Abstract
Activation of the DNA damage response (DDR) is critical for genomic integrity and tumor suppression. The occurrence of DNA damage quickly evokes the DDR through ATM/ATR-dependent signal transduction, which promotes DNA repair and activates the checkpoint to halt cell cycle progression. The shut off process of the DDR upon satisfaction of DNA repair, also known as “checkpoint recovery,” involves deactivation of DDR elements, but the mechanism is poorly understood. Greatwall kinase (Gwl) has been identified as a key element in the G2/M transition and helps maintain M phase through inhibition of PP2A/B55δ, the principal phosphatase for Cdk-phosphorylated substrates. Here, we show that Gwl also promotes recovery from DNA damage and is itself directly inhibited by the DNA damage response (DDR). In Xenopus egg extracts, immunodepletion of Gwl increased the DDR to damaged DNA, whereas addition of wild-type, but not kinase-dead Gwl, inhibited the DDR. The removal of damaged DNA from egg extracts leads to recovery from checkpoint arrest and entry into mitosis, a process impaired by Gwl depletion and enhanced by Gwl overexpression. Moreover, activation of Cdk1 after the removal of damaged DNA is regulated by Gwl. Collectively, these results defines Gwl as a new regulator of the DDR, which plays an important role in recovery from DNA damage.
Key words: Greatwall,; DNA damage; checkpoint recovery
Introduction
How cells recover from activation of the DDR is starting to be revealed from studies in several laboratories. Many, if not all, key DDR factors associate with phosphatase complexes that mediate their dephosphorylation and inactivation during recovery.1 Regulated proteolysis is also involved in checkpoint recovery, as best illustrated by degradation of Claspin and Wee1 through the β-TrCP-SCF ubiquitin ligase pathway.2–4 Recognition of Claspin and Wee1 by β-TrCP-SCF requires Plk1-dependent phosphorylation, consistent with other evidence for a critical role of Plk1 in checkpoint inactivation and recovery during G2 phase.5–9 Deactivation of checkpoint signaling, including Claspin, Chk1, Chk2, etc., supports activation of Cdc25 phosphatases, which promote activation of MPF (maturation-promoting factor, Cdc2/cyclin B) and thus cell cycle progression into mitosis.10,11
Greatwall (Gwl) was first identified in Drosophila as a nuclear protein required for proper chromosome condensation and mitotic progression.12,13 Further studies in the Xenopus system showed that Gwl is a protein kinase activated during mitosis; depletion of Gwl from mitotic extracts rapidly lowers MPF activity through accumulation of inhibitory phosphorylation on Cdc2, and Gwl depletion from interphase extracts prevents entry into M phase.14,15 Moreover, activated Gwl accelerates the mitotic G2/M transition in cycling egg extracts and induces meiotic maturation in G2-arrested Xenopus oocytes in the absence of progesterone.16 Activated Greatwall can induce phosphorylation of Cdc25 independently of Cdc2, Plx1, MAPK or PKA.16 Recent evidence in both Xenopus and human cells revealed the mechanism that underlies Gwl function: it promotes inactivation of PP2A/B55δ, a phosphatase directed against Cdk phosphorylation sites whose activity governs mitotic entry and exit.17–23
Results
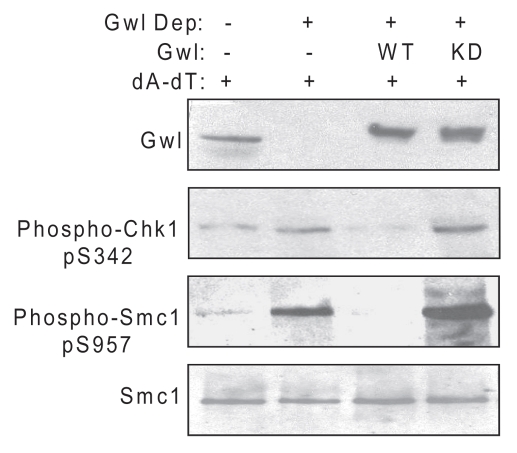
In interphase egg extracts, the addition of double-stranded oligonucleotides (dA-dT) to mimic double-stranded breaks in DNA leads to activation of the DDR, as judged by increased phosphorylation of the ATM/ATR targets Chk1 and Smc1 (Figs. 1 and 2A and reviewed in ref. 24–28). To test directly the potential involvement of Gwl in the process, we immunodepleted Gwl from the extract and observed elevated levels of Chk1 and Smc1 phosphorylation induced by dA-dT. This effect was suppressed by addition of recombinant wild-type, but not kinase-dead, Gwl (Fig. 1A). These results establish Gwl as a negative regulator of DDR signaling. Interestingly, kinase-dead Gwl consistently increased the checkpoint signals induced by dA-dT, suggesting action as a dominant negative regulator of the DDR. It should be noted that depletion of Gwl was not sufficient to activate the DDR in extracts without the addition of dA-dT (data not shown).
Figure 1.
Gwl negatively regulates the DNA damage response. Interphase Xenopus egg extracts were mock-treated (beads alone), immunodepleted for Gwl or Gwl-depleted and then reconstituted with purified wild-type (WT) or kinase-dead (KD) Gwl, prepared as described previously.15 Extracts were then supplemented with double-stranded oligonucleotides (dA-dT) at 20 ug/ml, incubated for 30 min at room temperature and analyzed for the DDR by western blotting using the indicated antibodies.
Figure 2.
DNA damage inhibits Gwl activity. (A) Interphase Xenopus egg extracts with or without dA-dT (20 ug/ml) were incubated at room temperature for 15 (−), 30 or 90 min, as indicated. Fifteen min before harvest, the extract was transferred to a tube containing a FLAG-Gwl bead pellet corresponding to the amount of Gwl expressed in three oocytes. At the end of the incubation, Gwl beads were spun down and used for kinase assay as described in Materials and Methods. The supernatant was supplemented with SDS-PAGE sample buffer and then analyzed by western blotting for phospho-Smc1, Smc1 and phospho-Cdc2. An autoradiograph of phosphorylated MBP is shown. (B) Interphase egg extracts were supplemented with or without dA-dT and caffeine (10 mM) as indicated for 10 min. Then extract was added to a tube containing a FLAG-Gwl bead pellet corresponding to the amount of Gwl expressed in three oocytes. After a further 20 min incubation, the beads were re-isolated by centrifugation and used for determination of Gwl kinase activity as described in Materials and Methods. The supernatant was analyzed by western blotting for phospho-Smc1 and Smc1. Equal loading in the immune-complex kinase assay was verified by western blotting of the assay for FLAG-Gwl and by Coomassie staining of the assay substrate (MBP). Liquid scintillation counting of the excised MBP bands indicates approximately 80% inhibition of Gwl activity after 30 min of DNA damage (data not shown).
Activation of the DNA damage checkpoint prevents mitotic entry through inhibition of key mitotic kinases including MPF and Plk1.29–32 Recent studies identified Gwl as an essential regulator of mitosis. In particular, active Gwl is sufficient to drive cell cycle progression into mitosis independently of activation of other mitotic kinases.16 These findings prompted us to investigate whether Gwl is a target of the DDR. Interphase egg extracts supplemented with recombinant Gwl were treated with or without dA-dT, and Gwl kinase activity was measured at various time points. In the absence of dA-dT, Gwl kinase activity increased as the extract entered mitosis, as indicated by dephosphorylation of Tyr15 in Cdc2 (Fig. 2A). In the presence of dA-dT, checkpoint arrest in G2 occurred as judged both by phosphorylation of Smc1 and by continued inhibitory phosphorylation of Cdc2 on Tyr 15. Under these conditions, mitotic activation of Gwl was not evident. However, more interestingly, the level of Gwl kinase activity declined progressively after dA-dT addition, suggesting negative regulation by the DDR (Fig. 2A). Gwl inhibition after dA-dT treatment was reversed by the addition of caffeine, an inhibitor of ATM/ATR. These results suggest that Gwl is a target of ATM/ATR-dependent signal transduction (Fig. 2B).
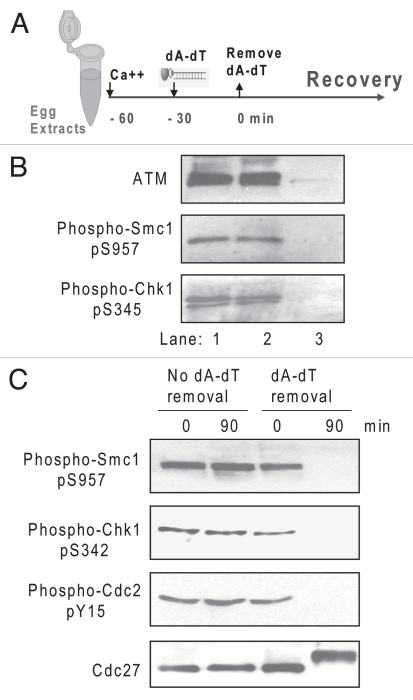
The regulation of Gwl activity by DNA damage and Gwl's ability to inactivate checkpoint signaling suggested that this kinase might be involved in recovery from DNA damage. Recovery is normally associated with completion of DNA repair and can be monitored by the dephosphorylation of a variety of proteins in the ATM/ATR-dependent pathway that mediates cell cycle arrest.1,10,11 In Xenopus egg extracts, removal of damaged DNA does not co-deplete ATM or phosphorylated Chk1 or Smc1 (Fig. 3A and B). In fact, the DNA damage pathway remains active for a considerable period after removal of damaged DNA from the system, as shown previously.24 In the present work, we have refined this system further and now observe that by 60–90 min after removal of dA-dT, the system has “recovered” from cell cycle arrest, as judged by dephosphorylation of Smc1 and Chk1 and entry into M phase, as monitored by the phosphorylation states of Cdc2 and Cdc27 (Fig. 3A and C). In contrast, the control extract without removal of dA-dT exhibits persistent checkpoint signaling and cell cycle arrest (Fig. 3C).
Figure 3.
Recovery from the DNA damage response in extracts. (A) The cell-free checkpoint recovery system. Metaphase II-arrested CSF extracts were released into interphase for 30 min by addition of Ca++. Magnetic beads conjugated with dA-dT were added to 20 µg/ml and incubated in the extract for 30 min then removed with a magnet as described in Materials and Methods to mimic the completion of DNA repair. (B) Analysis of dA-dT beads removed from extracts. As in (A), extracts before and after dA-dT removal, as well as the removed beads were analyzed by western blotting using the indicated antibodies. Lane 1, extract before dA-dT removal; lane 2, extract after dA-dT removal; lane 3, dA-dT beads removed from lane 1. (C) The removal of dA-dT from extracts depicted in (A) enables deactivation of DNA damage signaling and re-entry into mitosis, as judged by western blotting of Smc1, Chk1, Cdc2 and Cdc27. Control extracts without removal of dA-dT (left) sustained the checkpoint arrest.
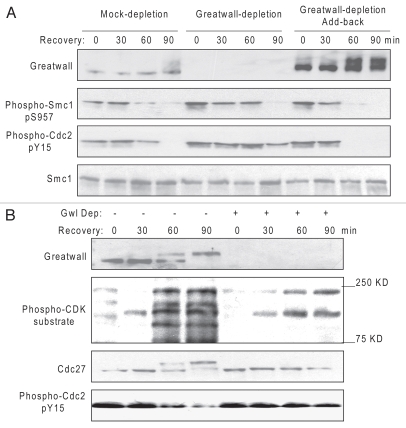
To assess the role of Gwl specifically in checkpoint recovery, the protein was co-depleted simultaneously with dA-dT. As shown in Figure 4A, recovery was greatly reduced in the absence of Gwl, as judged by the delayed dephosphorylation of Smc1 at ATM/ATR-targeted sites and the continued phosphorylation of Cdc2 at its inhibitory site. Consistent with a role in recovery, when an excess of recombinant Gwl was added back to depleted extracts, recovery was accelerated by at least 30 min (Fig. 4A). The ectopic Gwl also became highly activated as the extract entered M phase, as indicated by a change in its electrophoretic mobility (Fig. 4A).
Figure 4.
Gwl promotes checkpoint recovery. (A) As in Figure 3A, biotinylated dA-dT oligos bound to M-280 streptavidin beads were added to extracts for 30 min to activate the DNA damage checkpoint and then removed with a magnet to allow recovery. As indicated, these extracts had also been either mock-treated with Protein G Dynabeads or depleted of Gwl with anti-Gwl antibody bound to Protein G Dynabeads as described in Materials and Methods, or Gwl-depleted and then reconstituted with purified Gwl. Recovery in the extracts was then analyzed at 90 min by western blotting with the indicated antibodies. (B) As in (A) extracts with or without depletion of Gwl were monitored for Cdk activation by western blotting with the indicated antibodies at various time points during recovery.
While Gwl depletion impedes dephosphorylation of Smc1 and Cdc2 following removal of DNA damage, Gwl is required for Cdk activation during recovery. The increased phosphorylation of Cdk substrates that occurred in concert with Gwl activation in the control was not evident with Gwl depletion (Fig. 4B). It has been reported that, in a normal cell cycle, Gwl promotes mitotic entry upon Cdk1 activation by preventing dephosphorylation of Cdk1-phosphorylated substrates,20 and its own activation is partially blocked by roscovitine, a Cdk inhibitor.16 Our data presented here are consistent with these previous reports and support the existence of a positive feedback loop between Gwl and Cdk1 in the context of checkpoint recovery. The molecular relationships between Gwl and Cdk1 remain to be clarified further during both normal cell cycles and checkpoint recovery.
Discussion
These results define a new assay with Xenopus egg extracts for assessing recovery from DNA damage. The system complements existing methods and has several apparent advantages. For example, the removal of damaged DNA mimics the completion of DNA repair, thus distinguishing this process from adaptation, a situation in which cell cycle progression resumes with unrepaired DNA damage. Moreover, the use of extracts allows the recovery process to proceed synchronously after removal of damaged DNA and facilitates other biochemical manipulations. Simultaneous depletion of a protein and damaged DNA can also separate involvement in checkpoint recovery from any role in initial activation of the checkpoint. In this study, the assay has led to identification of a role for Gwl in promoting recovery. This role could be seen both in loss-of-function experiments, in which depletion of Gwl delayed recovery, and in gain-of-function experiments, in which ectopic expression of wild-type Gwl enhanced recovery.
The participation of Gwl in the DNA damage response was also evident in the inhibition of Gwl activity upon addition of damaged DNA to the extracts. This inhibition not only prevented the activation of Gwl as G2 arrest was induced but also led to a time-dependent decrease in basal Gwl activity. The mechanism is not clear at present, but it does not involve degradation of Gwl (Fig. 2B). Rather, Gwl inhibition is a consequence of ATM/ATR-dependent signaling, as addition of caffeine completely reversed the inhibition (Fig. 2B). The data place Gwl, like Cdks and Plk1, as a target of DNA damage checkpoint activation and likely reflect a “multiple stops” model of checkpoint execution in which inhibition of Gwl reinforces other pathways of DNA damage-induced G2 arrest. Additionally, as a negative regulator of the DDR, Gwl inhibition by DNA damage may also contribute to full activation of the DDR response.
The new role of Gwl in promoting checkpoint recovery raises an intriguing question about the significance of “basal” kinase activity, as Gwl activity is lower in interphase than in mitosis.15 The same question is evident for Plk1, which regulates recovery from the DDR in G2 phase.8,33 Interestingly, a recent study from the Medema lab showed that during checkpoint recovery in mammalian cells, partial activation of Plk1 occurs a few hours before mitosis.9 We have also observed substantial activation of Gwl before dephosphorylation of Smc1 and Chk1 after removal of damaged DNA (data not shown). DNA damage signaling is enhanced with Gwl depletion or addition of KD Gwl, suggesting that basal activation of Gwl may partially neutralize DNA damage signals before the checkpoint is established to prevent mitotic entry. We thus hypothesize that antagonizing actions between certain mitotic kinases (e.g., Plk1, Gwl) and DDR elements determine checkpoint activation and deactivation. Persistent DNA damage signals suppress full activation of Plk1 and Gwl, whereas the completion of DNA repair (or removal of damaged DNA) allows progressive activation of Plk1 and Gwl to initiate recovery. Finally, it is unclear whether the apparently similar roles of Plk1 and Gwl in recovery are achieved through comparable mechanisms. Plk1-dependent phosphorylation primes DDR factors for destruction,2–4,7 whereas the substrates of Gwl that mediate recovery remain to be identified.
Materials and Methods
Antibodies.
Antibodies against human Chk1 phospho-Ser 345 (Xenopus Chk1 Ser 342), Cdc2 phospho-Tyr 15 and phospho-CDK substrates were obtained from Cell Signaling Technology, (Beverly, MA). Antibodies to Smc1 and Smc1 phospho-Ser 957 were purchased from Bethyl Labs (Montgomery, TX). Cdc27 antibody was purchased from BD Transduction Laboratories, (San Jose, CA). Biotinylated dA-dT oligonucleotides were purchased from Integrated DNA Technologies (Coralville, IA). Rabbit polyclonal antibody against Xenopus Gwl was generated as previously described.15
Greatwall kinase assay.
Flag-Gwl was constructed by inserting full-length Xenopus Gwl into a pCS-Flag vector using LIC cloning (Novagen). Flag-Gwl mRNA was produced using a mMessage Machine kit (Ambion), and 40 nl mRNA (0.25 mg/ml) was microinjected into immature oocytes followed by incubation overnight at 18°C. Oocytes were homogenized in 5 µl per oocyte of ice cold EB (80 mM β-glycerophosphate, 20 mM EGTA, 5 mM MgCl2, 20 mM Hepes, pH 7.5) and the cytosol collected after centrifugation at 10,000 × g for 1 min. at 4°C. A two-fold volume of a 50% slurry of anti-FLAG agarose beads (Sigma) was added, incubated for 60 min at 4°C, and the beads were then washed three times in EB. After resuspension in EB, beads corresponding to the amount of Gwl expressed in three oocytes were aliquoted into tubes. The anti-FLAG beads were spun down and then resuspended in 30µ40 µl of egg extract, treated as indicated. After 15 min (Fig 2A) or 20 min (Fig 2B) incubation, the beads were spun down by centrifugation and washed three times with EB and once with kinase buffer (20 mM HEPES, 10 mM MgCl2, 3 mM β-mercaptoethanol, pH 7.6). The kinase reaction was performed by resuspending the beads for 15 minutes at 30°C in 30 µl of kinase buffer supplemented with 10 µg myelin basic protein (MBP) and 100 µM [γ-32P]-ATP (5 µCi per reaction). The reaction was stopped by addition of 8 µl of 4 × SDS gel sample buffer, and half of the reaction was loaded onto a 4–20% gradient gel (Criterion) and analyzed by autoradiography.
Immunodepletion.
For Gwl immunodepletion, protein G Dynabeads (Invitrogen) were conjugated with Gwl antibody according to the manufacturer's protocol. Beads were then added to extracts and removed with a magnet after incubation for 20 min. The remaining extract after bead removal was used as immunodepleted extract, and the efficiency of depletion assessed by western blot. Mock-depleted extract was prepared similarly with Protein G Dynabeads not conjugated with antibody. Recombinant wild-type and kinase-dead Gwl were purified from okadaic-acid treated Sf9 cells infected with baculovirus encoding Xenopus Gwl, as previously described.15
Xenopus egg extracts.
Cytostatic factor (CSF) extracts were freshly prepared as previously described,24 stably released into interphase by supplementation with 0.4 mM CaCl2 and incubated for 30 min at room temperature. For checkpoint activation and recovery, biotinylated dA-dT oligos were pre-bound to M-280 streptavidin Dynabeads (Invitrogen) following the standard protocol provided by the manufacturer, and the beads were then added to the extracts to produce a final concentration of 20 ug/ml dA-dT. After 30 min, the beads were removed with a magnet to initiate checkpoint recovery, and the removal point is the 0 min time point of recovery (Fig. 3A). In experiments that assess the involvement of Gwl, Gwl was co-depleted together with damaged DNA by mixing dA-dT-bound M-280 Dynabeads with protein G-Dynabeads bound to Gwl antibody.
Acknowledgements
This work was supported by the Howard Hughes Medical Institute and by a grant from the NIH to M.L.G. (GM48430). A.P. and T.M.M. are Research Associates and J.L.M. an Investigator of the Howard Hughes Medical Institute.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/13632
References
- 1.Peng A, Maller JL. Serine/threonine phosphatases in the DNA damage response and cancer. Oncogene. 2010;29:5977–5988. doi: 10.1038/onc.2010.371. [DOI] [PubMed] [Google Scholar]
- 2.Mailand N, Bekker-Jensen S, Bartek J, Lukas J. Destruction of claspin by SCFbeta TrCP restrains Chk1 activation and facilitates recovery from genotoxic stress. Mol Cell. 2006;23:307–318. doi: 10.1016/j.molcel.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Mamely I, van Vugt MA, Smits VA, Semple JI, Lemmens B, Perrakis A, et al. Polo-like kinase-1 controls proteasome-dependent degradation of Claspin during checkpoint recovery. Curr Biol. 2006;16:1950–1955. doi: 10.1016/j.cub.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Peschiaroli A, Dorrello NV, Guardavaccaro D, Venere M, Halazonetis T, Sherman NE, et al. SCFbetaTrCP-mediated degradation of Claspin regulates recovery from the DNA replication checkpoint response. Mol Cell. 2006;23:319–329. doi: 10.1016/j.molcel.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Toczyski DP, Galgoczy DJ, Hartwell LH. CDC5 and CKII control adaptation to the yeast DNA damage checkpoint. Cell. 1997;90:1097–1106. doi: 10.1016/s0092-8674(00)80375-x. [DOI] [PubMed] [Google Scholar]
- 6.Yoo HY, Kumagai A, Shevchenko A, Shevchenko A, Dunphy WG. Adaptation of a DNA replication checkpoint response depends upon inactivation of Claspin by the Polo-like kinase. Cell. 2004;117:575–588. doi: 10.1016/s0092-8674(04)00417-9. [DOI] [PubMed] [Google Scholar]
- 7.van Vugt MATM, Bras A, Medema RH. Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells. Mol Cell. 2004;15:799–811. doi: 10.1016/j.molcel.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 8.van Vugt MA TM, Medema RH. Checkpoint adaptation and recovery: Back with polo after the break. Cell Cycle. 2004;3:1383–1386. doi: 10.4161/cc.3.11.1248. [DOI] [PubMed] [Google Scholar]
- 9.Macurek L, Lindqvist A, Lim D, Lampson MA, Klompmaker R, Freire R, et al. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 2008;455:119–123. doi: 10.1038/nature07185. [DOI] [PubMed] [Google Scholar]
- 10.Clemenson C, Marsolier-Kergoat MC. DNA damage checkpoint inactivation: Adaptation and recovery. DNA Repair. 2009;8:1101–1109. doi: 10.1016/j.dnarep.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Bartek J, Lukas J. DNA damage checkpoints: From initiation to recovery or adaptation. Curr Opin Cell Biol. 2007;19:238–245. doi: 10.1016/j.ceb.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Yu J, Fleming SL, Williams B, Williams EV, Li Z, Somma P, et al. Greatwall kinase: A nuclear protein required for proper chromosome condensation and mitotic progression in Drosophila. J Cell Biol. 2004;164:487–492. doi: 10.1083/jcb.200310059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Archambault V, Zhao X, White-Cooper H, Carpenter ATC, Glover DM. Mutations in drosophila Greatwall/Scant reveal its roles in mitosis and meiosis and interdependence with polo kinase. Plos Genet. 2007;3:e2163–e2179. doi: 10.1371/journal.pgen.0030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson PK. Climbing the Greatwall to mitosis. Mol Cell. 2006;22:156–157. doi: 10.1016/j.molcel.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Yu J, Zhao Y, Li ZX, Galas S, Goldberg ML. Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol Cell. 2006;22:83–91. doi: 10.1016/j.molcel.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Haccard O, Wang R, Yu J, Kuang J, Jessus C, et al. Roles of Greatwall kinase in the regulation of cdc25 phosphatase. Mol Biol Cell. 2008;19:1317–1327. doi: 10.1091/mbc.E07-11-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castilho PV, Williams BC, Mochida S, Zhao Y, Goldberg ML. The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55delta, a phosphatase directed against CDK phosphosites. Mol Biol Cell. 2009;20:4777–4789. doi: 10.1091/mbc.E09-07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vigneron S, Brioudes E, Burgess A, Labbé JC, Lorca T, Castro A. Greatwall maintains mitosis through regulation of PP2A. EMBO J. 2009;28:2786–2793. doi: 10.1038/emboj.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgess A, Vigneron S, Brioudes E, Labbé JC, Lorca T, Castro A. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc Natl Acad Sci USA. 2010;107:12564–12569. doi: 10.1073/pnas.0914191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg ML. Greatwall kinase protects mitotic phosphosites from barbarian phosphatases. Proc Natl Acad Sci USA. 2010;107:12409–12410. doi: 10.1073/pnas.1006046107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mochida S, Ikeo S, Gannon J, Hunt T. Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J. 2009;28:2777–2785. doi: 10.1038/emboj.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorca T, Bernis C, Vigneron S, Burgess A, Brioudes E, Labbé JC, et al. Constant regulation of both the MPF amplification loop and the Greatwall-PP2A pathway is required for metaphase II arrest and correct entry into the first embryonic cell cycle. J Cell Sci. 2010;123:2281–2291. doi: 10.1242/jcs.064527. [DOI] [PubMed] [Google Scholar]
- 23.Voets E, Wolthuis RM. MASTL is the human orthologue of Greatwall kinase that facilitates mitotic entry, anaphase and cytokinesis. Cell Cycle. 2010;9:3591–3601. doi: 10.4161/cc.9.17.12832. [DOI] [PubMed] [Google Scholar]
- 24.Peng A, Lewellyn AL, Maller JL. Undamaged DNA transmits and enhances DNA damage checkpoint signals in early embryos. Mol Cell Biol. 2007;27:6852–6862. doi: 10.1128/MCB.00195-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng A, Lewellyn AL, Schiemann WP, Maller JL. Repo-Man controls a protein phosphatase 1-dependent threshold for DNA damage checkpoint activation. Curr Biol. 2010;20:387–396. doi: 10.1016/j.cub.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumagai A, Guo ZJ, Emami KH, Wang SX, Dunphy WG. The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J Cell Biol. 1998;142:1559–1569. doi: 10.1083/jcb.142.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo Z, Dunphy WG. Response of Xenopus Cds1 in cell-free extracts to DNA templates with double-stranded ends. Mol Biol Cell. 1999;11:1535–1546. doi: 10.1091/mbc.11.5.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lupardus PJ, Van C, Cimprich KA. Analyzing the ATR-mediated checkpoint using Xenopus egg extracts. Methods. 2007;41:222–231. doi: 10.1016/j.ymeth.2006.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou BBS, Elledge SJ. The DNA damage response: Putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 30.Melo J, Toczyski D. A unified view of the DNA-damage checkpoint. Curr Opin Cell Biol. 2002;14:237–245. doi: 10.1016/s0955-0674(02)00312-5. [DOI] [PubMed] [Google Scholar]
- 31.Smits VA, Klompmaker R, Arnaud L, Rijksen G, Nigg EA, Medema RH. Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat Cell Biol. 2000;2:672–676. doi: 10.1038/35023629. [DOI] [PubMed] [Google Scholar]
- 32.van Vugt MATM, Smits VAJ, Klompmaker R, Medema RH. Inhibition of polo-like kinase-1 by DNA damage occurs in an ATM- or ATR-dependent fashion. J Biol Chem. 2001;276:41656–41660. doi: 10.1074/jbc.M101831200. [DOI] [PubMed] [Google Scholar]
- 33.Eckerdt F, Yuan JP, Strebhardt K. Polo-like kinases and oncogenesis. Oncogene. 2005;24:267–276. doi: 10.1038/sj.onc.1208273. [DOI] [PubMed] [Google Scholar]