice.
Table 1.
One-Tailed p-Values Obtained After Pairwise Within-Group Comparisons Using One-Way Repeated Measures ANOVAs for Object Contacts and the Time Spent in Object Zones on Indicated Days
|
Number of Contacts
|
Time Spent in Object Zone
|
||||
|---|---|---|---|---|---|
| Objects | Days | WT | HDC-KO | WT | HDC-KO |
| A < B | 3 to 8 | p = 0.014 | p = 0.240 | p = 0.007 | p = 0.0025 |
| A < C | 5 to 8 | p = 0.002 | p = 0.215 | p = 0.006 | p = 0.038 |
| A < D | 7 and 8 | p < 0.0005 | p = 0.040 | p = 0.0005 | p = 0.005 |
| B < C | 5 to 8 | p = 0.102 | p = 0.454 | p = 0.360 | p = 0.227 |
| B < D | 7 and 8 | p = 0.003 | p = 0.149 | p = 0.0005 | p = 0.086 |
| C < D | 7 and 8 | p = 0.042 | p = 0.287 | p = 0.002 | p = 0.105 |
Figure 2.
Episodic object memory. Rank order of summed contacts with objects A, B, C, D on days 7 + 8. (A) Wild-type mice. Mean and sem sum of contacts with objects on days 7 + 8. (B) HDC-KO mice. Mean and sem total contacts with objects A, B, C, and D on days 7 + 8. P-values represent t-tests for dependent samples.
After a retention interval of 6 d, the animals were again presented with the Objects A, B, C, D.
During the long-term memory test for temporal inter-object relationships, the HDC-KO mice again showed fewer contacts with Objects C and D but not A and B compared with the wild-type mice (A: p = 0.138; B: p = 0.057; C: p = 0.026; D: p < 0.001; t-test for independent samples; Fig. 1B,C).
The wild-type mice contacted Object D more frequently than the other three objects (Fig. 1B; see also Table 2 for respective p-values) and contacted Object A less frequently than B and C. The contact numbers of Objects B and C were similar.
Table 2.
One-Tailed p-Values Obtained After Pairwise Within-Group Comparisons Using t-Tests for Dependent Samples for Object Contacts and the Time Spent in Object Zones During the Test for Long-Term Memory on Day 15
| Number of contacts
|
Time spent in object zone
|
|||
|---|---|---|---|---|
| Objects | WT | HDC-KO | WT | HDC-KO |
| A < B | p = 0.034 | p = 0.293 | p = 0.124 | p = 0.064 |
| A < C | p = 0.036 | p = 0.247 | p = 0.084 | p = 0.183 |
| A < D | p = 0.001 | p = 0.156 | p = 0.009 | p = 0.073 |
| B < C | p = 0.142 | p = 0.464 | p = 0.265 | p = 0.333 |
| B < D | p = 0.001 | p = 0.245 | p = 0.004 | p = 0.334 |
| C < D | p = 0.008 | p = 0.263 | p = 0.038 | p = 0.227 |
On the contrary, HDC-KO mice contacted the four objects to similar extents (Fig. 1C; see also Table 2 for respective p-values). These results confirm the above finding (days 3 to 8) that HDC-KO mice are unable to relate the number of previous encounters with one object to those of another, and, thus, have not formed a long-term memory for temporal inter-object relationships.
Because the above results might be the consequence of reduced general activity or the inability to discriminate different objects visually by HDC-KO mice, we additionally assessed the time the mice spent in the object zones (Fig. 1D,E). As can be seen in Figure 1D and from the p-values depicted in Table 1, the wild-type mice spent significantly more time in Object zone D relative to the remaining object zones. Furthermore, they spent more time in Object zones B and C relative to A. Thus, the time spent and contact number parameters yielded similar results for wild-type mice. On the contrary, the HDC-KO mice only spent less time in Object zone A relative to the other zones, but the values for the remaining comparisons were similar (Fig. 1E; Table 1 for respective p-values). Thus, the “time spent in object zone” parameter indicates that the HDC-KOs were able to discriminate at least Object A from the other ones visually and regarding temporal relationships. However HDC-KO mice were not able to discriminate the temporal relationships between Objects B, C, and D. These results demonstrate that the deficit of HDC-KO mice is not related to their low activity level or to sensory impairments.
On day 15 after a retention interval of 6 d, the control mice still spent more time in Object zone D relative to the remaining object zones (see Fig. 1D and Table 2 for p-values). Again, as with the “contact” parameter, no differences were found for the HDC-KO mice (see Fig. 1E and Table 2 for p-values). Thus, these results clearly indicate that the HDC-KO mice indeed show deficient object discrimination on the base of temporal relationships (days 3 to 8), and, thus, deficient relational object memory (day 15).
Reinforced Relational Spatial Memory
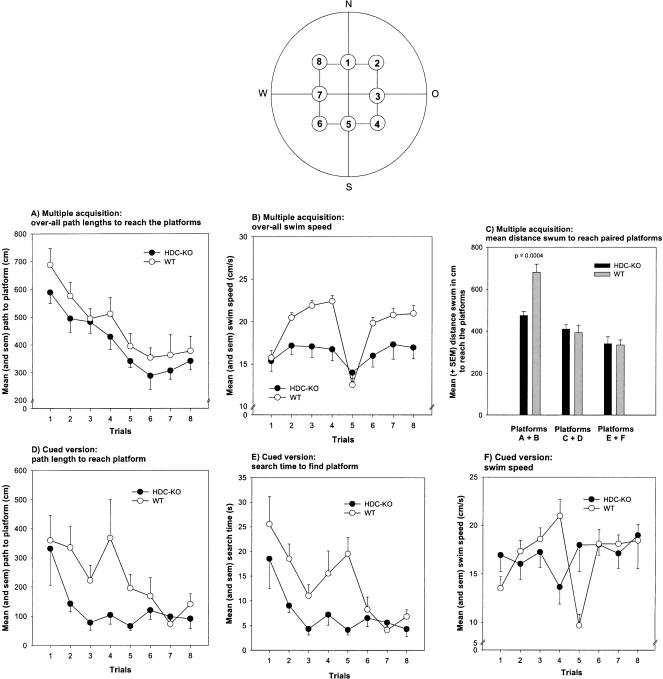
Reinforced relational spatial memory was assessed with a water-maze hidden-platform task, in which the mice were required to associate different platform locations with extra-maze cues to efficiently escape from forced swimming. Both groups showed reductions in search times and path lengths to reach the six hidden platforms across the eight trials (HDC-KO: search times, F(7,63) = 9.854, p < 0.001; path length, F(7,63) = 6.778, p < 0.001; WT: search times, F(7,77) = 15.039, p < 0.001; path length, F(7,77) = 5.194, p < 0.001; one-way ANOVA). The mean distance the animals swam to locate the first two platform positions was lower in the HDC knockouts compared with controls (p = 0.0004; t-test for independent samples; Fig. 3C); this was not the case for the remaining platforms (all ps > 0.1). When the distance traveled to reach the six different platform locations were averaged for each subject yielding eight data points, the HDC-KO mice swam a shorter distance to reach the platforms compared with controls (Fig. 3A). However, this difference failed to reach a p-value smaller than 0.05 (F(1,20) = 3.753, p = 0.067). Thus, as hypothesized, the HDC gene disruption indeed improved initial hidden-platform water-maze performance (platforms A + B). The overall search times to locate the platforms were similar between groups (F(1,20) = 0.425, p = 0.522; data not shown), possibly because of the higher swim speed of the controls (F(1,20) = 5.220, p = 0.033; Fig. 3B).
Figure 3.
Effects of the HDC gene disruption on negatively reinforced water-maze performance during the multiple acquisition (A-C) and signaled platform tasks (D-F). (Insert top) Arrangement of possible platform positions. (A) Mean and sem overall path lengths to reach the six hidden platforms during the multiple acquisition task. (B) Mean and sem overall swim speeds during the multiple acquisition task. (C) Mean and sem path lengths to reach the hidden platforms A + B, C + D, and E + F during the multiple acquisition task. (D) Mean and sem path lengths to reach the signaled platforms during the cued version. (E) Mean and sem search time to reach the signaled platforms. (F) Mean and sem swim speeds during the signaled platform task. P = HDC-KO versus WT, t-test for independent samples.
Stimulus-Response Learning
During two consecutive days, the submerged platform was shifted from trial to trial and was signaled by an easily perceptible cue. The animals were required to learn the association between the cue and the hidden platform. Both groups showed reductions in search times and path lengths to reach the signaled platform locations across the eight trials (HDC-KO: search times, F(7,63) = 3.125, p = 0.007; path length, F(7,63) = 5.307, p = 0.032; WT: search times, F(7,77) = 5.055, p < 0.001; path length, F(7,77) = 2.556, p = 0.020; one-way ANOVA). The HDC-KO mice performed superior to controls, exhibiting shorter path lengths (F(1,20) = 5.307, p = 0.032; Fig. 3D) and search times (F(1,20) = 10.122, p = 0.005; Fig. 3E) to reach the platform, while having similar swim speeds (F(1,20) = 0.011, p = 0.918; Fig. 3F). These results indicate that the HDC gene disruption had improved performance in a simple stimulus-response task.
Striatal Dopamine Concentrations and Metabolism
HDC-KO mice had higher dihydrophenylacetic acid concentrations (p = 0.054; Table 3) and a higher dihydrophenylacetic acid/dopamine ratio in the neostriatum (p = 0.088). In the ventral striatum, the dihydrophenylacetic acid/dopamine (p = 0.044; Table 4) and 3-methoxytyramine/dopamine ratios (p = 0.046) were higher relative to the wild-type mice. No further differences were observed (all p-values > 0.1). These results indicate that histamine deficiency altered dopamine metabolisms in the neo- and ventral striata known to be involved in brain reward and reinforcement.
Table 3.
Mean (and SEM) Concentration (Picograms/Milligram) of Dopamine (DA), Dihydrophenylacetic Acid (DOPAC), Homovanillic Acid (HVA), and 3-Methoxytyramine (3-MT) and Metabolite/Transmitter Ratios in the Neostriatum of HDC-KO and Wild-Type Mice. P = HDC-KO Versus Wild-Type Mice t-Test for Independent Samples
| DA | DOPAC | HVA | 3-MT | DOPAC/DA | HVA/DA | 3-MT/DA | |
|---|---|---|---|---|---|---|---|
| HDC-knockout mice | |||||||
| Mean | 29,395.54 | 1666.32 | 1617.21 | 1174.81 | 0.0564 | 0.0551 | 0.0401 |
| SEM | 513.88 | 133.51 | 54.34 | 20.87 | 0.0042 | 0.0018 | 0.0011 |
| Wild-type mice | |||||||
| Mean | 28,463.30 | 1258.73 | 1591.82 | 1205.59 | 0.0443 | 0.0561 | 0.0424 |
| SEM | 354.75 | 133.10 | 61.29 | 37.89 | 0.0047 | 0.0023 | 0.0012 |
| P = | 0.160 | 0.054 | 0.775 | 0.528 | 0.088 | 0.769 | 0.213 |
Table 4.
Mean (and SEM) Concentration (Picograms/Milligram) of Dopamine (DA), Dihydrophenylacetic Acid (DOPAC), Homovanillic Acid (HVA), and 3-Methoxytyramine (3-MT) and Metabolite/Transmitter Ratios in the Ventral Striatum of HDC-KO and Wild-Type Mice. P = HDC/KO Versus Wild-Type Mice t-Test for Independent Samples
| DA | DOPAC | HVA | 3-MT | DOPAC/DA | HVA/DA | 3-MT/DA | |
|---|---|---|---|---|---|---|---|
| HDC-knockout mice | |||||||
| Mean | 5569.85 | 840.58 | 874.00 | 403.27 | 0.1604 | 0.1765 | 0.0732 |
| SEM | 628.32 | 65.84 | 47.16 | 38.72 | 0.0128 | 0.0212 | 0.0032 |
| Wild-type mice | |||||||
| Mean | 5923.83 | 713.84 | 834.78 | 362.14 | 0.1273 | 0.1516 | 0.0627 |
| SEM | 473.15 | 33.12 | 21.74 | 25.43 | 0.0081 | 0.0118 | 0.0034 |
| P = | 0.667 | 0.101 | 0.456 | 0.394 | 0.044 | 0.320 | 0.046 |
DISCUSSION
In the present study, we investigated the effects of a HDC gene disruption in the mouse on two relational memory tasks, a nonreinforced object exploration task and a negatively reinforced water-maze task, as well as on neo- and ventro-striatal dopamine systems. HDC-KO mice had higher dihydrophenylacetic acid concentrations and a higher dihydrophenylacetic acid/dopamine ratio in the neostriatum. In the ventral striatum, the dihydrophenylacetic acid/dopamine and 3-methoxytyramine/dopamine ratios were higher in HDC-KO mice. Thus, histamine deficiency altered dopamine metabolism in the neo- and ventral striata known to be involved in brain reward and reinforcement (Fibinger and Phillips 1988; Di Chiara et al. 1991). As expected, the HDC-KO mice showed improved water-maze performance during both hidden and cued platform tasks, but surprisingly deficient object discrimination based on temporal relationships. Our data imply that disruption of brain histamine synthesis can have both memory promoting and suppressive effects apparently via distinct and independent mechanisms, and further indicate that these opposed effects are related to the task inherent reinforcement contingencies.
Drugs with rewarding and reinforcing properties increase dopamine release in the neo- and ventral striata (Fibinger and Phillips 1988; Wise 1996). TM lesions as well as histamine receptor blockade in rats lower the threshold for rewarding brain stimulation (Wagner et al. 1993; Zimmermann et al. 1999). Furthermore, antihistaminergic drugs induce place preference (Unterwald et al. 1984) and potentiate the rewarding effects of addictive drugs, such as amphetamines (Masukawa et al. 1993) and opioids (Shannon and Su 1982). These findings indicate that the brain histamine system exerts inhibitory effects on the brain's reinforcement respective reward system reciprocal to mesolimbic dopamine systems (Wise 1996; Huston et al. 1997). Here, we found changes in dopamine metabolites and turnover ratios in the neo- and ventral striata in HDC-KO mice. Our results indicate that dopamine turnover in these brain areas was increased in HDC-KO mice, possibly because of increased dopamine release (Wood and Altar 1988; Schlicker et al. 1993; Dringenberg et al. 1998; Maisonnette et al. 1998; Galosi et al. 2001). In future studies, we will examine whether HDC-KO mice show changes in cocaine- and morphine-induced place preference to test the hypothesis that reward and reinforcement processes are actually disinhibited in HDC-KO mice. However, if lack of neuronal histamine in HDC-KO mice has a disinhibitory effect on the brain's reinforcement system, it should also have a beneficial effect on performance in learning and memory tasks in which specific behaviors are positively or negatively reinforced (Huston et al. 1997). As hypothesized, the HDC-KO mice showed improved performance not only in the hidden but also in the cued platform water-maze task, possibly because in both tasks escape to the platform is negatively reinforced. Furthermore, the HDC-KO mice showed decreased swim speeds during the hidden platform task. This finding, however, stands in contrast with an increased motivation to escape from forced swimming. Interestingly, the wild-type mice showed an increase in swimming speed across the four daily trials during hidden and cued platform tasks, leading to a drop in swimming speed on the fifth trial on the second days of hidden platform and cued version performance. However, the basis for this effect remains obscure and awaits further research.
Although these above findings are in accord with results showing that TM lesions (Frisch et al. 1998) and systemic blockade of histaminergic receptors (Hasenöhrl et al. 1999) or histamine synthesis (Sakai et al. 1998) facilitate performance in several positively or negatively reinforced learning and memory tasks, there is also evidence for impaired learning performance after inhibition of histaminergic neurotransmission (Chen et al. 1999; Taga et al. 2001). The low specificity of the lesion techniques (Airaksinen et al. 1992) and the pharmacological tools used (Hill et al. 1997) might have contributed to this discrepancy. However, one should also take into account that past research on the role of histamine in learning and memory processes almost exclusively used tasks that bear an explicit reinforcing event. Only a handful of studies used nonassociative memory tasks, yielding diverging results.
We further hypothesized that HDC-KO mice would not exhibit performance changes in a nonreinforced relational object memory task. We surprisingly found deficient episodic object memory performance of HDC-KO mice. Here, the HDC-/- mice showed normal habituation to object stimuli but were strongly impaired when they had to discriminate different objects varying regarding their familiarity, or in other words, in dependence on the number of previous encounters with those objects. Moreover, after a retention interval of 6 d, the wild-type mice, but not the HDC-/- mice, still recognized the lastly presented Object D as “novel” relative to the other objects. It seems that the HDC gene disruption had selectively impaired episodic object memory. As outlined below, this finding might involve effects on NMDA receptor-mediated synaptic plasticity and on memory-related intracellular second messenger cascades activated after H1 and H2 receptor stimulation.
NMDA receptors were implicated in certain types of synaptic long-term potentiation (LTP) and some types of memory (Martin and Morris 2002). Histamine enhances NMDA-receptor responses and hippocampal LTP (Vorobjev et al. 1993; Brown et al. 1995). Among the NMDA-receptor subtypes, those containing the NR2B subunit show biophysical properties well suited for LTP induction (Thomas et al. 1996; Williams et al. 1998; Tang et al. 1999). Histamine facilitates NR2B containing NMDA-receptor activation directly via binding to polyamine sites, indirectly via H1-receptor induced C-terminal phosphorylation through PKC, and through reduced voltage sensitivity (Bekkers 1993; Vorobjev et al. 1993; Williams 1994; Payne and Neumann 1997). Furthermore, H2 receptor activation was linked to cAMP and PKA production; both were implicated in the persistent postsynaptic structural consequences of LTP (Selbach et al. 1997). H1 receptors (via the induction of the retrograde messengers nitric oxide and arachidonic acid) might also be involved in the presynaptic changes seen after LTP induction (Brown and Haas 1999; Haas and Panula 2003). Therefore, the impaired performance of HDC-KO mice in the nonreinforced relational object memory task might be related to the absence of the facilitating effect of brain histamine on both NMDA-receptor-dependent hippocampal synaptic plasticity and histamine-receptor-dependent activation of retrograde and second messenger systems. However, it remains to be determined whether hippocampal NMDA-receptor-dependent LTP is actually altered in the brains of HDC-KO mice.
Given the importance of NMDA receptors and the second messenger systems activated after histamine receptor stimulation for certain types of synaptic plasticity and possibly memory, the question arises, why did the HDC-KO mice not also show impaired water-maze performance? The simplest answer to this question might be that the beneficial effect of histamine on NMDA-receptor activation and the second messenger systems involved after histamine receptor stimulation might be critically involved in relational object memory based on the establishment of temporal relationships between distinct objects, but possibly not in water-maze performance. Even if the modulatory effect of brain histamine on NMDA receptors is crucial for their functioning, water-maze performance might be preserved. For example, hippocampal NMDA-receptor blockade (Bannerman et al. 1995; Hoh et al. 1999), and even hippocampal LTP-saturation (Otnaess et al. 1999) do not necessarily impair water-maze performance (but see also Steele and Morris 1999). Furthermore, the synaptic plasticity subserving water-maze performance might also be triggered by metabotropic G-protein-coupled glutamate receptors or voltage-gated calcium channels that mediate a NMDA-receptor-independent form of hippocampal LTP (Cavus and Teyler 1998; Grover and Yan 1999).
Another possibility might be that the disinhibition of the brain's reinforcement system in HDC-deficient mice is not only sufficient for leveling the concomitant memory-impairing effect of histamine synthesis disruption, but instead overcompensates it.
Brain histamine was also implicated in arousal mechanisms and the regulation of sleep-wake cycles (for review, see Lin 2000). Accordingly, the HDC-/- mice showed alterations in cortical-EEG and sleep-wake cycle and fell asleep after ∼18.4 ± 1.8 min in a novel environment (Parmentier et al. 2002) and showed reduced activity in an accustomed environment (Kubota et al. 2002). It was suspected that histamine deficiency reduces exploratory activity in HDC-/- mice via the inability to stay awake or deregulated arousal mechanisms and should therefore generally interfere with performance in learning and memory tasks (Parmentier et al. 2002). Here, we showed that HDC-KO mice despite showing reduced activity are nevertheless able to habituate to object stimuli. Furthermore, in an open field, the HDC-KO mice showed reduced exploratory behaviors, which, however, did not prevent spatial habituation (E. Dere, M.A. De Souza-Silva, B. Topic, H.L. Haas, J.P. Huston, unpubl.). Our findings indicate that deregulated arousal mechanisms in HDC-KO mice do not per se prevent nonreinforced memory formation.
Residual Brain Histamine in HDC-KO Mice?
It was reported that the HDC gene disruption prevented the HDC-gene expression at the transcriptional level. However, in HDC-KO mice that were fed a low-histamine diet, some residual histamine levels were found in brains, but not several other organs, of the HDC-/- mice (HDC-/-: 18.41 ± 2.74 pmole/g; wild type: 58.67 ± 9.83 pmole/g), possibly through absorption from the digestive tract (Ohtsu et al. 2001). Because it is thought that histamine cannot easily permeate the blood-brain barrier (Schwarz et al. 1991), it was assumed that this residual brain histamine is likely to be nonneuronal and located outside the blood-brain barrier (Parmentier et al. 2002).
General Limitations of the Knockout Approach
Similar to other techniques in neuroscience, the classical knockout approach has its limitations. To avoid the interpretation problems of knockout studies due to mixed genetic backgrounds (for review, see Gerlai 1999), the HDC-deficient mice and their wild-type littermates were kept on a pure 129/Sv genetic background. However, the HDC deficiency might have induced subtle aberrations in brain development and might also have initiated compensatory mechanisms that are not easily detectable. However, HDC-deficient mice were fertile and born at the expected Mendelian frequency (Ohtsu et al. 2001), and no overt morphological or neurochemical abnormalities have been described in HDC-deficient mice. Indicators of general health status, such as fur appearance and skin color, were not different from controls at the analyzed ages. Nevertheless, it should be considered that the HDC-gene disruption affects all cells in the body that synthesize histamine. Therefore, peripheral effects cannot be excluded. Nevertheless, the dissociation found for episodic object memory and negatively reinforced water-maze performance supports the argument against such gross peripheral effects. However, the final behavioral phenotype of the knockout mice model is always the consequence of various interacting processes, which might be affected by the lack of endogenous histamine. It is obvious that the classical knockout approach cannot be regarded as the ultimate tool to clarify the controversy regarding the involvement of brain histamine in different types of memory; it can, however, provide complementary information. For the future, it might be promising to generate a brain-specific inducible conditional HDC-knockout utilizing the already available cre-loxP recombinase technique (Tsien 1998) to exclude developmental and peripheral effects.
In conclusion, our present results clearly demonstrate that disruption of brain histamine synthesis can promote and suppress performance in memory tasks, possibly via different mechanisms, and dependent on the task-inherent reinforcement contingencies.
MATERIALS AND METHODS
Animals
The HDC-KO and wild-type mice used in the present study were generated by Ohtsu et al. (2001) and were the progeny of the colony maintained at the Department of Experimental Medicine, Claude Bernard University of Lyon, France. The procedures for creating a null allele of the HDC gene, generation of HDC-deficient mice, loss of HDC activity and reduction of histamine levels in organs of homozygous HDC-deficient mice were described previously in detail (Ohtsu et al. 2001). Both the HDC-deficient and wild-type mice had a pure 129/Sv genetic background. The mice used were 5- month-old male HDC-KO (n = 10) and wild-type mice (n = 12). The mice were obtained from the animal breeding division of the Heinrich-Heine University of Düsseldorf. The animals were single-housed and accustomed to the housing conditions for 1 wk prior to the beginning of the behavioral experiments. During this adaptation period, the animals were habituated to handling. The mice were held in standard Makrolon cages (type 2, 22 × 16 × 13 cm) with metal covers and had continuous access to rodent chow (Ssniff, Spezialdiäten GMBH) and tap water. The mice were maintained on a 12-h light/dark cycle and were tested during the light phase between 9 a.m. and 4 p.m.
Habituation to Object Stimuli
The mice were exposed to four equivalent objects (type A), made of glass with a height of 12 cm and a maximum diameter of 4 cm, placed in the corners of a familiar open field (30 × 30 × 40 cm). The mice were free to explore these four objects during 5-min sessions for two consecutive days (test days 1 and 2). This test was performed to ensure that the mice were able to habituate to object stimuli after a delay of 24 h, prior to the assessment of object discrimination on the basis of temporal relationships.
Nonreinforced Relational Object Memory6
To measure relational object memory, we demanded the animals to relate the frequency of previous encounters with a specific object to the frequency of previous encounters with other objects, thus adding a time factor to an object discrimination task (see insert in Fig. 1). In this task, which does not involve an explicit reinforcing event, the animals learn about the temporal relationships between objects. Therefore, possible effects of the HDC gene disruption on brain reinforcement processes as indicated by pharmacological and lesion studies (Huston et al. 1997) might not be decisive in this task. Thus, we expected unaltered performance of HDC-deficient mice.
One day after the object habituation task (test day 3), one of the four type A objects was replaced by a novel glass object (type B) with similar height, color, and smell, but a different shape and surface texture. Although this is a more difficult task to solve than the discrimination between objects differing regarding a multitude of dimensions (different materials, surface textures, smell, colors, size, shape, and height), it nevertheless controls more strictly for gene deletion effects on specific sensory modalities or the preference for certain materials. On test days 5 (C) and 7 (D), another two novel glass objects replaced two old ones of the A type. Thus, on days 7 and 8, four different objects were presented with different degrees of “familiarity” (see insert in Fig. 1). Once a given novel object was introduced to a specific corner, it was kept in this location over the following days. It was expected that on days 3 to 8 the animals would contact “novel” objects more frequently and spend more time in the “novel” object zones relative to “familiar” ones, dependent on the number of encounters with an object on previous days. Because the performance of the mice in this task not only demands the dichotomic distinction, novel versus familiar, between two different objects, but also requires the distinction of relative novelty and familiarity among at least four different objects, for example, requires the establishment of temporal inter-object relationships, this task can also be considered as a test for relational, but nonreinforced, learning, respective memory. After a retention interval of 6 d, the same spatial constellation of objects as on days 7 and 8 was presented to assess long-term memory of temporal inter-object relationships. After each trial, the apparatus and the objects were cleaned with water containing 0.1% acetic acid. The number of object contacts with forepaws or vibrissae were scored. Furthermore, the time spent (seconds) in the four corner squares where objects were placed (10 × 10 cm each) was measured using an automated tracking system (EthoVision, Noldus).
Reinforced Relational Spatial Memory
We used the Morris water-maze, hidden-platform paradigm to measure relational spatial memory of HDC-KO mice. In this task, animals acquire relational spatial memories after negative reinforcement. Therefore, it is expected that the possible disinhibition of the brain's reinforcement system after HDC gene disruption would improve water-maze performance.
Apparatus
The water maze used was a black, painted, circular tank with 112 cm diameter, and 40 cm height. It was filled to a depth of 25 cm with water (19°-20°C) made opaque white by the addition of 1 L of durable milk. The escape platform, made of transparent Plexiglas, had a diameter of 10 cm and was height-adjustable. The room was diffusely illuminated by ceiling lamps. Several potential visual cues surrounded the water maze, including doors, racks, apparatus, and ceiling texture. A spatially fixed broad-spectrum noise generator provided masking noise and possibly an auditory spatial cue for orientation in the maze. To assess relational learning, the animals were required to find a submerged platform at six sequenced different locations. Each new location was presented for two consecutive days with four trials a day. For each animal, the platform was submerged 0.5 cm beneath the water surface in one of eight possible platform locations (see insert in Fig. 2). For the first location to be learned, all possible platform locations were used at least once in both groups. Thereafter, the platform was shifted every 2 d 180°, 90°, 180°, 225°, and 180° in the clockwise direction. Mice were placed into the maze from four equally spaced points (N, S, W, O) along the perimeter of the pool in a semirandom sequence. After reaching the platform, the animals were allowed to stay on it for 30 sec. If an animal failed to escape within 60 sec, it was placed manually onto the platform. During the 60-sec intertrial interval, the mice were placed into a resting cage beside the pool. The digitized image of the animal's path was analyzed with a semiautomated tracing device (EthoVision, Noldus). The search time (seconds) and the path length (centimeters) to reach the hidden platform as well as the mean swim speed (centimeters/second) were analyzed. Two days after the hidden platform task, the platform was indicated by a black-and-white striped narrow rod (diameter 0.5 cm, height 22 cm), and was shifted in a quasirandomized fashion from trial to trial to a new position. This was done to assess simple stimulus-response learning. Each animal received four trials on two consecutive days with the same procedure as on previous days. For each subject, the mean hidden platform task performance (search times, distance moved, and swim speed) across the six platform locations was computed by building the mean of corresponding trials, yielding eight data points per variable. Additionally, the mean distance to reach the platforms, A + B, C + B, and E + F (mean performance across the 16 trials), was calculated for each subject.
Neo- and Ventral Striatal Dopamine Concentrations and Metabolism
After behavioral testing, dopamine and its metabolites were analyzed in the neo- and ventral striata to determine whether brain histamine deficiency altered dopaminergic systems related to brain reward and reinforcement (Fibinger and Phillips 1988; Wise 1996). The animals were sacrificed by cervical dislocation followed by decapitation (Sethy and Francis 1988); their brains were quickly removed, and placed in an ice-cold brain matrix. Coronal sections were made following landmarks on the base of the brain, and the neo- and ventral striata were dissected out bilaterally onto an ice-cold platform. Thereafter, the brain tissue was weighed, homogenized in ice-cold 0.5 N perchloric acid containing ethylhomocholine as an internal standard, centrifuged, filtered, and kept at -70°C until analyzed. Samples were analyzed for dopamine (DA), dihydrophenylacetic acid (DOPAC), homovanillic acid (HVA), and 3-methoxytyramine (3-MT) levels using high-performance liquid chromatography with electro-chemical detection (for technical details, see De Souza-Silva et al. 1997). To determine dopamine turnover in the neo- and ventral striata, DOPAC/DA, HVA/DA, and 3-MT/DA ratios were computed (Irifune et al. 1995).
Statistics
For statistical analyses, repeated measures one-way ANOVAs and t-tests for independent and dependent samples were used. Unless otherwise indicated, the p-values given are two-tailed, and represent measures of effect.
Acknowledgments
We are very grateful to Dr. Hiroshi Ohtsu from the Department of Cellular Pharmacology, Tohoku University, Japan, and Dr. Jian Sheng Lin from the Department of Experimental Medicine, Claude Bernard University, Lyon, France, for the supply of the HDC-KO and wild type mice. This work was supported by the Deutsche Forschungsgemeinschaft (DFG) with grants HU306/24-1 to JPH and HA1525/6-3 to HLH as well as by funds of the European Union EU QLG3-CT-2002-00826 to HLH.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.67603.
Footnotes
The term “nonreinforced” refers to the fact that a specific reaction of the animal is not immediately followed by the application, termination, or nonoccurrence of an explicit aversive stimulation; nor is the animal explicitly rewarded, for example, by palatable food or liquid delivery, for exerting a certain reaction.
References
- Airaksinen, M.S., Alanen, S., Szabat, E., Visser, T.J., and Panula, P. 1992. Multiple neurotransmitters in the tuberomammillary nucleus: Comparison of rat, mouse, and guinea pig. J. Comp. Neurol. 323: 103-116. [DOI] [PubMed] [Google Scholar]
- Alvarez, E.O. and Banzan, A.M. 1996. Hippocampus and learning: Possible role of histamine receptors. Medicina 56: 155-160. [PubMed] [Google Scholar]
- Bannerman, D.M., Good, M.A., Butcher, S.P., Ramsay, M., and Morris, R.G. 1995. Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature 378: 182-186. [DOI] [PubMed] [Google Scholar]
- Bekkers, J.M. 1993. Enhancement by histamine of NMDA-mediated synaptic transmission in the hippocampus. Science 261: 104-106. [DOI] [PubMed] [Google Scholar]
- Blandina, P., Giorgetti, M., Bartolini, L., Cecchi, M., Timmerman, H., Leurs, R., Pepeu, G., and Giovannini, M.G. 1996. Inhibition of cortical acetylcholine release and cognitive performance by histamine H3 receptor activation in rats. Br. J. Pharmacol. 119: 1656-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, R.E. and Haas, H.L. 1999. On the mechanism of histaminergic inhibition of glutamate release in the rat dentate gyrus. J. Physiol. 515: 777-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, R.E., Fedorov, N.B., Haas, H.L., and Reymann, K.G. 1995. Histaminergic modulation of synaptic plasticity in area CA1 of rat hippocampal slices. Neuropharmacology 34: 181-190. [DOI] [PubMed] [Google Scholar]
- Brown, R.E., Stevens, D.R., and Haas, H.L. 2001. The physiology of brain histamine. Prog. Neurobiol. 63: 637-672. [DOI] [PubMed] [Google Scholar]
- Cavus, I. and Teyler, T.J. 1998. NMDA receptor-independent LTP in basal versus apical dendrites of CA1 pyramidal cells in rat hippocampal slice. Hippocampus 8: 373-379. [DOI] [PubMed] [Google Scholar]
- Chen, Z., Sugimoto, Y., and Kamei, C. 1999. Effects of intracerebroventricular injection of α-fluoromethylhistidine on radial maze performance in rats. Pharmacol. Biochem. Behav. 64: 513-518. [DOI] [PubMed] [Google Scholar]
- De Souza-Silva, M.A., Mattern, C., Häcker, R., Nogueira, P.J., Huston, J.P., and Schwarting, R.K. 1997. Intranasal administration of the dopaminergic agonists L-DOPA, amphetamine, and cocaine increases dopamine activity in the neostriatum: A microdialysis study in the rat. J. Neurochem. 68: 233-239. [DOI] [PubMed] [Google Scholar]
- Di Chiara, G., Acquas, E., and Carboni, E. 1991. Role of mesolimbic dopamine in the motivational effects of drugs: Brain dialysis and place preference studies. In The mesolimbic dopamine system: From motivation to action (eds. P. Willner and J. Scheel-Krüger), pp. 367-384. Wiley, Chichester, UK.
- Dringenberg, H.C., De Souza-Silva, M.A., Schwarting, R.K., and Huston J.P. 1998. Increased levels of extracellular dopamine in neostriatum and nucleus accumbens after histamine H1 receptor blockade. Naunyn Schmiedebergs Arch. Pharmacol. 358: 423-429. [DOI] [PubMed] [Google Scholar]
- Fibinger, H.C. and Phillips, A.G. 1988. Mesocorticolimbic dopamine systems and reward. In The mesocorticolimbic dopamine system (ed. P.W. Kalivas), Vol. 537, pp. 206-215. New York Academy of Sciences, NY. [DOI] [PubMed] [Google Scholar]
- Flood, J.F., Uezu, K., and Morley, J.E. 1998. Effect of histamine H2 and H3 receptor modulation in the septum on post-training memory processing. Psychopharmacology 140: 279-284. [DOI] [PubMed] [Google Scholar]
- Frisch, C., Hasenöhrl, R.U., Haas, H.L., Weiler, H.T., Steinbusch, H.W.M., and Huston, J.P. 1998. Facilitation of learning after lesions of the tuberomammillary nucleus region in adult and aged rats. Exp. Brain Res. 118: 447-456. [DOI] [PubMed] [Google Scholar]
- Frisch, C., Hasenöhrl, R.U., and Huston, J.P. 1999. Memory improvement by post-trial injection of lidocaine into the tuberomammillary nucleus, the source of neuronal histamine. Neurobiol. Learn. Mem. 72: 69-77. [DOI] [PubMed] [Google Scholar]
- Galosi, R., Lenard, L., Knoche, A., Haas, H., Huston, J.P., and Schwarting, R.K. 2001. Dopaminergic effects of histamine administration in the nucleus accumbens and the impact of H1-receptor blockade. Neuropharmacology 40: 624-633. [DOI] [PubMed] [Google Scholar]
- Gerlai, R. 1999. Ethological approaches in behavioral neurogenetic research. In Handbook of molecular-genetic techniques for brain and behavior research; techniques in the behavioral and neural science (eds. W.E. Crusio and R.T. Gerlai), Vol. 13, pp. 605-613. Elsevier Science B.V., New York. [Google Scholar]
- Grover, L.M. and Yan, C. 1999. Evidence for involvement of group II/III metabotropic glutamate receptors in NMDA receptor-independent long-term potentiation in area CA1 of rat hippocampus. J. Neurophysiol. 82: 2956-2969. [DOI] [PubMed] [Google Scholar]
- Haas, H. and Panula, P. 2003. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat. Rev. Neurosci. 4: 121-130. [DOI] [PubMed] [Google Scholar]
- Hasenöhrl, R.U., Weth, K., and Huston, J.P. 1999. Intraventricular infusion of the histamine H1 receptor antagonist chlorpheniramine improves maze performance and has anxiolytic-like effects in aged hybrid Fischer 344xBrown Norway rats. Exp. Brain Res. 128: 435-440. [DOI] [PubMed] [Google Scholar]
- Hill, S.J., Ganellin, C.R., Timmerman, H., Schwarz, J.C., Shankley, N.P., Young, J.M., Schunack, W., Levi, R., and Haas, H.L. 1997. International Union of Pharmacology. XIII. Classification of histamine receptors. Pharmacol. Rev. 49: 253-278. [PubMed] [Google Scholar]
- Hoh, T., Beiko, J., Boon, F., Weiss, S., and Cain, D.P. 1999. Complex behavioral strategy and reversal learning in the water maze without NMDA receptor-dependent long-term potentiation. J. Neurosci. 19: RC2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston, J.P. and Oitzl, M.S. 1989. The relationship between reinforcement and memory: Parallels in the rewarding and mnemonic effects of the neuropeptide substance P. Neurosci. Biobehav. Rev. 13: 171-180. [DOI] [PubMed] [Google Scholar]
- Huston, J.P., Wagner, U., and Hasenöhrl, R.U. 1997. The tuberomammillary nucleus in the control of learning, memory and reinforcement processes: Evidence for an inhibitory role. Behav. Brain Res. 83: 97-105. [DOI] [PubMed] [Google Scholar]
- Irifune, M., Nomoto, M., and Fukuda, T. 1995. Effects of GBR 12909 on locomotor activity and dopamine turnover in mice: Comparison with apomorphine. Eur. J. Pharmacol. 272: 79-85. [DOI] [PubMed] [Google Scholar]
- Kamei, C., Okumura, Y., and Tasaka, K. 1993. Influence of histamine depletion on learning and memory recollection in rats. Psychopharmacology 111: 376-382. [DOI] [PubMed] [Google Scholar]
- Köhler, C., Swanson, L.W., Haglund, L., and Wu, J.Y. 1985. The cytoarchitecture, histochemistry and projections of the tuberomammillary nucleus in the rat. Neuroscience 16: 85-110. [DOI] [PubMed] [Google Scholar]
- Kubota, Y., Ito, C., Sakurai, E., Sakurai, E., Watanabe, T., and Ohtsu, H. 2002. Increased methamphetamine-induced locomotor activity and behavioral sensitization in histamine-deficient mice. J. Neurochem. 83: 837-845. [DOI] [PubMed] [Google Scholar]
- Lin, J.S. 2000. Brain structures and mechanisms involved in the control of cortical activation and wakefulness, with emphasis on the posterior hypothalamus and histaminergic neurons. Sleep Med. Rev. 4: 471-503. [DOI] [PubMed] [Google Scholar]
- Maisonnette, S., Huston, J.P., Brandao, M., and Schwarting, R.K. 1998. Behavioral asymmetries and neurochemical changes after unilateral lesions of tuberomammillary nucleus or substantia nigra. Exp. Brain Res. 120: 273-282. [DOI] [PubMed] [Google Scholar]
- Martin, S.J. and Morris, R.G. 2002. New life in an old idea: The synaptic plasticity and memory hypothesis revisited. Hippocampus 12: 609-636. [DOI] [PubMed] [Google Scholar]
- Masukawa, Y., Suzuki, T., and Misawa, M. 1993. Differential modification of the rewarding effects of methamphetamine and cocaine by opioids and antihistamines. Psychopharmacology 111: 139-143. [DOI] [PubMed] [Google Scholar]
- Ohtsu, H., Tanaka, S., Terui, T., Hori, Y., Makabe-Kobayashi, Y., Pejler, G., Tchougounova, E., Hellman, L., Gertsenstein, M., Hirasawa, N., et al. 2001. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 502: 53-56. [DOI] [PubMed] [Google Scholar]
- Onodera, K., Yamatodani, A., and Watanabe, T. 1992. Effects of α-fluoromethylhistidine on locomotor activity, brain histamine and catecholamine contents in rats. Exp. Clin. Pharmacol. 14: 97-105. [PubMed] [Google Scholar]
- Onodera, K., Yamatodani, A., Watanabe, T., and Wada, H. 1994. Neuropharmacology of the histaminergic neuron system in the brain and its relationship with behavioral disorders. Prog. Neurobiol. 42: 685-702. [DOI] [PubMed] [Google Scholar]
- Otnaess, M.K., Brun, V.H., Moser, M.B., and Moser, E.I. 1999. Pretraining prevents spatial learning impairment after saturation of hippocampal long-term potentiation. J. Neurosci. 19: RC49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier, R., Ohtsu, H., Djebbara-Hannas, Z., Valatx, J.L., Watanabe, T., and Lin, J.S. 2002. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: Evidence for the role of brain histamine in behavioral and sleep-wake control. J. Neurosci. 22: 7695-7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne, G.W. and Neuman, R.S. 1997. Effects of hypomagnesia on histamine H1 receptor-mediated facilitation of NMDA responses. Br. J. Pharmacol. 121: 199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio, S., Begega, A., Santin, L.J., and Arias, J.L. 2002. Improvement of spatial memory by (R)-α-methylhistamine, a histamine H3-receptor agonist, on the Morris water-maze in rat. Behav. Brain Res. 129: 77-82. [DOI] [PubMed] [Google Scholar]
- Sakai, N., Sakurai, E., Sakurai, E., Yanai, K., Mirua, Y., and Watanabe, T. 1998. Depletion of brain histamine induced by α-fluoromethylhistidine enhances radial maze performance in rats with modulation of brain amino acid levels. Life Sci. 62: 989-994. [DOI] [PubMed] [Google Scholar]
- Schlicker, E., Fink, K., Detzner, M., and Gothert, M. 1993. Histamine inhibits dopamine release in the mouse striatum via presynaptic H3 receptors. J. Neural. Transm. Gen. Sect. 93: 1-10. [DOI] [PubMed] [Google Scholar]
- Schwarz, J.C., Arrang, J.M., Garbarg, M., Pollard, H., and Ruat, M. 1991. Histaminergic transmission in the mamalian brain. Physiol. Rev. 71: 1-51. [DOI] [PubMed] [Google Scholar]
- Selbach, O., Brown, R.E., and Haas, H.L. 1997. Long-term increase of hippocampal excitability by histamine and cyclic AMP. Neuropharmacology 36: 1539-1548. [DOI] [PubMed] [Google Scholar]
- Sethy, V.M. and Francis, J.W. 1988. Regulation of brain acetylcholine concentration by muscarinic receptors. J. Pharmacol. Exp. Ther. 246: 243-248. [PubMed] [Google Scholar]
- Shannon, H.E. and Su, T.P. 1982. Effects of the combination of tripelennamine and pentazocine at the behavioral and molecular levels. Pharmacol. Biochem. Behav. 17: 789-795. [DOI] [PubMed] [Google Scholar]
- Steele, R.J. and Morris, R.G.M. 1999. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus 9: 118-136. [DOI] [PubMed] [Google Scholar]
- Taga, C., Sugimoto, Y., Nishiga, M., Fujii, Y., and Kamei, C. 2001. Effects of vasopressin on histamine H1 receptor antagonist-induced spatial memory deficits in rats. Eur. J. Pharmacol. 423: 167-170. [DOI] [PubMed] [Google Scholar]
- Tang, Y.P., Shimiizu, E., Dube, G.R., Rampon, C., Kerchner, G.A., Zhuo, M., Liu, G., and Tsien, J.Z. 1999. Genetic enhancement of learning and memory in mice. Nature 401: 63-69. [DOI] [PubMed] [Google Scholar]
- Thomas, K.L., Davis, S., Hunt, S.P., and Laroche, S. 1996. Alterations in the expression of specific glutamate receptor subunits following hippocampal LTP in vivo. Learn. Mem. 3: 197-208. [DOI] [PubMed] [Google Scholar]
- Tsien, J.Z. 1998. Behavioral genetics: Subregion- and cell type-restricted gene knockout in mouse brain. Pathol. Biol. 46: 699-700. [PubMed] [Google Scholar]
- Unterwald, E.M., Kucharski, L.T., Williams, J.E., and Kornetsky, C. 1984. Tripelennamine: Enhancement of brain-stimulation reward. Life Sci. 34: 149-153. [DOI] [PubMed] [Google Scholar]
- Vorobjev, V.S., Sharonova, I.N., Walsh, I.B., and Haas, H.L. 1993. Histamine potentiates N-methyl-D-aspartate responses in acutely isolated hippocampal neurons. Neuron 11: 837-844. [DOI] [PubMed] [Google Scholar]
- Wada, H., Inagaki, N., Yamatodani, A., and Watanabe, T. 1991. Is the histaminergic neuron system a regulatory center for whole-brain activity? Tends Neurosci. 14: 415-418. [DOI] [PubMed] [Google Scholar]
- Wagner, U., Weiler, H.T., and Huston, J.P. 1993. Amplification of rewarding hypothalamic stimulation following a unilateral lesion in the region of the tuberomammillary nucleus. Neuroscience 52: 927-932. [DOI] [PubMed] [Google Scholar]
- Watanabe, T., Yamatodani, A., Maeyama, K., and Wada, H. 1990. Pharmacology of α-fluoromethylhistidine, a specific inhibitor of histidine decarboxylase. Trends Pharmac. Sci. 11: 363-367. [DOI] [PubMed] [Google Scholar]
- Williams, J.M., Mason-Parker, S.E., Abraham, W.C., and Tate, W.P. 1998. Biphasic changes in the levels of N-methyl-D-aspartate receptor-2 subunits correlate with the induction and persistence of long-term potentiation. Mol. Brain Res. 60: 21-27. [DOI] [PubMed] [Google Scholar]
- Williams, K. 1994. Subunit-specific potentiation of recombinant N-methyl-D-aspartate receptors by histamine. Mol. Pharmacol. 46: 531-541. [PubMed] [Google Scholar]
- Wise, R.A. 1996. Addictive drugs and brain stimulation reward. Annu. Rev. Neurosci. 19: 319-340. [DOI] [PubMed] [Google Scholar]
- Wood, P.L. and Altar, C.A. 1988. Dopamine release in vivo from nigrostriatal, mesolimbic, and mesocortical neurons: Utility of 3-methoxytyramine measurements. Pharmacol. Rev. 40: 163-187. [PubMed] [Google Scholar]
- Yamatodani, A., Inagaki, N., Panula, P., Itowi, N., Watanabe, T., and Wada, H. 1991. Structure and functions of the histaminergic neurone system. In Histamine and histamine antagonists (ed. B. Uvnäs), pp. 243-283. Springer-Verlag, Berlin.
- Yanai, K., Son, L.Z., Endou, M., Sakurai, E., and Watanabe, T. 1998a. Targeting disruption of histamine H1 receptors in mice: Behavioral and neurochemical characterization. Life Sci. 62: 1607-1610. [DOI] [PubMed] [Google Scholar]
- Yanai, K., Son, L.Z., Endou, M., Sakurai, E., Nakagawasai, O., Tadano, T., Kisara, K., Inoue, I., Watanabe, T., and Watanabe, T. 1998b. Behavioural characterization and amounts of brain monoamines and their metabolites in mice lacking histamine H1 receptors. Neuroscience 87: 479-487. [DOI] [PubMed] [Google Scholar]
- Zimmermann, P., Privou, C., and Huston, J.P. 1999. Differential sensitivity of the caudal and rostral nucleus accumbens to the rewarding effects of a H1-histaminergic receptor blocker as measured with place-preference and self-stimulation behavior. Neuroscience 94: 93-103. [DOI] [PubMed] [Google Scholar]