Abstract
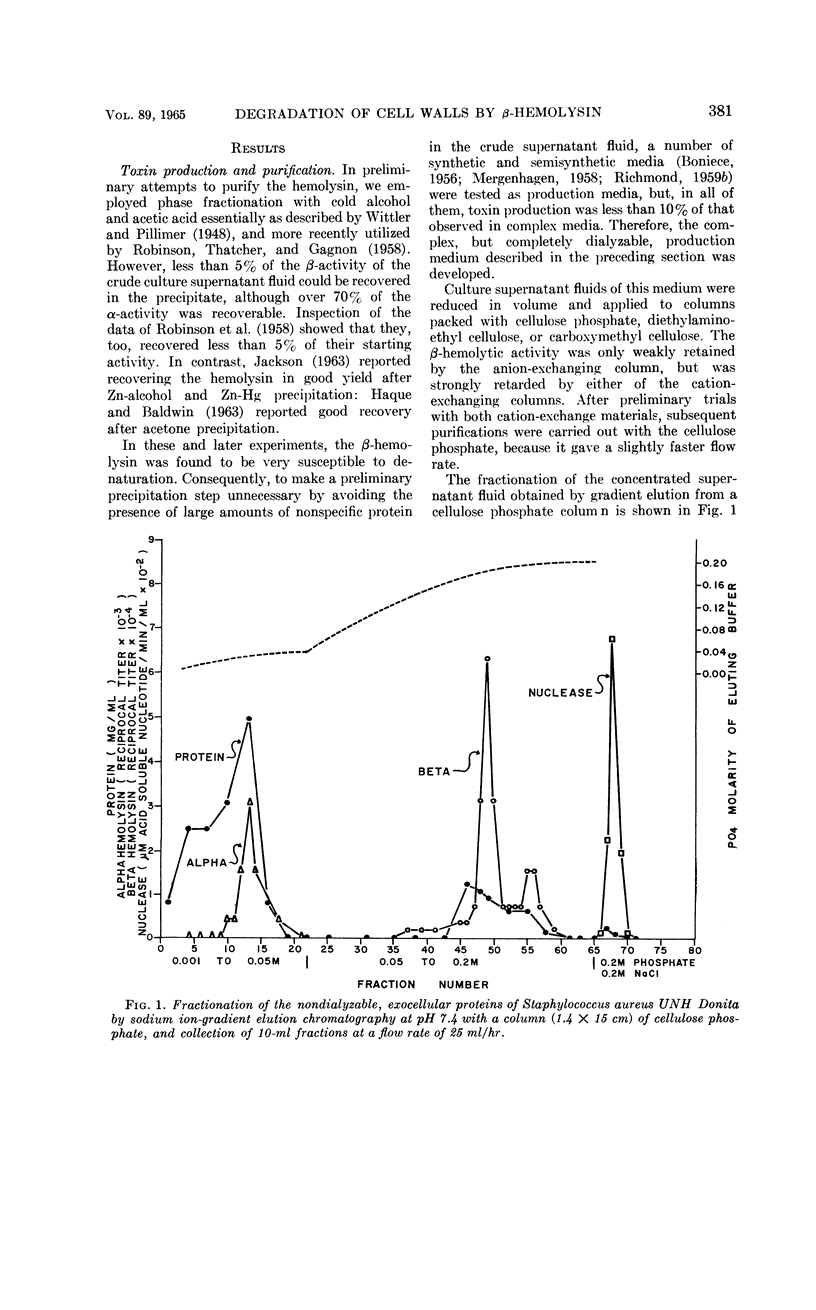
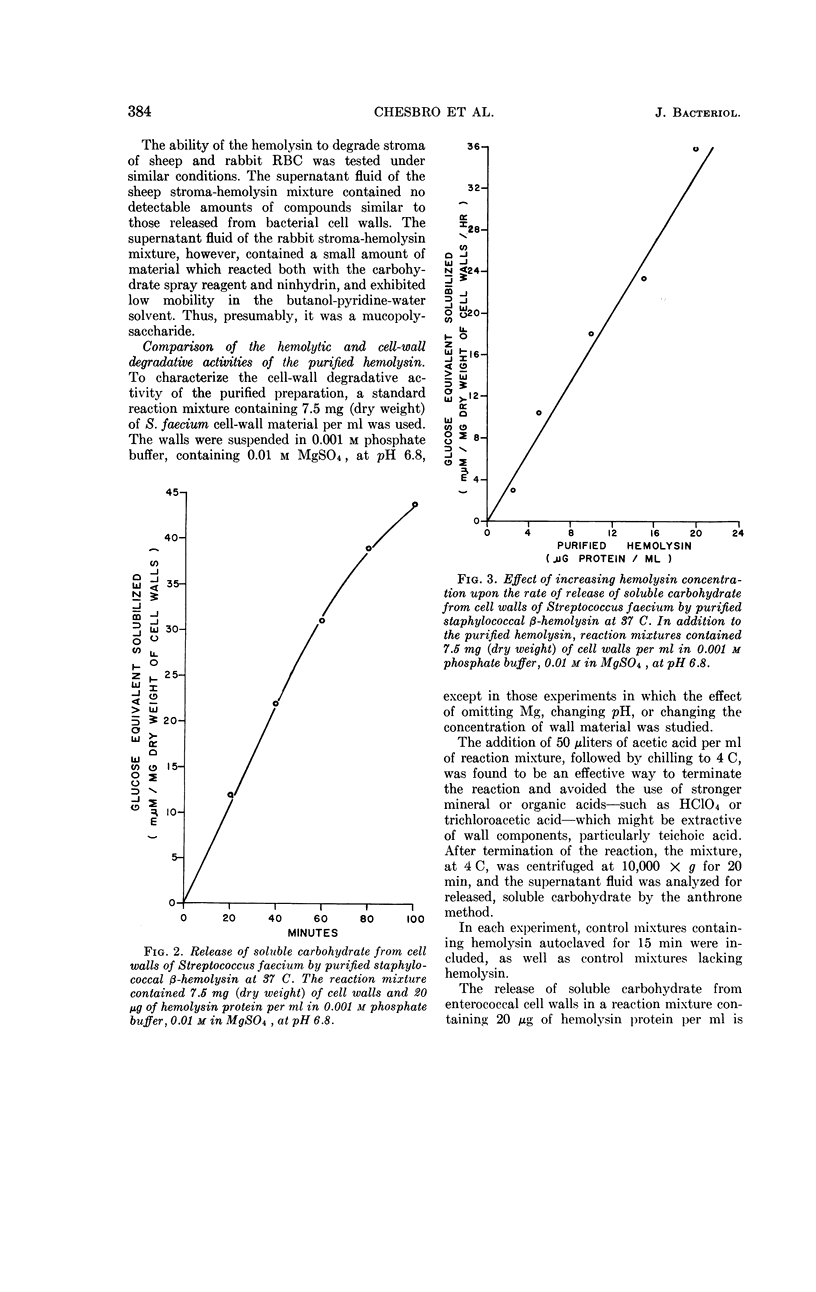
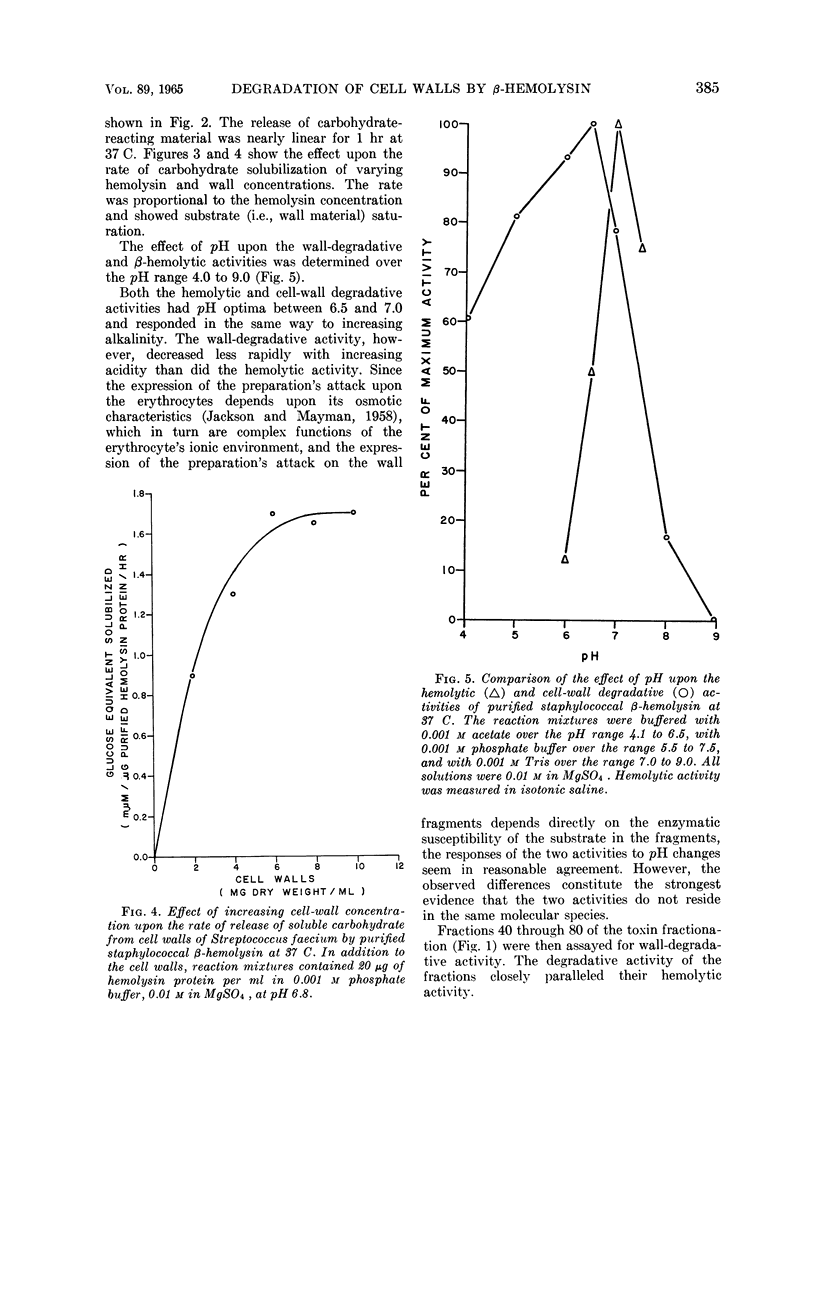
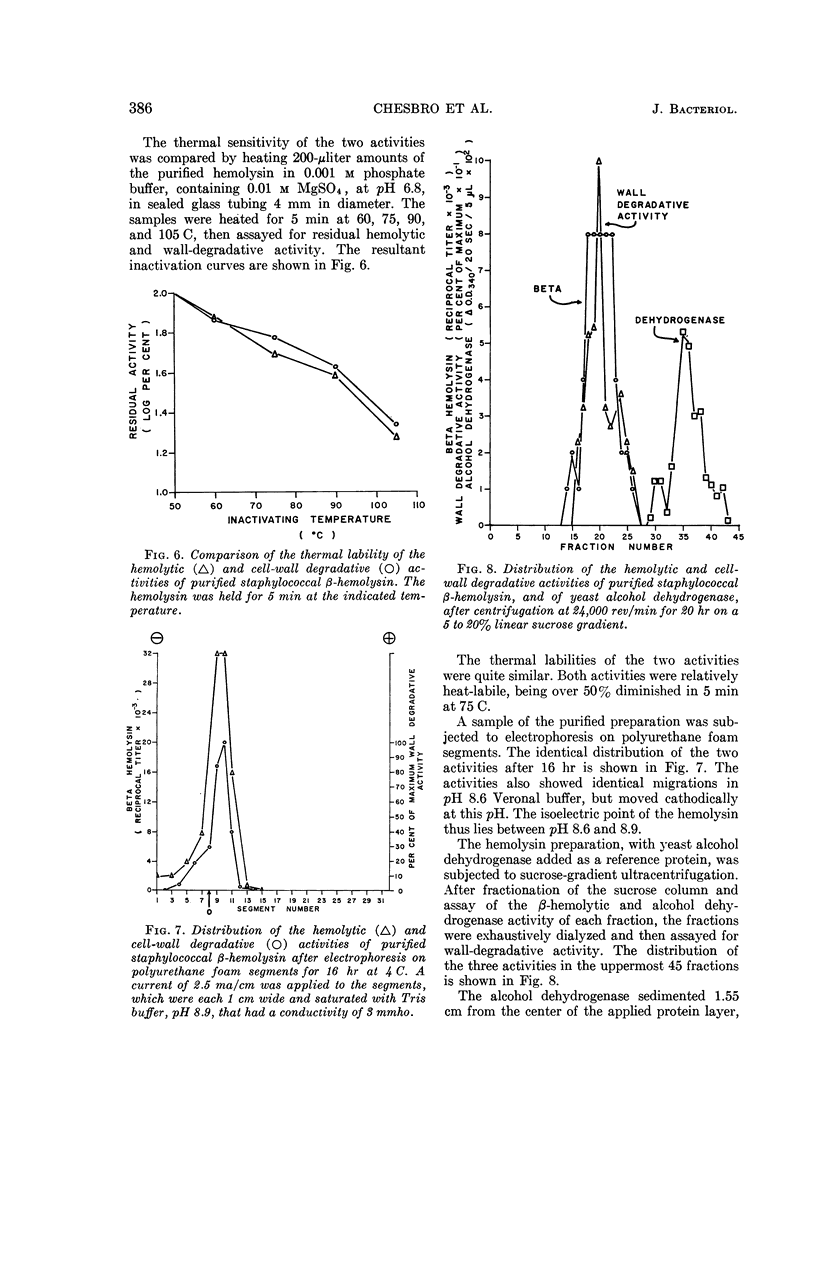
Chesbro, William R. (University of New Hampshire, Durham), Fred P. Heydrick, Roland Martineau, and Gail N. Perkins. Purification of staphylococcal β-hemolysin and its action on staphylococcal and streptococcal cell walls. J. Bacteriol. 89:378–389. 1965.—After growth of bovine-derived strains of Staphylococcus aureus in a completely dialyzable medium, the β-hemolysin in the culture supernatant fluids was purified by gradient-elution chromatography on cellulose phosphate. The purified hemolysin contained two components, demonstrable by immunodiffusion or electrophoresis, but was free from α-hemolysin, coagulase, Δ-hemolysin, enterotoxins A and B, glucuronidase, hyaluronidase, lipase, muramidase, Panton-Valentine leukocidin, phosphatase, and protease. The hemolysin was heat-labile and sulfhydryl-dependent, and the preparation was leukocidal for guinea pig macrophages. When rabbit red blood cell (RBC) stroma and staphylococcal or enterococcal cell walls were treated with the purified hemolysin, it liberated mucopolysaccharides from the rabbit RBC stroma, polysaccharides and mucopolysaccharides (or mucopeptides) from the staphyloccoal cell walls, and rhamnose, glucose, an unidentified monosaccharide, N-acetylglucosamine, and at least two polysaccharides from the enterococcal cell walls. The hemolytic and cell-wall degradative activities had similar thermal inactivation kinetics, pH optima, sedimentation coefficients, and chromatographic and electrophoretic mobilities; both required Mg and were inhibited by thiol-inactivating agents. Consequently, it seems likely that both activities are expressions of the same enzyme.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER M., HEPPEL L. A., HURWITZ J. The purification and properties of micrococcal nuclease. J Biol Chem. 1961 Nov;236:3014–3019. [PubMed] [Google Scholar]
- BERNHEIMER A. W., SCHWARTZ L. L. Isolation and composition of staphylococcal alpha toxin. J Gen Microbiol. 1963 Mar;30:455–468. doi: 10.1099/00221287-30-3-455. [DOI] [PubMed] [Google Scholar]
- BURNS J., HOLTMAN D. F. Biochemical properties of virulent and avirulent staphylococci. Ann N Y Acad Sci. 1960 Nov 21;88:1115–1124. doi: 10.1111/j.1749-6632.1960.tb20101.x. [DOI] [PubMed] [Google Scholar]
- CHESBRO W. R., EVANS J. B. Uptake and transmination of amino acids by Streptococcus faecium. Biochim Biophys Acta. 1962 Apr 23;58:538–549. doi: 10.1016/0006-3002(62)90064-1. [DOI] [PubMed] [Google Scholar]
- CROWLE A. J. A simplified micro double-diffusion agar precipitin technique. J Lab Clin Med. 1958 Nov;52(5):784–787. [PubMed] [Google Scholar]
- DAVIDSON H. M. Experiments on electrophoresis in segmental systems composed of polyurethane foam. Biochim Biophys Acta. 1959 Jul;34:67–78. doi: 10.1016/0006-3002(59)90233-1. [DOI] [PubMed] [Google Scholar]
- GLADSTONE G. P., VAN HEYNINGEN W. E. Staphylococcal leucocidins. Br J Exp Pathol. 1957 Apr;38(2):123–137. [PMC free article] [PubMed] [Google Scholar]
- HOFSTEE B. H. Direct and continuous spectrophotometric assay of phosphomonoesterases. Arch Biochem Biophys. 1954 Jul;51(1):139–146. doi: 10.1016/0003-9861(54)90461-0. [DOI] [PubMed] [Google Scholar]
- IKAWA M. The partial chemical degradation of the cell walls of Lactobacillus plantarum, Streptococcus faecalis, and Lactobacillus casei. J Biol Chem. 1961 Apr;236:1087–1092. [PubMed] [Google Scholar]
- ISQUITH A. J., CHESBRO W. R. POOLS, CONFLUXES AND TRANSPORT OF AMINO ACIDS IN STREPTOCOCCUS FAECIUM. Biochim Biophys Acta. 1963 Sep 10;74:642–658. doi: 10.1016/0006-3002(63)91416-1. [DOI] [PubMed] [Google Scholar]
- JACKSON A. W., LITTLE R. M. Leucocidal effect of staphylococcal delta-lysin. Can J Microbiol. 1957 Feb;3(1):101–102. doi: 10.1139/m57-012. [DOI] [PubMed] [Google Scholar]
- JACKSON A. W., MAYMAN D. Staphylococcal toxins. IV. Factors affecting hemolysis by delta-lysin. Can J Microbiol. 1958 Oct;4(5):477–486. doi: 10.1139/m58-051. [DOI] [PubMed] [Google Scholar]
- JONES N. R. The estimation of free sugars in skeletal muscle of codling (Gadus callarias) and herring (Clupea harengus). Biochem J. 1958 Apr;68(4):704–708. doi: 10.1042/bj0680704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MERGENHAGEN S. E. Effect of certain amino acids and peptides on hyaluronidase production by Staphylococcus aureus. Proc Soc Exp Biol Med. 1958 Mar;97(3):703–706. doi: 10.3181/00379727-97-23852. [DOI] [PubMed] [Google Scholar]
- RALSTON D. J., BAER B., LIEBERMAN M., KRUEGER A. P. Virolysin, a virus-induced lysin: its appearance and function in phage-infected staphylococci. J Gen Microbiol. 1961 Mar;24:313–325. doi: 10.1099/00221287-24-3-313. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H. Lytic enzymes of Staphylococcus aureus 524. Biochim Biophys Acta. 1959 Feb;31(2):564–565. doi: 10.1016/0006-3002(59)90041-1. [DOI] [PubMed] [Google Scholar]
- ROBINSON J., THATCHER F. S., MONTFORD J. Studies with staphylococcal toxins. V. Possible identification of alpha hemolysin with a proteolytic enzyme. Can J Microbiol. 1960 Apr;6:183–194. doi: 10.1139/m60-020. [DOI] [PubMed] [Google Scholar]
- Richmond M. H. The differential effect of arginine and canavanine on growth and enzyme formation in Staphylococcus aureus 524 SC. Biochem J. 1959 Sep;73(1):155–167. doi: 10.1042/bj0730155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALTON M. R. An improved method for the detection of N-acetylamino sugars on paper chromatograms. Biochim Biophys Acta. 1959 Aug;34:308–312. doi: 10.1016/0006-3002(59)90284-7. [DOI] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- SLANETZ L. W., BARTLEY C. H., ALLEN F. E. The immunization of dairy cattle against staphylococcal mastitis. J Am Vet Med Assoc. 1959 Feb 15;134(4):155–161. [PubMed] [Google Scholar]
- SLANETZ L. W., BARTLEY C. H. The diagnosis of staphylococcal mastitis, with special reference to the characteristics of mastitis staphylococci. J Infect Dis. 1953 Mar-Apr;92(2):139–151. doi: 10.1093/infdis/92.2.139. [DOI] [PubMed] [Google Scholar]
- WILSON M. W., PRINGLE B. H. Experimental studies of the agar-plate precipitin test of Ouchterlony. J Immunol. 1954 Oct;73(4):232–243. [PubMed] [Google Scholar]
- WOODIN A. M. Fractionation of a leucocidin from Staphylococcus aureus. Biochem J. 1959 Oct;73:225–237. doi: 10.1042/bj0730225. [DOI] [PMC free article] [PubMed] [Google Scholar]



