Abstract
In a recent study, rats with hippocampal lesions performed as well as did unoperated rats on an olfactory memory span task, performing ∼80% correct even when the span length reached 24 odors. This finding seems potentially at odds with demonstrations that memory-impaired patients typically fail tasks in which large amounts of information must be retained. Accordingly, we have assessed recognition memory span performance for line drawings of objects, designs, and odors in amnesic patients with damage thought to be limited to the hippocampal region. The patients were impaired on all three tasks. We consider possible explanations for the difference between the findings for humans and rats, including the fact that olfactory function is particularly well-developed in rodents.
Bilateral damage to the medial temporal lobe causes severe and selective memory impairment (Scoville and Milner 1957). The hallmark of the condition is anterograde amnesia, a failure to form long-term memories for new facts and events (Squire 1992). In contrast, immediate memory is intact. Thus, patients with severe memory impairment can carry on a conversation and can hold a limited amount of information in the mind, provided they are not distracted. In formal tests, amnesic patients were found to have an intact digit span, even when repeated testing allowed the span to be estimated at nearly one-decimal-point precision (Baddeley and Warrington 1970; Cave and Squire 1992). Further, patients with damage to the hippocampal formation exhibited intact immediate memory for nonverbal material. They were able to recall the position of a dot on a line, retain the size of an angle, and judge whether a just-presented array of three-by-three black-and-white squares now appeared as a correct mirror reversal (Cave and Squire 1992).
So long as only a limited amount of information is presented and it is easy to rehearse (e.g., a three-digit number), a severely impaired patient may succeed at recall even after a delay of several minutes (Milner et al. 1998). Conversely, if a large amount of information is presented, patients may perform poorly after even a minimal delay, especially if the material is difficult to rehearse. For example, in a test of paired associate learning, the presentation of 10 word pairs was followed immediately by presentation of the first word in each pair and instructions to recall the second word. Six patients with damage limited to the hippocampal region averaged only 1.7 items correct (controls, 6.0 items; Manns et al. 2003). Similarly, when four complex designs were presented in sequence and a single recognition probe was subsequently presented, memory-impaired patients exhibited intact recognition memory at delays of 0-2 sec but impaired performance at delays as brief as 6-10 sec (Buffalo et al. 1998).
The idea that retention will fail even at short delays if too much information is presented (i.e., an amount of information that exceeds the “immediate-memory span”) can also account for the poor performance of memory-impaired patients on extended span tasks. For example, in one study, controls learned to repeat back digit strings of progressively increasing length, needing fewer than five repetitions at any string length to reach a string length of 10 digits. Patients with large medial temporal lobe lesions were able to achieve a string length of only 8.6 digits on average, despite receiving up to 25 repetitions at each string length (Drachman and Arbit 1966).
Similarly, amnesic patients with Korsakoff's syndrome and demented patients were impaired on the delayed recognition span test, in which a single item is presented, then two, then three, and so on, with the instruction on each trial to choose the new item (Moss et al. 1986). The number of items continues to increase until an error is made. Unlike the digit span task, in this span task there is no requirement to remember the order in which items are presented. The participant must only identify the newly added item at each span length. Despite the fact that only 10 sec separated each trial, patients had abnormally short spans for colors, words, patterns, faces, and spatial locations. When the same test was adapted for the monkey, monkeys with bilateral lesions of the hippocampal formation were impaired on span tests for spatial location, colors, and objects (Beason-Held et al. 1999).
These results for memory span tests stand in striking contrast to the findings from a recent study of odor span recognition memory in rats (Dudchenko et al. 2000). Rats with hippocampal lesions were as good as control animals at identifying the novel odor each time a new odor was added to the display. In the most dramatic test, new odors were added to the display one at a time until 25 odors were present, and rats were rewarded on each trial for choosing the new odor. Across all the trials, both operated and control rats averaged ∼80% correct choices.
Considering the findings that have been reported previously for patients given visual, auditory, and spatial memory span tests, there appear to be three ways to understand the finding in rats. One possibility is that patients with large medial temporal lobe lesions, or other lesions, might be impaired at recognition memory span tasks, but as in rats, performance will be spared when damage is limited to the hippocampal region. A second possibility is that the olfactory memory span task is different in some important way from memory span tasks in other modalities. Accordingly, olfactory memory span performance may be intact after hippocampal lesions even though span performance is impaired in other modalities. A third possibility is that species differences are important and that the role of the hippocampus in olfactory memory span tasks is different in rats and humans. To choose among these alternatives, we have carried out two studies of recognition memory span performance in memory-impaired patients with bilateral lesions thought to be limited to the hippocampal region. In the first experiment, we tested visual memory span performance for drawings of common objects and for kaleidoscope-like designs. In the second experiment, we tested memory span performance for odors.
RESULTS
Experiment 1: Visual Span
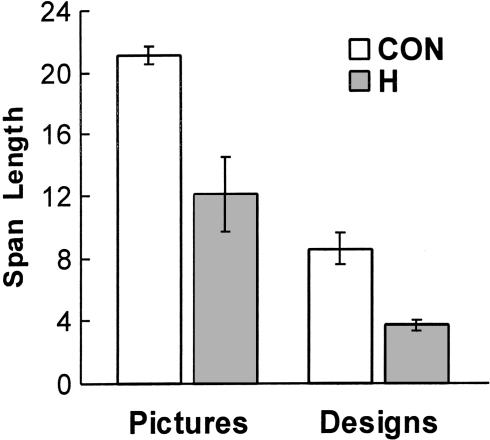
Figure 1 shows the mean span length for line drawings of common objects and for designs. The controls performed better than did the amnesic patients on both tests (mean span length for the line drawings: 21.1 ± 0.5 versus 12.1 ± 2.4, respectively, t[18] = 5.57, P < 0.01; mean span length for the designs: 8.6 ± 1.0 versus 3.7 ± 0.3, t[18] = 2.76, P = 0.01).
Figure 1.
Mean visual span length for control volunteers (CON; n = 15) and for amnesic patients with damage thought to be limited to the hippocampal region (H; n = 5) on two visual span tasks. In one task (left two bars), the stimuli were line drawings of common objects. In the second task (right two bars), the stimuli were kaleidoscope-like colored designs. Brackets indicate standard error of the mean.
In the interview, most controls indicated that they made their choices by looking for the new unfamiliar item (12 of 15 in the line drawing test and 10 of 15 in the designs test). Thus, participants often reported that on each trial, they simply looked for the image that “popped out” as being new.
The amnesic patients, like the controls, reported that they looked for the new unfamiliar item (four of five for the line drawings test and three of five for the designs test). Performance did not differ as a function of how participants reported approaching the task (P > 0.1).
Experiment 2: Olfactory Span
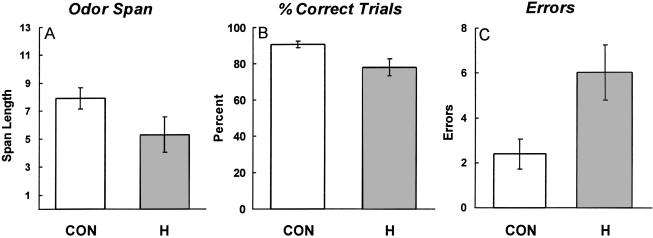
Figure 2 shows the results for the olfactory span task. The controls performed better than did the amnesic patients overall. Thus, the controls achieved more correct trials than did the patients in the course of reaching a span length of 13 odors (90.6 ± 1.8% correct versus 77.7 ± 4.8% correct, t[19] = 2.52, P < 0.01). Controls also committed fewer total errors in the course of reaching a span length of 13 odors (2.4 ± 0.7 versus 6.0 ± 1.2, t[19] = 2.56, P < 0.05). Because participants continued to sample the vials on each trial until the new odor was identified, participants could make more than one error on a given trial. Finally, the controls achieved a numerically longer span length than did the patients (7.9 ± 0.8 versus 5.3 ± 1.3), but this difference did not reach significance (t[19] = 1.78, P = 0.11). The measure of span length appeared to be less stable than were the other two measures, because a participant could make an error early in the series but then do well in the remainder of the test. Thus, eight of 16 controls exhibited large differences (more than five) in span length scores across the two test sessions, whereas only two controls obtained large differences in their percent correct score (>15%) or in number of errors (more than four).
Figure 2.
Olfactory span performance for control volunteers (CON; n = 16) and for amnesic patients with damage thought to be limited to the hippocampal region (H; n = 5). (A) Mean olfactory span length. (B) Percentage of trials performed correctly in the course of reaching a span length of 13 odors. (C) Mean number of errors accumulatedin the course of reaching a span length of 13 odors. Brackets indicate standard error of the mean.
The controls had a slightly better butanol threshold score than did the patients (7.2 and 6.7, respectively), but this difference did not approach significance (t[19] = 0.9, P > 0.1). Accordingly, it is quite unlikely that the difference in memory performance resulted from a difference in olfactory acuity.
DISCUSSION
Five amnesic patients with bilateral damage thought to be limited to the hippocampal region were given three recognition memory span tasks. Each task began by presenting one item, then two items, then three items, and so on, and the instruction at each stage was to select the novel item, that is, the one that had just been added to the display. The first task involved line drawings of common objects, the second task involved kaleidoscope-like designs, and the third task involved odors. The patients were impaired on all three tasks.
Impaired recognition span performance (for visual and spatial material) was reported previously for patients with Alzheimer's disease, Huntington's disease, or Korsakoff's syndrome (Moss et al. 1986). The present findings extend these findings in two ways. First, memory span performance was impaired in patients with circumscribed memory impairment and damage thought to be limited to the hippocampal region. Second, olfactory recognition memory span was impaired just as visual recognition memory span was impaired. The finding that recognition memory span performance was broadly impaired after hippocampal lesions supports the idea that the recognition span task is fundamentally similar to other tasks typically failed by memory-impaired patients (e.g., recognition memory tasks that either involve a substantial retention interval or present so much information that the material to be learned exceeds immediate memory capacity).
Accordingly, the findings for visual and olfactory memory span are best understood as additional evidence for the importance of the human hippocampus in recognition memory (Reed and Squire 1997; Manns et al. 2003). To the extent that performance on the two visual tasks depended on simple judgments of familiarity (and it seems reasonable to suppose that these tasks did depend on familiarity in the case of those participants who reported simply looking for which item seemed new or which item popped out as being new), the results count against the view that hippocampal damage selectively impairs the capacity for recollection but spares the capacity for making familiarity judgments (Brown and Aggleton 2001; Yonelinas et al. 2002).
How then can one understand the finding that rats with hippocampal lesions were intact on the olfactory memory span task, performing well even at a span length of 24 odors (Dudchenko et al. 2000)? The present results help to rule out two possible explanations of the findings in rats. First, it appears not to be the case that recognition memory span performance is generally spared when damage is limited to the hippocampal region. The patients in our study have radiological evidence of damage limited to the hippocampal region, but they were impaired on all three memory span tests. Second, the findings rule out the idea that the olfactory memory span task is different from other memory span tasks in some important way, with the result that olfactory memory span performance will always be spared after hippocampal lesions. The patients were impaired on the olfactory memory span task, just as they were impaired on the two visual memory span tasks.
It is difficult to exclude the possibility that some participants attached labels to the odors. If this were done extensively, the deficit exhibited by patients might be due in part to poor verbal memory. Yet, it is interesting that performance by controls on the olfactory span task (average span length = 7.9) was similar to performance on the span task for designs (average span length = 8.6), which were difficult to label verbally. Further, the deficits obtained by patients in these two tests appeared comparable, as assessed by z-scores (designs test: z - 1.26; olfactory test: span length, z - 0.85; percentage correct, z - 1.81; number of errors, z - 1.36).
If the difference between the findings for humans and rats cannot be explained by the extent or locus of the lesion or by the sensory modality being tested, then the explanation may lie in species differences, for example, differences in how rats and humans with hippocampal lesions accomplish the olfactory memory span task. One possibility is that the rats were able to perform the olfactory span task within working memory. Although the amount of information that would need to be held in working memory is considerable (and beyond the capacity of the amnesic patients), one could suppose that olfactory function is so well-developed in the rat that rats can use olfactory information in ways that humans cannot.
A second possibility is that the span task is a relatively easy task of hippocampus-dependent recognition memory. It is noteworthy that the same rats that succeeded at the olfactory span task were marginally impaired (P = 0.061) at an olfactory delayed nonmatching to sample task at delays of 30 and 60 min. Twelve odors were presented one at a time, and recognition was assessed by presenting each of the sample odors together with a novel odor (Dudchenko et al. 2000). Unlike the delayed nonmatching to sample task, which involved presenting each odor just once, in the span task the odors that were presented early in the series appear again and again, and the animal effectively must learn only one new odor on each trial and retain it for a relatively short time. Perhaps the repetition given each odor is sufficient to support performance. This interpretation of how rats, but not humans, succeeded at the olfactory span task also depends on the idea that olfactory function is particularly well-developed in the rat.
A third possibility is that rats with hippocampal lesions can retain information for a longer period of time after learning than can humans with hippocampal lesions, whether they are tested in the olfactory modality or in the visual modality. Examples have been reported of good memory performance by rats with hippocampal lesions at 30 sec (Clark et al. 2001), 5 min (Baker and Kim 2002), and even 15 min (Dudchenko et al. 2000) after learning, even though in these cases memory was impaired at longer intervals. This possibility can be tested by asking whether rats with hippocampal lesions can perform a visual memory span task as well as they can perform the olfactory memory span task, or whether their good memory span performance is unique to olfaction.
Whatever the explanation is for the good olfactory memory span performance of rats with hippocampal lesions, the findings reported here for humans indicate that the recognition memory span task is best understood as a hippocampus-dependent task and that it has this characteristic because the material to be learned and operated on exceeds what can be held in immediate memory.
MATERIALS AND METHODS
Experiment 1: Visual Span
Participants
Five amnesic patients (four men and one woman) with damage limited primarily to the hippocampal region (CA fields, dentate gyrus, and subicular complex) participated (Table 1). All the patients had moderately severe memory impairment. Their average scores for copy and delayed (12 min) reproduction of the Rey-Osterrieth figure (maximum score = 36; Osterrieth 1944) were 28.8 and 2.6, respectively (controls = 30.3 and 20.6; Squire et al. 1989). Immediate (12 min) and delayed recall of a short prose passage (Gilbert et al. 1968) averaged 4.6 and 0.4 segments, respectively (15 controls = 8.3 and 7.1).
Table 1.
Characteristics of Amnesic Patients
| Age (years)
|
Education (years)
|
WAIS-III IQ
|
WMS-R
|
|||||
|---|---|---|---|---|---|---|---|---|
| Patient | Attention | Verbal | Visual | General | Delay | |||
| J.R.W. | 38 | 12 | 90 | 87 | 65 | 95 | 70 | <50 |
| G.W. | 42 | 12 | 108 | 105 | 67 | 86 | 70 | <50 |
| R.S. | 45 | 12 | 99 | 99 | 85 | 81 | 82 | <50 |
| L.J. | 64 | 12 | 101 | 105 | 83 | 60 | 69 | <50 |
| A.B. | 64 | 20 | 107 | 87 | 62 | 72 | 54 | <50 |
The Wechsler Adult Intelligence Scale III (WAIS-III) and the Wechsler Memory Scale-Revised (WMS-R) yield mean scores of 100 in the normal population with 15 SD. The WMS-R does not provide numerical scores for individuals who score <50. IQ scores for J.R.W. and R.S. are from the Wechsler Adult Intelligence Scale-Revised.
Patients A.B. and J.R.W. became amnesic after an anoxic episode associated with cardiac arrest (in 1976 for A.B. and 1990 for J.R.W.). Patients G.W. and R.S. became amnesic after a drug overdose and associated respiratory failure (in 2001 for G.W. and 1998 for R.S.). Patient L.J. became amnesic in 1988 during a 6-month period with no known precipitating event. Her memory impairment has remained stable since that time.
For four of the five patients, new estimates of the extent of medial temporal lobe damage were obtained, based on a larger number of control brains than had been available previously. Magnetic resonance imaging (MRI) was done in a 1.5T clinical scanner (for illustrations of MRI scans, see Manns et al. 2003). The volume of the full anterior-posterior length of the hippocampus and the volume of the parahippocampal gyrus were measured by using criteria based on histological analysis of healthy brains (Amaral and Insausti 1990; Insausti et al. 1998a,b). Volumes were normalized by intracranial volume (ICV) to correct for between-subject variability in brain size. Relative to age- and gender-matched healthy controls (19 males and 11 females), patients L.J., R.S., G.W., and J.R.W. have an average bilateral reduction in hippocampal size of 46%, 33%, 48%, and 44%, respectively. The parahippocampal gyrus was relatively unaffected (mean reduction = 3%, range from 12% smaller to 8% larger). None of the patients had focal lesions in the entorhinal cortex or significant reductions in its volume (mean reduction = 12%). The fifth patient (A.B.) was unable to participate in MRI studies but was thought to have hippocampal damage on the basis of etiology (anoxia) and a neurologic examination indicating well-circumscribed amnesia. In addition, high-resolution computed tomography (CT) images obtained in 2001 were consistent with restricted damage to the hippocampal region (Schmolck et al. 2002).
Fifteen healthy volunteers (nine men and six women) were also tested. They averaged 51.4 ± 3.3 years of age (patients = 50.6 ± 5.6 years) and 14.4 ± 0.5 years of education (patients = 13.6 ± 1.6 years).
Materials
Two different span tests were constructed, one involving line drawings of common objects (Snodgrass and Vanderwart 1980) and one involving computer-generated, kaleidoscope-like designs (Buffalo et al. 1998). Both tests were presented on a computer. Half the participants were given the object span test first, and half were given the design span test first.
Procedure
Both span tests were presented in the same way. On the first trial, a single image was presented, and participants selected it with the computer mouse. A white box then appeared around the image, and the screen went blank for a 3-sec intertrial interval. For the second trial, two images were presented on the screen in new locations: the image from trial 1 and a new image. The participant's task was to select the new image. When the choice was correct, a white box appeared around the correct image. For the third trial, a new image was presented together with the two images from the first two trials, again in new locations. Trials continued in this way, always with the instruction to select the new image, until a maximum of 24 images had been presented. When the choice was incorrect, an error message appeared. The task was then restarted with a different set of images until the task had been given a total of 10 times.
On the first trial, the image was presented for a minimum of 5 sec. That is, selecting the image before 5 sec had elapsed produced a white box around the image, but the image remained on the screen. When the image was selected after 5 sec, the white box appeared for 0.5 sec, and the screen then went blank for the 3-sec intertrial interval. On each subsequent trial, the minimum duration of the display increased by 0.5 sec, such that by the 24th trial, the minimum duration of the display was 16.5 sec. This procedure encouraged participants to inspect all the images on the screen. The average control participant took ∼4.5 min to complete each of the 10 runs of the object span task and ∼1.5 min to complete each run of the design span task.
Scoring
For each participant, the memory span for objects and for designs was the average across 10 tests of the largest number of images that could be correctly distinguished from the new image. For example, if an error was made when seven images were present, the span for that series of trials was five (five familiar images were correctly distinguished from the new image on the previous trial). For a similar scoring procedure, see Dudchenko et al. (2000).
Interview
At the end of each testing session, participants were asked to indicate which of two statements more accurately reflected their strategy: “I chose the new design [object] by remembering the designs [objects] that I had seen previously and eliminating them as choices.” or “I chose the new design [object] by finding the design [object] that I felt I had never seen before.”
Experiment 2: Olfactory Span
Participants
The same five amnesic patients were tested as in experiment 1. Sixteen healthy volunteers (12 men and four women) were also tested, five of whom also participated in experiment 1. An additional male participant was excluded from the study after failing the olfactory threshold test (see below). The controls averaged 52.5 ± 2.8 years of age (patients = 50.6 ± 5.6 years) and 14.8 ± 0.8 years of education (patients = 13.6 ± 1.6 years). Their immediate and delayed recall scores for the short prose passage were 7.7 and 6.8 (patients = 4.6 and 0.4). By self report, all participants had normal olfactory function.
Materials
The stimuli consisted of 28 common foods, condiments, and household items (e.g., garlic powder, almond extract, shoe polish), all of which had a distinctive odor. The 28 odors were used to construct two sets of 14 odors. Stimuli were presented in opaque glass vials (9.8 cm tall) and were not visible to participants at any time. Half of the participants received one set of odors first, and half received the other set first.
Procedure
For testing, vials were presented in a cardboard bottle holder, with 10 cm between vial slots. On the first trial, a single open vial was presented for smelling. It was then capped and removed from the holder, and the second trial was immediately prepared. For the second trial, the original vial and a new one were placed in the holder, randomly with respect to left-right position, and participants were asked to identify the new odor. Both vials were then capped and removed and were subsequently returned to the holder along with a third new vial (again randomly with respect to relative position). Trials continued in this fashion until all 14 vials had been presented. Thus, on each trial, one new vial was added to the previous array, and participants were asked to identify the new odor. Approximately 45 min were needed to administer the test. The initial trials were accomplished quickly (e.g., the interval between the presentation of a single vial and two vials was ∼15 sec). The later trials required more time (e.g., the interval between the presentation of 13 vials and 14 vials was ∼2 min). The time needed to administer the task and the interval between trials appear to be similar to the time involved when the olfactory memory span task was given to rats (Dudchenko et al. 2000).
Participants were first asked to sample the vials in order from left to right and then, if they had not yet made a choice, in whatever order they preferred. To prevent mixing of odors, only one vial was uncapped at a time, and vials were recapped after they had been sampled. When a correct choice was made, the next trial was presented as soon as the vials could be rearranged. When the choice was incorrect, the incorrect vial was removed from the display, and participants continued to choose among the remaining vials until the new odor was correctly identified. The test was given twice on two separate occasions (median interval = 3 d), using the two different sets of odors.
Scoring
Three scores were derived from the two administrations of the odor span task. The span length was the number of consecutive trials on which participants correctly identified the new odor on their first try (maximum = 13, after the method of Dudchenko et al. 2000; e.g., a correct choice on the second trial followed by an error on the third trial was counted as a span length of one). The percentage of correct trials was the total number of trials, out of 13, in which the first choice at each span length was correct. The error score was the total number of errors committed by a participant during all 13 test trials (participants could make more than one error on each trial). Each participant's score for each of these three measures was averaged across the two different administrations of the test.
Olfactory Threshold
At the completion of testing, olfactory threshold was assessed using a two-alternative forced-choice method (Murphy et al. 1990). Ten 60-mL solutions of n-butyl alcohol in deionized water (beginning with a 4.0% solution) were prepared in 250-mL squeezable polyethylene bottles. Each successive dilution was one-third the concentration of the preceding dilution. In each test trial, a bottle containing odorant and a second bottle of deionized water (no smell) were presented one at a time, beginning with the most dilute solution. Participants sampled from each pair of bottles with a single nostril and indicated which bottled contained the odorant (45-sec interval between samplings). Which nostril was tested first and the left-right presentation of the bottles were random across participants. After a correct choice, the same pair of bottles was presented again up to a maximum of five correct trials. After an error, the next highest concentration of butanol was presented. Threshold was defined as the most dilute concentration (nine, most dilute; zero, least dilute) at which five consecutive correct choices were made. One control who did not succeed at detecting the odor after the fifth dilution of n-butyl alcohol was considered hyposmic (Murphy et al. 1990) and was excluded from the study.
Acknowledgments
Supported by the Medical Research Service of the Department of Veterans Affairs, NIMH grant 24600, NIA grant AG05131 to the Alzheimer's Disease Research Center at UCSD, and the Metropolitan Life Foundation. We thank Jennifer Frascino and Leah Swalley for assistance; Terry Jernigan, Ph.D., and her colleagues for providing control data for the MRI analyses; and Claire Murphy, Ph.D., for advice on olfactory testing.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.66703.
References
- Amaral, D.G. and Insausti, R. 1990. The hippocampal formation. In The human nervous system (ed. G. Paxinos), pp. 711-755. Academic Press, San Diego, CA.
- Baddeley, A.P. and Warrington, E.K. 1970. Amnesia and the distinction between long and short-term memory. J. Verbal Learn. Verbal Behav. 9: 176-189. [Google Scholar]
- Baker, K.B. and Kim, J.J. 2002. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learn. Mem. 9: 58-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beason-Held, L.L., Rosene, D.L., Killiany, R.J., and Moss, M.B. 1999. Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus 9: 562-574. [DOI] [PubMed] [Google Scholar]
- Brown, M.W. and Aggleton, J.P. 2001. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2: 51-61. [DOI] [PubMed] [Google Scholar]
- Buffalo, E.A., Reber, P.J., and Squire, L.R. 1998. The human perirhinal cortex and recognition memory. Hippocampus 8: 330-339. [DOI] [PubMed] [Google Scholar]
- Cave, C. and Squire, L.R. 1992. Intact verbal and non-verbal short-term memory following damage to the human hippocampus. Hippocampus 2: 151-163. [DOI] [PubMed] [Google Scholar]
- Clark, R.E., West, A.N., Zola, S.M., and Squire, L.R. 2001. Rats with lesions of the hippocampus are impaired on the delayed nonmatching-to-sample task. Hippocampus 11: 176-186. [DOI] [PubMed] [Google Scholar]
- Drachman, D.A. and Arbit, J. 1966. Memory and the hippocampal complex, II: Is memory a multiple process? Arch. Neurol. 15: 52-61. [DOI] [PubMed] [Google Scholar]
- Dudchenko, P.A., Wood, E.R., and Eichenbaum, H. 2000. Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span, recognition, and alternation. J. Neurosci. 20: 2964-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, J.G., Levee, R.F., and Catalano, F.L. 1968. A preliminary report on a new memory scale. Percept. Motor Skills 27: 277-278. [DOI] [PubMed] [Google Scholar]
- Insausti, R., Insausti, A.M., Sobreviela, M.T., Salinas, A., and Martinez-Penuela, J.M. 1998a. Human medial temporal lobe in aging: Anatomical basis of memory preservation. Microscopy Res. Technique 43: 8-15. [DOI] [PubMed] [Google Scholar]
- Insausti, R., Juottonen, K., Soininen, H., Insausti, A.M., Partanen, K., Vainio, P., Laakso, M.P., and Pitkanen, A. 1998b. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. Am. J. Neuroradiol. 19: 659-671. [PMC free article] [PubMed] [Google Scholar]
- Manns, J.R., Hopkins, R.O., Reed, J.M., Kitchener, E.G., and Squire, L.R. 2003. Recognition memory and the human hippocampus. Neuron 37: 171-180. [DOI] [PubMed] [Google Scholar]
- Milner, B., Squire, L.R., and Kandel, E.R. 1998. Cognitive neuroscience and the study of memory. Neuron 20: 445-468. [DOI] [PubMed] [Google Scholar]
- Moss, M.B., Albert, M.S., Butters, N.L., and Payne, M. 1986. Differential patterns of memory loss among patient with Alzheimer's disease, Huntington's disease, and alcoholic Korsakoff's syndrome. Arch. Neurol. 43: 239-246. [DOI] [PubMed] [Google Scholar]
- Murphy, C., Gilmore, M.M., Seery, C.S., Salmon, D.P., and Lasker, B.R. 1990. Olfactory thresholds are associated with degree of dementia in Alzheimer's disease. Neurobiol. Aging 11: 465-469. [DOI] [PubMed] [Google Scholar]
- Osterrieth, P.A. 1944. Le test de copie d'une figure complexe. Arch. Psychol. 30: 206-356. [Google Scholar]
- Reed, J.M. and Squire, L.R. 1997. Impaired recognition memory in patients with lesions limited to the hippocampal formation. Behav. Neurosci. 111: 667-675. [DOI] [PubMed] [Google Scholar]
- Schmolck, H., Kensinger, E.A., Corkin, S., and Squire, L.R. 2002. Semantic knowledge in patient H. M. and other patients with bilateral medial and lateral temporal lobe lesions. Hippocampus 12: 520-533. [DOI] [PubMed] [Google Scholar]
- Scoville, W.B. and Milner, B. 1957. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20: 11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass, J.G. and Vanderwart, M. 1980. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. J. Learn. Mem. Human Mem. Cognit. 6: 174-215. [DOI] [PubMed] [Google Scholar]
- Squire, L.R. 1992. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 99: 195-231. [DOI] [PubMed] [Google Scholar]
- Squire, L.R., Amaral, D.G., Zola-Morgan, S., Kritchevsky, M., and Press, G. 1989. Description of brain injury in amnesic patient N.A. based on magnetic resonance imaging. Exper. Neurol. 105: 25-35. [DOI] [PubMed] [Google Scholar]
- Yonelinas, A.P., Kroll, N.E., Quamme, J.R., Lazzara, M.M., Sauve, M.J., Widaman, K.F., and Knight, R.T. 2002. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat. Neurosci. 5: 1236-1241. [DOI] [PubMed] [Google Scholar]