Abstract
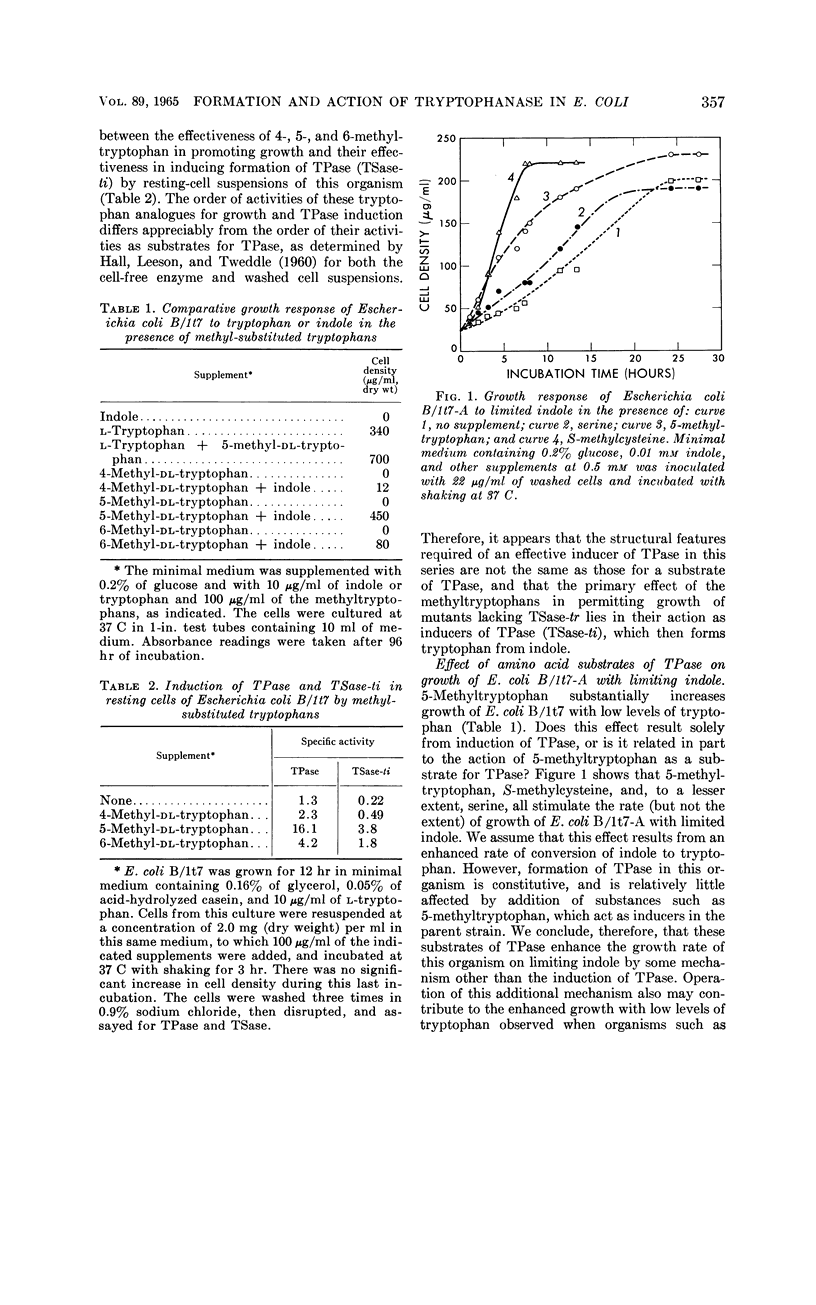
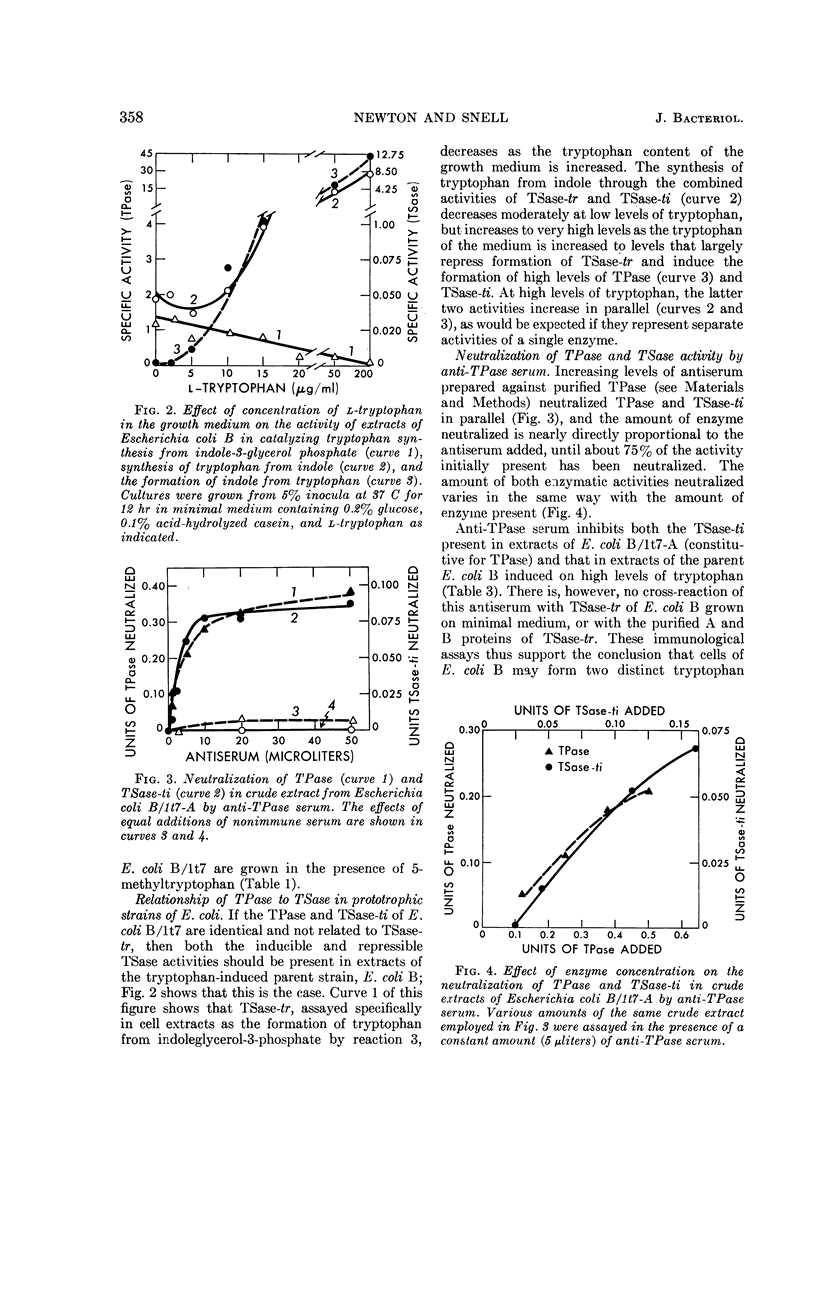
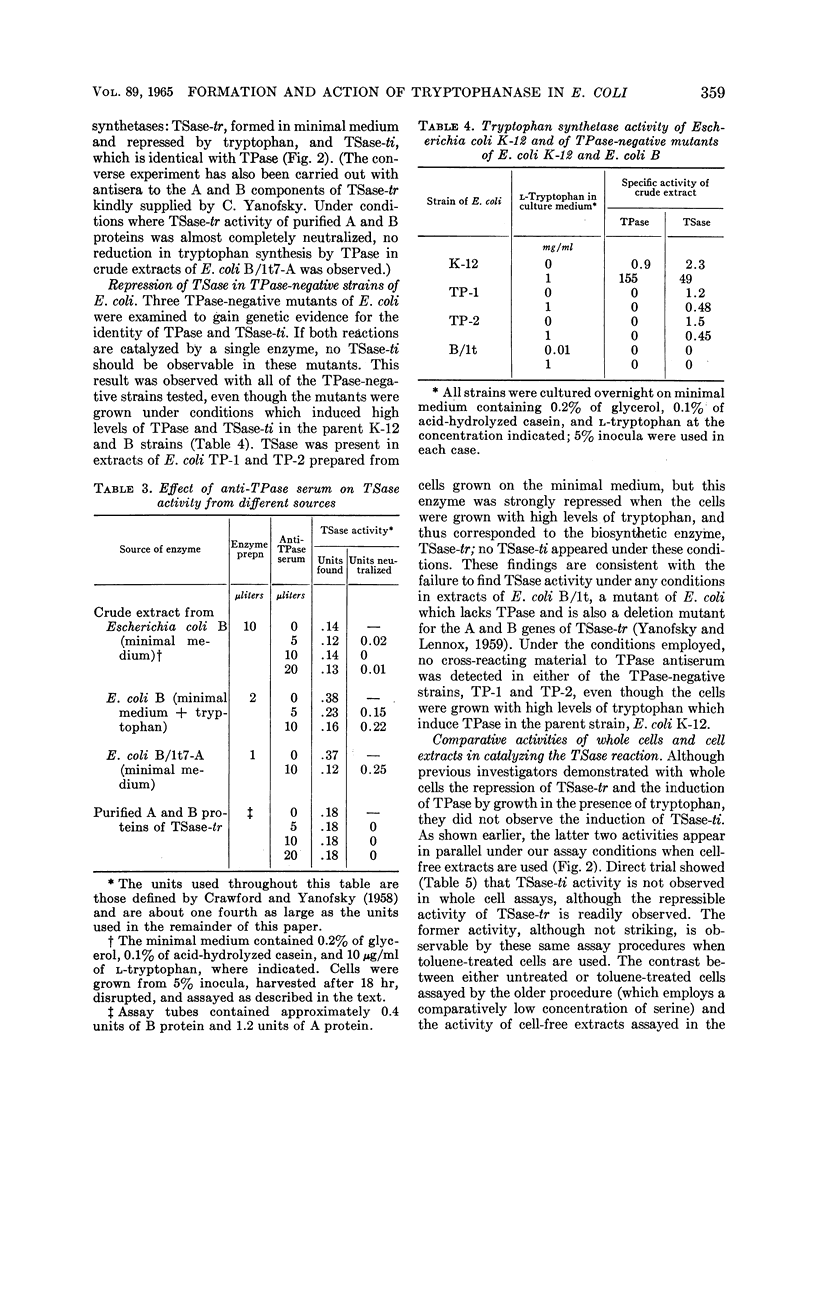
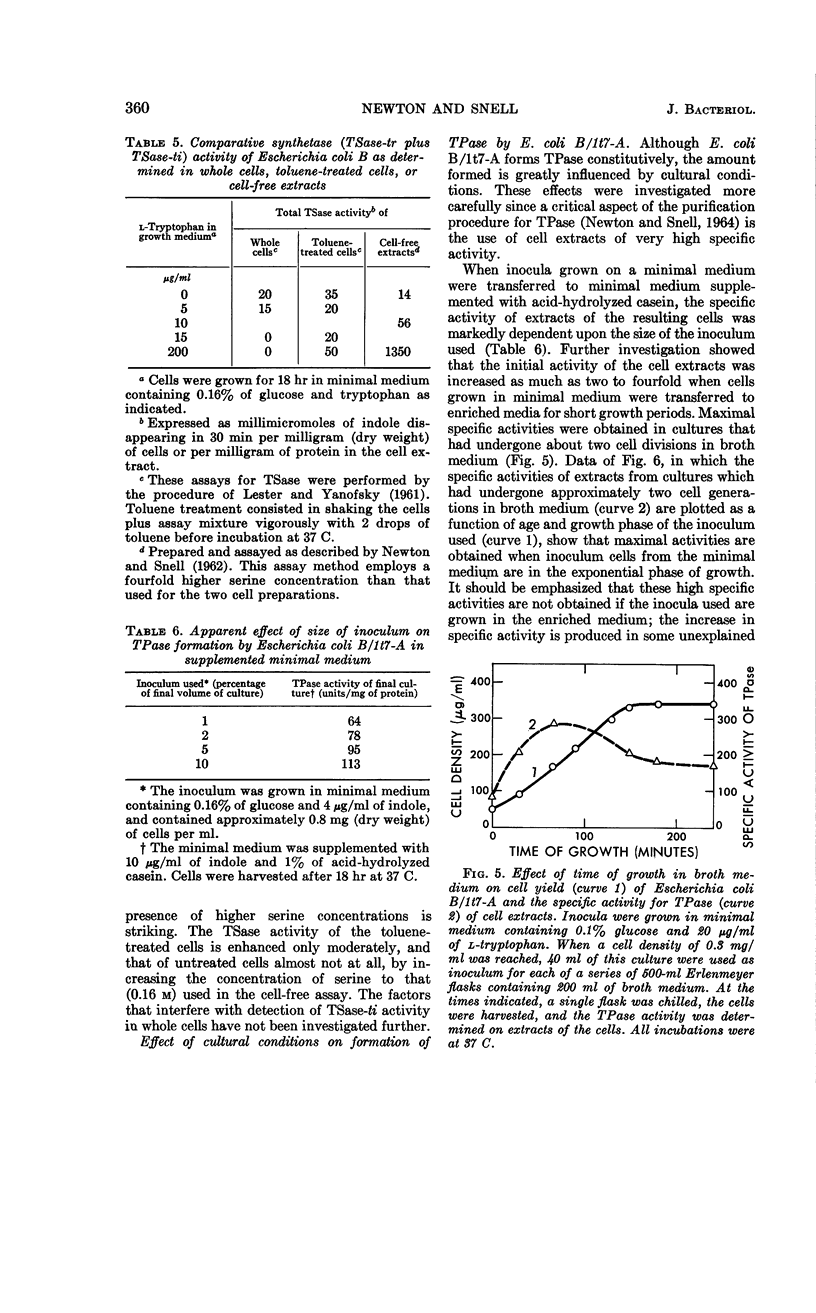
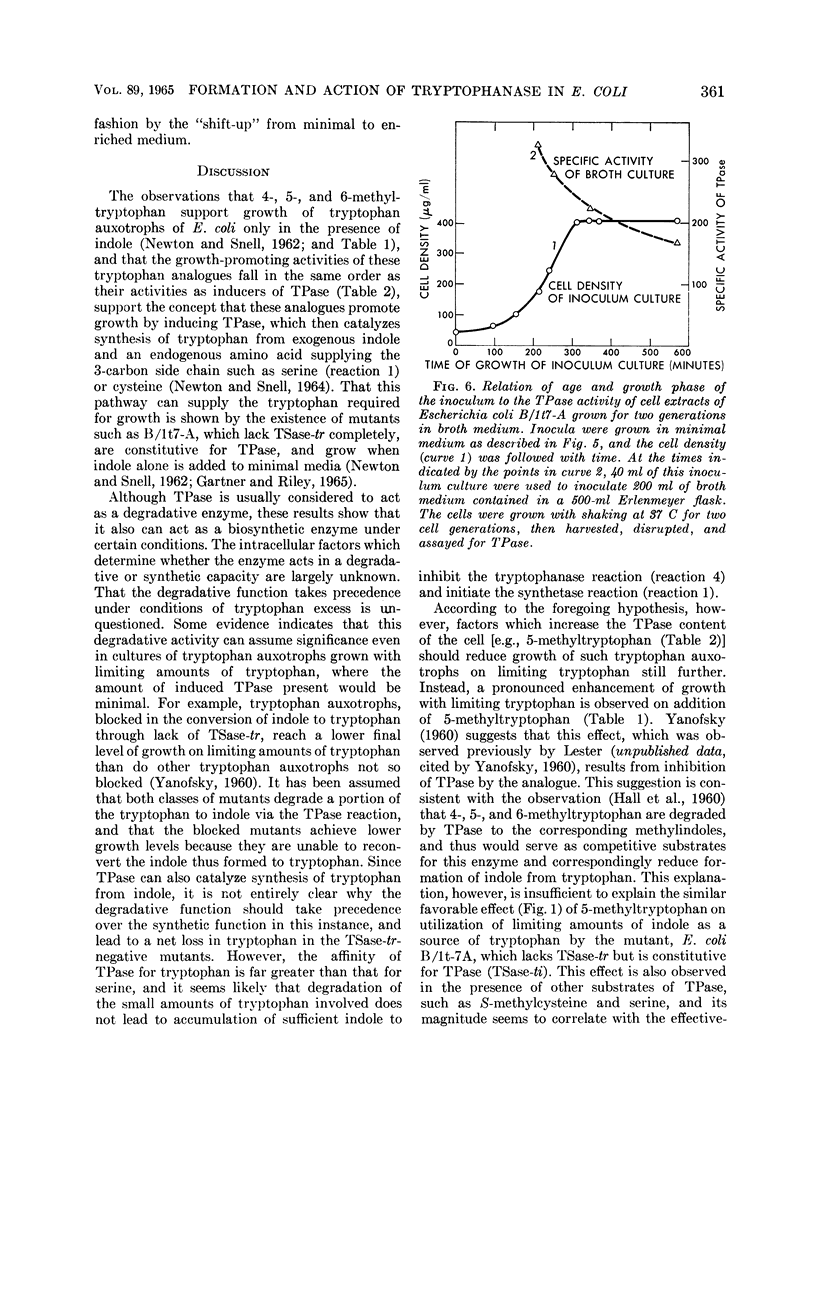
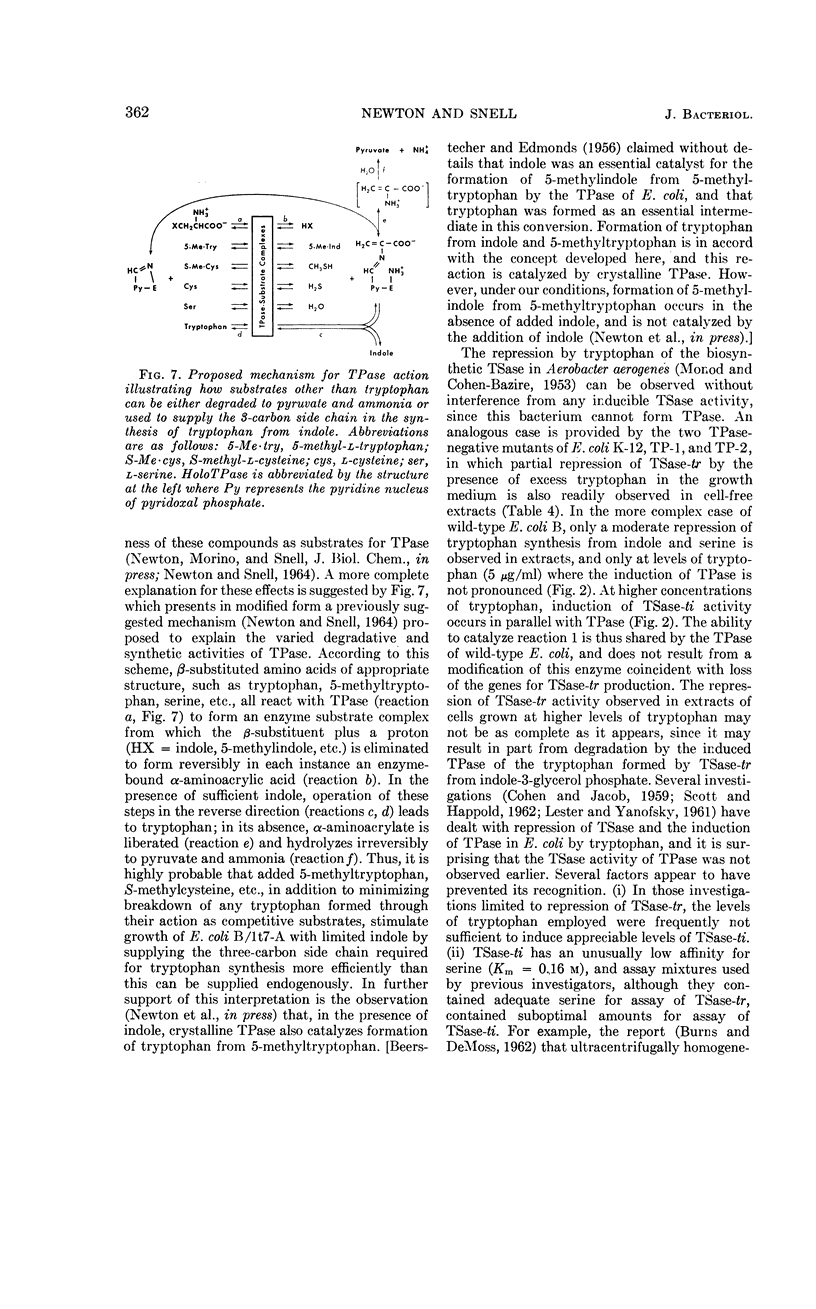
Newton, W. Austin (University of California, Berkeley), and Esmond E. Snell. Formation and interrelationships of tryptophanase and tryptophan synthetases in Escherichia coli. J. Bacteriol. 89:355–364. 1965.—In addition to the classical tryptophan-repressible tryptophan synthetase (TSase-tr), tryptophan auxotrophs of Escherichia coli contain another distinct tryptophan synthetase (TSase-ti) which is induced by tryptophan and is identical with tryptophanase (TPase). Escherichia coli B (wild type) forms only TSase-tr when the growth medium lacks tryptophan. When tryptophan is supplied, parallel induction of TPase and TSase-ti occurs while TSase-tr is repressed. Antiserum prepared against purified TPase neutralized TPase and TSase-ti equally, but not TSase-tr. TPase-negative strains of E. coli do not form TSase-ti. Unlike TSase-tr, TSase-ti is not readily detected by whole-cell assays. In the tryptophan auxotroph, E. coli B/1t7, a direct correlation exists between the effectiveness of 4-, 5-, and 6-methyl-tryptophan in inducing TPase and in promoting growth in the presence of indole. In a mutant of this organism, E. coli B/1t7-A, which is constitutive for TPase, 5-methyl-tryptophan and other substrates of TPase increased the rate of growth on limiting indole, a result ascribed to their ability to inhibit degradation of tryptophan and to supply the 3-carbon side chain for synthesis of tryptophan by TPase. This organism produced maximal amounts of TPase when inocula from log-phase cells grown in tryptophan-supplemented minimal medium were allowed to undergo two cell generations in an enriched broth medium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNS R. O., DEMOSS R. D. Properties of tryptophanase from Escherichia coli. Biochim Biophys Acta. 1962 Dec 4;65:233–244. doi: 10.1016/0006-3002(62)91042-9. [DOI] [PubMed] [Google Scholar]
- COHEN G., JACOB F. Sur la répression de la synthèse des enzymes intervenant dans la formation du tryptophane chez Escherichia coll. C R Hebd Seances Acad Sci. 1959 Jun 15;248(24):3490–3492. [PubMed] [Google Scholar]
- FREUNDLICH M., LICHSTEIN H. C. Inhibitory effect of glucose on tryptophanase. J Bacteriol. 1960 Nov;80:633–638. doi: 10.1128/jb.80.5.633-638.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARTNER T. K., RILEY M. ISOLATION OF MUTANTS AFFECTING TRYPTOPHANASE PRODUCTION IN ESCHERICHIA COLI. J Bacteriol. 1965 Feb;89:313–318. doi: 10.1128/jb.89.2.313-318.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL A. N., LEESON J. A., RYDON H. N., TWEDDLE J. C. The degradation of some Bz-substituted tryptophans by Escherichia coli tryptophanase. Biochem J. 1960 Feb;74:209–216. doi: 10.1042/bj0740209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- LERNER P., YANOFSKY C. An immunological study of mutants of Escherichia coli lacking the enzyme tryptophan synthetase. J Bacteriol. 1957 Oct;74(4):494–501. doi: 10.1128/jb.74.4.494-501.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER G., YANOFSKY C. Influence of 3-methylanthranilic and anthranilic acids on the formation of tryptophan synthetase in Escherichia coli. J Bacteriol. 1961 Jan;81:81–90. doi: 10.1128/jb.81.1.81-90.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G. L'effet d'inhibition spécifique dans la biosynthèse de la tryptophane-desmase chez Aerobacter aerogenes. C R Hebd Seances Acad Sci. 1953 Feb 2;236(5):530–532. [PubMed] [Google Scholar]
- NEWTON W. A., SNELL E. E. An inducible tryptophan synthetase in tryptophan auxotrophs of Escherichia coli. Proc Natl Acad Sci U S A. 1962 Aug;48:1431–1439. doi: 10.1073/pnas.48.8.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON W. A., SNELL E. E. CATALYTIC PROPERTIES OF TRYPTOPHANASE, A MULTIFUNCTIONAL PYRIDOXAL PHOSPHATE ENZYME. Proc Natl Acad Sci U S A. 1964 Mar;51:382–389. doi: 10.1073/pnas.51.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICKENBERG H. V., YANOFSKY C., BONNER D. M. Enzymatic deadaptation. J Bacteriol. 1953 Dec;66(6):683–687. doi: 10.1128/jb.66.6.683-687.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT T. A., HAPPOLD F. C. Studies on the inhibition of induced tryptophanase synthesis in Escherichia coli by the simultaneous presence of fermentable carbohydrate and aromatic amino acids during growth. Biochem J. 1962 Mar;82:407–412. doi: 10.1042/bj0820407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANOFSKY C., LENNOX E. S. Transduction and recombination study of linkage relationships among the genes controlling tryptophan synthesis in Escherichia coli. Virology. 1959 Aug;8:425–447. doi: 10.1016/0042-6822(59)90046-7. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C. The tryptophan synthetase system. Bacteriol Rev. 1960 Jun;24(2):221–245. doi: 10.1128/br.24.2.221-245.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]


