Abstract
Objective
The purpose of this study was to investigate the association between large-for-gestational age (LGA) infants and the development of childhood obesity in an inner-city, primarily African-American population.
Study Design
Maternal, neonatal, socioeconomic and nutritional histories were collected for mothers with children age 2–5 years old. Associations between Alexander and customized birth weight (BW) percentiles and body mass index (BMI)-for-age of the child were examined.
Results
195 mother-child pairs were enrolled; childhood obesity rate was 18%. Increasing Alexander and customized BW percentiles were related to increasing obesity. LGA newborns were 2.5 times more likely to be obese in childhood than average size newborns. Maternal smoking was also associated with childhood obesity.
Conclusion
LGA infants have the highest likelihood of childhood obesity in this inner-city, predominantly African-American population. Customized growth percentiles perform best in identifying the highest risk population.
Keywords: Childhood obesity, Customized growth percentiles, Large-for-gestational age infants, Maternal smoking
Introduction
Obesity has increased to and remained at epidemic levels in recent years, around the world and specifically in the US, where adult obesity rates are above 30% in all adult subgroups and higher than 45% in non-white women.1 Perhaps even more concerning are the rising rates of childhood obesity in the US; so much so that this has become a focus of public health initiatives, including First Lady Michelle Obama's recently unveiled “Let's Move” campaign against childhood obesity. While these initiatives are critical in the fight against childhood obesity, growing evidence regarding the association between birth outcomes and risks of future obesity may lead some of the focus to shift to the impact of pregnancy on these concerning outcomes.2
Knowledge of the relationship between in-utero exposures and early infant outcomes with later adult morbidities is not new. An association between small-for-gestational-age infants and adult morbidities, such as hypertension, type II diabetes, and cardiovascular disease, was first proposed by Barker following his large epidemiologic studies and has been validated in some subsequent work.3–5 Increasing BW's relationship to childhood obesity has also been noted in several studies.6,7 Our objective in this study was to determine the current levels of childhood obesity in an inner-city African-American population, and to evaluate whether an association exists between large-for-gestational age (LGA) infants and childhood obesity and overweight. We went further by using a previously validated customized growth percentile model to control for additional confounders to best elucidate those infants that are truly overgrown, and thus, we believe, at the highest risk.
Materials and Methods
This is a longitudinal case-control study consisting of obese (cases) and normal weight children (controls) age 2–5 years old. Mothers who presented to the well-child clinics of Children's Hospital of Michigan with children between the ages of 2 and 5 years old were identified and enrolled, beginning in January 2009. The child's records were reviewed and his/her height, weight, and other pertinent clinical data were recorded. In addition, the mother was asked to complete an extensive questionnaire that assessed data not consistently found in the prenatal chart, including maternal marital and education status, nutrition, pre-pregnancy exercise frequency, economic and employment status, use of WIC or food stamps, and substance misuse history, as well as an extensive nutrition history of the infant/child. Finally, maternal delivery and prenatal records were reviewed for pertinent pregnancy and delivery data. Patients who did not deliver at one of four hospitals included in the study (Hutzel Women's Hospital, Sinai-Grace Hospital, Henry Ford Hospital, and St. John Riverview Hospital) were excluded. Additional exclusion criteria included those whose delivery records were not available for review, and children born with major congenital malformations. Institutional review board approval was obtained from the Wayne State University Human Investigation Committee, and permission for review of maternal delivery charts were obtained from the two additional hospital systems.
Age- and sex-specific BMI percentiles for each enrolled child were calculated and categorized using Centers for Disease Control-defined groupings: childhood obesity (BMI-for-age ≥ 95 percentile), overweight (BMI-for-age ≥ 85 percentile to BMI-for-age < 95 percentile) and healthy weight (BMI-for-age > 5 percentile to BMI-for-age < 85 percentile).8 Underweight children (BMI-for-age < 5th percentile) were not included in this analysis, as the comparison of interest was the obese group to healthy weight/overweight group. Risk (independent) variables included BW percentile subgroups, which were defined by the standard Alexander growth curve, which is a US national reference for fetal growth that determines BW percentile subgroup by BW per gestational age at delivery. Gestational age was obtained from delivery record documentation and was generally determined by LMP and/or first or second trimester ultrasound. Based on the method of Alexander et al, data was trimmed to exclude BW inconsistent with gestational age.9 Additionally, the GROW (Gestation related optimal weight) calculator (available for free download at www.gestation.net)10, was utilized to calculate customized BW percentiles. These percentiles are calculated from infant BW, adjusted for gestational age at delivery, infant gender, maternal height, maternal weight at first prenatal visit, race and parity, and yield an individualized growth trajectory, indicating how much a given fetus is under- or over-grown. The calculated percentiles for both Alexander and GROW were sub-grouped into <10th percentile, 10–50th percentile, 50th–90th percentile, and >90th percentile. Covariates evaluated included variables gleaned from the questionnaire and delivery chart, including but not limited to maternal delivery and medical history, socioeconomic factors, and measures of infant feeding.
Pearson chi-square and one-way ANOVA with Student-Newman-Keuls post-hoc comparisons were used to compare maternal characteristics between child BMI groups. Bivariate analyses of the independent variables and covariates versus the two dependent variables (childhood obese and overweight) were performed using Fisher's exact test or Pearson chi-square for categorical variables and Student's T-test for continuous variables. To obtain a small group of potential predictors of childhood obesity and overweight outcomes, all factors with a p<0.1 were included in logistic regression. A probability value of <.05 in the stepwise logistic regression was considered significant.
Results
At the time of analysis, 195 mother-child pairs met study criteria, were enrolled and all pertinent data were collected. 35 children (18.3%) were obese and an additional 22 (11.5%) were overweight. Table 1 compares maternal demographics and clinical characteristics between the two groups and the healthy weight group (n = 120), where continuous measures were analyzed with one-way ANOVA with Student Newman-Keuls post-hoc comparisons, and categorical variables were analyzed with Pearson chi-square. No significant differences were noted between groups.
Table 1.
Maternal demographics and clinical characteristics of child obese, overweight, and health weight groups
| Maternal characteristics | Obese (n = 35) | Overweight (n = 22) | Healthy weight (n = 120) | p |
|---|---|---|---|---|
| Maternal age (y)* | 24 (15–38) | 23 (16–34) | 22 (15–38) | |
| Race | ||||
| Black | 34 (97.1%) | 18 (94.7%) | 106 (90.6%) | .29 |
| Single marital status | 30 (85.7%) | 20 (90.9%) | 108 (90.0%) | .55 |
| Nulliparity | 19 (54.3%) | 12 (54.5%) | 53 (44.2%) | .40 |
| Gestational age (wks)** | 38.8 ± 1.7 | 38.8 ± 2.3 | 38.8 ± 2.6 | .18 |
| Pregnancy wt gain** | 37.6 ± 17.6 | 36.3 ± 13.7 | 32.2 ± 15.7 | .25 |
| BMI at delivery** | 35.4 ± 9.3 | 36.0 ± 7.4 | 32.6 ± 7.3 | .09 |
| Smoking during pregnancy | 10 (28.6%) | 4 (18.2%) | 17 (14.2%) | .12 |
| Fast food servings/d during pregnancy** | 1.2 ± 1.6 | .75 ± .73 | .94 ± 1.1 | .59 |
Median with range
Mean (± standard deviation)
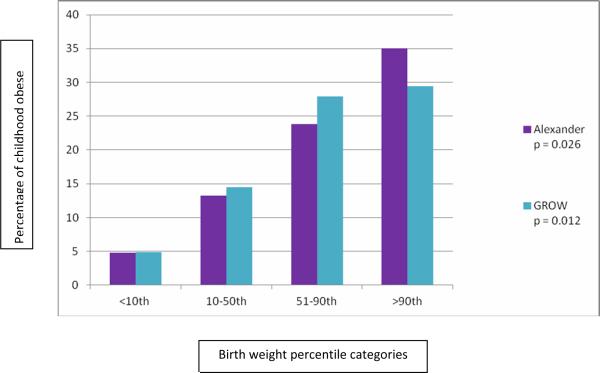
Birth weights for the obese group were evaluated. Increasing BW by Alexander percentiles as well as by customized growth percentiles using the GROW calculator was related to increasing obesity at age 2–5 years old (Figure 1). Of 75 independent variables and covariates, six, including the two independent variables Alexander > 90% and GROW>90%, which reflected fetal growth, and four covariates, smoking during pregnancy, number of fruit servings daily during pregnancy, number of vegetable servings daily during pregnancy, and number of servings of juice imbibed daily by the child, had p<0.1 in a bivariate setting. These six variables were entered into a stepwise logistic regression (Table 2). Only GROW>90% and maternal smoking were significant determinants of childhood obesity at age two to five; jointly they explained 14% of the variance in childhood obesity -- GROW (R square = .106) contributed over twice the variance that smoking did (R square = .034). Interactions were not significant. Newborns who were <10th percentile GROW customized growth percentile had an OR of 0.25 (95th CI .08–.8) for childhood obesity compared to the entire group; those with a >90th GROW customized growth percentile had an OR of 2.5 (CI 1.001–6.2) for childhood obesity. In other words, for babies underweight at birth (small for gestational age), the risk for childhood obesity was ¼ that of the average size newborn, but LGA newborns were 2.5 times as likely as average to be obese in childhood.
Figure.
Increasing bodyweight by Alexander percentiles and by customized growth percentiles using the gestation-related optimal weight (GROW) calculator
Table 2.
Multiple logistic regression for risk factors for childhood obesity
| Risk factor | B | S.E. | Odds ratio | 95% CI | p |
|---|---|---|---|---|---|
| GROW > 90% | .91 | .46 | 2.48 | 1.001–6.146 | 0.03 |
| Smoking during pregnancy | 1.14 | .47 | 3.13 | 1.24–7.89 | 0.02 |
|
| |||||
| Alexander > 90% | .33 | .65 | 1.39 | .386–4.964 | 0.62 |
| # of vegetables/d pregnancy | .16 | .14 | 1.17 | .897–1.522 | 0.25 |
| # of fruits/d pregnancy | .12 | .12 | 1.12 | .882–1.429 | 0.35 |
| # of servings juice/d child | .30 | .19 | 1.34 | .921–1.961 | 0.13 |
Risk factors for childhood overweight were GROW>90%, Alexander>90%, smoking during pregnancy, and number of fruit servings during pregnancy. GROW>90% and smoking during pregnancy were significant in the stepwise.logistic regression.
Comment
The major finding in our study is that LGA newborns, >90th BW percentile as identified by the use of the GROW customized growth percentile calculator, are associated with childhood obesity (BMI ≥ 95%) in the age group of 2–5 years old. Previous studies have suggested a relationship between increasing BW and increased risk of childhood obesity. Our study, however, adds to the literature in several important ways.
One way that our study differs from published studies on this topic is the population, here a primarily inner-city African-American population. Specifically, the study population was 90% African-American, 90% single mothers, with noted low education and household income levels. This is in contrast to publications from a major study, self-described as having a population with “educational and income levels [that] were relatively high”; with results that were “not generalizable to more socioeconomically disadvantaged populations.”11–13 African-Americans, along with Hispanics, have the highest rates of adult, as well as childhood, obesity in the United States,1 According to the most recent National Health and Nutrition Examination Survey (2007–2008), 9.1% of non-Hispanic white children age 2–5 years old have a BMI ≥ 95%, vs. 11.4% of non-Hispanic black children and 13.7% of Mexican American children.14 Our study showed the rate of BMI ≥ 95% in 2–5 year olds to be a remarkably high 18.3%. Of the work done previously looking at racial/ethnic differences in early risk factors for childhood obesity, it was again noted that “educational and income levels of our study population is relatively high.”15 While an increased risk for childhood obesity appears to exist in African-Americans regardless of socioeconomic status, several studies have noted that socioeconomic status is associated with obesity outcomes.16–19 Therefore the combination of factors of inner-city, disadvantaged, and predominant African-American likely present one of the highest risk populations in the United States for the obesity epidemic, one that is very important for further study and aggressive intervention if modifiable factors can be established.
Oken and Gillman have noted that while several previous studies show a relationship between increasing BW and childhood obesity, many had limited data on gestational age, parental body size, tobacco use, and socioeconomic factors.20 This is not the case with our study. First, Alexander growth charts were used to adjust for gestational age.9 We then went further by using customized growth charts to further adjust BW, using the US version of the freely available customized centile calculator software program10. These customized growth charts were first developed by Gardosi for European population, and have been utilized in previous studies.21–26 The value of the use of customized growth percentiles is the ability to adjust for physiological variables, such as maternal size, parity, and ethnic origin, and not for pathologic factors, thus allowing an improvement in identification of those fetuses that are truly pathologically grown. While studies have been published looking at SGA identification using the customized growth charts, both in the European23, 25, 26 and in the US population27; these studies have demonstrated that customized growth charts performed better at identifying babies at increased risk for morbidity and mortality. This is the first study we are aware of that has evaluated the identification of LGA infants. We found that customized growth percentiles performed better in isolating those infants most associated with childhood obesity, suggesting that this method successfully isolated those infants were pathologically abnormally grown, eliminating the Alexander-adjusted BW percentiles in a multivariate setting. One limitation of this model may be the adjustment for maternal BMI, which may allow for adjustment of genetic influence on neonatal BW, but may also adjust out the some of the influence of the (pathologic) environment on BW as well, and should therefore be evaluated with caution.20 Nonetheless, based on our results, customized growth percentiles such as those developed by Gardosi may perform better than BW simply adjusted for gestational age in separating out those infants that are pathologically big and therefore at greatest risk for childhood obesity
The positive relationship between maternal smoking and childhood obesity/overweight, on the surface surprising, has nonetheless been well-documented in previous studies27–29 and evaluated in a systematic review/meta-analysis.30 Our study also showed that this association was persistent in a less affluent population, where a high percentage of the obese children were exposed to smoking during pregnancy (28.6%), much higher than the average rate of smoking during pregnancy in the US (14.1%).31 We will confirm this high level of smoking and the association with childhood obesity upon completion of our study. Smoking appears to remain an independent risk factor for childhood obesity, and future studies are needed in this area to better elucidate the underlying association of this modifiable risk factor.
Limitations of this study include the retrospective review of prenatal and delivery data, as well as the obtaining of some data by self-report. Infant body composition data has been suggested as a better measure than BW in delineating high-risk groups32; this information was not available to us. Whether customized growth percentiles, as an indirect but simpler measure, can compete with infant body composition data in determining truly pathologic infants remains to be seen. This study is the first of a series of planned studies of obstetric risk and childhood obesity. It was not powered at this time to analyze the association of modifiable risk factors with childhood obesity. Our study does, though, add validity to findings in previous longitudinal studies11–13, 15 in a unique, high-risk population, and adds validity to the use of a simple tool, customized growth percentiles, to better identify those kids at risk.23–26
The US has been working at a public health level on interventions aimed at preventing childhood obesity, including those related to school food and physical activity, taxes, food marketing, and reducing screen time for children.14 While these lifestyle changes play an important role in reducing the large numbers of young obese, the evidence continues to grow suggesting that the in-utero environment may also plays a critical role in these outcomes. Therefore, more work needs to be done to not only determine modifiable factors, but to educate women preconceptionally and during pregnancy to the associations between pregnancy effects and childhood obesity, as this may be the true starting point of a lifelong propensity to obesity.
Condensation.
Large-for-gestational-age infants are associated with childhood obesity in an inner-city African-American population; and are best defined using customized growth charts.
Acknowledgments
Supported in part by Grant no. K12 HD-01254 (S.H.M.) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting presentation: Central Association of Obstetricians and Gynecologists 77th Annual Meeting, Las Vegas, Nevada Oral presentation, October 30, 2010
No reprints available.
References
- 1.Flegel KM, Carroll MD, Ogden CL. Prevalence and trends in obesity among US adults. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Wojcicki JM, Heyman MB. Let's move—childhood obesity prevention from pregnancy and infancy onward. N Engl J Med. 2010;362:1457–9. doi: 10.1056/NEJMp1001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker DJP, Osmond C, Simmonds SJ, Wield GA. The relation of head size and thinness at birth to death from cardiovascular disease in adult life. Br Med J. 1993;306:422–426. doi: 10.1136/bmj.306.6875.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DJP, Gluckman PD, Godfrey KM, Harding J, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–41. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 5.Barker D. Fetal origins of coronary heart disease. BMJ. 1995;311:171–4. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331:929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hui LL, Schooling CM, Leung SS, et al. Birth weight, infant growth, and childhood body mass index. Arch Pediatr Adolesc Med. 2008;162:212–8. doi: 10.1001/archpediatrics.2007.62. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control Defining childhood overweight and obesity. [Accessed June 24, 2010]; Available at: http://www.cdc.gov/obesity/childhood/defining.html.
- 9.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 10.Gardosi J, Francis A. Software program for the calculation of customised birth weight percentiles. [Accessed January 2010];2008 (Version 5.15_US) www.gestation.net.
- 11.Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123:1177–83. doi: 10.1542/peds.2008-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oken E, Kleinman KP, Belfort MB, Hammitt JK, Ogden CL, Gillman MW. Associations of gestational weight gain with short- and longer-term maternal and child health outcomes. Am J Epidemiol. 2009;170:173–80. doi: 10.1093/aje/kwp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Olsen SF, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obset Gynecol. 2007;196:322e1–8. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–9. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 15.Taveras EM, Gillman MW, Kleinman K, Rich-Edwards JW, Rifas-Shiman SL. Racial/ethnic differences in early-life risk factors for childhood obesity. Pediatrics. 2010;125:686–95. doi: 10.1542/peds.2009-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song YM. Commentary: Varying relation of socioeconomic status with obesity between countries at different stages of development. Int J Epidemiol. 2006;35:112–3. doi: 10.1093/ije/dyi227. [DOI] [PubMed] [Google Scholar]
- 17.Jeffrey RW, French SA. Socioeconomic status and weigh control practices among 20-to 45-year old women. Am J Public Health. 1996;86:1005–10. doi: 10.2105/ajph.86.7.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dietz WH. Does hunger cause obesity? Pediatrics. 1995;95:766–7. [PubMed] [Google Scholar]
- 19.Townsend MS, Peerson J, Love B, Acherberg C, Murphy SP. Food insecurity is positively related to overweight in women. J Nutr. 2001;131:1738–45. doi: 10.1093/jn/131.6.1738. [DOI] [PubMed] [Google Scholar]
- 20.Oken E, Gillman MW. Fetal origins of obesity. Obesity Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 21.Zeitlin J, Ancel PY, Saurel-Cubizolles MJ, Papiernik E. The relationship between intrauterine growth restriction and preterm delivery: an empirical approach using data from European case-control study. BJOG. 2000;107:750–8. doi: 10.1111/j.1471-0528.2000.tb13336.x. [DOI] [PubMed] [Google Scholar]
- 22.Groom KM, Poppe KK, North RA, McCowan ME. Small-for-gestational age infants classified by customised or population birthweight centiles: impact of gestational age at delivery. Am J Obstet Gynecol. 2007;197:239e1–5. doi: 10.1016/j.ajog.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 23.de Jong CLD, Gardosi J, Dekker GA, Collenbrander GJ, van Geijn HP. Application of a customised birthweight standard in the assessment of perinatal outcome in a high risk population. BJOG. 1998;105:531–35. doi: 10.1111/j.1471-0528.1998.tb10154.x. [DOI] [PubMed] [Google Scholar]
- 24.Clausson B, Gardosi J, Francis A, Cnattingius S. Perinatal outcome in SGA births defined by customised versus population-based birthweight standards. BJOG. 2001;108:830–4. doi: 10.1111/j.1471-0528.2001.00205.x. [DOI] [PubMed] [Google Scholar]
- 25.McCowan L, Harding JE, Stewart AW. Customised birthweight centiles predict SGA pregnancies with perinatal morbidity. BJOG. 2005;112:1026–33. doi: 10.1111/j.1471-0528.2005.00656.x. [DOI] [PubMed] [Google Scholar]
- 26.Ego A, Subtil D, Grange G, et al. Customised versus population-based birth weight standards for identifying growth restricted infants: a French multicenter study. Am J Obstet Gynecol. 2006;194:1042–9. doi: 10.1016/j.ajog.2005.10.816. [DOI] [PubMed] [Google Scholar]
- 27.Oken E, Huh SY, Taveras EM, Rich-Edwards JW, Gillman MW. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes Res. 2005;13:2021–8. doi: 10.1038/oby.2005.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorog K, Pattenden S, Antova T, et al. Maternal smoking during pregnancy and childhood obesity: Results from the CESAR study. 2009. Matern Child Health J. doi: 10.1007/s10995-009-0543-5. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki K, Ando D, Sato M, Tanaka T, Kondo N, Yamagata Z. The association between maternal smoking during pregnancy and childhood obesity persists to age of 9–10 years. J Epidemiol. 2009;19:136–42. doi: 10.2188/jea.JE20081012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes (Lond) 2008;32:201–10. doi: 10.1038/sj.ijo.0803760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong VT, Jones JR, Dietz PM, D'Angelo D, Bombard JM, Centers for Disease Control and Prevention Trends in smoking before, during, and after pregnancy—Pregnancy risks assessment monitoring system (PRAMS), United States, 31 sites, 2000–2005. MMWR Surveill Surv. 2009;58:1–29. [PubMed] [Google Scholar]
- 32.Catalano PM, Farrell K, Thomas A, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90:1303–13. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]