Abstract
P38αMAPK (p38α) is usually activated in response to various stresses and plays a role in the inhibition of cell proliferation and tumor progression, but little is known about its roles in meiotic spindle assembly. In this study, we characterized the dynamic localization of p38α and explored its function in mouse oocyte meiotic maturation. P38α specifically colocalized with γ-tubulin and Plk1 at the center of MTOCs and spindle poles. Depletion of p38α by specific morpholino injection resulted in severely defective spindles and misaligned chromosomes probably via MK2 dephosphorylation. Notably, depletion of p38α led to significant spindle pole defects, spindle elongation, non-tethered kinetochore microtubules and increased microtubule tension. The disruption of spindle stability was coupled with decreased γ-tubulin and Plk1 at MTOCs. Overexpression of Eg5, a conserved motor protein, also caused spindle elongation and its morpholino injection almost completely rescued spindle elongation caused by p38α depletion. In addition, p38α-depletion decreased BubR1 and interfered with spindle assembly checkpoint (SAC), which resulted in aneuploid oocytes. Together, these data indicate that p38α is an important component of MTOCs, which regulates spindle assembly and spindle length, as well as stabilizes the spindle and spindle poles. Perturbed SAC and abnormal microtubule tension may be responsible for the misaligned chromosomes and high aneuploidy in p38α-depleted mouse oocytes.
Key words: p38α, meiosis, mouse oocyte, spindle assembly, microtubule organization center (MTOC), Eg5, spindle assembly checkpoint
Introduction
The assembly of a functional bipolar spindle is critical for accurate chromosome segregation in mammalian oocytes.1,2 Segregation errors during mitosis in somatic cells cause genomic instability and contribute to the development and progression of cancer.3,4 Chromosome missegregation during meiosis is a major source of embryonic aneuploidy in mammals and accounts for most spontaneous abortion and birth defects in human.5,6 Therefore, understanding the mechanism that governs meiotic spindle assembly in mammals remains an important and challenging goal. Microtubule organizing centers (MTOCs), which control the formation and anchorage of microtubules, include centrosomes in vertebrates and spindle pole bodies (SPB) in yeast. Mouse oocytes contain over 80 microtubule-organizing centers (MTOCs) and rely on the self-organization of numerous acentriolar MTOCs that are functional equivalents of centrosomes in mitosis.7 MTOCs contain the pericentriolar material components: γ-tubulin and pericentrin. Mouse oocyte MTOCs have similar properties as centrosomes, which nucleate microtubules from the γ-tubulin ring complexes containing γ-tubulin. The sites of γ-tubulin binding to the spindle may represent the location of capped microtubule minus-ends. The poles of the spindles are major focal points for the minus ends of spindle microtubules that are critical for chromosome segregation. In mouse oocytes, multiple microtubule focal points are seen after MTOC aggregation is complete. Eg-5 is required for spindle elongation and spindle bipolarization.7–9 However, how these microtubules are captured and tethered at the poles has not yet been determined. In particular, the means for sustained anchoring of microtubules at spindle poles containing two broad γ-tubulin bands7 and the role of MTOC in maintenance of spindle pole integrity are not yet established. MTOCs are composed of hundreds of proteins. Although significant research has focused on centrosome function in mitotic cells, little is known about the function of MTOC-associated proteins in the acentrosomal meiotic spindle assembly in mouse oocytes.
Many proteins including motor proteins, microtubule-associated proteins (MAPs), protein kinases and phosphatases, as well as MTOC components become enriched at spindle poles during meiosis. The mitogen-activated protein kinase (MAPK) superfamily comprises classical MAPK (also called ERK), c-Jun amino-terminal or stress-activated protein kinase (JNK or SAPK) and p38, all of which are highly conserved in all eukaryotic systems.10 MAPK (ERK) plays important roles in stabilizing and facilitating pole and chromosome separation.11 In addition, MEK, the upstream regulator of ERK participates in spindle assembly and chromosome alignment.12,13 p38 mitogen-activated protein kinase (p38 MAPK) is one of the three major members of the MAPK family that regulates cellular responses including apoptosis, cell proliferation and immune response as well as cell growth, differentiation and cytoskeletal rearrangements.14 Activation of p38 MAPK is usually correlated with cell cycle arrest including G1 and G2/M arrest in mammalian cells, M phase arrest in Xenopus cleaving embryos and G2/M arrest in sea star oocytes.15,16 The p38 protein is known to be phosphorylated by MKK3 and MKK6 for activation in response to cell stress and in turn it phosphorylates a number of substrates, including MAPKAP kinase 2 (MK2). p38α forms a stable heterodimer with MK2 17,18 and mediates the phosphorylation and functions of MK2 in vitro and in vivo.19,20
Spindle assembly checkpoint (SAC) represents a surveillance mechanism that arrests cells in M-phase in the presence of unattached chromosomes. p38 MAPK has been reported to participate in antephase checkpoint, G2/M cell cycle checkpoint as well as spindle assembly checkpoint after DNA damage and mitotic arrest.16,21,22 However, it is controversial whether p38 MAPK functions as a component of the spindle assembly checkpoint in mitosis. Matsusaka and Shiromizu determined that p38 MAPK participated in the antephase checkpoint rather than in the spindle assembly checkpoint.22,23 Moreover, a recent study showed that p38 MAPK signaling could mediate SAC activation, which accelerates ubiquitination and proteolysis of Cdc20 that is essential for prometaphase arrest in human cells.24 In addition, the observation from mitosis suggests that loss of p38 MAPK makes it more difficult for cell to satisfy the mitotic checkpoint, but is not required for a functional mitotic checkpoint.25 However, there are few reports about the role of p38 MAPK in mouse meiotic maturation.
Earlier studies indicated that p38 MAPK is a microtubule associated protein (MAP) and regulates cytoskeletal organization.26–28 In mammalian cells, p38 MAPK consists of four informs-α, β, γ and δ; p38α knockout mice are lethal, while knockouts of p38β, γ and δ are viable and fertile.20,29,30 Although p38 MAPK signaling in cumulus cells may be involved in meiotic resumption in porcine oocytes,31 whether p38 MAPK participates in meiotic spindle assembly and subsequent accurate chromosome segregation remains unknown.
In the present study, we for the first time investigated the localization and functions of p38α during mouse oocyte meiotic maturation. Our results strongly suggest that p38α is a component of the MTOC and plays a crucial role in spindle assembly, spindle pole formation and chromosome alignment. The depletion of p38α decreased recruitment of γ-tubulin to MTOCs and reduced spindle microtubule stability, resulting in multiple ectopic poles. Moreover, p38α could indirectly generate an inward force towards the midzone of the spindle and partly antagonize the outward force produced by Eg5. Therefore, depletion of p38α causes microtubule force imbalance resulting in increase in spindle tension and spindle elongation.32,33 p38α depletion also disturbed SAC, resulting in production of oocytes with abnormal spindles and misaligned chromosomes, which may be responsible for the high aneuploidy of MII oocytes.
Results
Expression and subcellular localization of phospho-p38α MAPK (p-p38α) during mouse oocyte meiotic maturation.
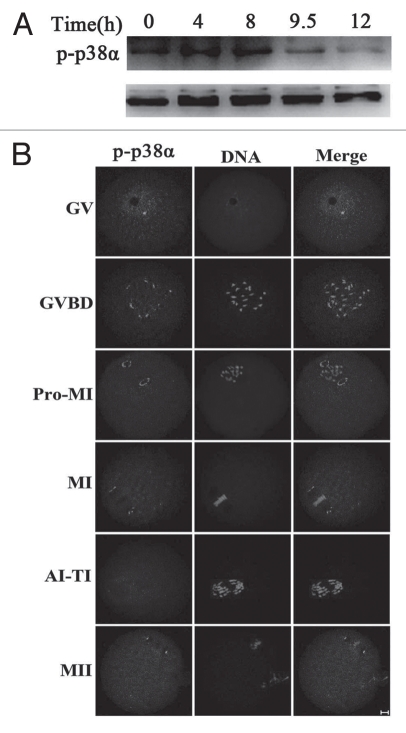
To investigate the role of p38α during oocyte meiotic maturation, we examined the expression and subcellular localization of this protein. Samples of mouse oocytes at different stages of meiosis were collected after oocytes had been cultured for 0, 4, 8, 9.5 and 12 hours, corresponding to germinal vesicle (GV), prometaphase I (Pro-MI), metaphase I (MI), anaphase/telophase I (ATI) and metaphase II (MII) stages, respectively. As shown in Figure 1A, the expression level of p-p38α remained stable from GV to MI stage, and then become reduced at the ATI and MII stages.34 For subcellular localization of p-p38α, oocytes at different stages of meiosis were processed for immunofluorescent staining and a subcellular distribution pattern of p-p38α was observed during the progression of meiosis (Fig. 1B). At the GV stage, p-p38α was localized in the germinal vesicle; after GVBD, multiple bright foci of p-p38α were detected near the individual chromosomes. By Pro-MI when chromosomes began to migrate to the equator of the spindle, p-p38α was gradually translocated to the spindle poles. Notably, p-p38α were specifically concentrated at the spindle poles at the MI stage. At the poles, p-p38α was typically observed in an “O”- or “C”-shaped configuration. No evident signals of p-p38α were labeled in anaphase/telophase oocytes, however, p-p38α reappeared at the spindle poles in oocytes at the MII stage.
Figure 1.
Expression and subcellular localization of p-p38α during mouse oocyte meiotic maturation. (A) Expression of p-p38α and p38 during meiotic maturation at 0, 4, 8, 9.5 and 12 h, corresponding to GV, pro-MI, MI, AI-TI and MII stages, respectively. The molecular mass of p-p38α is 43 kDa and that of p38 is 43 kDa. Proteins from 300 oocytes were loaded for each sample. (B) Confocal microcopy showing immunostaining of p-p38α (green) and DNA (red) in mouse oocytes at GV (germinal vesicle), GVBD (germinal vesicle breakdown), pro-MI (first prometaphase), MI (first metaphase), AI-TI (first anaphase and telophase) and MII (second metaphase) stages. Bar = 10 µm.
p-p38α colocalizes with γ-tubulin at cytoplasmic MTOCs and spindle poles in mouse oocytes.
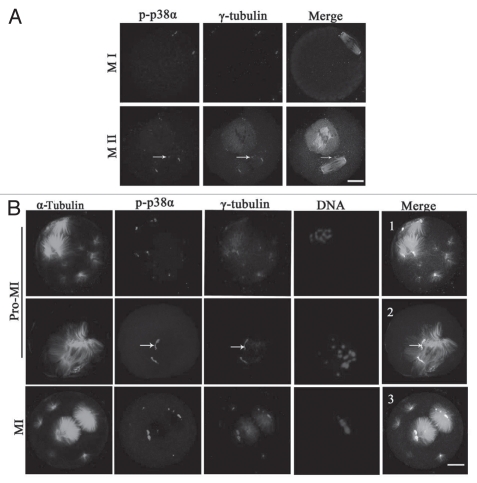
Based on the above data showing that p38α localized at the spindle poles, we considered that p38α may be a new component of spindle poles or MTOCs. As shown in Figure 2A, p-p38α was colocalized with γ-tubulin at the spindle poles and cytoplasmic MTOCs in oocytes at MI and MII stages. To further clarify the correlation between p38α and microtubule dynamics, taxol, a microtubule-stabilizing drug, was employed for oocyte treatment. When spindle organization started after GVBD, microtubule fibers in taxol-treated oocytes became excessively polymerized leading to significantly enlarged spindles together with numerous asters in the cytoplasm (Fig. 2B). In this case, p-p38α was specifically colocalized with γ-tubulin at the poles of the abnormal spindles, the center of MTOCs, and at the center of the cytoplasmic asters in oocytes at Pro-MI, MI and MII stages. Specifically, p-p38α was strictly colocalized with a broad “C”-shaped γ-tubulin configuration unattached at the poles (Fig. 2B, 2 and arrows). These data suggest that p-p38α was associated with MTOCs and may be a component of acentriolar meiotic mouse spindle MTOCs.
Figure 2.
p-p38α colocalizes with γ-tubulin at meiotic oocyte MTOCs and spindle poles. (A) Representative images of oocytes at MI and MII stages during meiotic maturation. p-p38 (purple) colocalization with γ-tubulin (red) was assessed, together with α-tubulin (green) and DNA (blue) staining. Arrows indicate cytoplasmic asters. Bar = 10 µm. (B) Colocalization of p-p38α and γ-tubulin at MTOCs in mouse oocytes treated with taxol. Oocytes were incubated in M2 medium containing 200 µg/ml taxol for 45 min at pro-MI and MI stages, respectively. Oocytes were stained with antibodies against p-p38α (purple), γ-tubulin (red), α-tubulin (green) and DNA (blue) dye Hoechst 33258. Arrows indicate that p-p38α is strictly colocalized with typical “C”-shaped γ-tubulin at unattached poles off spindles. Bar = 10 µm.
Depletion of p38α resulted in abnormal spindles and misaligned chromosomes.
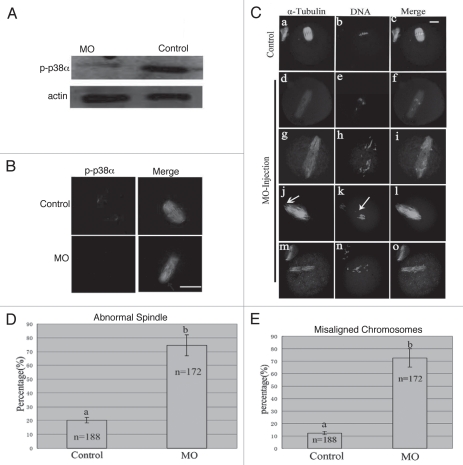
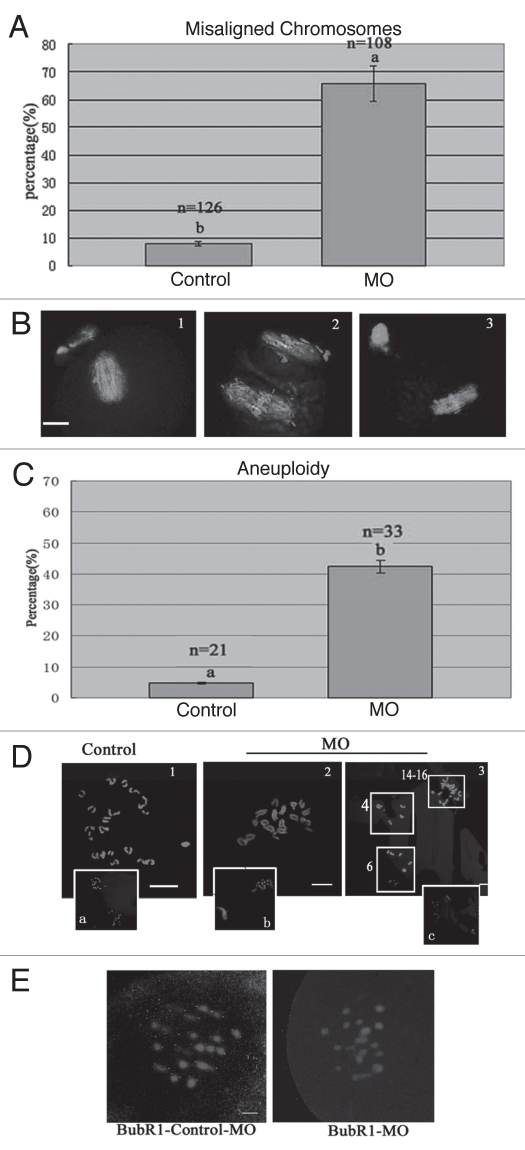
To explore the roles of p38α, we employed a morpholino-based gene-silencing approach to deplete p38α in oocytes. Using a specific p38α-targeting morpholino (termed p38αMO), we depleted most of the endogenous p-p38α protein in oocytes (Fig. 3A). As shown in Figure 3B, p38α signal was clearly observed at the spindle poles in the oocytes injected with control morpholino (control MO), while p38α was not detectable in p38αMO-injected oocytes. The data revealed the successful knockdown of p-p38α. p-p38α depletion did not affect meiotic cell cycle progression and rates of polar body extrusion (see results below). Downregulation of p-p38α resulted in significant defects in spindle formation and chromosomes alignment. Aberrant spindle organization (Fig. 3C) included elongated spindles (Fig. 3Cd, g, j and m), and various defective poles including multipolar spindles (Fig. 3Cg) and disintegrated spindle poles (Fig. 3Cj). The rate of abnormal spindle formation in the p38αMO-injected group was 74.4% (n = 172) (Fig. 3D), which was considerably higher than that of the control MO-injected group (21.2%, n = 188). p38α-depleted oocytes displayed severe defects in chromosome alignment, showing lagging chromosomes and irregularly scattered chromosomes (Fig. 3C). The incidence of misaligned chromosomes in the p38αMO-injected group was up to 75% (n = 172), much higher than that in the control group (10.2%, n = 188) (Fig. 3E). These results suggest that p38α is required for regulating spindle organization, spindle pole integrity and chromosome alignment.
Figure 3.
p38α depletion leads to defective spindles, abnormal spindle poles and misaligned chromosomes. (A) Expression of p-p38α in the p38αMO microinjected oocytes. GV oocytes were microinjected with standard control MO and p38αMO, respectively, and incubated for 24 h in M2 medium containing 2.5 µM Milrinone before oocytes were collected for western blotting. Control MO: 300 oocytes microinjected with standard control MO; MO: 300 oocytes microinjected with p38αMO. (B) Clear p-p38α signal was found at spindle poles in control MO injected oocytes; no p-p38α signal was detected in p38αMO-injected oocytes (MO). p-p38α (red); α-tubulin (green); DNA (blue). Bar = 10 µm. (C) Spindle morphology and chromosome alignment after microinjection of MO standard control and p38αMO in mouse oocytes. In the control group (n = 188), most oocytes showed normal spindle morphology and chromosome alignment (C, a and b), while in the MO injection group (n = 172), most oocytes showed severely defective spindle and chromosome alignment. Straggled (h and n) or lagging chromosomes (e and k) were found in oocytes at the MI, AI, MII stages. Spindle elongation (d, g, j and m); multiple poles (g); disintegrated poles (d, g, j and m) were observed in oocytes at MI and MII. Arrow in “k” indicates the misaligned chromosomes; Arrow in “j” shows non-tethered pole. α-tubulin (green); DNA (red). Bar = 10 µm. (D and E) Percentage of oocytes with abnormal spindles and misaligned chromosomes between the control and p38αMO groups. Data are expressed as mean ± SEM of at least three independent experiments and different letters indicate statistically significant difference (p < 0.05).
p38α depletion may compromise meiotic spindle organization and chromosome alignment via the p38α/MK2 signaling pathway.
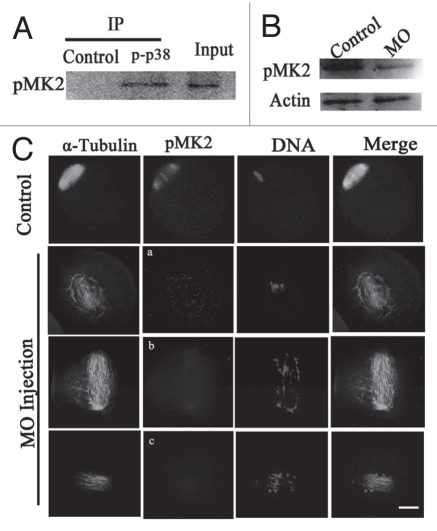
To further address the mechanism of p38α in meiotic spindle assembly, we explored the relationship between p38α and MK2. Firstly, we tested a possible interaction between endogenous p-p38α and p-MK2 by coimmunoprecipitation with p-p38α antibody in mouse MI oocytes extracts and then performed immunoblot analysis with the anti-p-MK2 antibody. As shown in Figure 4A, p-MK2 was detected as a specific band in the oocyte extract lane (Input). In the control lane, the immunoprecipitation was carried out with an irrelevant antibody (anti-β actin) instead of anti-p-p38α antibody and no band was detected. By contrast, in the p-p38α lane, p-MK2 can be detected in the immunoprecipitates and appeared at the same position as the mouse extract lane (Input).
Figure 4.
Analysis of the interaction of p38α with MK2. (A) Coimmunoprecipitation of p38α and MK2 was analyzed in extracts of 2,000 MI oocytes. Immunoprecipitation experiments were performed with a polyclonal anti-p-p38 antibody or a control irrelevant antibody (anti-β actin). Input was loaded with about 18 µl oocyte extract sample. pMK2 was immunodetected by a polyclonal antibody against pMK2. (B) Expression of pMK2 in the p38α-MO microinjected oocytes. GV oocytes were microinjected with standard control MO and p38α-MO respectively, and incubated for 24 h in M2 medium containing 2.5 µM Milrinone before oocytes were collected for western blotting. (C) Localization of pMK2 in the p38α-MO microinjected oocytes. In control-MO injected oocytes, pMK2 was clearly localized at spindle poles and chromosomes while pMK2 was disassociated from spindles in p38α-MO oocytes. Abnormal spindles and misaligned chromosomes were found in the p38α-MO group. α-Tubulin (green); pMK2 (red); DNA (blue). Bar = 10 µm.
Secondly, we focused on whether p38α phosphorylates MK2 during oocyte meiosis. We examined the protein level of phosphorylated MK2 in p38α-depleted oocytes by western blot. As shown in Figure 4B, p-MK2 was clearly decreased in the p38α-depleted oocytes compared with the control group, indicating that p38α could phosphorylate and activate MK2. Subsequently, we examined the p-MK2 localization after p38α depletion and demonstrated that p-MK2 was dissociated from the spindle poles and chromosomes, and were scattered in the cytoplasm in the p38α-depleted oocytes. Interestingly, dispersal of p-MK2 around the spindle occurred into an oval-shape formation (Fig. 4Ca). Moreover, there was little p-MK2 signal (if any) localized in the significantly disrupted spindle (Fig. 4Cb and c).
Clearly, p38α depletion did affect the localization and phosphorylation of MK2 in oocytes and is at least partly responsible for the aberrant spindle assembly.
p38α is required for recruitment of γ-tubulin to MTOCs and stabilization of spindle bipolarity.
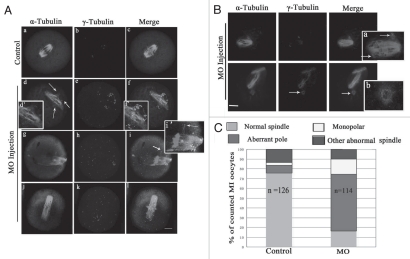
In p38α-depleted MI oocytes, spindle organization was disrupted in over 70% of oocytes (n = 114; Fig. 5A–C). The typical defects in spindle assembly included aberrant poles (58.2%, n = 114), with striking additional small poles around spindles or near the main poles (Fig. 5Ad and g). These aberrant poles contained γ-tubulin labeling (Fig. 5Af and i). Moreover, individual microtubules associated with the spindles were dissociated from the spindle poles and the minus-ends of individual microtubules also were labeled with γ-tubulin (Fig. 5Ai). However, γ-tubulin signal at the main poles became faint (Fig. 5A). Interestingly, oocytes with spread chromosomes showed elongated spindles (Figs. 3C and 5Af, i and l). Unexpectedly, about 20% of oocytes displayed detachment of intact “O”-shaped γ-tubulin rings from the ends of meiotic spindles (Fig. 5B and arrow).
Figure 5.
Depletion of p-p38α disrupts spindle pole structure at MI stage. (A and B) Representative defective spindle poles were observed in control-MO (n = 126) and p38αMO injected oocyte group (n = 114). Normal barrel-shaped bipolar spindles were found in the control-MO group; various abnormal poles were found in the p38αMO injected group: multiple poles in (A, d and g); untethered poles in A (n); uncoupled poles with spindle in (B). Abnormal distribution of γ-tubulin was observed in (A). Arrow in (A, d) indicates the ectopic poles; (d', f' and i') in (A) indicate the magnification of (d, f and i). Arrows in (B, a) and (B, b) indicate uncoupled poles labeled with γ-tubulin. (A): α-tubulin (green); γ-tubulin (purple); DNA (red). Bar = 10 µm. (B): α-tubulin (purple); γ-tubulin (green); DNA (blue). (C) Frequencies of abnormal morphology of MI meiotic spindles in control-MO and p38αMO injected groups. More than 100 oocytes were examined. The number of spindles analyzed is shown in the columns. The difference is significant (p < 0.05).
Furthermore, as shown in Figure 5Ae, h and k, significant γ-tubulin signals were observed in the cytoplasm in p38α-depleted MI oocytes. Moreover, MTOC asters labeled with γ-tubulin loosely dispersed within the abnormal spindles or in the cytoplasm in MI oocytes, which indicates that activation of p38α is required for recruitment of cytoplasmic MTOCs to spindle poles.7 The oocytes with abnormal spindles displayed multiple bright foci loosely dispersed at the defective spindle poles. It is possible that p38α depletion reduced recruitment of γ-tubulin to MTOCs and cytoplasmic MTOCs to spindle poles which destabilized spindle bipolarity.35
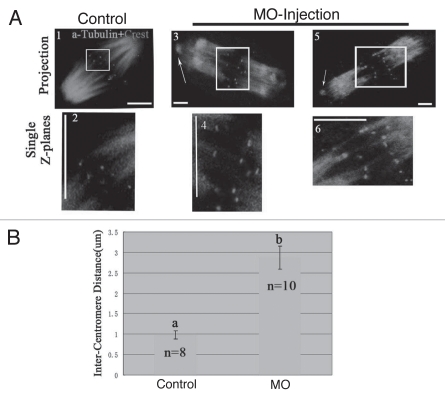
Preferential depolymerization of non-kinetochore microtubules by cold treatment confirmed that kinetochore fibers remained stably attached to kinetochores in p38α-disrupted oocytes even when becoming completely defocused at poles (Fig. 6A3–6).36,37 When treated with cold, microtubule-nucleating components became dissociated from the spindle ends in the p38α-depleted oocytes (Fig. 6A3 and 5), which was identical to phenotypes shown in Figure 5B. Additionally, the minus ends of kinetochore microtubules were not tethered at the spindle poles. Surprisingly, the remaining kinetochore fibers showed higher spreading at the microtubule minus ends after cold treatment compared to that in spindle poles of pretreated p38α-depleted oocytes (Figs. 6A and 5Ad, g and j), in support of our data above.
Figure 6.
Increased spindle tension and obvious non-tethered kinetochore microtubules in the absence of p38α. Oocytes were incubated on ice for 15 min to selectively depolymerize non-kinetochore microtubules and the distance between homologous kinetochores in MI oocytes was measured. (A) Merge of spindle (green) with Crest (kinetochore marker) (red). A (2, 4 and 6) respectively indicate region magnified in (A, 1, 3 and 5). Arrows indicate the detached nucleation structures from the poles. Images represent single projection of z sections spanning the entire spindle width. Kinetochore pairs were identified in single z sections by the relative positioning of kinetochore and associated kinetochore fibers. Bar = 10 µm. (B) Relative average distance between kinetochore pairs in control (n = 8) and p38α- depleted spindles (n = 10). Data are expressed as mean ± SEM and different letters indicate statistically significant difference. Error bars represent one standard error. The difference is significant (p < 0.05).
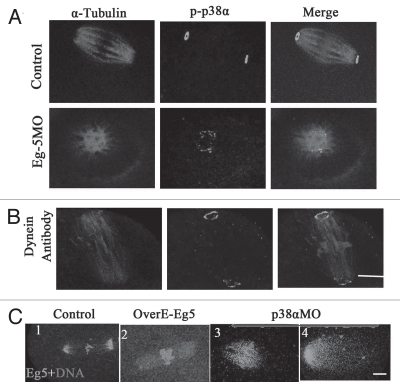
Plk1 is a centrosomal kinase localized at the spindle poles, involved in centrosome maturation and spindle assembly in mitosis and meiosis.37,38 In this study, we investigated whether the functions of p38α and Plk1 were correlated in meiosis. As shown in Supplemental Figure 1A, p38α was colocalized with Plk1 at MTOCs and spindle poles in oocytes treated with taxol and in control oocytes. In p38α-depleted oocytes, Plk1 was either completely dissociated from the spindle poles and chromosomes or Plk1 signals became faint (Sup. Fig. 1B). These results show that p38α is required for Plk1 localization to the spindle poles.
p38α and Eg5 play opposite roles in determining spindle tension and length.
p38α-depleted oocytes displayed elongated spindles and increased tension between homologous kinetochores in pole-defocused spindles. The distance between homologous kinetochores in pole-defocused MI spindles of p38α-depleted oocytes (2.88 µm, s.e.m = 0.05, n = 10 spindles, 64 kinetochore pairs) was significantly increased compared to that of control oocytes (0.99 µm, s.e.m = 0.04, n = 8 spindles, 52 kinetochore pairs) (Fig. 6A and B), indicating that the tension between homologous kinetochores in pole-defocused spindles was notably increased.
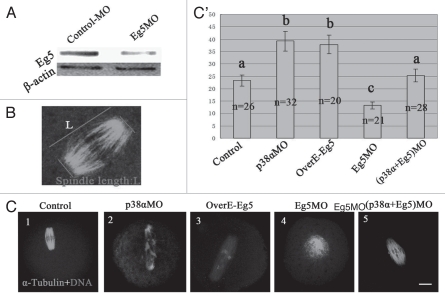
Meiotic spindle assembly requires various motor proteins including Eg5 and dynein and the motor proteins could generate antagonistic forces during bipolar spindle assembly. We propose that p38α may interact with Eg5 to generate a balance of forces between the two poles by indirectly generating an inward force. Using a specific Eg5-targeting morpholino (termed Eg5MO), we depleted most of the endogenous Eg5 protein in oocytes (Fig. 7A). To quantify spindle length, we measured the spindle length as shown in the schematic (Fig. 7B). As shown in Figure 7C1, C2 and C', the spindles were 50% longer in p38αMO-injected oocytes than those of control MO-injected oocytes. Interestingly, spindle elongation was observed in Eg5- overexpressed oocytes (Fig. 7C3 and C'). In contrast, monopolar spindles were observed in Eg5 knocked-down oocytes (Fig. 7C4). We further injected oocytes with both p38αMO and Eg5MO. As expected, in p38αMO and Eg5MO co-injected oocytes, bipolar spindle morphology and length were rescued (Fig. 7C5 and C').39
Figure 7.
p38α/Eg5 antagonism. (A) Expression of Eg5 in the Eg5MO-microinjected oocytes. GV oocytes were microinjected with standard control MO and Eg5MO respectively, and incubated for 24 h in M2 medium containing 2.5 µM Milrinone before oocytes were collected for western blotting. (B) Diagram shows the measurement of spindle length. Spindle length indicates pole-pole distance. (C) Spindle with normal length was found in the control-MO injected group (C1); p38α depletion (C2) and Eg5 overpression (C3) similarly affect MI spindle length; p38α (C2) and Eg5 (C4) depletion inversely affect metaphase spindle length; Co-depletion of p38α and Eg5 rescues the above spindle length (A4). Bar = 10 µm. (C') Bar grap reports the average length of fixed spindles, measured as shown in (B). More than 20 spindles for each group were measured. Data are expressed as mean ± SEM and different letters indicate statistically significant difference. Error bars represent one standard error. The difference is significant (p < 0.05).
p38α depletion affected the distribution of Eg5 along microtubules in abnormal spindles.
To further explore the mechanism of p38α function on spindle length, we examined the localization of p38α after downregulation of Eg5 or dynein. The results indicated that Eg5 or dynein depletion did not affect the localization of p38α, and p38α was still localized at spindle poles as shown in Figure 8A and B. However, p38α depletion significantly affected the distribution of Eg5 along the spindles. As shown in Figure 8C1, Eg5 was localized at microtubules and concentrated at spindle poles in control MO-injected oocytes. In Eg5-overexpressed oocytes, Eg5 was localized to microtubules but not concentrated at the poles (Fig. 8C2). However, in p38α-depleted oocytes, the expression of Eg5 was decreased at poles, but increased on the spindle microtubules, notably at the midzone of the spindle (Fig. 8C3). In some spindles, Eg5 signal was only detected in half of the whole spindle (Fig. 8C4).
Figure 8.
The localization of p38α is independent of Eg5 and dynein, but the p38α depletion and Eg5 overexpression affect the localization of Eg5 in spindles. (A and B) In control groups (standard control MO and rabbit IgG), p-p38α was localized at the poles; the localization of p-p38α was found at the monopoles in the Eg5MO-injected group; the localization of p-p38α was observed at the poles in the dynein-antibody injection group. α-tubulin (red); p38α (green); DNA (blue). Bar = 10 µm. (C) In control-MO injected oocytes, Eg5 concentrated along microtubules at poles (C1); in the Eg5-overexpressed group, Eg5 was localized at microtubules (C2); p38α depletion affected the localization of Eg5 in the spindle: obvious distribution in the midzone of spindles and unfocused on the poles (C3). Eg5 (green); DNA (red). Bar = 10 µm.
P38α depletion inactivated SAC and increased frequencies of aneuploidy.
We observed that the ratio of polar body extrusion (PBE) in p38α-depleted oocytes (78.2%, n = 234) was not different from that of the control group (79.8%, n = 196). However, p38α-delepted MI and MII oocytes showed obviously misaligned chromosomes (Fig. 9A and B). Thus, chromosome spreads were performed in p38α-depleted MII oocytes. As known for MII, the number of single chromosomes (univalents) in mouse oocytes is 20 (control, Fig. 9D1), which is the prerequisite for genomic integrity. Our results showed that p38α-depleted MII oocytes typically displayed incorrect numbers (more or less than 20) of univalents (Fig. 9D2 and 3). The frequency of aneuploidy of p38α-depleted MII oocytes was significantly higher than that in control oocytes (Fig. 9C and D). This prompted us to speculate that p38α depletion may affect SAC.
Figure 9.
p38αMO affects the localization of BubR1, leading to aneuploidy in MII oocytes. (A and B) Percentage of oocytes with misaligned chromosomes between control-MO (control) and p38αMO (MO) groups in MII oocytes, respectively. Normal aligned chromosomes were found in the control-MO group; straggled chromosomes were detected in p38αMO groups. α-tubulin (green); DNA (red). (C and D) Chromosome spread was performed in control-MO and p38αMO-injected oocytes. The numbers of univalents in the oocytes in D1–D3 are 20, 18, 24–26 respectively. Da–Dc are the minification of D1–D3 including chromosomes in Pbs. Different letters indicate statistically significant difference. The difference is significant (p < 0.05). Bar = 10 µm. (E) Recruitment of BubR1 to kinetochores in p38α-depleted pre-MI oocytes with defective spindles. In control-MO injected oocytes, BubR1 was localized at kinetochores, while no staining was found in oocytes in the p38αMO group. BubR1 (red); DNA (blue). Bar = 10 µm.
Next, we analyzed the localization of BubR1, an important component of SAC. We observed that BubR1 was enriched at numerous kinetochores in control MO-injected oocytes at the prometaphase stage. However, no BubR1 signal was detected in 85% (38/45) of p38αMO-injected oocytes (Fig. 9E). The results imply that decreased BubR1 in p38αMO-injected oocytes may lead to high frequencies of aneuploidy.
Discussion
In the present study, we provide evidence that p38α is a component of acentriolar MTOCs, and participates in the recruitment of cytoplasmic MTOCs and γ-tubulin to spindle poles in mouse oocytes. These data indicate that depletion of p38α in mouse oocytes impairs spindle assembly, leads to abnormal poles formation, increases microtubule tension, spindle elongation and misaligned chromosomes. It is possible that p38α maintains normal spindle morphology by generating an inward force to antagonize the anti-parallel sliding force produced by Eg5. p38α depletion affected the distribution of Eg5 in the spindle, which may lead to defective spindle poles and spindle elongation. Spindles in p38α-depleted oocytes failed to retain appropriate tension across homologous kinetochores and to align chromosomes at the metaphase plate. Furthermore, depletion of p38α perturbed SAC by dissociating BubR1 from the kinetochores, resulting in high frequencies of aneuploidy in the oocytes.40,41
p38α is a component of acentriolar MTOC s and required for spindle assembly in mouse oocytes.
p38 has been reported to play roles in inhibition of cell cycle progression, cell proliferation and tumor progression in mitosis.30 In mitosis, p38α is a microtubule-associated protein colocalized with γ-tubulin at centrosomes.42 Chromosome lagging was observed in Hela cells treated with p38α inhibitors.42,43 In denuded porcine oocytes, inhibition of p38α activity blocked the MI/MII transition.31 In the present study, p-p38α was shown to colocalize with γ-tubulin and Plk1 at the spindle poles and cytoplasmic MTOCs in oocytes at MI and MII stages, which is similar to many proteins involved in spindle formation, such as aurora-kinase A (AURKA), PKC and NEDD1 in mitosis44,45 and meiosis.46,47 In addition, p38α was colocalized with both γ-tubulin and Plk1 at the centers of acentriolar MTOCs and cytoplasmic asters in oocytes after taxol treatment. The expression of p38α at the MTOC was reduced at anaphase and reappeared at the MII stage. The dynamic pattern of p38α localization at MTOCs is similar to that of γ-tubulin.48 These data indicate that p38α is a component of MTOCs in mouse oocyes and may contribute to the meiotic spindle assembly in mouse oocytes. The abnormal spindle morphology and chromosome alignment in p38α-MO-injected oocytes provide evidences that p38α is indispensable for meiotic spindle assembly. However, it is not clear how p38α functions in spindle assembly.
γ-Tubulin is part of the γ-tubulin ring complex at centrosomes in mitosis; the high local concentration of γ-tubulin in its ringlike configuration may play an important role in microtubule nucleation in the vicinity of the condensed chromosomes.49 In most animal cells, recruitment of γ-tubulin to the centrosomes becomes increased and is needed for the formation of new spindle microtubules. Moreover, γ-tubulin becomes relocated along spindle microtubules in MI mouse oocytes in a pattern similar to that in meiotic spindles of Xenopus oocyte extracts, suggesting that γ-tubulin may act as a microtubule stabilization protein.50 Disruption of γ-tubulin in mouse oocytes caused spindle microtubule defects and chromosome segregation errors.51 In this study, γ-tubulin was disassociated from the spindle poles and dispersed into the cytoplasm in p38α-depleted oocytes, suggesting that p38α is required for the regulation of γ-tubulin recruitment to MTOCs. Furthermore, γ-tubulin was scattered in the cytoplasm indicating that p38α depletion affected recruitment of MTOCs to the area around chromosomes and disrupted microtubule nucleation and stability. Thus, our data show that p38α participates in the meiotic spindle assembly by recruiting γ-tubulin and MTOC in mouse oocytes. But we also observed that part of γ-tubulin was still localized at the minus-ends of microtubules. Residual γ-tubulin at MTOCs may reflect incomplete p38α knockdown in oocytes, and it is also possible that recruitment of γ-tubulin to the oocyte MTOCs is not only dependent on p38α, but also regulated by other proteins.
The recruitment of γ-tubulin is also regulated by protein kinases such as aurora A and Plk1.45,52,53 In mitosis, Plk1 is involved in the p38 MAPK pathway.42 It has been well established that phosphorylation of Plk1 is required for centrosome maturation, subsequent bipolar spindle formation and chromosome cohesion.45,54 In the present study, our data also showed that p38α was colocalized with Plk1 at MTOCs and the spindle poles. Moreover, knockdown of p38 affected the localization of Plk1 at spindle poles. These data imply that p38α participates in γ-tubulin recruitment to MTOCs by interaction with Plk1.
In somatic cells, p38α exists in a stable complex with its downstream substrate, the kinase MK2.17 p38α phosphorylates and activates MK2, and the p38α/MK2 signaling complex is thought to participate in centrosome maturation and chromosome alignment.55 But it is not clear whether the p38α/MK2 pathway plays a role in spindle assembly in oocyte meiosis. In mitosis, p-p38α is colocalized with pMK2 at spindle poles.42 However, we observed that p-p38α and pMK2 were only partially colocalized at the spindle poles of MI and MII oocytes. Furthermore, we demonstrated that there was a direct interaction between p38α and pMK2 in mouse oocytes by using coimmunoprecipation analysis of endogenous proteins. Also, the results that pMK2 protein level was reduced by down-regulating p-p38α imply that p38α may phosophorylate and activate MK2 in mouse oocytes. Moreover, it was observed that pMK2 was detached from spindle poles and chromosomes or that it was below the detection level in p38α-depleted oocytes. MK2 is also involved in spindle assembly in mouse oocyte maturation and plays an important role in maintaining spindle stability and chromosome alignment.56 The results provide support that p38α may participate in spindle assembly by regulating phosphorylation and localization of MK2 in mouse oocyte meiotic maturation.
p38α is required for stabilizing spindles and spindle poles.
In the present study, we demonstrated that disintegrated spindle poles were predominant in defective spindles including spindles with multiple poles, monopoles and un-tethered poles instead of bipolar spindles in p38α-depleted oocytes. Interestingly, some foci and ectopic poles still contained γ-tubulin. Mouse meiotic spindle poles contained γ-tubulin foci and γ-tubulin contributed to the polymerization of spindle microtubules by capping the microtubule-minus ends.35 In mouse oocyte meiosis, the progressive aggregation of MTOCs forms the spindle poles.57 The reduced MTOC recruitment to the chromosome area and γ-tubulin recruitment to MTOCs affect microtubule nucleation, which is associated with depolymerization of spindle microtubules at spindle poles in p38α-depleted oocytes. Interestingly, γ-tubulin-containing poles were observed to detach from the spindles, implying that p38α may be required for coupling polar microtubules and acentrosomal MTOCs with microtubule-minus ends.58,59 These results imply that p38α is involved in stabilizing the spindle and spindle poles. The microtubules forming the ectopic poles can temporarily retain an independent orientation from existing spindle microtubules possibly due to compromised microtubule nucleation.
In vertebrate meiotic spindles, non-kinetochore microtubules comprise about 95% of the spindle microtubules.60 We performed cold treatment which disrupts the non-kinetochore microtubules36 to observe the function of p38α on kinetochore fiber regulation. Unexpectedly, kinetochore fibers were not tethered at the poles though most kinetochore microtubules were connected with centromeres in p38α-depleted oocytes. The fragments of nucleating material and bright foci in p38α-depleted oocytes were more apparent than in oocytes without cold treatment. More notably frayed spindle poles were observed in p38α-depleted MI oocytes after cold treatment. The data indicate that p38α is required to tether kinetochore microtubule minus ends at the poles of bipolar spindles and stabilize the spindle poles. In meiosis, non-kinetochore microtubules contribute to the dynamics of kinetochore microtubules and to the establishment of proper spindle size.59 Some spindle pole-associated protein such NuMA can contribute to the transport of the K-fiber minus end toward the spindle pole through a motor-dependent activity in mitosis.61 Whether p38α directly tethers kinetochore microtubules or is indirectly involved in focusing kinetochore microtubules by regulating non-kinetochore microtubules remains to be explored by influencing the motor protein.
p38α and Eg5 play opposite roles in determining spindle tension and length.
The constant length of spindles is important for spindle function, because it influences the distance over which chromosomes are segregated.62,63 Microtubule dynamics is the major determinant for metaphase spindle length and sister-chromatid cohesion is also critical to restrain the length.62 Kendra proposed a slide-and-cluster model that robustly forms bipolar spindles with sharp poles and a stable steady-state length.39 This modulation showed that the pole-to-pole length of bipolar spindles significantly depended on Eg5.63 A recent study also showed that Eg5 was responsible for the ejection and clustering of multiple poles as well as spindle elongation in mouse oocytes.7 Inter-kinetochore stretching is one readout of the forces generated by the spindle on single chromosomes.64 Therefore, we speculated that p38α was involved in spindle length by indirectly influencing Eg5. In the present study, spindle elongation and increased spindle tension were obvious in p38α-depleted MI oocytes. Eg5 overexpression lead to diffusive distribution of Eg5 in the spindle and caused spindle elongation similar to the phenotype caused by p38α depletion, while depletion of Eg5 caused short monopolar spindle formation. When we injected both p38αMO and Eg5MO into oocytes, the elongated spindle caused by p38α depletion was almost rescued by Eg5 depletion. The result confirms the presence of an antagonistic relationship between Eg5 and p38α in mouse oocytes. Given that Eg5 generates an outward force by sliding antiparallel microtubules, we considered the possibility that p38α may affect other minus proteins, which generate an inward force and partially antagonized Eg5. Thus, Eg5 could be responsible for spindle elongation with increased tension in the absence of p38α. p38α depletion led to imbalance in force between spindle poles, which may be responsible for the abnormal poles in the absence of p38α.
How should p38a antagonize Eg5 function in the spindle? The variation of Eg5 dynamics with its position in the spindle is indicative of position-dependent functions of this motor protein.65,66 Eg5 located at the half-zone and pole of the spindle could contribute to parallel microtubule cross-linking; however, Eg5 in the midzone cross-links antipalallel microtubules and slides them apart where antiparallel microtubules overlap. The function of the poleward accumulation of Eg5 could contribute to microtubule bundling or even clustering of microtubules by connecting parallel microtubules close the pole.65 We observed that p38α remained localized at the pole of monopolar spindles in Eg5-depleted oocytes, and this indicates that p38α is required for kinetochore fibers to focus toward the monopole. Moreover, p38α may partially function in monopole fusion by indirectly generating a force that pulls the poles together in Eg5-depleted oocytes.39 However, the localization of Eg5 was changed after p38α depletion. In p38α-depleted oocytes, the expression of Eg5 was reduced at the non-tethered poles, but increased in the midzone of the spindle. We speculate that the abnormal distribution of Eg5 in the spindle may disrupt the poleward flux.65 Reduced Eg5 at the pole compromises microtubule clustering, resulting in non-tethered poles. The increase of Eg5 in the midzone contributes to the antiparallel separation and cross-links kinetochore microtubules, which may lead to spindle elongation and microtubule tension increase. A recent study indicated that p38 MAPK depletion increased spindle length but showed normal spindles and chromosomes in mitosis, which speculated that loss of p38 MAPK might influence motor protein dynamics and produce longer spindles.25 However, our data also showed that p38α depletion led spindle elongation but microtubule tension increase, which is dependent on Eg5 in mouse oocyte meiosis. But as an important kinase, our results could not exclude the effect of p38α on minus motor proteins in spindle poles and indirectly antagonize the Eg5.
p38α regulates SAC in mouse oocytes.
We observed that p38α depletion caused significant chromosome misalignment, but did not affect PBE rates in p38α-depleted oocytes. Moreover, chromosome spreads of p38α-depleted MII oocytes showed high frequencies of aneuploidy. These results prompted us to investigate whether p38 MAPK depletion may affect SAC. Spindle assembly checkpoint (SAC) is the mechanism to prevent anaphase onset until all kinetochores of chromosomes are properly attached to the spindle to prevent chromosome mis-segregation and aneuploidy. The checkpoints contain Mad2, Bub1, BubR1, Cdc20, APC/C and other molecules67–69 during oocyte meiotic maturation. Cdc20 degradation is observed during MI.70 Whether p38α is a component of SAC or regulates SAC in mitosis is still controversial. A recent research indicated that the p38 activity is a component of SAC that is essential for Cdc20 proteolysis via Cdh1-independent APC/C ubiquitination to prevent premature anaphase entry under spindle injury in mitosis.24 However, Rieder reported that p38 can satisfy the mitotic checkpoint but is not required for the fidelity of chromosome segregation.25 Furthermore, little is known about p38 association with SAC in meiosis. BubR1, an important component of SAC, is only enriched at kinetochores of chromosomes that are either not attached to the spindle or not fully occupied by microtubules.69,71 Our data indicated that BubR1 failed to be localized to centromeres in p38α-depleted oocytes at the Pro-MI stage. Plk1 phosphorylates BubR1 and facilitates chromosome alignment during prometaphase through BubR1 in vitro and in vivo in mitosis.72 It needs to be clarified whether p38α-depletion indirectly disturbs BubR1 localization at kinetochores in pro-MI oocytes by affecting the localization of Plk1 or influences the other SAC. On the whole, our results showed that impairment of p38α disturbed the localization of BubR1 at the kinetochores of chromosomes and compromised the SAC, which caused misaligned chromosome and subsequent aneuploidy in mouse oocyte meiosis.
Materials and Methods
All chemicals and culture media were purchased from Sigma Chemical Company (St. Louis, MO) unless stated otherwise.
Antibodies.
Rabbit polyclonal anti-p-p38α MAP Kinase (Thr180/Tyr182) antibody, Rabbit polyclonal anti-phospho- MK2 (Thr180/Tyr182) antibody, Rabbit polyclonal anti-p38 MAP Kinase antibody were purchased from Cell Signaling Technology (Beverly, MA); Rabbit polyclonal anti-Eg5 and Sheep anti-BubR1 antibody were purchased from Abcam Co., (Cambridge); mouse monoclonal anti-α-tubulin-FITC antibody, mouse monoclonal anti-γ-tubulin and mouse monoclonal anti-Plk1 antibody were obtained from Sigma-Aldrich Co., (St. Louis, MO). Cy5-conjugated goat anti-rabbit IgG (H + L) and Cy5-conjugated goat anti-Human IgG (H + L) were purchased from Jackson Co., FITC-conjugated goat anti-rabbit IgG (H + L), TRITC-conjugated goat anti-rabbit IgG (H + L) and TRITC-conjugated goat anti-mouse IgG (H + L) were purchased from Zhongshan Golden Bridge Biotechnology Co., LTD (Beijing).
Oocyte collection and culture.
ICR mice care and handling were conducted in accordance with the Animal Research Committee guidelines promulgated by the Ethics Committee of the Institute of Zoology, Chinese Academy of Sciences. Immature oocytes were collected from ovaries of 6-week-old female ICR mice. GV oocytes were cultured in M2 medium under liquid paraffin oil at 37°C in an atmosphere of 5% CO2 in air. After specific treatments, oocytes were washed thoroughly and cultured in M2 undergoing GV (0 hour), germinal vesicle breakdown (GVBD) (2 hour), Pro-MI (5 hour), MI (8 hour), ATI (9.5 hour) and MII (12–14 hour) stages. At different times of culture, oocytes were collected for immunostaining, drug treatment, western blotting or coimmunoprecipitation.
Taxol treatment and cold treatment of oocytes.
For taxol treatment, 5 mM taxol in DMSO stock was diluted in M2 medium to give a final concentration of 10 µM and oocytes at different stages were incubated for 45 minutes. After treatment, oocytes were washed thoroughly and used for immunofluorescence staining. Control oocytes were treated with the same concentration of DMSO in the medium. For cold treatment, p38αMO was injected into cytoplasm of GV stage oocytes. Oocytes were arrested at GV stage in M2 medium supplemented with 2.5 µM Milrinone for 24 hours to prevent meiosis resumption. Oocytes were first cultured for 8 hours when most depleted-p38α oocytes were in the MI stage. Then the oocytes were transferred to M2 medium which was precooled to 4°C, and cultured for 20 minutes at this temperature, followed by immunofluorescent staining.
Construction of plasmids for wild-type Eg5 and in vitro transcription of RNA.
Total RNA was extracted from 150 mouse oocytes using RNeasy micro purification kit (QIAGEN) at the GV stage, and then reversely transcribed to cDNA with oligo dT primer using PrimeScript 1st Strand cDNA Synthesis Kit (TaKaRa). The full length Eg5 coding sequence was amplified by Nest PCR with the following primers: F1: AGG AGG TTC GTC CTG TCC, R1: AAA TCC CAA ATG ATA CCC, F2: TCA GGC CGG CCG ATG GCG TCC CAG CCG A, R2: GTT GGC GCG CCC TAG AGG TTT ATG GAG GTG TGA AG, and then cloned at Fse and Asc of pCS2 plus vector. The pCS plus vector allows in vitro transcription of polyadenylated mRNA from SP6 promoter.
In vitro synthesis of capped RNAs was performed using linearized plasmids with the mMessage mMachine kit (Ambion). The mRNAs were purified on RNeasy columns (QIAGEN) and eluted in H2O.
Morpholino oligonucleotides, myc-Eg5 mRNA and antibody microinjection.
The antisense morpholino oligonucleotide spanning the start codon of p38α gene (5′-TCT CCT GCG ACA TCT TCC AGC GGC A-3′), Eg5 gene (5′-GAC GCC ATG ACG GTC GAG CCA AAA C-3′) and a missense N-morpholino control oligonucleotide (5′-CCT CTT ACC TCA GTT ACA ATT TAT A-3′) were purchased from Gene Tools LLC (Philomath, OR). GV oocytes were microinjected with N-morpholino oligonucleotides to assess the effects of p38α and Eg5 knockdown. Microinjections were performed using an Eppendorf microinjector (Hamburg, Germany) and completed within 30 minutes. For knockdown studies, the N-morpholinos were diluted to 2 mM. Antisense or missense oligonucleotides (approximately 0.5 ng/oocyte) or morpholino control were injected into cytoplasm of GV stage oocytes. Oocytes were arrested at the GV stage in M2 medium supplemented with 2.5 µM Milrinone for 24 hours to prevent meiosis resumption, then cultured in fresh M2 medium to resume meiosis. The control was injected with MO standard control.
For myc-Eg5 expression, 2.5 mg/ml mRNA solution was injected into cytoplasm of GV stage oocytes. The same amount of myc mRNA was injected as control. Oocytes were arrested at the GV stage in M2 containing 2.5 µM Milrinone for 6 h and then released in M2 culture medium.
About 7 pl anti-dynein (0.5 mg/ml) antibody was microinjected into the cytoplasm of a fully grown GV oocyte. The oocytes were kept in M2 medium supplemented with 2.5 µM Milrinone (Sigma) to prevent GV breakdown during the injection period. Control oocytes were microinjected with the same amount of rabbit immunoglobulin G (IgG). Each experiment was repeated three to five times.
Immunofluorescence, confocal microscopy and chromosome spreading.
Immunofluorescence was performed as described previously.73 For double staining of proteins, oocytes were fixed in 4% paraformaldehyde in PBS (pH 7.4) for at least 30 min at room temperature. After being permeabilized with 0.5% Triton X-100 at room temperature for 20 min, oocytes were blocked in 1% BSA-supplemented PBS for 1 h and incubated overnight at 4°C with the primary antibodies: rabbit anti-p-p38 antibody (1:100); rabbit anti-p-MK2 antibody (1:100); mouse anti-Plk1 antibody (1:100); mouse anti-γ-tubulin antibody (1:100); sheep anti-BubR1 (1:50); goat anti-Eg5 (1:100); human anti-Crest (1:150). After three washes in PBS containing 0.1% Tween 20 and 0.01% Triton X-100 for 5 minutes each, the oocytes were labeled with second antibody for 1 hour at room temperature. After three washes in PBS containing 0.1% Tween 20 and 0.01% Triton X-100, the oocytes were co-stained with propidium iodide (PI; 10 µg/ml in PBS). Finally, the oocytes were mounted on glass slides and examined with a confocal laser scanning microscope (Zeiss LSM 510 META, Germany). Each experiment was repeated at least three times.
For chromosome spreading, MII oocytes were left for 15 minutes in 1% sodium citrate at room temperature and then fixed with fresh methanol: glacial acetic acid (3:1). 10 mg/ml PI was used for chromosome staining. Oocytes were examined with a Confocal Laser Scanning Microscope (Zeiss LSM 510 META, Germany). Instrument settings were kept constant for each replicate.
Immunoprecipitation and immunoblotting analysis.
western blotting: Mouse oocytes at appropriate stages during meiotic maturation and oocytes injected with p38αMO, Eg5MO, control-MO were collected in SDS sample buffer. A total of 300 oocytes were collected for each sample. Immunoblotting was performed as described previously.74 First, the proteins were separated in 10% acrylamide gels containing 0.1% SDS, and then transferred onto hydrophobic PVDF membranes (Amersham, Piscataway, NJ). Membranes were blocked in TBST (TBS supplemented with 0.1% Tween-20) containing 5% skimmed milk for 2 h at room temperature, then incubated with anti-p-p38α, anti-MK2 and anti-Eg5 with dilutions of 1:500, 1:750, 1:1,000, respectively, for overnight at 4°C, followed by three (10 minute) washes in TBST (TBS with 0.1% Tween-20). A peroxide-conjugated secondary antibody was added for 1 h at 37°C prior to using an ECL-plus detection system (Amersham).75
Coimmunoprecipitation: Lysates were prepared from MI-stage oocytes (n = 2,000) collected from superovulated female mice. Analysis was carried out using Fro Found™ Mammalian Co-Immunoprecipitation Kit (Pierce, Rockford, IL) in accordance with the manufacturer's instructions. Briefly, rabbit anti-p-p38α was dialyzed against PBS, and immobilized on the coupling gel. An irrelevant antibody (anti-β actin) was used as an immunoprecipitate control. Cell lysates were prepared, immediately transferred onto the antibody-coupled gel and incubated for 2 h. The coimmunoprecipitation complex was eluted and processed for SDS-PAGE analysis. The immune-complex samples were dissolved in sample buffer, resolved on 10% SDS gel, and analyzed by western blotting.76 Following protein transfer, the membranes were blocked in TBST with 5% BSA overnight at 4°C, and then probed with anti-MK2.
Statistical analysis.
Image processing, spindle length and kinetochore measurements were performed using an LSM Image Browser. Data (mean ± SEM) were from at least three replicates per experiment and analyzed by ANOVA using SPSS software (SPSS Inc., Chicago, IL) followed by the Fisher's LSD test. The number of oocytes observed was labeled in parentheses as (n). Difference at p < 0.05 were considered to be statistically significant and different superscripts indicate the statistical difference.
Acknowledgements
We are grateful to Shi-Wen Li, Yi Hou for their technical assistance, Drs. Shu-Tao Qi, Jing-Shan Tong, Lei Guo for insightful suggestions on the manuscript. This work was supported by the National Natural Science Foundation of China (No. 30930065 ) and National Basic Research Program of China (2006CB944001, 2006CB504004) to Q.Y.S.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/13389
Supplementary Material
References
- 1.Compton DA. Spindle assembly in animal cells. Annu Rev Biochem. 2000;69:95–114. doi: 10.1146/annurev.biochem.69.1.95. [DOI] [PubMed] [Google Scholar]
- 2.Gatlin JC, Matov A, Groen AC, Needleman DJ, Maresca TJ, Danuser G, et al. Spindle fusion requires dynein-mediated sliding of oppositely oriented micro-tubules. Curr Biol. 2009;19:287–296. doi: 10.1016/j.cub.2009.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor SS, Scott MI, Holland AJ. The spindle checkpoint: a quality control mechanism which ensures accurate chromosome segregation. Chromosome Res. 2004;12:599–616. doi: 10.1023/B:CHRO.0000036610.78380.51. [DOI] [PubMed] [Google Scholar]
- 4.Kastan MB, Bartek J. Cell cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 5.Martin RH. Meiotic errors in human oogenesis and spermatogenesis. Reprod Biomed Online. 2008;16:523–531. doi: 10.1016/s1472-6483(10)60459-2. [DOI] [PubMed] [Google Scholar]
- 6.Wang WH, Sun QY. Meiotic spindle, spindle checkpoint and embryonic aneuploidy. Front Biosci. 2006;11:620–636. doi: 10.2741/1822. [DOI] [PubMed] [Google Scholar]
- 7.Schuh M, Ellenberg J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell. 2007;130:484–498. doi: 10.1016/j.cell.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Brunet S, Polanski Z, Verlhac MH, Kubiak JZ, Maro B. Bipolar meiotic spindle formation without chromatin. Curr Biol. 1998;8:1231–1234. doi: 10.1016/s0960-9822(07)00516-7. [DOI] [PubMed] [Google Scholar]
- 9.Schatten G. The centrosome and its mode of inheritance: the reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev Biol. 1994;165:299–335. doi: 10.1006/dbio.1994.1256. [DOI] [PubMed] [Google Scholar]
- 10.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 11.Saavedra HI, Fukasawa K, Conn CW, Stambrook PJ. MAPK mediates RAS-induced chromosome instability. J Biol Chem. 1999;274:38083–38090. doi: 10.1074/jbc.274.53.38083. [DOI] [PubMed] [Google Scholar]
- 12.Sun SC, Xiong B, Lu SS, Sun QY. MEK1/2 is a critical regulator of microtubule assembly and spindle organization during rat oocyte meiotic maturation. Mol Reprod Dev. 2008;75:1542–1548. doi: 10.1002/mrd.20891. [DOI] [PubMed] [Google Scholar]
- 13.Yu LZ, Xiong B, Gao WX, Wang CM, Zhong ZS, Huo LJ, et al. MEK1/2 regulates microtubule organization, spindle pole tethering and asymmetric division during mouse oocyte meiotic maturation. Cell Cycle. 2007;6:330–338. doi: 10.4161/cc.6.3.3805. [DOI] [PubMed] [Google Scholar]
- 14.Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki K, Chiba K. Induction of apoptosis in starfish eggs requires spontaneous inactivation of MAPK (extracellular signal-regulated kinase) followed by activation of p38MAPK. Mol Biol Cell. 2004;15:1387–1396. doi: 10.1091/mbc.E03-06-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takenaka K, Moriguchi T, Nishida E. Activation of the protein kinase p38 in the spindle assembly checkpoint and mitotic arrest. Science. 1998;280:599–602. doi: 10.1126/science.280.5363.599. [DOI] [PubMed] [Google Scholar]
- 17.ter Haar E, Prabhakar P, Liu X, Lepre C. Crystal structure of the p38alpha-MAPKAP kinase 2 heterodimer. J Biol Chem. 2007;282:9733–9739. doi: 10.1074/jbc.M611165200. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Levy R, Leighton IA, Doza YN, Attwood P, Morrice N, Marshall CJ, et al. Identification of novel phosphorylation sites required for activation of MAPKAP kinase-2. EMBO J. 1995;14:5920–5930. doi: 10.1002/j.1460-2075.1995.tb00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larochelle S, Suter B. The Drosophila melanogaster homolog of the mammalian MAPK-activated protein kinase-2 (MAPKAPK-2) lacks a proline-rich N-terminus. Gene. 1995;163:209–214. doi: 10.1016/0378-1119(95)00279-f. [DOI] [PubMed] [Google Scholar]
- 20.Allen M, Svensson L, Roach M, Hambor J, McNeish J, Gabel CA. Deficiency of the stress kinase p38alpha results in embryonic lethality: characterization of the kinase dependence of stress responses of enzyme-deficient embryonic stem cells. J Exp Med. 2000;191:859–870. doi: 10.1084/jem.191.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikhailov A, Shinohara M, Rieder CL. Topoisomerase II and histone deacetylase inhibitors delay the G2/M transition by triggering the p38 MAPK checkpoint pathway. J Cell Biol. 2004;166:517–526. doi: 10.1083/jcb.200405167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsusaka T, Pines J. Chfr acts with the p38 stress kinases to block entry to mitosis in mammalian cells. J Cell Biol. 2004;166:507–516. doi: 10.1083/jcb.200401139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiromizu T, Goto H, Tomono Y, Bartek J, Totsukawa G, Inoko A, et al. Regulation of mitotic function of Chk1 through phosphorylation at novel sites by cyclin-dependent kinase 1 (Cdk1) Genes Cells. 2006;11:477–485. doi: 10.1111/j.1365-2443.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- 24.Yen AH, Yang JL. Cdc20 proteolysis requires p38 MAPK signaling and Cdh1-independent APC/C ubiquitination during spindle assembly checkpoint activation by cadmium. J Cell Physiol. 2010;223:327–334. doi: 10.1002/jcp.22038. [DOI] [PubMed] [Google Scholar]
- 25.Lee K, Kenny A, Rieder CL. P38 MAP Kinase Activity Is Required during Mitosis for Timely Satisfaction of the Mitotic Checkpoint but Not for the Fidelity of Chromosome Segregation. Mol Biol Cell. 2010;21:2150–2160. doi: 10.1091/mbc.E10-02-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Puscheck EE, Wang F, Trostinskaia A, Barisic D, Maniere G, et al. Serine-threonine kinases and transcription factors active in signal transduction are detected at high levels of phosphorylation during mitosis in preimplantation embryos and trophoblast stem cells. Reproduction. 2004;128:643–654. doi: 10.1530/rep.1.00264. [DOI] [PubMed] [Google Scholar]
- 27.Paliga AJ, Natale DR, Watson AJ. p38 mitogen-activated protein kinase (MAPK) first regulates filamentous actin at the 8–16 cell stage during preimplantation development. Biol Cell. 2005;97:629–640. doi: 10.1042/BC20040146. [DOI] [PubMed] [Google Scholar]
- 28.Guay J, Lambert H, Gingras-Breton G, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci. 1997;110:357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- 29.Aouadi M, Binetruy B, Caron L, Le Marchand-Brustel Y, Bost F. Role of MAPKs in development and differentiation: lessons from knockout mice. Biochimie. 2006;88:1091–1098. doi: 10.1016/j.biochi.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773:1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Villa-Diaz LG, Miyano T. Activation of p38 MAPK during porcine oocyte maturation. Biol Reprod. 2004;71:691–696. doi: 10.1095/biolreprod.103.026310. [DOI] [PubMed] [Google Scholar]
- 32.Goshima G, Wollman R, Stuurman N, Scholey JM, Vale RD. Length control of the metaphase spindle. Curr Biol. 2005;15:1979–1988. doi: 10.1016/j.cub.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 33.Brust-Mascher I, Sommi P, Cheerambathur DK, Scholey JM. Kinesin-5-dependent poleward flux and spindle length control in Drosophila embryo mitosis. Mol Biol Cell. 2009;20:1749–1762. doi: 10.1091/mbc.E08-10-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang ZB, Ou XH, Tong JS, Li S, Wei L, Ouyang YC, et al. The SUMO pathway functions in mouse oocyte maturation. Cell Cycle. 2010;9:2640–2646. doi: 10.4161/cc.9.13.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joshi HC, Palacios MJ, McNamara L, Cleveland DW. Gamma-tubulin is a centrosomal protein required for cell cycle-dependent microtubule nucleation. Nature. 1992;356:80–83. doi: 10.1038/356080a0. [DOI] [PubMed] [Google Scholar]
- 36.Rieder CL. The structure of the cold-stable kinetochore fiber in metaphase PtK1 cells. Chromosoma. 1981;84:145–158. doi: 10.1007/BF00293368. [DOI] [PubMed] [Google Scholar]
- 37.Salmon ED, Begg DA. Functional implications of cold-stable microtubules in kinetochore fibers of insect spermatocytes during anaphase. J Cell Biol. 1980;85:853–665. doi: 10.1083/jcb.85.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haren L, Stearns T, Luders J. Plk1-dependent recruitment of gamma-tubulin complexes to mitotic centrosomes involves multiple PCM components. PLoS One. 2009;4:5976. doi: 10.1371/journal.pone.0005976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burbank KS, Mitchison TJ, Fisher DS. Slide-and-cluster models for spindle assembly. Curr Biol. 2007;17:1373–1383. doi: 10.1016/j.cub.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 40.Vogt E, Kirsch-Volders M, Parry J, Eichenlaub-Ritter U. Spindle formation, chromosome segregation and the spindle checkpoint in mammalian oocytes and susceptibility to meiotic error. Mutat Res. 2008;651:14–29. doi: 10.1016/j.mrgentox.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 41.Malmanche N, Maia A, Sunkel CE. The spindle assembly checkpoint: preventing chromosome missegregation during mitosis and meiosis. FEBS Lett. 2006;580:2888–2895. doi: 10.1016/j.febslet.2006.03.081. [DOI] [PubMed] [Google Scholar]
- 42.Tang J, Yang X, Liu X. Phosphorylation of Plk1 at Ser326 regulates its functions during mitotic progression. Oncogene. 2008;27:6635–6645. doi: 10.1038/onc.2008.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cha H, Wang X, Li H, Fornace AJ., Jr A functional role for p38 MAPK in modulating mitotic transit in the absence of stress. J Biol Chem. 2007;282:22984–22992. doi: 10.1074/jbc.M700735200. [DOI] [PubMed] [Google Scholar]
- 44.Hannak E, Kirkham M, Hyman AA, Oegema K. Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J Cell Biol. 2001;155:1109–1116. doi: 10.1083/jcb.200108051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barr FA, Sillje HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–440. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- 46.Yao LJ, Fan HY, Tong C, Chen DY, Schatten H, Sun QY. Polo-like kinase-1 in porcine oocyte meiotic maturation, fertilization and early embryonic mitosis. Cell Mol Biol (Noisy-le-grand) 2003;49:399–405. [PubMed] [Google Scholar]
- 47.Fan HY, Tong C, Teng CB, Lian L, Li SW, Yang ZM, et al. Characterization of Polo-like kinase-1 in rat oocytes and early embryos implies its functional roles in the regulation of meiotic maturation, fertilization and cleavage. Mol Reprod Dev. 2003;65:318–329. doi: 10.1002/mrd.10283. [DOI] [PubMed] [Google Scholar]
- 48.Meng XQ, Fan HY, Zhong ZS, Zhang G, Li YL, Chen DY, et al. Localization of gamma-tubulin in mouse eggs during meiotic maturation, fertilization and early embryonic development. J Reprod Dev. 2004;50:97–105. doi: 10.1262/jrd.50.97. [DOI] [PubMed] [Google Scholar]
- 49.Raynaud-Messina B, Merdes A. Gamma-tubulin complexes and microtubule organization. Curr Opin Cell Biol. 2007;19:24–30. doi: 10.1016/j.ceb.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Lee J, Miyano T, Moor RM. Spindle formation and dynamics of gamma-tubulin and nuclear mitotic apparatus protein distribution during meiosis in pig and mouse oocytes. Biol Reprod. 2000;62:1184–1192. doi: 10.1095/biolreprod62.5.1184. [DOI] [PubMed] [Google Scholar]
- 51.Barrett SL, Albertini DF. Allocation of gamma-tubulin between oocyte cortex and meiotic spindle influences asymmetric cytokinesis in the mouse oocyte. Biol Reprod. 2007;76:949–957. doi: 10.1095/biolreprod.106.057141. [DOI] [PubMed] [Google Scholar]
- 52.Berdnik D, Knoblich JA. Drosophila Aurora-A is required for centrosome maturation and actin-dependent asymmetric protein localization during mitosis. Curr Biol. 2002;12:640–647. doi: 10.1016/s0960-9822(02)00766-2. [DOI] [PubMed] [Google Scholar]
- 53.Barr AR, Gergely F. Aurora-A: the maker and breaker of spindle poles. J Cell Sci. 2007;120:2987–2996. doi: 10.1242/jcs.013136. [DOI] [PubMed] [Google Scholar]
- 54.Sumara I, Gimenez-Abian JF, Gerlich D, Hirota T, Kraft C, de la Torre C, et al. Roles of polo-like kinase 1 in the assembly of functional mitotic spindles. Curr Biol. 2004;14:1712–1722. doi: 10.1016/j.cub.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 55.Ben-Levy R, Hooper S, Wilson R, Paterson HF, Marshall CJ. Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr Biol. 1998;8:1049–1057. doi: 10.1016/s0960-9822(98)70442-7. [DOI] [PubMed] [Google Scholar]
- 56.Yuan J, Xu BZ, Qi ST, Tong JS, Wei L, Li M, et al. MAPK-activated protein kinase 2 is required for mouse meiotic spindle assembly and kinetochore-microtubule attachment. PLoS One. 2010;5:11247. doi: 10.1371/journal.pone.0011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maro B, Howlett SK, Webb M. Non-spindle microtubule organizing centers in metaphase II-arrested mouse oocytes. J Cell Biol. 1985;101:1665–1672. doi: 10.1083/jcb.101.5.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silk AD, Holland AJ, Cleveland DW. Requirements for NuMA in maintenance and establishment of mammalian spindle poles. J Cell Biol. 2009;184:677–690. doi: 10.1083/jcb.200810091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Houghtaling BR, Yang G, Matov A, Danuser G, Kapoor TM. Op18 reveals the contribution of nonkinetochore microtubules to the dynamic organization of the vertebrate meiotic spindle. Proc Natl Acad Sci USA. 2009;106:15338–15343. doi: 10.1073/pnas.0902317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohi R, Burbank K, Liu Q, Mitchison TJ. Nonredundant functions of Kinesin-13s during meiotic spindle assembly. Curr Biol. 2007;17:953–959. doi: 10.1016/j.cub.2007.04.057. [DOI] [PubMed] [Google Scholar]
- 61.Khodjakov A, Copenagle L, Gordon MB, Compton DA, Kapoor TM. Minus-end capture of preformed kinetochore fibers contributes to spindle morphogenesis. J Cell Biol. 2003;160:671–683. doi: 10.1083/jcb.200208143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wuhr M, Chen Y, Dumont S, Groen AC, Needleman DJ, Salic A, et al. Evidence for an upper limit to mitotic spindle length. Curr Biol. 2008;18:1256–1261. doi: 10.1016/j.cub.2008.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mitchison TJ, Maddox P, Gaetz J, Groen A, Shirasu M, Desai A, et al. Roles of polymerization dynamics, opposed motors and a tensile element in governing the length of Xenopus extract meiotic spindles. Mol Biol Cell. 2005;16:3064–3076. doi: 10.1091/mbc.E05-02-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waters JC, Mitchison TJ, Rieder CL, Salmon ED. The kinetochore microtubule minus-end disassembly associated with poleward flux produces a force that can do work. Mol Biol Cell. 1996;7:1547–1558. doi: 10.1091/mbc.7.10.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uteng M, Hentrich C, Miura K, Bieling P, Surrey T. Poleward transport of Eg5 by dynein-dynactin in Xenopus laevis egg extract spindles. J Cell Biol. 2008;182:715–726. doi: 10.1083/jcb.200801125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang G, Cameron LA, Maddox PS, Salmon ED, Danuser G. Regional variation of microtubule flux reveals microtubule organization in the metaphase meiotic spindle. J Cell Biol. 2008;182:631–639. doi: 10.1083/jcb.200801105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wassmann K, Niault T, Maro B. Metaphase I arrest upon activation of the Mad2-dependent spindle checkpoint in mouse oocytes. Curr Biol. 2003;13:1596–1608. doi: 10.1016/j.cub.2003.08.052. [DOI] [PubMed] [Google Scholar]
- 68.Yin S, Wang Q, Liu JH, Ai JS, Liang CG, Hou Y, et al. Bub1 prevents chromosome misalignment and precocious anaphase during mouse oocyte meiosis. Cell Cycle. 2006;5:2130–2137. doi: 10.4161/cc.5.18.3170. [DOI] [PubMed] [Google Scholar]
- 69.Wei L, Liang XW, Zhang QH, Li M, Yuan J, Li S, et al. BubR1 is a spindle assembly checkpoint protein regulating meiotic cell cycle progression of mouse oocyte. Cell Cycle. 2010;9:1112–1121. doi: 10.4161/cc.9.6.10957. [DOI] [PubMed] [Google Scholar]
- 70.Reis A, Madgwick S, Chang HY, Nabti I, Levasseur M, Jones KT. Prometaphase APCcdh1 activity prevents non-disjunction in mammalian oocytes. Nat Cell Biol. 2007;9:1192–1198. doi: 10.1038/ncb1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fan HY. BubR1, a spindle assembly checkpoint protein in mammalian oocyte meiosis. Cell Cycle. 2010;9:1456–1465. [PubMed] [Google Scholar]
- 72.Elowe S, Hummer S, Uldschmid A, Li X, Nigg EA. Tension-sensitive Plk1 phosphorylation on BubR1 regulates the stability of kinetochore microtubule interactions. Genes Dev. 2007;21:2205–2219. doi: 10.1101/gad.436007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li M, Li S, Yuan J, Wang ZB, Sun SC, Schatten H, et al. Bub3 is a spindle assembly checkpoint protein regulating chromosome segregation during mouse oocyte meiosis. PLoS One. 2009;4:7701. doi: 10.1371/journal.pone.0007701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun SC, Wei L, Li M, Lin SL, Xu BZ, Liang XW, et al. Perturbation of survivin expression affects chromosome alignment and spindle checkpoint in mouse oocyte meiotic maturation. Cell Cycle. 2009;8:3365–3372. doi: 10.4161/cc.8.20.9855. [DOI] [PubMed] [Google Scholar]
- 75.Gaetz J, Kapoor TM. Dynein/dynactin regulate metaphase spindle length by targeting depolymerizing activities to spindle poles. J Cell Biol. 2004;166:465–471. doi: 10.1083/jcb.200404015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuan J, Li M, Wei L, Yin S, Xiong B, Li S, et al. Astrin regulates meiotic spindle organization, spindle pole tethering and cell cycle progression in mouse oocytes. Cell Cycle. 2009;8:3384–3395. doi: 10.4161/cc.8.20.9885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.