Abstract
The role of autophagy in tumorigenesis is controversial. Both autophagy inhibitors (chloroquine) and autophagy promoters (rapamycin) block tumorigenesis by unknown mechanism(s). This is called the “Autophagy Paradox.” We have recently reported a simple solution to this paradox. We demonstrated that epithelial cancer cells use oxidative stress to induce autophagy in the tumor microenvironment. As a consequence, the autophagic tumor stroma generates recycled nutrients that can then be used as chemical building blocks by anabolic epithelial cancer cells. This model results in a net energy transfer from the tumor stroma to epithelial cancer cells (an energy imbalance), thereby promoting tumor growth. This net energy transfer is both unilateral and vectorial, from the tumor stroma to the epithelial cancer cells, representing a true host-parasite relationship. We have termed this new paradigm “The Autophagic Tumor Stroma Model of Cancer Cell Metabolism” or “Battery-Operated Tumor Growth.” In this sense, autophagy in the tumor stroma serves as a “battery” to fuel tumor growth, progression and metastasis, independently of angiogenesis. Using this model, the systemic induction of autophagy will prevent epithelial cancer cells from using recycled nutrients, while the systemic inhibiton of autophagy will prevent stromal cells from producing recycled nutrients—both effectively “starving” cancer cells. We discuss the idea that tumor cells could become resistant to the systemic induction of autophagy by the upregulation of natural, endogenous autophagy inhibitors in cancer cells. Alternatively, tumor cells could also become resistant to the systemic induction of autophagy by the genetic silencing/deletion of pro-autophagic molecules, such as Beclin1. If autophagy resistance develops in cancer cells, then the systemic inhibition of autophagy would provide a therapeutic solution to this type of drug resistance, as it would still target autophagy in the tumor stroma. As such, an anti-cancer therapy that combines the alternating use of both autophagy promoters and autophagy inhibitors would be expected to prevent the onset of drug resistance. We also discuss why anti-angiogenic therapy has been found to promote tumor recurrence, progression and metastasis. More specifically, anti-angiogenic therapy would induce autophagy in the tumor stroma via the induction of stromal hypoxia, thereby converting a non-aggressive tumor type to a “lethal” aggressive tumor phenotype. Thus, uncoupling the metabolic parasitic relationship between cancer cells and an autophagic tumor stroma may hold great promise for anti-cancer therapy. Finally, we believe that autophagy in the tumor stroma is the local microscopic counterpart of systemic wasting (cancer-associated cachexia), which is associated with advanced and metastatic cancers. Cachexia in cancer patients is not due to decreased energy intake, but instead involves an increased basal metabolic rate and increased energy expenditures, resulting in a negative energy balance. Importantly, when tumors were surgically excised, this increased metabolic rate returned to normal levels. This view of cachexia, resulting in energy transfer to the tumor, is consistent with our hypothesis. So, cancer-associated cachexia may start locally as stromal autophagy and then spread systemically. As such, stromal autophagy may be the requisite precursor of systemic cancer-associated cachexia.
Key words: caveolin-1, autophagy, cancer associated fibroblasts, hypoxia, mitophagy, oxidative stress, DNA damage, genomic instability, tumor stroma, wasting (cancer cachexia), Warburg effect
Introduction
We have recently proposed a new paradigm for understanding tumor progression. We have termed this new paradigm “The Autophagic Tumor Stroma Model of Cancer.”1–5 In this model, cancer cells induce oxidative stress in adjacent cancer-associated fibroblasts (and possibly other stromal cell types).2 Oxidative stress in the tumor micro-environment activates an autophagic program, leading to the production of recycled nutrients that can then be used as “fuel” to promote the anabolic growth and aggressive progression of tumor epithelial cells.2 Another way to think about this process is to envision the autophagic stroma as a “battery” that provides the necessary energy source for tumor growth.
Oxidative stress in the tumor microenvironment also has mutagenic consequences.2 We have shown that ROS production in cancer-associated fibroblasts, via a bystander effect, induces DNA damage and aneuploidy in adjacent epithelial cancer cells, indicative of the onset of genomic instability. So, oxidative stress in the tumor microenvironment serves as a catalyst for the random mutagenesis of tumor cells and for tumor-stroma co-evolution.2
Finally, we also see that autophagy in cancer-associated fibroblasts dramatically protects tumor cells against apoptotic cell death,2,4 probably because it provides cancer cells with a steady stream of recycled nutrients (chemical building blocks) to feed their large anabolic appetite. As such, uncoupling the metabolic parasitic relationship between cancer cells and an autophagic tumor stroma may hold great promise for anti-cancer therapy.
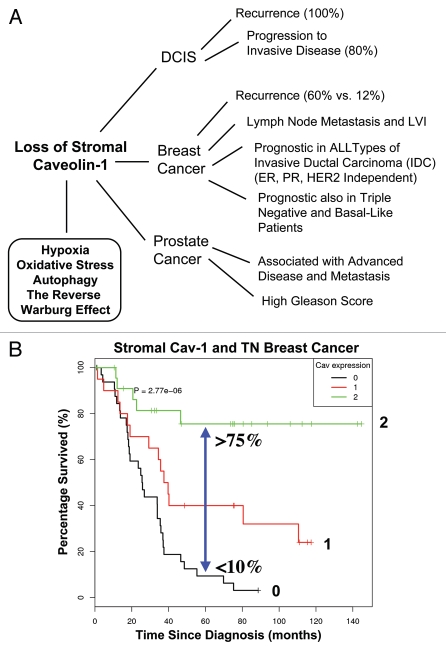
The discovery of the “Autophagic Tumor Stroma Model of Cancer” was largely based on the identification of a stromal biomarker known as caveolin-1 (Cav-1). Thus, we also discuss the powerful prognostic value of a loss of stromal Cav-1 in breast and prostate cancers (Fig. 1).6–10 We now know that a loss of stromal Cav-1 is a biomarker for chronic hypoxia, oxidative stress and autophagy in the tumor micro-environment and is predictive of early tumor recurrence, lymph node metastasis and poor clinical outcome.1,2,4,11,12 For recent reviews on autophagy, please see the following references 13–16.
Figure 1.
Prognostic value of caveolin-1 (Cav-1) as a stromal biomarker for DCIS, breast cancer and prostate cancer. (A) Schematic diagram summarizing that a loss of stromal Cav-1 is a new biomarker for hypoxia, oxidative stress, autophagy and the “Reverse Warburg Effect.” Importantly, a loss of stromal Cav-1 effectively predicts poor clinical outcome in DCIS (ductal carcinoma in situ), breast cancer and prostate cancer patients. Given that most human tumors have a stromal component, a loss of stromal Cav-1 may have prognostic value in a wide variety of different types of human cancers. Thus, a loss of stromal Cav-1 could be used to identify high-risk cancer patients at diagnosis, facilitating treatment stratification and clinical management. LVI, lympho-vascular invasion. (B) Kaplan-Meier analysis of stromal Cav-1 predicts overall survival in triple negative (TN) breast cancer patients. Patients with high levels of stromal Cav-1 (score = 2) had a good clinical outcome, with 75.5% of the patients remaining alive during the follow-up period (nearly 12 years). In contrast, the median survival for patients with absent stromal Cav-1 staining (score = 0) was 25.7 months. The results of this analysis were highly statistically significant (p = 2.8 × 10−6). (B) was reproduced from reference 8, with permission from the publisher (Landes Bioscience).
Caveolin-1 (Cav-1) as a Biomarker for Cancer Prognosis and Treatment Stratification
Several recent lines of experimental and clinical evidence support the idea that the tumor stroma plays a direct and critical role in determining patient outcome.17–20 Although the tumor stroma is complex and is composed of a plethora of cell types, it appears that stromal fibroblasts play a dominant role. For example, tumor stromal fibroblasts [a.k.a, cancer-associated fibroblasts (CAFs)] promote an EMT in adjacent cancer cells and can also enhance the metastatic dissemination of cancer cells to distant organ sites.21,22 Thus, it is of critical importance to understand exactly how CAFs promote tumor progression and metastasis.
Recently, we identified that a loss of stromal Cav-1 in the tumor fibroblast compartment23 is a powerful single independent predictor of clinical outcome in human breast cancer patients (Fig. 1).9 More specifically, a loss of stromal Cav-1 protein expression was specifically associated with early tumor recurrence, lymph-node metastasis and tamoxifen-resistance, resulting in poor clinical outcome.8,9 Interestingly, these results were independent of epithelial markers status (ER, PR and HER), directly demonstrating that a loss of Cav-1 has prognostic value in all of the most common sub-types of human breast cancer [invasive ductal carcinoma (IDC)].9 A loss of stromal Cav-1 was particularly valuable in triple-negative (TN) and basal-like breast cancer patients.8 In TN patients, high stromal Cav-1 levels predict a >75% overall survival rate at 12 years post-diagnosis.8 In striking contrast, an absence of stromal Cav-1 reduces overall survival to <10% at 5 years post-diagnosis.8 Thus, it is imperative to mechanistically understand the prognostic value of stromal Cav-1, as it could have important implications for the therapeutic stratification of breast cancer patients. The prognostic value of a loss of stromal Cav-1 in human breast cancers has now been validated in three independent patient cohorts, using three different anti-caveolin-1 antibodies.6,8,9,24
Stromal Cav-1 levels also have prognostic value in DCIS patients (Fig. 1).7 For example, a loss of Cav-1 in DCIS patients was associated with a 100% recurrence rate and 80% of these patients progressed to invasive breast cancer.7 Thus, a loss of stromal Cav-1 is a marker of DCIS recurrence and progression to a more invasive phenotype.
Finally, given that most tumors have a stromal component, we also assessed the behavior of stromal Cav-1 in prostate cancer patients (Fig. 1). Interestingly, our results showed that a loss of stromal Cav-1 was specifically associated with advanced prostate cancer and progression to metastatic disease and a high Gleason score, indicative of a poor prognosis.10 Thus, stromal Cav-1 may be a useful biomarker for predicting clinical outcome in many different types of epithelial cancer, possibly independently of the organ site.
To begin to understand the prognostic value of stromal Cav-1 as a novel biomarker, we turned to Cav-1 (-/-) null mice.25 As CAFs are thought to originate from the bone marrow (mesenchymal stem cells), we prepared bone marrow-derived stromal cells from WT and Cav-1 (-/-) mice and subjected them to an unbiased proteomics analysis, as well as genome-wide transcriptional profiling.26 Proteomics analysis revealed the upregulation of (1) eight myofibroblasts markers (such as vimentin, calponin and tropomyosin); (2) eight glycolytic enzymes (including PKM2 and LDHA, among others); and (3) two markers of oxidative stress (catalase and peroxiredoxin1).26 Interestingly, these proteomics findings were directly supported by transcriptional profiling data, indicating that a loss of stromal Cav-1 has a strong effect on transcriptional control. Many of the markers that we identified by proteomics were also upregulated in the tumor stromal compartment of human breast cancers lacking stromal Cav-1, providing an indication that our findings may have direct clinical relevance.26 Based on these findings, we proposed that a loss of Cav-1 is sufficient to induce both a myofibroblastic phenotype and a glycolytic phenotype in stromal fibroblasts.26 This provides an indication that a loss of stromal Cav-1 induces aerobic glycolysis (a.k.a, the Warburg Effect) in CAFs.26 Interestingly, aerobic glycolysis is induced under normoxia via the stabilization of HIF1a via oxidative stress. Consistent with this idea, we observed that two markers of oxidative stress were increased in Cav-1 (-/-) stromal cells.26
As a consequence of these findings, we proposed a new model termed the “Reverse Warburg Effect,” where aerobic glycolysis in CAFs generates energy-rich metabolites (such as lactate and pyruvate) that are transferred to adjacent cancer cells, where they then enter the TCA cycle, promote oxidative phosphorylation and result in increased ATP production.11,12,26–28
The Autophagic Tumor Stroma Model of Cancer Metabolism: “Battery-Operated Tumor Growth”
To begin to understand the cellular processes that control the induction of aerobic glycolysis in fibroblasts, we performed an extensive informatics analysis on the transcriptional profiling data generated from WT and Cav-1 (-/-) null stromal cells.11 Via this analysis, we determined that a loss of stromal Cav-1 induces target genes associated with oxidative stress, HIF1-alpha and NFκB transcriptional activation.11
To validate these findings, we next created a co-culture system to study how breast cancer cells (MCF7) induce a loss of stromal Cav-1 in adjacent fibroblasts.29 Importantly, co-culture of MCF7 cells with human fibroblasts is sufficient to promote the onset of a CAF-like phenotype, with the induction of myo-fibroblast markers, the over-production of extracellular matrix proteins and activated TGFβ signaling.29 Under these conditions, we also observed that Cav-1 protein expression was significantly downregulated, and fibroblasts lost their mitochondria, while adjacent cancer cells showed a corresponding increase in mitochondrial mass/biogenesis.2,4,29 Mechanistically, a loss of Cav-1 was prevented by the administration of anti-oxidants (such as N-acetyl-cysteine (NAC), metformin and quercetin) or lysosomal inhibitors.2,4,29 This is consistent with the idea that, under these co-culture conditions, certain cell organelles (such as caveolae and mitochondria) in fibroblasts are targeted for “self-digestion” via autophagy. The effects of cancer cells on fibroblasts could be mimicked simply by subjecting fibroblasts to hypoxia or oxidative stress (via depletion of reduced glutathione), resulting in a loss of Cav-1 expression and the induction of autophagy.2,4,29 Conversely, the effects of fibroblasts on cancer cells could be mimicked by the addition of L-lactate to the culture media, resulting in increased mitochondrial mass/biogenesis in cancer cells.2,4,29 As such, we believe that cancer cells induce oxidative stress in adjacent fibroblasts, resulting in the onset of a myofibroblastic pro-autophagic phenotype.2,4,29 This pro-autophagic phenotype in fibroblasts then leads to a loss of mitochondria via autophagy (a.k.a, mitophagy), forcing tumor associated fibroblasts to undergo aerobic glycolysis (the “Reverse Warburg Effect”) (Fig. 2). The products of aerobic glycolysis (such as L-lactate) are then used by cancer cells for oxidative mitochondrial metabolism, resulting in increased mitochondrial mass in cancer cells.2,4,29 Thus, a loss of stromal Cav-1 is a biomarker for oxidative stress, hypoxia and autophagy in the tumor stromal micro-environment.2,4,29 We have termed this new idea “The Autophagic Tumor Stroma Model of Cancer Metabolism” (Fig. 2). In this sense, the “Reverse Warburg Effect” is a direct consequence of oxidative stress in fibroblasts driving the autophagic destruction of mitochondria, thereby committing these cells to aerobic glycolysis under conditions of normoxia.2,4,29
Figure 2.
The autophagic tumor stroma model of cancer metabolism: Role of oxidative stress, recycled nutrients and random mutagenesis. (A) Oxidative Stress, Recycled Nutrients and Resistance to Apoptosis. We have shown that human breast cancer cells induce ROS (reactive oxygen species) production in adjacent cancer-associated fibroblasts (CAFs), leading to the onset of stromal oxidative stress. Oxidative stress in CAFs, in turn, drives autophagy via HIF1 induction and NFκB activation, resulting in the autophagic destruction of mitochondria (mitophagy) and caveolin-1 (Cav-1). Stromal autophagy and mitophagy provides recycled nutrients via catabolism and aerobic glycolsysis to feed the appetite of adjacent cancer cells. These recycled chemical building blocks (derived from autophagy and aerobic glycolysis) “fuel” oxidative mitochondrial metabolism in cancer cells and provide resistance against apoptosis. Mechanistically, fibroblasts induce the expression of TIGAR in adjacent cancer cells, which shuts down autophagy and apoptosis in these cancer cells. (B) Random mutagenesis and tumor-stroma co-evolution. Oxidative stress in cancer-associated fibroblasts (CAFs) also produces a local DNA damage response, with increased ROS production. ROS amplification in the tumor stroma then leads to an antioxidant defense in adjacent cancer cells. In addition, stromal ROS production also leads to DNA damage in adjacent cancer cells via a “Bystander Effect”. As a consequence, stromal ROS promotes aneuploidy and genomic instability in cancer cells, driving tumor-stroma co-evolution. CAFs, cancer-associated fibroblasts; ROS, reactive oxygen species.
In these experiments, we also showed that oxidative stress in fibroblasts affects the apoptotic and genomic status of adjacent cancer cells (Fig. 2). For example, oxidative stress in fibroblasts specifically protected adjacent cancer cells against apoptosis via the induction of an antioxidant defense and the upregulation of antioxidant molecules, such as peroxiredoxin 1.2,4 Protection against apoptosis was probably also due to the recycled nutrients (amino acids, nucleotides and lactate/pyruvate) produced via stromal autophagy, leading to better overall mitochondrial “health” in cancer cells.2,4 This protection against apoptosis was even further increased by co-culturing cancer cells with Cav-1-deficient fibroblasts.2,4
Despite the fact that oxidative stress in fibroblast protects adjacent cancer cells from apoptosis, it also drives genomic instability in cancer cells. Oxidatives stress in fibroblasts amplifies ROS production, which, via a bystander effect, then induces DNA damage and aneuploidy/tetraploidy in cancer cells.2,4 As such, oxidative stress in adjacent fibroblasts drives random mutagenesis in cancer cells, resulting in tumor-stroma co-evolution.2,4
In summary, we propose a new mechanism for understanding the process of tumor-stroma co-evolution (Fig. 2). Cancer cells induce oxidative stress in adjacent fibroblasts, leading to the induction of stromal autophagy.2,4 This has at least three consequences: (1) induces genomic instability in cancer cells; (2) protects cancer cells against apoptosis; and (3) provides cancer cells with recycled nutrients, fueling the anabolic growth of the tumor.
Validating the Autophagic Tumor Stroma Model of Cancer
Metabolic, transcriptional and micro-RNA (miR) profiling of a “lethal” tumor microenvironment.
We next used Cav-1 (-/-) null mice as a pre-clinical model for a “lethal tumor microenvironment.”1 Unbiased metabolic profiling of Cav-1 (-/-) mammary fat pads revealed the upregulation of nearly 100 metabolites, indicative of a major catabolic phenotype. These findings were consistent with the induction of oxidative stress and autophagy/mitophagy.1 The two most prominent metabolites were ADMA (asymmetric dimethyl arginine) and BHB (β-hydroxybutyrate), which are markers of oxidative stress and mitochondrial dysfunction.1 Transcriptional profiling of human tumor stroma from breast cancer patients directly supported an association with oxidative stress and autophagy/mitophagy, as well as ADMA and ketone production.1 MicroRNA profiling of Cav-1 (-/-) stromal cells revealed the upregulation of two key miRs.1 Consistent with our model, these miRs are associated with oxidative stress (miR-34c) or activation of the hypoxic response/HIF1α (miR-31), which are both sufficient to drive authophagy/mitophagy. As such, these candidate biomarkers (ADMA, ketones and miR-31/34c) could be used to identify high-risk cancer patients at diagnosis.1 Autophagy/mitophagy in the tumor stromal compartment provides an effective means by which epithelial cancer cells can directly “feed off” of stromal-derived recycled nutrients and energy-rich metabolites, driving tumor progression and metastasis.1
To test this hypothesis, we evaluated whether the end products of aerobic glycolysis (3-hydroxy-butyrate and L-lactate) stimulated tumor growth and experimental metastasis using MDA-MB-231 breast cancer xenografts.3 Administration of 3-hydroxy-butryate (a ketone body) increased tumor growth by ∼2.5-fold, without any increase in tumor vascularization.3 Both 3-hydroxy-butyrate and L-lactate functioned as chemo-attractants, stimulating cancer cell migration.3 L-lactate did not increase primary tumor growth but stimulated lung metastasis by ∼10-fold.3 Thus, ketones and lactate fuel tumor growth and metastasis, providing functional evidence to support the “Reverse Warburg Effect.”3,27
The idea that a loss of caveolin-1 (Cav-1) in adipocytes triggers autophagy was also recently independently reported by Le Lay et al.30 More specifically, they showed that constitutive autophagy in mature adipocytes derived from Cav-1 (-/-) null mice may underlie their lipoatrophic whole-body phenotype, with global alterations in protein turn-over, accelerated degradation of long-lived proteins and increased lipidation of the LC3 autophagy marker.30 However, they did not address the possible implications of their findings for the pathogenesis of cancer.
Identification of the signaling pathway(s) regulating stromal autophagy: hypoxia, HIF1 induction and NFκB activation in the tumor microenvironment.
The signaling mechanism(s) underlying “The Autophagic Tumor Stroma Model of Cancer” remained largely unknown. Interestingly, hypoxia is sufficient to induce the autophagic degradation of Cav-1 in fibroblasts.4 Based on a series of inhibitor-based studies, we observed that hypoxia and oxidative stress both mediate the induction of HIF1- and NFκB-activation in fibroblasts, driving the autophagic degradation of Cav-1.4 In support of this hypothesis, MCF7 cancer cells activated HIF-1α- and NFκB-driven luciferase reporters in adjacent cancer-associated fibroblasts.4 In addition, acute knockdown of Cav-1 in stromal fibroblasts, using an siRNA approach, was sufficient to induce autophagy, with the upregulation of both lysosomal and mitophagy markers.4 Moreover, a loss of Cav-1 in stromal fibroblasts protected adjacent cancer cells against apoptotic cell death. Thus, autophagy in cancer-associated fibroblasts (1) provides recycled nutrients for cancer cell metabolism but (2) also prevents the death of adjacent epithelial cancer cells.4 For this purpose, cancer-associated fibroblasts upregulate the expression of TIGAR in adjacent epithelial cancer cells, conferring resistance to apoptosis and autophagy.4 These findings directly support the “Autophagic Tumor Stroma Model of Cancer Metabolism,” and explain the exceptional prognostic value of a loss of stromal Cav-1 in cancer patients.1,2 As such, a loss of fibroblast Cav-1 is a biomarker for hypoxia, oxidative stress and autophagy in the tumor stroma.2 Cancer patients lacking stromal Cav-1 would likely benefit from HIF-inhibitors, NFκB-inhibitors, antioxidant therapies as well as autophagy/lysosomal inhibitors.2
Autophagy in cancer associated fibroblasts fuels tumor growth, while autophagy in cancer cells retards tumor growth.
We hypothesized that a loss of stromal caveolin-1 (Cav-1) expression and HIF1-α activation could drive the cancer-associated fibroblast phenotype, through the paracrine production of nutrients via autophagy and aerobic glycolysis.2,11 To this end, we expressed activated HIF1α in fibroblasts and examined their ability to promote tumor growth using a xenograft model that employs human breast cancer cells (MDA-MB-231).5 Fibroblasts with activated HIF1α showed reductions in Cav-1 protein levels and a shift towards aerobic glycolysis with a loss of mitochondrial activity and increased lactate production.5 Consistent with these phenotypic changes, activated HIF1α induced BNIP3 and BNIP3L, both markers for the autophagic destruction of mitochondria.5 Functionally, fibroblasts expressing activated HIF1α increased tumor mass by ∼2-fold and tumor volume by ∼3-fold, without an increase in angiogenesis.5 Similarly, HIF1α-transfected fibroblasts increased the lymph node metastasis of cancer cells. Driving NFkB activation in fibroblasts, another inducer of autophagy, yielded virtually identical results. As such, activated HIF1α functionally confers the cancer-associated fibroblast phenotype.5
HIF1α expression is also required for the induction of autophagy in cancer cells. Thus, we directly expressed activated HIF1α in MDA-MB-231 cells and assessed its effects on tumor growth. Surprisingly, activated HIF1α in cancer cells dramatically suppressed tumor growth, resulting in a two-fold reduction in tumor mass and a three-fold reduction in tumor volume.5 As such, HIF1α activation in different cell types can either promote or repress tumorigenesis.5 Based on these studies, autophagy in cancer-associated fibroblasts promotes tumor growth via the paracrine production of recycled nutrients that can “feed” cancer cells. However, autophagy in cancer cells represses tumor growth via their “self-digestion.” These results provide direct experimental support for the “Autophagic Tumor Stroma Model of Cancer.”5
Implications of the “Autophagic Tumor Stroma Model” for Cancer-Associated Cachexia (Wasting) and Cancer Chemotherapy
Cancer cells activate autophagy in the tumor stromal compartment via paracrine mechanisms (Fig. 3). Autophagy in the tumor stroma, especially in cancer-associated fibroblasts, then provides cancer cells with a steady stream of recycled nutrients and energy-rich metabolites, which are then re-used by cancer cells to drive tumor growth and metastasis. Thus, stromal catabolism “fuels” anabolic tumor growth.1,2,4 Bone marrow-derived mesenchymal stem cells could be continually be recruited to the tumor and induced to undergo autophagy to satisfy the tumor's appetite.
Figure 3.
Understanding energy transfer in cancer metabolism: Stromal autophagy and cancer-associated cachexia. Diagram summarizing that autophagy in the tumor stroma is used by adjacent epithelial cells to fuel tumor growth via oxidative mitochondrial metabolism. This local phenomenon may spread systemically and explains the onset of cancer-associated cachexia, characterized by systemic wasting and a negative net energy balance. A+ signifies stromal autophagy, which results in fuel production. A− signifies an absence of autophagy in cancer cells. AR signifies genetic resitance to autophagy in cancer cells, e.g., when Beclin1 gene expression is silenced. Both A− and AR would be associated with fuel consumption by cancer cells. The direction of energy flow, from the autophagic tumor stroma to the anabolic cancer cells, is indicated by the direction of a blue arrow.
Extension of this idea from a local to a systemic phenomenon may explain the onset of cancer-associated cachexia, which is associated with chronic malignancy (Fig. 3).31–35 Autophagy in the tumor stroma may be the local microscopic counterpart of systemic wasting (cancer-associated cachexia). Cachexia in cancer patients is not due to decreased energy intake, but instead involves an increased basal metabolic rate and increased energy expenditures, resulting in a negative energy balance.31–35 Importantly, when tumors were surgically excised, this increased metabolic rate returned to normal levels. This view of cachexia, resulting in energy transfer to the tumor, is consistent with our hypothesis. So, cachexia may start locally, as stromal autophagy, and then spread systemically (local autophagy versus systemic autophagy) (Fig. 3).
In support of this model, epithelial tumor cells induce autophagy in cancer-associated fibroblasts via oxidative stress, driving the autophagic/lysosomal degradation of Cav-1.2,4,29 Under these conditions, Cav-1 degradation in cancer-associated fibroblasts was inhibited by antioxidants (such as N-acetyl-cysteine) or autophagy/lysosomal inhibitors (such as chloroquine).2,4,29 Similarly, acute knock-down of Cav-1 in fibroblasts using an siRNA approach was sufficient to induce ROS production, oxidative stress, mitochondrial dysfunction and autophagy/mitophagy.2,4,29 Thus, loss of stromal Cav-1 is both up-stream and down-stream of oxidative stress and autophagy in cancer-associated fibroblasts.
Similarly, others have proposed that cancer-associated cachexia is due to systemic oxidative stress (reflected by decreased glutathione levels), which can be successfully treated with antioxidants, such as N-acetyl-cysteine.36–43 Work with animal models also directly supports the idea that cancer-associated cachexia is driven by oxidative stress (ROS) and nitrosative stress (RNS) (a.k.a., a redox imbalance).44–46 As such, stromal autophagy may be the requisite precursor of systemic cancer-associated cachexia.
This new model of “stromal autophagy” also provides a rational basis for designing new therapeutic intervention(s). Thus, inhibition of autophagy in the tumor stroma could halt or reverse tumor growth. This would explain the effectiveness of known autophagy inhibitors as anti-tumor agents, such as chloroquine and 3-methyladenine. Conversely, induction of autophagy in epithelial cancer cells would block or inhibit tumor growth. This idea would explain the anti-tumor activity of agents that activate autophagy, such as mTOR inhibitors (like rapamycin). Rapamycin derivatives temsirolimus and everolimus are effective anti-cancer therapies and have now been approved for the treatment of renal cell carcinoma in the US.47,48 Thus, using this model, compounds that either systemically block or systemically activate autophagy would have the same net effect: disrupting the metabolic coupling between the epithelial cancer cells and the tumor stromal fibroblasts (Fig. 4). As such, this model directly resolves the long-lived “Autophagy Paradox,” that both systemic inhibition of autophagy and systemic stimulation of autophagy have the same net effect, which is to inhibit tumor growth. This new model requires further experimental validation; however, it does provide a new paradigm and rationale for drug development, driving new metabolic therapeutic interventions.
Figure 4.
A simple solution to the autophagy paradox: “Battery Operated Tumor Growth”. The role of autophagy in tumorigenesis is controversial. Both autophagy inhibitors and autophagy promoters block tumorigenesis. This is called the “Autophagy Paradox”. We have reported a simple solution to this paradox. Mechanistically, we have shown that autophagy/catabolism in the tumor stroma drives the anabolic growth of adjacent cancer cells and tumor progression. In this sense, autophagy in the tumor stroma serves a “battery” to fuel tumor growth, progression and metastasis, independently of angiogenesis. Using this model, systemic induction of autophagy (with rapamycin) will prevent epithelial cancer cells from using recycled nutrients, while the systemic inhibiton of autophagy (with chloroquine) will prevent stromal cells from producing recycled nutrients—both effectively “starving” cancer cells. Tumor cells could become resistant to the systemic induction of autophagy by the upregulation of natural endogenous autophagy inhibitors in cancer cells (such as TIGAR). Alternatively, tumor cells could also become resistant to the systemic induction of autophagy by the genetic silencing of pro-autophagic molecules, such as Beclin1. If this occurs, then the systemic inhibition of autophagy would provide a therapeutic solution to this new type of drug resistance. CAFs, cancerassociated fibroblasts.
This new model may also have implications for understanding the development of drug resistance (Fig. 4). For example, tumor cells could become resistant to the systemic induction of autophagy by the upregulation of natural endogenous autophagy inhibitors in cancer cells, such as TIGAR.2 Conversely, tumor cells could also become resistant to the systemic induction of autophagy by the genetic silencing/deletion of pro-autophagic molecules, such as Beclin1.49–53 If this occurs, then the systemic inhibition of autophagy would provide a therapeutic solution to this new type of drug resistance. As such, an anti-cancer therapy that combines the alternating use of both autophagy promoters and autophagy inhibitors would be expected to prevent the onset of drug resistance.
Anti-Angiogenic Therapy Promotes Tumor Progression and Metastasis by Inducing Hypoxia and Autophagy in the Tumor Micro-Enviroment, Thereby Fueling Tumor-Stroma Co-evolution
Anti-angiogenic therapy54,55 has been found to promote tumor recurrence, progression and metastasis.56–65 A major contributing factor appears to be that anti-angiogenic therapy induces severe hypoxia54,55 in the tumor micro-environment.56–69
For example, phase III trials of antiangiogenic therapy with bevacizumab have shown mixed results.70,71 In fact, the Oncologic Drugs Advisory Committee (ODAC) of the FDA on July 20, 2010 voted 12 to 1 against the use of bevacizumab in combination with chemotherapy for metastatic breast cancer, which had been granted accelerated approval in 2008 pending further studies (www.cancer.gov/ncicancerbulletin/072710/page2).
The “Autophagic Tumor Stroma Model of Cancer” now provides a rational explanation for understanding this phenomenon. More specifically, anti-angiogenic therapy would drive autophagy in the tumor stroma via the induction of stromal hypoxia, thereby converting a non-aggressive tumor type to a “lethal” aggressive tumor phenotype (Fig. 5).
Figure 5.
Understanding how anti-angiogenic therapy increases tumor progression, recurrence and metastasis via hypoxia in the tumor stroma. (A) Flow diagram summarizing how anti-angiogenic therapy drives a hypoxic/autophagic response in the tumor stromal micro-environment, which experimentally leads to tumor progression, recurrence and metastasis. This view is also supported by the lack of efficacy of anti-angiogenesis inhibitors in a variety of clinical trials in humans. (B) Converting a non-aggressive tumor to a “lethal” tumor via anti-angiogenic therapy. We have shown that autophagy in the tumor stroma fuels the anabolic growth of cancer cells and tumor progression. Thus, a non-aggressive tumor would lack stromal autophagy. This premise is supported by the use of stromal Cav-1 as a biomarker of autophagy. High stromal Cav-1 would be predictive of an absence of stromal autophagy and good clinical outcome. Conversely, low or absent stromal Cav-1 would be predictive of a high rate of stromal autophagy and poor clinical outcome. This is what we observed experimentally in our pre-clinical models and in our translational biomarker studies. Based on our model of “Battery-Operated Tumor Growth,” anti-angiogenic therapy would induce hypoxia and autophagy in the tumor stromal micro-enviroment, promoting net energy transfer and tumor progression and leading to a “lethal” tumor phenotype.
This idea is supported by the use of stromal Cav-1 as a biomarker for autophagy. High stromal Cav-1 is predictive of an absence of stromal autophagy,2,4 a low rate of breast cancer recurrence and metastasis and good clinical outcome.8,9 Conversely, low or absent stromal Cav-1 is predictive of a high rate of stromal autophagy,2,4 with increased breast cancer recurrence and metastasis, resulting in poor clinical outcome.8,9
Opportunities for New Biomarker Discovery: Stromal Autophagy and Mitophagy
Given that a loss of stromal Cav-1 is a powerful independent biomarker for predicting poor clinical outcome in cancer patients, we should be able to use this to our advantage to discover new stromal biomarkers. For this purpose, we have extensively subjected Cav-1 (-/-) stromal cells and tissues to unbiased proteomic analyses and genome-wide transcriptional profiling. As mentioned above, this approach led to the identification of aerobic glycolysis and autophagy/mitophagy in Cav-1 (-/-) stromal cells. Thus, the stromal expression of (1) markers of aerobic glycolysis (such as PKM2 and LDH-B) and (2) markers of autophagy/mitophagy (such BNIP3L) may be effective new biomarkers to identifying high-risk cancer patients.4,26,27 In accordance with this hypothesis, PKM2, LDH-B and BNIP3L are highly expressed in the tumor stromal compartment of human breast cancer patients that lack the stromal expression of Cav-1 (Fig. 6). This provides further translational evidence to support “The Autophagic Tumor Stroma Model of Cancer Metabolism.”
Figure 6.
Aerobic glycolysis and mitophagy in the tumor stromal compartment. PKM2, LDH-B and BNIP3L are all highly expressed in the tumor stromal compartment of human breast cancers that lack the stromal expression of Cav-1. PKM2 and LDH-B are markers of aerobic glycolysis, while BNIP3L is a functional marker of the autophagic destruction of mitochondria (mitophagy). Images were reproduced from references 4, 26 and 27, with permission from the publisher (Landes Bioscience).
Conclusions
In summary, we believe that cancer is like a “Trojan horse” or “surprise attack.” Tumor cells send out a “cry for help,” a warning signal associated with cell injury. This represents oxidative stress. Then, cancer cells mount an antioxidant defense to protect themselves from autophagy and apoptosis (cell death). In the meantime, mesenchymal stem cells and fibroblasts are recruited to the false site of injury to feed the cancer. Once in the proximity of cancer cells, these stromal cancer-associated fibroblasts are hypnotized by oxidative stress and are forced to eat themselves (stromal autophagy) to feed the cancer cells. We believe that this can then extend from a local phenomenon to a systemic whole-body process, which is known as cancer-associated cachexia or wasting. In this regard, cancer-associated cachexia may represent a form of whole-body or total body autophagy, with the exception of the tumor. The “infectious” spread of autophagy from a local area to the rest of the body may be achieved by local oxidative stress and the resulting systemic inflammatory response produced by the body's innate immune reaction to this “surprise attack.”
Clinical Significance and Future Directions
In essence, we think that cancer starts as a seemingly innocuous point source of oxidative stress. This node or nodule of oxidative stress then uses stromal autophagy as a metabolic fuel source to transform itself into an highly aggressive tumor. Thus, effective cancer chemotherapy would involve cutting off this “fuel supply,” with powerful antioxidants (i.e., N-acetyl cysteine, quercetin or metformin) and systemic modulators of autophagy (i.e., chloroquine or rapamycin and its derivatives). The good news is that many of these compounds are already sitting on the shelf and are currently available as OTC (over-the-counter) dietary supplements or existing FDA-approved drugs. Thus, new clinical trials may be warranted.
Acknowledgements
M.P.L. and his laboratory were supported by grants from the NIH/NCI (R01-CA-080250; R01-CA-098779; R01-CA-120876; R01-AR-055660) and the Susan G. Komen Breast Cancer Foundation. F.S. was supported by grants from the W.W. Smith Charitable Trust, the Breast Cancer Alliance (BCA) and a Research Scholar Grant from the American Cancer Society (ACS). A.K.W. was supported by a Young Investigator Award from Breast Cancer Alliance, Inc. and a Susan G. Komen Career Catalyst Grant. R.G.P. was supported by grants from the NIH/NCI (R01-CA-70896, R01-CA-75503, R01-CA-86072 and R01-CA-107382) and the Dr. Ralph and Marian C. Falk Medical Research Trust. The Kimmel Cancer Center was supported by the NIH/NCI Cancer Center Core grant P30-CA-56036 (to R.G.P.). Funds were also contributed by the Margaret Q. Landenberger Research Foundation (to M.P.L.). This project is funded, in part, under a grant from the Pennsylvania Department of Health (to M.P.L.). The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. This work was also supported, in part, by a Centre grant in Manchester from Breakthrough Breast Cancer in the UK (to A.H.) and an Advanced ERC Grant from the European Research Council.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/13817
References
- 1.Pavlides S, Tsirigos A, Migneco G, Whitaker-Menezes D, Chiavarina B, Flomenberg N, et al. The autophagic tumor stroma model of cancer: Role of oxidative stress and ketone production in fueling tumor cell metabolism. Cell Cycle. 2010;9:3485–3505. doi: 10.4161/cc.9.17.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, Chiavarina B, Pavlides S, Wang C, et al. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: A new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle. 2010;9:3256–3276. doi: 10.4161/cc.9.16.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonuccelli G, Tsirigos A, Whitaker-Menezes D, Pavlides S, Pestell RG, Chiavarina B, et al. Ketones and lactate “fuel” tumor growth and metastasis: Evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle. 2010;9:3506–3514. doi: 10.4161/cc.9.17.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez-Outschoorn UE, Trimmer C, Lin Z, Whitaker-Menezes D, Chiavarina B, Zhou J, et al. Autophagy in cancer associated fibroblasts promotes tumor cell survival: Role of hypoxia, HIF1 induction and NFκB activation in the tumor stromal microenvironment. Cell Cycle. 2010;9:3515–3533. doi: 10.4161/cc.9.17.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiavarina B, Whitaker-Menezes D, Migneco G, Martinez-Outschoorn UE, Pavlides S, Howell A, et al. HIF1-alpha functions as a tumor promoter in cancer associated fibroblasts and as a tumor suppressor in breast cancer cells: Autophagy drives compartment-specific oncogenesis. Cell Cycle. 2010;9:3534–3551. doi: 10.4161/cc.9.17.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witkiewicz AK, Casimiro MC, Dasgupta A, Mercier I, Wang C, Bonuccelli G, et al. Towards a new “stromal-based” classification system for human breast cancer prognosis and therapy. Cell Cycle. 2009;8:1654–1658. doi: 10.4161/cc.8.11.8544. [DOI] [PubMed] [Google Scholar]
- 7.Witkiewicz AK, Dasgupta A, Nguyen KH, Liu C, Kovatich AJ, Schwartz GF, et al. Stromal caveolin-1 levels predict early DCIS progression to invasive breast cancer. Cancer Biol Ther. 2009;8:1167–1175. doi: 10.4161/cbt.8.11.8874. [DOI] [PubMed] [Google Scholar]
- 8.Witkiewicz AK, Dasgupta A, Sammons S, Er O, Potoczek MB, Guiles F, et al. Loss of stromal caveolin-1 expression predicts poor clinical outcome in triple negative and Basal-like breast cancers. Cancer Biol Ther. 2010;10:135–143. doi: 10.4161/cbt.10.2.11983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witkiewicz AK, Dasgupta A, Sotgia F, Mercier I, Pestell RG, Sabel M, et al. An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am J Pathol. 2009;174:2023–2034. doi: 10.2353/ajpath.2009.080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Vizio D, Morello M, Sotgia F, Pestell RG, Freeman MR, Lisanti MP. An absence of stromal caveolin-1 is associated with advanced prostate cancer, metastatic disease and epithelial Akt activation. Cell Cycle. 2009;8:2420–2424. doi: 10.4161/cc.8.15.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavlides S, Tsirigos A, Vera I, Flomenberg N, Frank PG, Casimiro MC, et al. Loss of stromal caveolin-1 leads to oxidative stress, mimics hypoxia and drives inflammation in the tumor microenvironment, conferring the “Reverse Warburg Effect”: A transcriptional informatics analysis with validation. Cell Cycle. 2010;9:2201–2219. doi: 10.4161/cc.9.11.11848. [DOI] [PubMed] [Google Scholar]
- 12.Pavlides S, Tsirigos A, Vera I, Flomenberg N, Frank PG, Casimiro MC, et al. Transcriptional evidence for the “Reverse Warburg Effect” in human breast cancer tumor stroma and metastasis: similarities with oxidative stress, inflammation, Alzheimer's disease and “Neuron-Glia Metabolic Coupling”. Aging. 2010;2:185–199. doi: 10.18632/aging.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Z, Klionsky DJ. An overview of the molecular mechanism of autophagy. Curr Top Microbiol Immunol. 2009;335:1–32. doi: 10.1007/978-3-642-00302-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yen WL, Klionsky DJ. How to live long and prosper: Autophagy, mitochondria and aging. Physiology. 2008;23:248–262. doi: 10.1152/physiol.00013.2008. [DOI] [PubMed] [Google Scholar]
- 16.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW. The organizing principle: Microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537–546. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghajar CM, Meier R, Bissell MJ. Quis custodiet ipsos custodies: Who watches the watchmen? Am J Pathol. 2009;174:1996–1999. doi: 10.2353/ajpath.2009.090363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ronnov-Jessen L, Bissell MJ. Breast cancer by proxy: Can the microenvironment be both the cause and consequence? Trends Mol Med. 2009;15:5–13. doi: 10.1016/j.molmed.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 22.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 23.Mercier I, Casimiro MC, Wang C, Rosenberg AL, Quong J, Allen KG, et al. Human beast cancer-associated fibroblasts (CAFs) show caveolin-1 downregulation and RB tumor suppressor functional inactivation: Implications for the response to hormonal therapy. Cancer Biol Ther. 2008;7:1212–1225. doi: 10.4161/cbt.7.8.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sloan EK, Ciocca D, Pouliot N, Natoli A, Restall C, Henderson M, et al. Stromal cell expression of caveolin-1 predicts outcome in breast cancer. Am J Pathol. 2009;174:2035–2043. doi: 10.2353/ajpath.2009.080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 26.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, et al. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 27.Bonuccelli G, Whitaker-Menezes D, Castello-Cros R, Pavlides S, Pestell RG, Fatatis A, et al. The Reverse Warburg Effect: Glycolysis inhibitors prevent the tumor promoting effects of caveolin-1 deficient cancer-associated fibroblasts. Cell Cycle. 2010;9:1960–1971. doi: 10.4161/cc.9.10.11601. [DOI] [PubMed] [Google Scholar]
- 28.Migneco G, Whitaker-Menezes D, Chiavarina B, Castello-Cros R, Pavlides S, Pestell RG, et al. Glycolytic cancer-associated fibroblasts promote breast cancer tumor growth, without a measurable increase in angiogenesis: Evidence for stromal-epithelial metabolic coupling. Cell Cycle. 2010;9:2412–2422. doi: 10.4161/cc.9.12.11989. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Outschoorn UE, Pavlides S, Whitaker-Menezes D, Daumer KM, Milliman JN, Chiavarina B, et al. Tumor cells induce the cancer-associated fibroblast phenotype via caveolin-1 degradation: Implications for breast cancer and DCIS therapy with autophagy inhibitors. Cell Cycle. 2010;9:2423–2433. doi: 10.4161/cc.9.12.12048. [DOI] [PubMed] [Google Scholar]
- 30.Le Lay S, Briand N, Blouin CM, Chateau D, Prado C, Lasnier F, et al. The lipoatrophic caveolin-1 deficient mouse model reveals autophagy in mature adipocytes. Autophagy. 2010;6:754–763. doi: 10.4161/auto.6.6.12574. [DOI] [PubMed] [Google Scholar]
- 31.Warnold I, Lundholm K, Schersten T. Energy balance and body composition in cancer patients. Cancer Res. 1978;38:1801–1807. [PubMed] [Google Scholar]
- 32.Bosaeus I, Daneryd P, Svanberg E, Lundholm K. Dietary intake and resting energy expenditure in relation to weight loss in unselected cancer patients. Int J Cancer. 2001;93:380–383. doi: 10.1002/ijc.1332. [DOI] [PubMed] [Google Scholar]
- 33.Fouladiun M, Korner U, Bosaeus I, Daneryd P, Hyltander A, Lundholm KG. Body composition and time course changes in regional distribution of fat and lean tissue in unselected cancer patients on palliative care—correlations with food intake, metabolism, exercise capacity and hormones. Cancer. 2005;103:2189–2198. doi: 10.1002/cncr.21013. [DOI] [PubMed] [Google Scholar]
- 34.Norton JA, Peacock JL, Morrison SD. Cancer cachexia. Crit Rev Oncol Hematol. 1987;7:289–327. doi: 10.1016/s1040-8428(87)80003-3. [DOI] [PubMed] [Google Scholar]
- 35.Barber MD, Ross JA, Fearon KC. Cancer cachexia. Surg Oncol. 1999;8:133–141. doi: 10.1016/s0960-7404(99)00045-6. [DOI] [PubMed] [Google Scholar]
- 36.Droge W, Hack V, Breitkreutz R, Holm E, Shubinsky G, Schmid E, et al. Role of cysteine and glutathione in signal transduction, immunopathology and cachexia. Biofactors. 1998;8:97–102. doi: 10.1002/biof.5520080117. [DOI] [PubMed] [Google Scholar]
- 37.Hack V, Schmid D, Breitkreutz R, Stahl-Henning C, Drings P, Kinscherf R, et al. Cystine levels, cystine flux and protein catabolism in cancer cachexia, HIV/SIV infection and senescence. Faseb J. 1997;11:84–92. doi: 10.1096/fasebj.11.1.9034170. [DOI] [PubMed] [Google Scholar]
- 38.Droge W, Gross A, Hack V, Kinscherf R, Schykowski M, Bockstette M, et al. Role of cysteine and glutathione in HIV infection and cancer cachexia: therapeutic intervention with N-acetylcysteine. Adv Pharmacol. 1997;38:581–600. doi: 10.1016/s1054-3589(08)61000-5. [DOI] [PubMed] [Google Scholar]
- 39.Ushmorov A, Hack V, Droge W. Differential reconstitution of mitochondrial respiratory chain activity and plasma redox state by cysteine and ornithine in a model of cancer cachexia. Cancer Res. 1999;59:3527–3534. [PubMed] [Google Scholar]
- 40.Mantovani G, Maccio A, Madeddu C, Mulas C, Massa E, Astara G, et al. Phase II study of subcutaneously administered interleukin-2 in combination with medroxyprogesterone acetate and antioxidant agents as maintenance treatment in advanced cancer responders to previous chemotherapy. Oncol Rep. 2002;9:887–896. [PubMed] [Google Scholar]
- 41.Mantovani G, Maccio A, Madeddu C, Mura L, Gramignano G, Lusso MR, et al. Antioxidant agents are effective in inducing lymphocyte progression through cell cycle in advanced cancer patients: assessment of the most important laboratory indexes of cachexia and oxidative stress. J Mol Med. 2003;81:664–673. doi: 10.1007/s00109-003-0476-1. [DOI] [PubMed] [Google Scholar]
- 42.Mantovani G, Maccio A, Madeddu C, Mura L, Massa E, Gramignano G, et al. Reactive oxygen species, antioxidant mechanisms and serum cytokine levels in cancer patients: impact of an antioxidant treatment. J Cell Mol Med. 2002;6:570–582. doi: 10.1111/j.1582-4934.2002.tb00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mantovani G, Madeddu C, Gramignano G, Lusso MR, Mocci M, Massa E, et al. Subcutaneous interleukin-2 in combination with medroxyprogesterone acetate and antioxidants in advanced cancer responders to previous chemotherapy: phase II study evaluating clinical, quality of life and laboratory parameters. J Exp Ther Oncol. 2003;3:205–219. doi: 10.1046/j.1359-4117.2003.01096.x. [DOI] [PubMed] [Google Scholar]
- 44.Marin-Corral J, Fontes CC, Pascual-Guardia S, Sanchez F, Olivan M, Argiles JM, et al. Redox balance and carbonylated proteins in limb and heart muscles of cachectic rats. Antioxid Redox Signal. 2010;12:365–380. doi: 10.1089/ars.2009.2818. [DOI] [PubMed] [Google Scholar]
- 45.Barreiro E, de la Puente B, Busquets S, Lopez-Soriano FJ, Gea J, Argiles JM. Both oxidative and nitrosative stress are associated with muscle wasting in tumour-bearing rats. FEBS Lett. 2005;579:1646–1652. doi: 10.1016/j.febslet.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 46.Argiles JM. Cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9:39–50. doi: 10.1016/j.ejon.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alpha or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 48.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 49.Li Z, Chen B, Wu Y, Jin F, Xia Y, Liu X. Genetic and epigenetic silencing of the beclin 1 gene in sporadic breast tumors. BMC Cancer. 2010;10:98. doi: 10.1186/1471-2407-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miracco C, Cosci E, Oliveri G, Luzi P, Pacenti L, Monciatti I, et al. Protein and mRNA expression of autophagy gene Beclin 1 in human brain tumours. Int J Oncol. 2007;30:429–436. [PubMed] [Google Scholar]
- 51.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 52.Edinger AL, Thompson CB. Defective autophagy leads to cancer. Cancer Cell. 2003;4:422–424. doi: 10.1016/s1535-6108(03)00306-4. [DOI] [PubMed] [Google Scholar]
- 53.Sun Q, Fan W, Zhong Q. Regulation of Beclin 1 in autophagy. Autophagy. 2009;5:713–716. doi: 10.4161/auto.5.5.8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blagosklonny MV. Hypoxia-inducible factor: Achilles' heel of antiangiogenic cancer therapy. Int J Oncol. 2001;19:257–262. doi: 10.3892/ijo.19.2.257. [DOI] [PubMed] [Google Scholar]
- 55.Blagosklonny MV. Antiangiogenic therapy and tumor progression. Cancer Cell. 2004;5:13–17. doi: 10.1016/s1535-6108(03)00336-2. [DOI] [PubMed] [Google Scholar]
- 56.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 57.Steeg PS. Angiogenesis inhibitors: motivators of metastasis? Nat Med. 2003;9:822–823. doi: 10.1038/nm0703-822. [DOI] [PubMed] [Google Scholar]
- 58.Grepin R, Pages G. Molecular mechanisms of resistance to tumour anti-angiogenic strategies. J Oncol. 2010;2010:835680. doi: 10.1155/2010/835680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mancuso MR, Davis R, Norberg SM, O'Brien S, Sennino B, Nakahara T, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 61.Rapisarda A, Melillo G. Role of the hypoxic tumor microenvironment in the resistance to anti-angiogenic therapies. Drug Resist Updat. 2009;12:74–80. doi: 10.1016/j.drup.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ide T, Kitajima Y, Miyoshi A, Ohtsuka T, Mitsuno M, Ohtaka K, et al. Tumor-stromal cell interaction under hypoxia increases the invasiveness of pancreatic cancer cells through the hepatocyte growth factor/c-Met pathway. Int J Cancer. 2006;119:2750–2759. doi: 10.1002/ijc.22178. [DOI] [PubMed] [Google Scholar]
- 63.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kerbel RS. Therapeutic implications of intrinsic or induced angiogenic growth factor redundancy in tumors revealed. Cancer Cell. 2005;8:269–271. doi: 10.1016/j.ccr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 67.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 68.Peinado H, Cano A. A hypoxic twist in metastasis. Nat Cell Biol. 2008;10:253–254. doi: 10.1038/ncb0308-253. [DOI] [PubMed] [Google Scholar]
- 69.Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 70.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 71.Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, et al. Randomized phase III trial of capecitabine compared with bevacizumb plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]