Abstract
The formation of the central spindle (or the spindle midzone) is essential for cytokinesis in animal cells. In this study, we report that coiled-coil domain-containing protein 69 (CCDC69) is implicated in controlling the assembly of central spindles and the recruitment of midzone components. Exogenous expression of CCDC69 in HeLa cells interfered with microtubule polymerization and disrupted the formation of bipolar mitotic spindles. Endogenous CCDC69 proteins were localized to the central spindle during anaphase. RNA interference (RNAi)-mediated knockdown of CCDC69 led to the formation of aberrant central spindles and disrupted the localization of midzone components such as aurora B kinase, protein regulator of cytokinesis 1 (PRC1), MgcRacGAP/HsCYK-4, and polo-like kinase 1 (Plk1) at the central spindle. Aurora B kinase was found to bind to CCDC69 and this binding depended on the coiled-coil domains at the C-terminus of CCDC69. Further, disruption of aurora B function in HeLa cells by treatment with a small chemical inhibitor led to the mislocalization of CCDC69 at the central spindle. Our results indicate that CCDC69 acts as a scaffold to regulate the recruitment of midzone components and the assembly of central spindles.
Key words: CCDC69, aurora B, Plk1, central spindles, midzone components, cytokinesis
Introduction
Antiparallel overlapping microtubules are bundled between the separating chromosomes during anaphase, forming the central spindle (or the spindle midzone) and leading to the assembly of the actomyosin contractile ring.1, 2 Regulators of central spindle formation and actomyosin contractile ring assembly are mostly restricted to the interdigitated microtubules of central spindles and they can be collectively called midzone components.3 Numerous regulators have been implicated in promoting antiparallel microtubule bundling at the central spindle. In particular, the chromosome passenger complex (CPC), the centralspindlin complex and protein regulator of cytokinesis 1 (PRC1) are considered to be the core regulators of central spindle assembly.4
The CPC contains aurora B, survivin, INCENP (inner centromere protein) and borealin.5–10 During the metaphase-to-anaphase transition, aurora B is translocated along with survivin, INCENP and borealin from centromeres to the central spindle. Perturbing the function of survivin, INCENP or borealin leads to the mislocalization of aurora B.11 The centralspindlin complex contains MgcRacGAP/HsCYK-4 (a GTPase-activating protein) and mitotic kinesin-like protein 1 (Mklp1).12–15 PRC1 can interact with kinesin family member 4A (Kif4A, a microtubule-based molecular motor), forming a PRC1-Kif4A complex.16–20 A line of evidence shows that the CPC, the centralspindlin complex, and PRC1, promote the assembly and bundling of antiparallel microtubules at the central spindle.4 The formation of central spindles ultimately leads to the recruitments of cytokinesis regulators such as polo-like kinase 1 (Plk1).21 It has been shown that Plk1 can phosphorylate and recruit critical cytokinesis regulators such as RhoGEFs to the central spindle,22–26 leading to the activation of RhoA at the cleavage furrow and the assembly of the actomyosin contractile ring. Therefore, the localization of Plk1 to the central spindle is a critical step during mitosis and cytokinesis.
Proper localizations of midzone components at the central spindle are important for central spindle assembly.4 It has been indicated that binding of midzone components to antiparallel overlapping microtubules is critical for their recruitments to the central spindle.4,27,28 Yet, localizations of midzone components to the central spindle are often interdependent. For instance, depletion PRC1 or Kif4A interferes with the localization of the centralspindlin complex and the CPC at the central spindle.19,29 In addition, disruption of centralspindlin function decreases the localization of the CPC.30 In turn, the CPC is required for the stable localization of the centralspindlin complex.31, 32 Therefore, it is likely that different midzone components are also interconnected and stabilized by scaffolds at the central spindle, thus contributing to the regulation of midzone component assembly at the central spindle. However, just how scaffolds are implicated in the assembly of midzone components at the central spindle is poorly understood.
It has been shown that microtubules at central spindles, while relatively more stable than those of the metaphase spindles, are still highly dynamic, especially during late anaphase.33 Microtubules polymerization is primarily regulated by the coordinated action of microtubule-stabilizing factors such as microtubule-associated proteins (MAPs) and microtubule-destabilizing factors such as kinesin-13, stathmin, and katanin.34–39 In particular, kinesin-13 family proteins can promote microtubule depolymerization and are key regulators of microtubule dynamics during mitosis.34,40,41 In addition, kinesin-13 proteins including Kif2a, Kif2b, and MCAK/Kif2c are localized to the central spindle and/or midbody during anaphase and telophase.42–44 However, it is still not clear how microtubule-destabilizing factors function at the central spindle to balance the microtubule-bundling activity of midzone components such as the CPC, the centralspindlin complex, and PRC1.
We found that coiled-coil domain containing protein 69 (CCDC69) can destabilize microtubules in transfected HeLa cells. CCDC69 localizes to the central spindle and physically interacts with aurora B. Depletion of CCDC69 delocalizes aurora B (a component of the CPC), MgcRacGAP (a component of the centralspindlin complex), and PRC1. Our results suggest that CCDC69 may act as a microtubule-destabilizing factor and a scaffold to control central spindle assembly as well as to recruit midzone components to the central spindle.
Results
Expression of coiled-coil domain containing protein 69 (CCDC69).
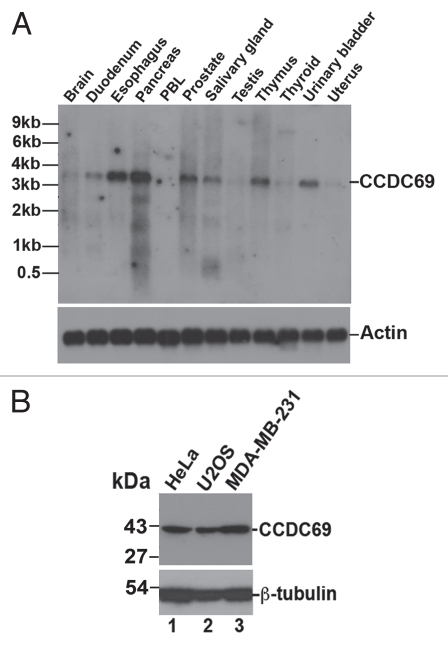
We originally identified CCDC69 as a potential downstream target for the homeodomain transcription factor Pitx2a using a tetracycline-inducible expression system.45,46 DNA microarrays and RT-PCR showed that exogenous expression of Pitx2a in HeLa cells increased the mRNA levels of CCDC69 (data not shown). Human CCDC69 (NM_015621) contains 296 amino acid residues with an expected molecular mass of ∼42 kDa. Mouse (NM_177471) and Xenopus (BC124990) orthologs of CCDC69 are also found in the database (Sup. Fig. 1). Northern blot analysis with full-length human CCDC69 cDNA as a probe showed that CCDC69 mRNA is highly expressed in duodenum, esophagus, pancreas, prostate, salivary gland, thymus, and urinary bladder (Fig. 1A). The size of CCDC69 mRNA in the blot is ∼3.5 kb, in agreement with that for the deposited CCDC69 mRNA in the NCBI database (NM_015621; 3416 bp in length). EST profile in the NCBI database also shows that CCDC69 is widely expressed in various tissues and organs (www.ncbi.nlm.nih.gov/UniGene/ESTProfileViewer.cgi?uglist=Hs.655336). Further, immunoblot analysis with an antibody specific for human CCDC69 showed that CCDC69 is expressed in various cancer cell lines such as HeLa, U2OS, and MDA-MB-231 (Fig. 1B). As shown in Supplemental Figure 1, mouse CCDC69 lacks the peptide sequence (CKPPKKKRQEPEPEQPPRPE) that was used to raise the human CCDC69 peptide antibody. Accordingly, immunoblot analysis using the CCDC69 peptide antibody did not detect any protein bands in the lysate from a mouse embryo (at embryonic day 10.5; data not shown), suggesting that the CCDC69 peptide antibody does not recognize mouse CCDC69 proteins.
Figure 1.
(A) Expression of CCDC69 mRNA in human tissues. A human poly(A+) RNA Northern blot was probed with P32-labeled human CCDC69 (upper panel; exposed for 24 h) or actin (lower panel; exposed for 7 h) cDNAs. (B) Expression of CCDC69 proteins in human cancer cell lines. The whole cell lysates were subjected to immunoblot analysis with antibodies specific for CCDC69 (upper panel) and β-tubulin (lower panel).
Exogenous expression of CCDC 69 destabilizes microtubules in transfected cells.
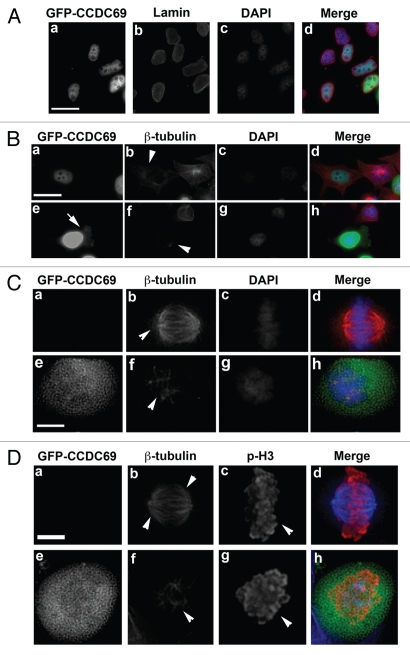
As one of the first attempts to explore the cellular function of CCDC69, we have exogenously expressed GFP-CCDC69 in HeLa cells and stained with antibodies against several cellular components including an antibody against b-tubulin. We noticed that, while GFP-CCDC69 was predominantly localized in the nucleus in interphase cells (Fig. 2A), exogenous expression of GFP-CCDC69 decreased microtubule staining in the cytoplasm (n=145/200 cells; arrowhead in Fig. 2Bd). This observation suggests that a small amount of exogenously expressed GFP-CCDC69 in the cytoplasm was responsible for such microtubule-destabilizing activity. Consistent with this speculation, in cells expressing high levels of GFP-CCDC69, a significant amount of GFP-CCDC69 remained in the cytoplasm (arrow in Fig. 2Be) and dramatically destabilized microtubules (n=105/105 cells; arrowhead in Fig. 2Bf).
Figure 2.
HeLa cells were transfected with a plasmid encoding GFP-CCDC69. 24 h after transfection, the transfected cells were fixed with paraformaldehyde and subjected to immunofluorescence staining. (A) The transfected HeLa cells were stained with anti-lamin and DAPI. (B–C) The transfected HeLa cells were stained with anti-β-tubulin and DAPI. Note that all images in B were collected using the same exposure time. (D) The transfected HeLa cells were stained with anti-β-tubulin and anti-phosphorylated histone 3 (p-H3). Bar, 20 µm (A–B) or 5 µm (C–D).
Exogenous expression of CCDC69 greatly affected the microtubule assembly during mitosis. Bipolar mitotic spindles are assembled in untransfected cells during mitosis (n=120/120 cells; arrowheads in Fig. 2Cb). However, mitotic cells expressing GFPCCDC69 failed to form bipolar mitotic spindles (n=95/102; arrowhead in Fig. 2Cf). To confirm that cells in panels e–h were at mitotic phase, immunofluorescence staining was done using antibodies specific for β-tubulin and the mitotic marker phosphorylated histone 3 (p-H3). As shown in Figure 2D, metaphase chromosomes of an untransfected cell were positively stained for p-H3 (arrowhead in panel c) and the bipolar mitotic spindles are nicely formed (arrowheads in panel b). However, chromosomes of a transfected cell were positively stained for p-H3 (arrowhead in panel g) but bipolar mitotic spindles of the cell failed to form (arrowhead in panel f). These results indicate that cells in Figure 2C and D are at mitotic phase. However, we did not observe cell death in interphase cells expressing GFP-CCDC69, suggesting that decreases in microtubule stability are not secondary to cell death. It should be noted that expression of GFP alone in HeLa cells had no impact on microtubule polymerization or mitotic spindle formation (data not shown).
The C-terminal half of CCDC 69 is required to destabilize microtubules in transfected cells.
The COIL program47 predicts that human CCDC69 contains four coiled-coil regions (amino acids 49–109, 122–159, 172–233, 237–269; see Figure 3A). Coiled-coil domains are also predicted in mouse and xenopus CCDC69. To identify the regions that are critical for the microtubule-destabilizing activity of CCDC69, we generated several truncated fragments of human CCDC69 (Fig. 3B). Plasmids encoding CCDC69 fragments were transfected into HeLa cells. 24 h after transfection, the transfected cells were processed for immunofluorescence to visualize GFP-tagged CCDC69 fragments and microtubules. Unlike the full-length CCDC69 that was predominantly localized to the nucleus (Figure 2A), the CCDC69 fragment 49–296 (containing all coiled-coil regions) showed diffuse distribution in the cytoplasm and it dramatically destabilized microtubules (arrowheads in Figure 3Cb; n=71/83). CCDC69 fragments 172–296 and 1–233 are predominantly localized to the nucleus and also showed microtubule-destabilizing activity (arrowheads in Figures 3Ce and 3Ck; for 172–296 fragment, n=50/80; for 1–233 fragment, n=37/76). In contrast, CCDC69 fragment 1–159 showed diffuse distribution in the cytoplasm but it had no obvious impact on microtubule polymerization (arrowhead in Figure 3Ch; n=45/47). These results indicate that amino acids 159–296 (containing coiled-coil regions 172–233 and 237–269) are critical for the microtubule-destabilizing activity of CCDC69.
Figure 3.
(A) The COIL program predicts that human CCDC69 contains four coiled-coil domains (amino acids 49–109, 122–159, 172–233, and 237–269). (B) Schematic diagram of CCDC69 fragments. (C) HeLa cells were transfected with plasmids encoding GFP-tagged CCDC69 fragments 49–296 (a–c), 172–296 (d–f), 1–159 (g–i), 1–233 (j–i). The transfected cells were fixed with paraformaldehyde and subjected to immunofluorescence staining with anti-β-tubulin. Bar, 20 µm.
Localization of endogenous CCDC69 during cell cycle progression.
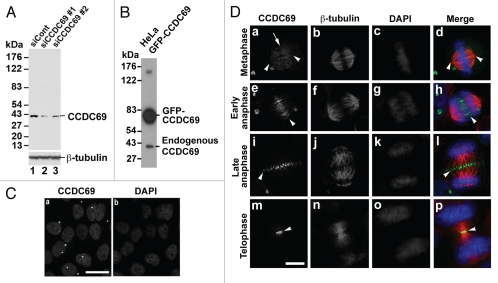
Immunoblot analysis with the CCDC69 peptide antibody detects a single band with a molecular mass of ∼42 kDa (Fig. 4A; lane 1). To further confirm the specificity of the CCDC69 peptide antibody, HeLa cells transfected with CCDC69 siRNAs were subjected to immunoblot analysis with the CCDC69 peptide antibody. Treatment of HeLa cells with CCDC69 siRNAs decreased the expression levels of the 42-kDa protein (Fig. 4A; compare lane 1 with lanes 2 and 3). Further, the CCDC69 peptide antibody also recognized GFP-CCDC69 from transfected HeLa cell lysate (Fig. 4B). These results suggest that the CCDC69 peptide antibody can specifically recognize human CCDC69 proteins. We then carried out immunofluorescence staining to examine the subcellular distribution of endogenous CCDC69 proteins during cell cycle progression. HeLa cells were fixed with paraformaldehyde and subjected to immunofluorescence staining with antibodies specific for CCDC69 and β-tubulin. CCDC69 showed nuclear staining in interphase HeLa cells (Fig. 4C). During metaphase, weak staining of CCDC69 was found along mitotic microtubules (arrow in Fig. 4Da). However, CCDC69 was not concentrated at the spindle poles (arrowheads in Fig. 4Da and d). During early anaphase, CCDC69 was localized along overlapping interpolar microtubules between the separating chromosomes (arrowheads in Fig. 4De and h). During late anaphase, CCDC69 formed a focused band at the center of central spindles (arrowheads in Fig. 4Di and l). Finally, CCDC69 was concentrated at the midbody during telophase (arrowheads in Fig. 4Dm and p). Thus, our findings indicate that CCDC69 is predominantly localized to antiparallel overlapping microtubules of the central spindle during anaphase. It should be noted that immunoblot analysis with the CCDC69 peptide antibody showed that CCDC69 protein levels remained constant during cell cycle progression (data not shown).
Figure 4.
(A) HeLa cells transfected with control (siCont) or CCDC69 (siCCDC69) siRNAs were subjected to immunoblot analysis with the CCDC69 peptide antibody. (B) HeLa cells transfected with a plasmid encoding GFP-CCDC69 were subjected to immunoblot analysis with the CCDC69 peptide antibody. (C) Untransfected HeLa cells were subjected to immunofluorescence staining with the CCDC69 peptide antibody and DAPI. (D) Untransfected HeLa cells were stained with anti-CCDC69, anti-β-tubulin and DAPI. Bar, 25 µm (C) or 5 µm (D).
Depletion of CCDC 69 leads to the formation of aberrant central spindles.
Exogenous expression of GFP-CCDC69 dramatically destabilized microtubules (Figs. 2 and 3). In addition, endogenous CCDC69 is concentrated to the central spindle during anaphase (Fig. 4). Therefore, we asked whether CCDC69 depletion has an impact on central spindle formation. HeLa cells depleted of CCDC69 by RNAi were subjected to immunofluorescence staining with antibodies specific for CCDC69 and β-tubulin. In anaphase cells depleted of CCDC69, we observed disorganized microtubule bundling at central spindles (n=11/11 cells; Figs. 5c–d). In these CCDC69-depleted cells, microtubule bundling was increased near the spindle poles (arrowheads in panels c and d) but not at the center of central spindles. These results suggest that CCDC69 is involved in regulating the integrity of central spindles. It should be noted that CCDC69 was not completely depleted by RNAi-mediated knockdown and CCDC69 proteins were still present in CCDC69 siRNA-treated cells (arrows in panels c–d).
Figure 5.
HeLa cells transfected with control (siCont; panels a–b) or CCDC69 (siCCDC69; panels c–d) siRNAs were subjected to immunofluorescence staining with anti-CCDC69 (green), anti-β-tubulin (red), and DAPI (blue). Bar, 5µm.
Depletion of CCDC 69 delocalizes RhoA at the cleavage furrow.
To determine the cellular function of CCDC69, we used time-lapse microscopy to monitor mitotic progression following RNAi-mediated depletion of CCDC69 in HeLa cells. We found that approximately 15% of CCDC69-depleted mitotic cells examined failed to advance to anaphase and eventually died (n=9/55 cells; control siRNA-treated cells: n=1/20). However, cytokinesis defects in CCDC69-depleted cells were not evident (n=3/50 cells showed furrow regression; control siRNA-treated cells: n=0/20), indicating that cells completely depleted of CCDC69 might fail to advance to anaphase. Another possibility is that the efficiency of CCDC69-depletion by RNAi is not high (see Fig. 2A; ∼55–65% knockdown) and the cells with low CCDC69 levels might still be able to complete cytokinesis. We have also used a human GIPZ lentiviral shRNAmir target gene set (Open Biosystems) specific for CCDC69 to carry out the knockdown experiments. Although the infected HeLa cells uniformly expressed the shRNAmir (judged by GFP signals), both immunoblot and immunofluorescence analyses showed that the knockdown efficiency did not improve (data not shown). We believe that this may be one of the reasons why whole genome screening did not identify CCDC69 as a regulator of central spindle formation and/or cytokinesis.
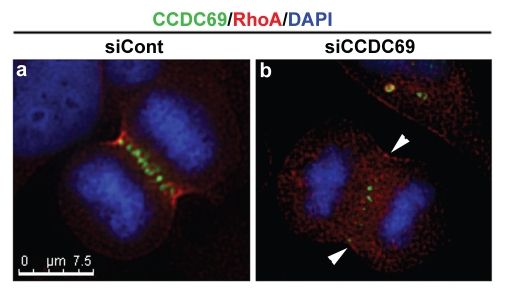
It is well established that activation of RhoA at the cleavage furrow is a key step during cytokinesis.48,49 Therefore, we carried out immunofluorescence staining to examine whether depletion of CCDC69 had an impact on RhoA staining at the cleavage furrow. HeLa cells transfected with control or CCDC69 siRNAs were fixed with TCA and then subjected to immunofluorescence staining with antibodies specific for CCDC69 and RhoA. As shown in Figure 6, RhoA staining was not concentrated at the equatorial cortex in HeLa cells depleted of CCDC69 (n=12 cells). Since cortical RhoA staining at the cleavage furrow has been used to measure RhoA activation during cytokinesis and since deficiency in equatorial RhoA activation leads to defective cytokinesis48,50,51, our results suggest that depletion of CCDC69 decreased RhoA activation at the cleavage furrow, resulting in cytokinesis defects.
Figure 6.
HeLa cells transfected with control (siCont; panel a) or CCDC69 (siCCDC69; panel b) siRNAs were fixed with TCA and subjected to immunofluorescence staining with anti-CCDC69 (green), anti-RhoA (red), and DAPI (blue). Bar, 7.5µm.
CCDC69 contributes to the concentration of aurora B at the central spindle.
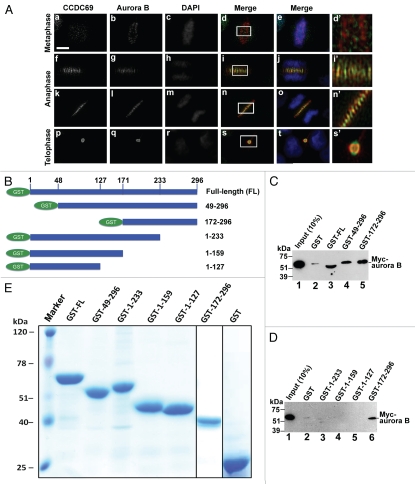
The chromosome passenger complex (CPC) contains aurora B, survivin, INCENP, and borealin.5–10 It has been shown that the CPC plays a central role in regulating central spindle formation.4 Depletion of CCDC69 resulted in the formation of aberrant central spindles, raising the possibility that CCDC69 may be implicated in controlling the localization of the CPC complex. To examine whether CCDC69 is colocalized with CPC components during mitosis and cytokinesis, HeLa cells were fixed and processed for immunofluorescence staining with antibodies specific for aurora B and CCDC69. As shown in Figure 7A, CCDC69 and aurora B were not colocalized at centromeres during metaphase. Nonetheless, CCDC69 appeared to be colocalized with aurora B at the central spindle during anaphase and at the midbody during telophase (Fig. 7A). We then asked whether CCDC69 could bind to CPC components. As shown in Figure 7C, lane 3, GST pull-down assays showed that GST-CCDC69 could pull down the in vitro translated Myc-aurora B, indicating that CCDC69 can physically interact with aurora B. To determine the regions in CCDC69 that are responsible for interaction with aurora B, we generated several truncated versions of CCDC69 (Fig. 7B). GST pull-down assays showed that GST-tagged CCDC69 fragments 49–296 and 172–296, but not fragments 1–233, 1–159, and 1–127, could precipitate Myc-aurora B (Fig. 7C and 7D), suggesting that amino acids 233–296, which contain one of the coiled-coil domains in CCDC69 (see Fig. 3A), are required for interaction with aurora B. Therefore, our results indicate that CCDC69 may interact with aurora B through its coiled-coil domain at the C-terminus.
Figure 7.
(A) Untransfected HeLa cells were subjected to immunofluorescence staining with anti-CCDC69 (green), anti-aurora B (red), and DAPI (blue). Panels e, j, o, and t show merge of three channels. Panels d', i', n', and s' are enlarged images that correspond to the insets in panels d, i, n, and s, respectively. Bar 5µm. (B) Schematic diagram of GST-tagged CCDC69 fragments that were used for GST pull-down assays in C and D. (C–D) GST-tagged CCDC69 full-length and fragments were used in GST pull-down assays to precipitate the in vitro translated Myc-aurora B. (E) A Coomassie staining gel of GST-tagged CCDC69 full-length and fragments that were used in C and D.
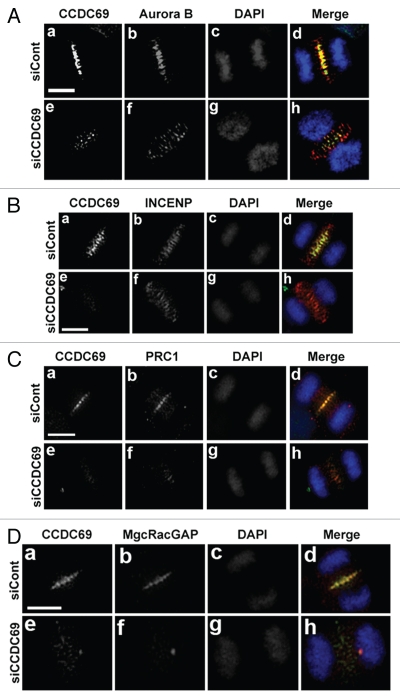
We then asked whether depletion of CCDC69 has an impact on the localization of the CPC complex. To this end, HeLa cells transfected with control or CCDC69 siRNAs were subjected to immunofluorescence staining for CCDC69 and CPC components (aurora B or INCENP). Depletion of CCDC69 did not interfere with the translocation of aurora B or INCENP from centromeres to the central spindle (Fig. 8A and 8B). However, both aurora B and INCENP staining appeared as broad bands at the central spindle (Fig. 8A and 8B).
Figure 8.
HeLa cells were transfected with control (siCont) or CCDC69 (siCCDC69) siRNAs. 72 h after transfection, the transfected cells were fixed with paraformaldehyde and subjected to immunofluorescence staining with antibodies as indicated. The chromatids were stained with DAPI. Bar, 5µm.
CCDC69 is required for the localization of PRC1 and MgcRacGAP at the central spindle.
In addition to the CPC complex, PRC1 and the centralspindlin complex also play a pivotal role in regulating the assembly of central spindles.16,17,30,52 Both PRC1 and the centralspindlin complex can stimulate microtubule bundling at the central spindle.17,30 Thus, we asked whether depletion of CCDC69 interfered with the localization of PRC1 and the centralspindlin complex at the central spindle. As shown in Figure 8C, depletion of CCDC69 in HeLa cells disrupted the localization of PRC1 to the central spindle. The centralspindlin complex is a heterotetrameric complex that contains a dimer of Mklp1 (a kinesin-6 motor protein) and a dimer of MgcRacGAP/HsCYK-4.5,52 Notably, Mklp1 and MgcRacGAP localize to the center of the central spindle as a complex, i.e. Mklp1 or MgcRacGAP alone does not localize to the central spindle.15 Figure 8D showed that depletion of CCDC69 also disrupted the localization of MgcRacGAP at the central spindle. Thus, our results indicate that CCDC69 is required for the localization of PRC1 and the centralspindlin complex at the central spindle. However, in vitro GST pull-down assays showed that GST-CCDC69 was not able to pull down Myc-tagged PRC1 or MgcRacGAP (data not shown).
CCDC69 is required for the localization of Plk1 to the central spindle.
Polo-like kinase 1 (Plk1) is a critical mitotic kinase that plays a central role in recruiting RhoGEFs to the central spindle, leading to the activation of RhoA and the assembly of the actomyosin contractile ring.23–26 We found that depletion of CCDC69 interfered with the localization of RhoA at the cleavage furrow (Fig. 6). Although it has been shown that PRC1 and Mklp2 are critical for the localization of Plk1 to the central spindle,53,54 it is suggested that other mechanisms may be involved as well.26 Thus, we asked whether CCDC69 had a role in localizing Plk1 to the central spindle. Immunofluorescence staining with antibodies specific for CCDC69 and Plk1 showed that CCDC69 and Plk1 were colocalized to the central spindle and midbody (Fig. 9Ah, l, and p), but not at the spindle poles (Fig. 9A). To determine whether CCDC69 is required for the localization of Plk1 to the central spindle, HeLa cells transfected with control or CCDC69 siRNA were subjected to immunofluorescence staining for CCDC69 and Plk1. As shown in Figure 9B, depletion of CCDC69 interfered with the localization of Plk1 to the central spindle. It has been shown that Plk1 can phosphorylate midzone components such as PRC1, Mklp2, and MgcRacGAP.26,53,54 Thus, we performed an in vitro kinase assay to determine whether CCDC69 also served as a substrate of Plk1. As shown in Figure 9C, GST-tagged full-length CCDC69 could be phosphorylated by Plk1. However, although aurora B interacts with CCDC69 (Fig. 7), it could not phosphorylate CCDC69 (Fig. 9C; lane 3).
Figure 9.
(A) Untransfected HeLa cells were fixed with acetone/methanol and subjected to immunofluorescence staining with anti-CCDC69 (green), anti-Plk1 (red), and DAPI (blue). (B) HeLa cells were transfected with control (siCont) or CCDC69 (siCCDC69) siRNAs. 72 h after transfection, the transfected cells were fixed with acetone/methanol and subjected to immunofluorescence staining with anti-CCDC69, anti-Plk1, and DAPI. Bar, 5µm. (C) Autoradiogram following SDS-PAGE of samples from in vitro kinase assays carried out to measure the activity of Plk1 and aurora B towards GST-CCDC69. (D) A Coomassie stained gel of GST-CCDC69 that was used in C. Bar, 5µm.
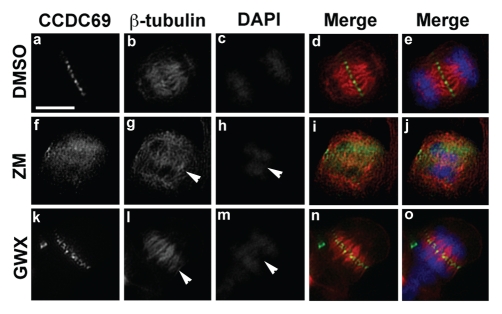
Inhibition of aurora B but not Plk1 disrupts the localization of CCDC69 to the central spindle.
It has been shown that localization of midzone components to the central spindle is often interdependent. For instance, Mklp2 is critical for the localization of CPC components INCENP and aurora B to the central spindle,55 whereas aurora B can phosphorylate centralspindlin components (Mklp1 and MgcRacGAP) and recruit them to the central spindle.56,57 Thus, we asked whether inhibition of aurora B or Plk1 had an impact on CCDC69 localization at the central spindle. HeLa cells treated with small chemical inhibitors of aurora B or Plk1 were subjected to immunofluorescence staining for CCDC69 and b-tubulin. Because of critical functional roles of aurora B and Plk1 during earlier mitosis, exposure of HeLa cells to these small chemical inhibitors does not allow them to advance to anaphase. However, we should still be able to identify cells that had already advanced to anaphase when they were exposed to the inhibitors for a short period of time (∼25 min). Consistent with previous reports,58 we found that inhibition of aurora B compromised the integrity of central spindles (Fig. 10; arrowhead in panel g) and caused defects in chromosome segregation (Fig. 10; arrowhead in panel h). Treatment of HeLa cells with an aurora B inhibitor disrupted the localization CCDC69 to the central spindle (Fig. 10; compare panels a–e with panels f–j). HeLa cells treated with a Plk1 inhibitor also showed defects in chromosome segregation (arrowhead in Fig. 10m). However, inhibition of Plk1 activation had little impact on central spindle formation (Fig. 10; arrowhead panel l) and did not interfere with CCDC69 localization at the central spindle (Fig. 10; compare panels a–e with panels k–o).
Figure 10.
Untransfected HeLa cells were treated with DMSO (panels a–e), ZM 447439 (ZM; panels f–j), or GW 843682X (GWX; panels k–o) for 25 min. The treated cells were fixed with paraformaldehyde and subjected to immunofluorescence staining with anti-CCDC69 (green), anti-β-tubulin (red), DAPI (blue). Merge of green and red channels is shown in d, i, n and merge of green, red, and blue channels is shown in e, j, o. Bar 7.5µm.
Discussion
In this article, we have demonstrated that the coiled-coil protein CCDC69 can destabilize microtubules. Knockdown of CCDC69 by RNAi leads to the formation of aberrant central spindles and interferes with the localization of midzone components such as aurora B, PRC1, MgcRacGAP, and Plk1. CCDC69 is a substrate for Plk1 and also physically interacts with aurora B through coiled-coil domain at the C-terminus of CCDC69. Inhibition of aurora B leads to the mislocalization of CCDC69 at the central spindle. Our results suggest that CCDC69 plays a critical role in regulating the assembly of central spindles and the recruitment of midzone components to the central spindle.
Effect of CCDC 69 on microtubule polymerization.
Endogenous CCDC69 is exclusively localized in the nucleus (Fig. 4C). Consistently, GFP-CCDC69 is also predominantly localized to the nucleus (Fig. 2A). However, exogenous expression of CCDC69 decreased microtubule staining in transfected interphase cells. We believe that some of the exogenously expressed GFP-CCDC69 remained in the cytoplasm, thus impacting microtubule stability. There are several findings that support this speculation. First, HeLa cells expressing high levels of GFP-CCDC69 showed a stronger GFP signal in the cytoplasm and, accordingly, microtubule staining in those cells is much weaker than that in cells expressing low levels of GFPCCDC69 (Fig. 2B). Second, a CCDC69 fragment 49–296 was predominantly localized to the cytoplasm and exhibited greater capability of destabilizing microtubules as compared with full-length CCDC69 (Compare Fig. 2Bb with Fig. 3Cb). Third, exogenous expression of GFP-CCDC69 dramatically destabilized microtubules during mitosis and disrupted the formation of bipolar mitotic spindles (Fig. 2C and 2D), suggesting that the breakdown of the nuclear envelope during mitosis and the release of GFP-CCDC69 from the nucleus may increase the impact of GFP-CCDC69 on microtubule polymerization. Consistent with these observations, we have never observed an anaphase cell expressing GFP-CCDC69 (data not shown). Thus, our results indicate that direct exposure of CCDC69 to microtubules is likely required for microtubule destabilization. Our results also indicate that, under physiological conditions, CCDC69 is sequestered in the nucleus during interphase and therefore prevented from affecting interphase microtubules. It would be interesting to know how the microtubule-destabilizing activity of CCDC69 is regulated once CCDC69 is released from the nucleus during mitosis.
Although exogenous expression of CCDC69 dramatically decreased microtubule staining in transfected HeLa cells (Fig. 2), we do not know at present whether CCDC69 can directly impact microtubule stability. The roles of microtubule-destabilizing factors (i.e. kinesin-13 proteins and stathmin/Op18) and the microtubule-severing protein katanin in regulating microtubule dynamics have been well established.44,59,60 In particular, kinesin-13 proteins are localized to the nucleus during interphase and then translocated to both mitotic spindles and central spindles.42,43,61 Exogenous expression of wild-type stathmin in transfected cells decreases microtubule staining during interphase and mitosis.62 Stathmin is distributed in the cytoplasm of the interphase cells and localizes to the mitotic spindle during mitosis.62 Therefore, it is possible that CCDC69 impacts microtubule polymerization via increasing the activity of microtubule-destabilizing factors such as kinesin-13 proteins and stathmin. On the other hand, a large number of microtubule-stabilizing/bundling factors including numerous midzone components promote microtubule polymerization.4,34 Thus, it is also possible that CCDC69 decreases microtubule stability through repressing the activity of microtubule-stabilizing factors.
Regulation of central spindle formation by CCDC69.
HeLa cells expressing GFP-CCDC69 failed to form bipolar mitotic spindles and were not able to advance to anaphase (Fig. 2C and 2D; data not shown). Thus, it is not clear whether exogenous expression of CCDC69 has an impact on antiparallel microtubule bundling at the central spindle during anaphase. Nonetheless, our results showed that RNAi-mediated knockdown of CCDC69 led to the formation of abnormal central spindles with increased microtubule bundling at or near the spindle poles (Fig. 5), indicating that CCDC69 may be involved in regulating microtubule bundling at the central spindle. However, over-whelming microtubule bundling was not observed at the central spindle of CCDC69-deficient cells. One possibility is that depletion of CCDC69 at the central spindle also delocalizes microtubule-bundling factors such as PRC1 and MgcRacGAP, i.e. depletion of CCDC69 leads to a decrease in both microtubule-destabilizing and -stabilizing activities at the central spindle. Although it appears that this is a futile cycle that does not lead to net changes in the balance between the activities of microtubule-stabilizing and -destabilizing factors, it may be an effective way to maintain optimal microtubule dynamics at the central spindle under physiological conditions. It is well established that aurora B, PRC1, and MgcRacGAP play a central role in regulating central spindle assembly. Thus, it is also possible that the formation of abnormal central spindles following CCDC69-depletion is due to the mislocalization of aurora B, PRC1, or MgcRacGAP.
Regulation of the assembly of midzone components at the central spindle by CCDC69.
Correct localization of midzone components during anaphase is essential for central spindle formation and the assembly of the actomyosin contractile ring.4 Our results showed that RNAi-mediated knockdown of CCDC69 led to the mislocalization of midzone components such as aurora B, PRC1, INCENP, MgcRacGAP, and Plk1. It has been shown that the translocation of aurora B from centromeres to the central spindle requires the coordinated action of survivin, INCENP or borealin.5,11,27 In particular, INCENP can target and activate aurora B by acting as a scaffold.28,32,63,64 We found that, in CCDC69-depleted cells, aurora B was successfully translocated from centromeres to the central spindle, but was not concentrated as a narrow band at the central spindle (Fig. 8). Consistently, CCDC69 and aurora B were colocalized at the central spindle but not at the centromeres (Fig. 7A). These results suggest that CCDC69 has a distinctive role in localizing aurora B at the central spindle. It would be interesting to know whether CCDC69 can regulate aurora B activation at the central spindle. CCDC69 is predicted to be coiled-coil protein and it can physically interact with aurora B, suggesting that CCDC69 may promote the assembly of aurora B at the central spindle by acting as a scaffold. However, we found that CCDC69 does not interact with survivin or borealin (data not shown). It is not clear whether CCDC69 can bind to INCENP.
There are at least two possibilities regarding how CCDC69 depletion impacts the localization of midzone components. One possibility is that CCDC69 acts as a scaffold at the central spindle to regulate the assembly of midzone components. For instance, CCDC69 binds to aurora B and is implicated in concentrating aurora B to the central spindle. It is of note that aurora B can phosphorylate centralspindlin components (Mklp1 and MgcRacGAP) and recruit them to the central spindle.56,57 Thus, mislocalization of MgcRacGAP at the central spindle following CCDC69 knockdown can be secondary to the mislocalization of aurora B. Another possibility is that CCDC69 may regulate microtubule polymerization at the central spindle, thus contributing to the regulation of central spindle formation. Our results show that CCDC69 depletion leads to abnormalities in central spindle assembly (Fig. 5). Thus, the effect of CCDC69 depletion on the mislocalization of midzone components can result from abnormalities in central spindle formation.
Regulation of Plk1 localization at the central spindle by CCDC69.
Plk1 can phosphorylate the midzone components PRC1 and MKlp2. Binding of Plk1 to PRC1 and Mklp2 promotes the timely recruitment of Plk1 to the central spindle during anaphase.53,54 In turn, Plk1 recruits RhoGEFs to the central spindle, leading to the activation of RhoA and the assembly of the actomyosin contractile ring.22–25 In particular, a recent study shows that phosphorylation of MgcRacGAP by Plk1 promotes the MgcARcGAP-Ect2 interaction and subsequently the recruitment of Ect2 to the central spindle.26 The study also indicates that, in addition to PRC1 and Mklp2, other mechanisms may also contribute to the regulation of Plk1 recruitment to the central spindle during anaphase.26 Our results indicate that CCDC69 knockdown interfered with the localization of Plk1 to the central spindle (Fig. 9B). Consistent with these observations, CCDC69 and Plk1 colocalized to the central spindle (Fig. 9A). Further, our results also showed that CCDC69 depletion decreased equatorial RhoA staining (Fig. 6). Therefore, it is likely that CCDC69 contributes to the recruitment of Plk1 to the central spindle. However, we cannot rule out the possibility that mislocalization of Plk1following CCDC69 knockdown is secondary to central spindle defects.
We have found that CCDC69 plays a critical role in controlling the localization of midzone components including aurora B at the central spindle. In turn, aurora B activity is required for the localization of CCDC69 to the central spindle. It has been shown that the localization and assembly of midzone components at the central spindle are, to a certain extent, interdependent.19,29–32 These observations are consistent with the concept that various midzone components and/or complexes are functionally and structurally connected through adaptors or scaffolds. Our results indicate that CCDC69 may be one such scaffold that provides physical interconnections among midzone components. Also, it is conceivable that antiparallel microtubule bundling at the central spindle is controlled by the coordinated action of microtubule- stabilizing/bundling and -destabilizing factors. Thus, a future direction will be to investigate whether CCDC69 is implicated in regulating microtubule polymerization at the central spindle.
Materials and Methods
Plasmids and cell culture.
The human CCDC69 cDNA was amplified from HeLa cell total RNA by RT-PCR with the following primer pair: 5'-CTCGAGATGGGCTGCAGACACAGCAGG-3' (forward primer; the underlined nucleotide sequence is the recognition site for XhoI) and 5'-GGATCC CTATGTGGCGAGGAAAGA-3' (reverse primer; the underlined nucleotide sequence is the recognition site for BamHI). The human CCDC69 cDNA was subcloned into pEGFP-C3 and pCS3+MT vectors to generate pEGFP-CCDC69 and pCS3-CCDC69. Different truncated versions of CCDC69 were also subcloned into pEGFP-C3 or pGEX-6p to generate plasmids encoding GFP- or GST-tagged polypeptides. HeLa cells were purchased from Clontech (Mountain View, CA). U2OS and MDA-MB-231 cells were purchased from ATCC (Manassas, VA). MDA-MB-231 cells were grown in Leibovitz's L-15 medium supplemented with 10% fetal bovine serum. HeLa and U2OS cells were cultured in DMEM supplemented with 10% fetal bovine serum. Transfection was done with Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). siRNAs specific for the human CCDC69 gene were purchased from Invitrogen (#1: GAG UCC AUU CUG AGC CGA AAC UAU A; #2: GCA CCA GAA GGA UAU AAC CAG AAU U).
Northern blot analysis.
The human poly(A+) RNA Northern blot (purchased from OriGene Technologies, Inc.) contains poly(A+) RNA samples from 12 major human tissues (brain, duodenum, esophagus, pancreas, PBL, prostate, salivary gland, testis, thymus, thyroid, urinary bladder, uterus). The full-length human CCDC69 cDNA was used as a probe. The probe was labeled with [αa-32P]dCTP (PerkinElmer Life and Analytical Sciences) using the DECAprime™ II Kit (Applied Biosystems/Ambion). The hybridization was carried out in the ULTRAhyb® Ultrasensitive Hybridization Buffer (Applied Biosystems/Ambion) according to the manufacturer's instructions. The membrane was exposed to x-ray films for 24 h at −75°C. After storage at −20°C for 2 months, the blot was re-probed using human actin cDNA as a probe.
Protein expression and in vitro translation.
The bacterial expression system was used to express GST-tagged polypeptides. BL21 bacterial cells expressing GST-fused polypeptides were homogenized by sonication and lysed in PBS containing 1% Triton X-100 for 1 h at 4°C. GST-tagged polypeptides were purified using glutathione-agarose beads. After elution with 100 mM Tris-HCl (pH 7.5) and 5 mM glutathione, the GST-polypeptides were dialyzed against 50mM Tris-HCl (pH 7.5), 50 mM NaCl. In vitro translated Myc-tagged Plk1 or aurora B protein was synthesized using the TNT SP6 quick coupled transcription/translation system (Promega) according to the manufacturer's instructions.
GST pull-down assays.
GST pull-down assays were carried out as described previously.65,66 Briefly, in vitro translated Myc-tagged proteins were incubated with the immobilized GST-fused polypeptides overnight at 4°C. After washing 4 times with binding buffer (50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 0.05% Triton-X-100, 10% glycerol, 0.2 mM EDTA and 1 mM DTT), the bound proteins were eluted into SDS-PAGE loading buffer.
Immunoblotting.
Cell lysates and GST pull-down proteins were separated by 7 or 4–12% SDS-PAGE gels, transferred to Immobilon-P transfer membranes (Millipore), blocked in 5% non-fat milk and incubated with primary antibodies as indicated. The following primary antibodies were used: mouse anti-Myc (9E10, 1:1000; Santa Cruz Biotechnology), rabbit anti-GFP (1:1000; Santa Cruz Biotechnology), rabbit anti-β-tubulin (1:2000; Santa Cruz Biotechnology), and rabbit anti-CCDC69 (1:500; rabbit anti-CCDC69 antibody was raised using the following peptide as antigen: CKPPKKKRQEPEPEQPPRPE; This peptide corresponds to amino acid residues 10–30 in human CCDC69). After washing three times, the blots were incubated with horseradish peroxide-conjugated secondary antibodies (1:5000; Santa Cruz Biotechnology) for 1 h at 23°C and visualized by SuperSignal West Pico Luminol/Enhancer solution (Pierce Biotechnology).
Immunofluorescence staining.
HeLa cells were transfected with plasmids (GFP-tagged CCDC69 full-length or fragments) or siRNAs (control or CCDC69 siRNAs). 24 h (plasmids) or 72 h (siRNAs) after transfection, the transfected cells were fixed with 4% paraformaldehyde to stain microtubules, CCDC69, aurora B, INCENP, MgcRacGAP, PRC1, lamin A+C, and phosphorylated histone 3 (p-H3). For RhoA staining, cells were fixed with 10% TCA for 10 min on ice. For Plk1 and CCDC69 co-staining, cells were fixed with methanol/acetone. To disrupt Plk1 or aurora B activation, HeLa cells were treated with vehicle (DMSO; Sigma), GW 843682X (1 µM; Tocris Bioscience) or ZM 447439 (10 µM; Tocris Bioscience) for 25 min and then fixed with 4% paraformaldehyde. The following primary antibodies were used for immunofluorescence staining: mouse monoclonal to aurora B (1:1000; Abcam); goat polyclonal to RACGAP1/MgcRacGAP (1:100; Abcam); mouse monoclonal to INCENP (1:500; Abcam); goat polyclonal to PRC1 (1:100; Santa Cruz Biotechnology); mouse monoclonal to RhoA (1:100; Santa Cruz Biotechnology); mouse monoclonal to β-tubulin (1:1000; Sigma); rabbit polyclonal to phospho-histone H3 (1:100; Millipore); mouse monoclonal to Plk1 (1:200; Millipore); mouse monoclonal to lamin A+C (1:100; Abcam). The secondary antibodies Alexa Fluor 594 donkey anti-mouse IgG (1:500), Alexa Fluor 350 donkey anti-mouse IgG (1:500), Alexa Fluor 594 donkey anti-goat IgG (1:500), Alexa Fluor 488 donkey anti-goat IgG (1:500), Alexa Fluor 594 donkey anti-rabbit IgG (1:500), and Alexa Fluor 488 donkey anti-rabbit IgG (1:500) were purchased from Invitrogen. The nuclei were visualized by DAPI (Sigma, St. Louis, MO). Images were taken using a Leica DMI 6000 B microscope (Leica, Deerfield, IL) and processed by blind deconvolution.
In vitro kinase assays.
For in vitro kinase assays, 5 µg of purified GST-tagged CCDC69 were incubated in 1X kinase assay buffer (5mM MOPS, pH 7.2, 2.5 mM β-glycerophosphate, 1mM EGTA, 0.4 mM EDTA, 5mM MgCl2, 0.05 µM DTT, 200 µM ATP) with 2.5 µg of His-tagged Plk1 (Cell Signaling) and 5 µCi [γ32] ATP. The total volume for the reactions is 50ul. The reaction mixtures were incubated at 30°C for 30 min, stopped by adding 2X SDS-PAGE loading buffer and boiling at 100°C for 10 min, resolved on a 10% SDS-PAGE, dried with the Gel Dryer Vacuum system (Fisher Scientific), and subjected to autoradiography.
Acknowledgements
We thank Dr. Robert S. Adelstein, Dr. Mary Anne Conti, and Dr. Richard H. Himes for critical reading and comments on the manuscript. This publication was made possible by NIH Grant Number P20 RR015563 from the National Center for Research Resources. This work was also supported by Terry C. Johnson Center for Basic Cancer Research. Contribution no.10-334-J from the Kansas Agricultural Experiment Station Manhattan, Kansas.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/13387
Supplementary Material
References
- 1.Wheatley SP, Wang Y. Midzone microtubule bundles are continuously required for cytokinesis in cultured epithelial cells. J Cell Biol. 1996;135:981–989. doi: 10.1083/jcb.135.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao LG, Wang YL. Signals from the spindle midzone are required for the stimulation of cytokinesis in cultured epithelial cells. Mol Biol Cell. 1996;7:225–232. doi: 10.1091/mbc.7.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oegema K, Mitchison TJ. Rappaport rules: cleavage furrow induction in animal cells. Proc Natl Acad Sci USA. 1997;94:4817–4820. doi: 10.1073/pnas.94.10.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glotzer M. The 3Ms of central spindle assembly: microtubules, motors and MAPs. Nat Rev Mol Cell Biol. 2009;10:9–20. doi: 10.1038/nrm2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeyaprakash AA, Klein UR, Lindner D, Ebert J, Nigg EA, Conti E. Structure of a Survivin-Borealin-INCENP core complex reveals how chromosomal passengers travel together. Cell. 2007;131:271–285. doi: 10.1016/j.cell.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 6.Terada Y, Tatsuka M, Suzuki F, Yasuda Y, Fujita S, Otsu M. AIM-1: a mammalian midbody-associated protein required for cytokinesis. EMBO J. 1998;17:667–676. doi: 10.1093/emboj/17.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooke CA, Heck MM, Earnshaw WC. The inner centromere protein (INCENP) antigens: movement from inner centromere to midbody during mitosis. J Cell Biol. 1987;105:2053–2067. doi: 10.1083/jcb.105.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li F, Ackermann EJ, Bennett CF, Rothermel AL, Plescia J, Tognin S, et al. Pleiotropic cell-division defects and apoptosis induced by interference with survivin function. Nat Cell Biol. 1999;1:461–466. doi: 10.1038/70242. [DOI] [PubMed] [Google Scholar]
- 9.Gassmann R, Carvalho A, Henzing AJ, Ruchaud S, Hudson DF, Honda R, et al. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J Cell Biol. 2004;166:179–191. doi: 10.1083/jcb.200404001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sampath SC, Ohi R, Leismann O, Salic A, Pozniakovski A, Funabiki H. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell. 2004;118:187–202. doi: 10.1016/j.cell.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Vagnarelli P, Earnshaw WC. Chromosomal passengers: the four-dimensional regulation of mitotic events. Chromosoma. 2004;113:211–222. doi: 10.1007/s00412-004-0307-3. [DOI] [PubMed] [Google Scholar]
- 12.Sellitto C, Kuriyama R. Distribution of a matrix component of the midbody during the cell cycle in Chinese hamster ovary cells. J Cell Biol. 1988;106:431–439. doi: 10.1083/jcb.106.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nislow C, Lombillo VA, Kuriyama R, McIntosh JR. A plus-end-directed motor enzyme that moves antiparallel microtubules in vitro localizes to the interzone of mitotic spindles. Nature. 1992;359:543–547. doi: 10.1038/359543a0. [DOI] [PubMed] [Google Scholar]
- 14.Toure A, Dorseuil O, Morin L, Timmons P, Jegou B, Reibel L, et al. MgcRacGAP, a new human GTPase-activating protein for Rac and Cdc42 similar to Drosophila rotundRacGAP gene product, is expressed in male germ cells. J Biol Chem. 1998;273:6019–6023. doi: 10.1074/jbc.273.11.6019. [DOI] [PubMed] [Google Scholar]
- 15.Mishima M, Kaitna S, Glotzer M. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev Cell. 2002;2:41–54. doi: 10.1016/s1534-5807(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 16.Jiang W, Jimenez G, Wells NJ, Hope TJ, Wahl GM, Hunter T, et al. PRC1: a human mitotic spindle-associated CDK substrate protein required for cytokinesis. Mol Cell. 1998;2:877–885. doi: 10.1016/s1097-2765(00)80302-0. [DOI] [PubMed] [Google Scholar]
- 17.Mollinari C, Kleman JP, Jiang W, Schoehn G, Hunter T, Margolis RL. PRC1 is a microtubule binding and bundling protein essential to maintain the mitotic spindle midzone. J Cell Biol. 2002;157:1175–1186. doi: 10.1083/jcb.200111052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu C, Jiang W. Cell cycle-dependent translocation of PRC1 on the spindle by Kif4 is essential for midzone formation and cytokinesis. Proc Natl Acad Sci USA. 2005;102:343–348. doi: 10.1073/pnas.0408438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurasawa Y, Earnshaw WC, Mochizuki Y, Dohmae N, Todokoro K. Essential roles of KIF4 and its binding partner PRC1 in organized central spindle midzone formation. EMBO J. 2004;23:3237–3248. doi: 10.1038/sj.emboj.7600347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mollinari C, Kleman JP, Saoudi Y, Jablonski SA, Perard J, Yen TJ, et al. Ablation of PRC1 by small interfering RNA demonstrates that cytokinetic abscission requires a central spindle bundle in mammalian cells, whereas completion of furrowing does not. Mol Biol Cell. 2005;16:1043–1055. doi: 10.1091/mbc.E04-04-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barr FA, Sillje HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–440. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- 22.Niiya F, Tatsumoto T, Lee KS, Miki T. Phosphorylation of the cytokinesis regulator ECT2 at G2/M phase stimulates association of the mitotic kinase Plk1 and accumulation of GTP-bound RhoA. Oncogene. 2006;25:827–837. doi: 10.1038/sj.onc.1209124. [DOI] [PubMed] [Google Scholar]
- 23.Asiedu M, Wu D, Matsumura F, Wei Q. Phosphorylation of MyoGEF on Thr-574 by Plk1 Promotes MyoGEF Localization to the Central Spindle. J Biol Chem. 2008;283:28392–28400. doi: 10.1074/jbc.M801801200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burkard ME, Randall CL, Larochelle S, Zhang C, Shokat KM, Fisher RP J, et al. Chemical genetics reveals the requirement for Polo-like kinase 1 activity in positioning RhoA and triggering cytokinesis in human cells. Proc Natl Acad Sci U S A. 2007;104:4383–4388. doi: 10.1073/pnas.0701140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petronczki M, Glotzer M, Kraut N, Peters JM. Polo-like kinase 1 triggers the initiation of cytokinesis in human cells by promoting recruitment of the RhoGEF Ect2 to the central spindle. Dev Cell. 2007;12:713–725. doi: 10.1016/j.devcel.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe BA, Takaki T, Petronczki M, Glotzer M. Polo-like kinase 1 directs assembly of the HsCyk-4 RhoGAP/Ect2 RhoGEF complex to initiate cleavage furrow formation. PLoS Biol. 2009;7:e1000110. doi: 10.1371/journal.pbio.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vader G, Medema RH, Lens SM. The chromosomal passenger complex: guiding Aurora-B through mitosis. J Cell Biol. 2006;173:833–837. doi: 10.1083/jcb.200604032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wheatley SP, Kandels-Lewis SE, Adams RR, Ainsztein AM, Earnshaw WC. INCENP binds directly to tubulin and requires dynamic microtubules to target to the cleavage furrow. Exp Cell Res. 2001;262:122–127. doi: 10.1006/excr.2000.5088. [DOI] [PubMed] [Google Scholar]
- 29.Verni F, Somma MP, Gunsalus KC, Bonaccorsi S, Belloni G, Goldberg ML, et al. Feo, the Drosophila homolog of PRC1, is required for central-spindle formation and cytokinesis. Curr Biol. 2004;14:1569–1575. doi: 10.1016/j.cub.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 30.Jantsch-Plunger V, Gonczy P, Romano A, Schnabel H, Hamill D, Schnabel R, et al. CYK-4: A Rho family gtpase activating protein (GAP) required for central spindle formation and cytokinesis. J Cell Biol. 2000;149:1391–1404. doi: 10.1083/jcb.149.7.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Severson AF, Hamill DR, Carter JC, Schumacher J, Bowerman B. The aurora-related kinase AIR-2 recruits ZEN-4/CeMKLP1 to the mitotic spindle at metaphase and is required for cytokinesis. Curr Biol. 2000;10:1162–1171. doi: 10.1016/s0960-9822(00)00715-6. [DOI] [PubMed] [Google Scholar]
- 32.Kaitna S, Mendoza M, Jantsch-Plunger V, Glotzer M. Incenp and an aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr Biol. 2000;10:1172–1181. doi: 10.1016/s0960-9822(00)00721-1. [DOI] [PubMed] [Google Scholar]
- 33.Shelden E, Wadsworth P. Interzonal microtubules are dynamic during spindle elongation. J Cell Sci. 1990;97(Pt 2):273–281. doi: 10.1242/jcs.97.2.273. [DOI] [PubMed] [Google Scholar]
- 34.Kline-Smith SL, Walczak CE. Mitotic spindle assembly and chromosome segregation: refocusing on microtubule dynamics. Mol Cell. 2004;15:317–327. doi: 10.1016/j.molcel.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Hunter AW, Wordeman L. How motor proteins influence microtubule polymerization dynamics. J Cell Sci. 2000;113(Pt 24):4379–4389. doi: 10.1242/jcs.113.24.4379. [DOI] [PubMed] [Google Scholar]
- 36.Quarmby L. Cellular Samurai: katanin and the severing of microtubules. J Cell Sci. 2000;113(Pt 16):2821–2827. doi: 10.1242/jcs.113.16.2821. [DOI] [PubMed] [Google Scholar]
- 37.Houseweart MK, Cleveland DW. Cytoskeletal linkers: new MAPs for old destinations. Curr Biol. 1999;9:R864–R866. doi: 10.1016/s0960-9822(00)80048-2. [DOI] [PubMed] [Google Scholar]
- 38.Drewes G, Ebneth A, Mandelkow EM. MAPs, MARKs and microtubule dynamics. Trends Biochem Sci. 1998;23:307–311. doi: 10.1016/s0968-0004(98)01245-6. [DOI] [PubMed] [Google Scholar]
- 39.Andersen SS. Spindle assembly and the art of regulating microtubule dynamics by MAPs and Stathmin/Op18. Trends Cell Biol. 2000;10:261–267. doi: 10.1016/s0962-8924(00)01786-4. [DOI] [PubMed] [Google Scholar]
- 40.Wordeman L. Microtubule-depolymerizing kinesins. Curr Opin Cell Biol. 2005;17:82–88. doi: 10.1016/j.ceb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Hunter AW, Caplow M, Coy DL, Hancock WO, Diez S, Wordeman L, et al. The kinesin-related protein MCAK is a microtubule depolymerase that forms an ATP-hydrolyzing complex at microtubule ends. Mol Cell. 2003;11:445–457. doi: 10.1016/s1097-2765(03)00049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ganem NJ, Compton DA. The KinI kinesin Kif2a is required for bipolar spindle assembly through a functional relationship with MCAK. J Cell Biol. 2004;166:473–478. doi: 10.1083/jcb.200404012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manning AL, Ganem NJ, Bakhoum SF, Wagenbach M, Wordeman L, Compton DA. The kinesin-13 proteins Kif2a, Kif2b, and Kif2c/MCAK have distinct roles during mitosis in human cells. Mol Biol Cell. 2007;18:2970–2979. doi: 10.1091/mbc.E07-02-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walczak CE, Mitchison TJ, Desai A. XKCM1: a Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell. 1996;84:37–47. doi: 10.1016/s0092-8674(00)80991-5. [DOI] [PubMed] [Google Scholar]
- 45.Wei Q, Adelstein RS. Pitx2a expression alters actinmyosin cytoskeleton and migration of HeLa cells through Rho GTPase signaling. Mol Biol Cell. 2002;13:683–697. doi: 10.1091/mbc.01-07-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei Q. Pitx2a binds to human papillomavirus type 18 E6 protein and inhibits E6-mediated P53 degradation in HeLa cells. J Biol Chem. 2005;280:37790–37797. doi: 10.1074/jbc.M502974200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 48.Yuce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol. 2005;170:571–582. doi: 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamijo K, Ohara N, Abe M, Uchimura T, Hosoya H, Lee JS, et al. Dissecting the role of Rho-mediated signaling in contractile ring formation. Mol Biol Cell. 2006;17:43–55. doi: 10.1091/mbc.E05-06-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishimura Y, Yonemura S. Centralspindlin regulates ECT2 and RhoA accumulation at the equatorial cortex during cytokinesis. J Cell Sci. 2006;119:104–114. doi: 10.1242/jcs.02737. [DOI] [PubMed] [Google Scholar]
- 51.Yonemura S, Hirao-Minakuchi K, Nishimura Y. Rho localization in cells and tissues. Exp Cell Res. 2004;295:300–314. doi: 10.1016/j.yexcr.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Pavicic-Kaltenbrunner V, Mishima M, Glotzer M. Cooperative assembly of CYK-4/MgcRacGAP and ZEN-4/MKLP1 to form the centralspindlin complex. Mol Biol Cell. 2007;18:4992–5003. doi: 10.1091/mbc.E07-05-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neef R, Gruneberg U, Kopajtich R, Li X, Nigg EA, Sillje H, et al. Choice of Plk1 docking partners during mitosis and cytokinesis is controlled by the activation state of Cdk1. Nat Cell Biol. 2007;9:436–444. doi: 10.1038/ncb1557. [DOI] [PubMed] [Google Scholar]
- 54.Neef R, Preisinger C, Sutcliffe J, Kopajtich R, Nigg EA, Mayer TU, et al. Phosphorylation of mitotic kinesin-like protein 2 by polo-like kinase 1 is required for cytokinesis. J Cell Biol. 2003;162:863–875. doi: 10.1083/jcb.200306009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gruneberg U, Neef R, Honda R, Nigg EA, Barr FA. Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J Cell Biol. 2004;166:167–172. doi: 10.1083/jcb.200403084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guse A, Mishima M, Glotzer M. Phosphorylation of ZEN-4/MKLP1 by aurora B regulates completion of cytokinesis. Curr Biol. 2005;15:778–786. doi: 10.1016/j.cub.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 57.Neef R, Klein UR, Kopajtich R, Barr FA. Cooperation between mitotic kinesins controls the late stages of cytokinesis. Curr Biol. 2006;16:301–307. doi: 10.1016/j.cub.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 58.Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, et al. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McNally FJ, Vale RD. Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell. 1993;75:419–429. doi: 10.1016/0092-8674(93)90377-3. [DOI] [PubMed] [Google Scholar]
- 60.Belmont LD, Mitchison TJ. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 1996;84:623–631. doi: 10.1016/s0092-8674(00)81037-5. [DOI] [PubMed] [Google Scholar]
- 61.Wordeman L, Mitchison TJ. Identification and partial characterization of mitotic centromere-associated kinesin, a kinesin-related protein that associates with centromeres during mitosis. J Cell Biol. 1995;128:95–104. doi: 10.1083/jcb.128.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gavet O, Ozon S, Manceau V, Lawler S, Curmi P, Sobel A. The stathmin phosphoprotein family: intracellular localization and effects on the microtubule network. J Cell Sci. 1998;111(Pt 22):3333–3346. doi: 10.1242/jcs.111.22.3333. [DOI] [PubMed] [Google Scholar]
- 63.Bishop JD, Schumacher JM. Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B Kinase stimulates Aurora B kinase activity. J Biol Chem. 2002;277:27577–27580. doi: 10.1074/jbc.C200307200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Honda R, Korner R, Nigg EA. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell. 2003;14:3325–3341. doi: 10.1091/mbc.E02-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu D, Asiedu M, Adelstein RS, Wei Q. A novel guanine nucleotide exchange factor MyoGEF is required for cytokinesis. Cell Cycle. 2006;5:1234–1239. doi: 10.4161/cc.5.11.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu D, Asiedu M, Wei Q. Myosin-interacting guanine exchange factor (MyoGEF) regulates the invasion activity of MDA-MB-231 breast cancer cells through activation of RhoA and RhoC. Oncogene. 2009;28:2219–2230. doi: 10.1038/onc.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.