Abstract
The Hedgehog (Hh) signaling pathway influences multiple stages of murine T-cell development. Hh signaling mediates transcriptional changes by the activity of the Gli family of transcription factors, Gli1, Gli2 and Gli3. Both Gli2 and Gli3 are essential for mouse development and can be processed to function as transcriptional repressors or transcriptional activators, whereas Gli1, itself a transcriptional target of Hh pathway activation, can only function as a transcriptional activator and is not essential for mouse development. Gli1-deficient mice are healthy and appear normal and non-redundant functions for Gli1 have been difficult to identify. Here we show that Gli1 is non-redundant in the regulation of T-cell development in the thymus, at multiple developmental stages. Analysis of Gli1-deficient embryonic mouse thymus shows a role for Gli1 to promote the differentiation of CD4−CD8− double negative (DN) thymocytes before pre-TCR signal transduction, and a negative regulatory function after pre-TCR signaling. In addition, introduction of a Class I-restricted transgenic TCR into the adult Gli1-deficient and embryonic Gli2-deficient thymus showed that both Gli1 and Gli2 influence its selection to the CD8 lineage.
Key words: Gli1, Gli2, thymus, T-cell, sonic hedgehog, thymocyte development
Introduction
During αβT-cell development in the thymus, thymocytes pass through a series of well-defined developmental stages, as they migrate through the thymus.1 Throughout this developmental program they receive signals from the thymic stroma which influence their survival, differentiation and expansion.2 CD4−CD8− double negative (DN) thymocytes give rise to the CD4+CD8+ double positive (DP) population, which differentiate into mature CD4 and CD8 single positive (SP) cells. The DN population can be further sub-divided by expression of cell surface CD44 and CD25. The earliest CD44+CD25− (DN1) cells give rise to the CD44+CD25+ (DN2) population that progresses to the CD25+CD44− (DN3) stage, and then to the CD44−CD25− (DN4) population,3 which differentiate to DP, often via a CD8 immature single positive intermediate.
Expression of rearranged TCRβ and formation of the pre-T Cell Receptor (TCR) is essential for full differentiation from DN3 to DP cell.4 Pre-TCR signal transduction leads to a series of events collectively known as β-selection, resulting in allelic exclusion of the TCRβ locus, expansion and differentiation. Transition from DP to SP cell requires signaling through the mature αβTCR and positive selection to ensure lineage-appropriate self-MHC-restriction, followed by negative selection to delete self-reactive clones.5,6 The secretion of Sonic Hedgehog (Shh) by thymic epithelial cells and Indian Hedgehog (Ihh) by both thymic epithelial cells and thymocytes has been shown to influence multiple stages of thymocyte development, including the differentiation of DN progenitors and TCR repertoire selection.7–21
There are three mammalian Hh proteins, Shh, Ihh and Desert Hedgehog, which share a common signaling pathway.22,23 They signal by binding to their cell surface receptor Patched. Hh protein binding to Patched releases the signal transduction molecule Smoothened to signal into the cell. At the end of the signaling pathway are the Gli family of transcription factors, Gli1, Gli2 and Gli3.22,23 Both Gli3 and Gli2 can be processed to function as activators or repressors of transcription in the presence or absence of Hh respectively.24–27 In vivo Gli2 functions primarily as an activator of transcription and is necessary to initiate the Hh signal,25,28 whereas Gli3 functions primarily as a transcriptional repressor, in the absence of Hh,29 and can function to limit the Hh signal, and act independently of Hh pathway activation.30,31 Both Gli2 and Gli3 have essential non-redundant, though partially overlapping, functions in mouse development.32–34 In contrast, Gli1 is not essential for mouse development, can only function as a transcriptional activator, and is itself an Hh-target gene.35,36 Adult Gli1−/− mice appear normal and in many tissues studied it has not been possible to identify any phenotype caused by Gli1-deficiency, or to identify a distinct non-redundant function for Gli1.35 Thus, the transcriptional activator function of Gli2 seems to substitute for Gli1, and the functions of Gli1 become apparent only on a Gli2-mutant background.28,35,37
Hh proteins can function as morphogens, which signal for distinct outcomes in a concentration-dependent manner and can regulate cell cycle progression.38–41 The interpretation of the Hh signal (and hence position in the morphogen gradient) by a given cell, which depends on both strength and duration of signal received, is determined by the balance of Gli Repressor (R) (Gli2R and Gli3R) and Gli Activator (Gli2A, Gli1, occasionally Gli3A) protein forms in the cell.40 Since Gli1 is itself a Hh target and can only function as an activator of transcription, it is assumed that its function in the signaling pathway would be to amplify an established signal, influencing the balance of GliA:GliR in the cell.
Analysis of mice mutant in genes encoding components of the Hh signaling pathway and in Hh proteins have revealed multiple functions for the Hh signaling pathway during thymocyte development.20,42 Studies of mutants of Smoothened, Shh, Gli3 and Gli2 have shown that Smoothened-dependent Shh signaling for differentiation and proliferation at the transition from DN1 to DN2 requires Gli2 and Gli3.10,13,15,21 Analysis of Gli2−/−, Shh−/−, Ihh−/−, Gli2ΔN2-transgenic and Gli2ΔC2-transgenic thymi have indicated that Hh pathway activation (by Shh and Ihh) in thymocytes negatively regulates pre-TCR induced differentiation from DN3 to DP, in a Gli2-dependent manner.16,17,21 In addition, studies on selection of a transgenic Class I-restricted TCR in Gli2ΔN2-transgenic, Shh−/− and Gli3+/− thymi have indicated that Hh pathway activation in DP thymocytes reduces both positive and negative selection, most likely by modulating TCR signal strength, whereas, reduction or inhibition of Hh pathway activation, increases the efficiency of selection of the transgenic TCR to the CD8 lineage.14,19 The relative contributions of Gli2 and Gli1 as transcriptional activators downstream of Hh signal transduction in its function to influence TCR repertoire selection are unknown.
The Gli proteins are differentially expressed in thymocyte populations. Gli3 is expressed in foetal, but not adult, thymocytes, with highest expression in the DN1 population.13 Gli1 and Gli2 are expressed in both foetal and adult thymocyte populations.15–17,20,21 Gli2 is most highly expressed in the DN1 and DN2 populations. Gli1 expression is highest in the DN2 and DN3 populations, downregulated in DN4 and DP, and then expressed more highly again in SP cells.17,19
Here we test if Gli1 has a non-redundant function in T-cell development. We show that the thymus is one of very few tissues in which Gli1 is required for normal development on a Gli2+/+ background. We show that Gli1 activity promotes foetal thymocyte development prior to pre-TCR signal transduction, but signals to negatively regulate the transition from DN to DP cell post pre-TCR signaling. In addition, we show that in the adult thymus Gli1 has a non-redundant function in Hh signaling for the modulation of TCR repertoire selection.
Results
Gli1 is not necessary for the transition from DN1 to DN2.
To test if Gli1 is necessary for the transition from DN1 to DN2, we analysed the Gli1−/− E13.5 foetal thymus, as E13.5 is the day on which DN2 cells appear. We found no significant difference in the proportion of DN1 and DN2 cells (Fig. 1A and B) and in the number of thymocytes between Gli1−/−, Gli1+/− and wild-type (WT) littermates (Fig. 1C), indicating that, in contrast to Gli2 and Gli3, Gli1 is not necessary at this developmental stage. Gli3 is most highly expressed in the DN1 population of thymocytes,13 and in other tissues, has been shown to function to limit Hh pathway activation, repressing Gli1 transcription. We therefore measured Gli1 transcription, by quantitative RT-PCR, in sorted DN1 cells from WT and Gli3−/− embryos (Fig. 1D). Gli1 transcription was increased twenty-fold in Gli3−/− DN1 cells compared to WT, showing that Gli3 repressed Gli1 transcription in the WT cells, and explaining the lack of influence of Gli1 on this developmental transition.
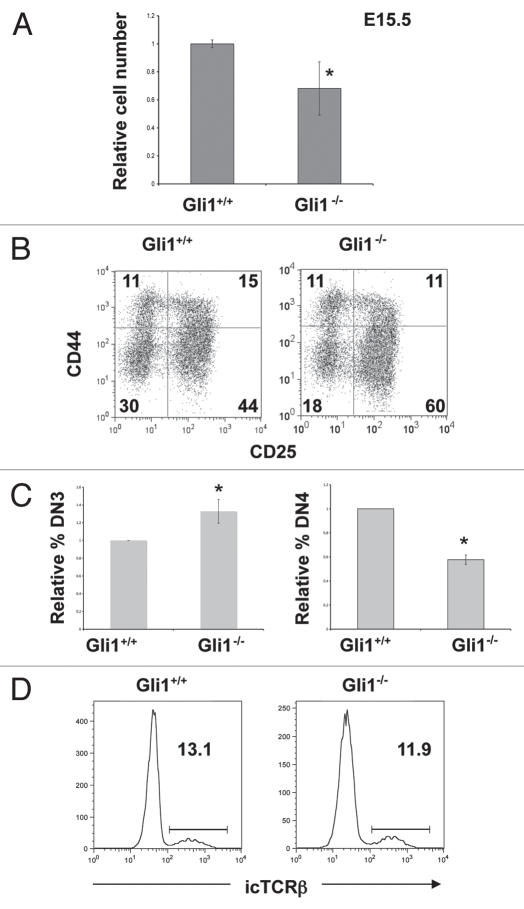
Figure 1.
Gli1−/− thymus on E13.5. (A) CD25 and CD44 expression in E13.5 thymocytes from Gli1+/+, Gli1+/− and Gli1−/− littermates. Percentage of DN1 and DN2 populations is given. Live cells were gated by FSC/SSC profile, and cells were stained with anti CD45.2, CD44 and CD25, and gated on CD45.2+ to identify thymocytes. Mean thymocyte number: 9.8 × 104 (WT); 9.37 × 104 (Gli1+/−); 1.09 × 105 (Gli1−/−). (B) The relative percentage of DN1 cells in E13.5 Gli1+/+, Gli1+/− and Gli1−/− thymus. To allow comparison between litters, the percentage of DN1 cells from each embryonic thymus was divided by the mean percentage of DN1 cells recovered from thymi from WT embryos from the same litter, to give a relative percentage for each embryo. There were no significant differences in the relative percentage of DN1 cells between the three genotypes of mice. (C) The scatter plot shows the relative number of thymocytes in E13.5 Gli1+/+, Gli1+/− and Gli1−/− thymi from 3 independent litters. To allow comparison between litters, the number of cells recovered from each embryonic thymus was divided by the mean number of cells recovered from WT embryonic thymi from the same litter, to give a relative cell number from three different E13.5 litters. There was no significant difference in relative cell number between the three genotypes of mice. (D) Relative transcription of Gli1 in DN1 cells sorted from WT and Gli3−/− foetal thymus. Live cells (by FSC/SSC profile) were sorted to be positive for CD45.2 and CD44, but negative for CD25. Gli1 transcription was measured by quantitative RT-PCR, and was normalised to Actβ transcription in each sample, and is shown relative to WT.
Gli1 promotes the differentiation of DN thymocytes.
To investigate the function of Gli1 at later stages of DN cell development, we compared thymocyte development in the E14.5 and E15.5 Gli1−/− and WT foetal thymus. We found no difference in cell number or the relative distribution of DN thymocyte subsets on E14.5 (data not shown). However, on E15.5, the Gli1−/− thymus contained significantly fewer thymocytes than WT (Fig. 2A) and there was an increase in the proportion of DN3 cells and decrease in the proportion of DN4 cells in the Gli1−/− thymus, compared to WT (Fig. 2B and C). The reduction in the DN4 population in the Gli1−/− thymus could have been the result of increased cell death of DN4 cells or could have been due to a partial arrest in differentiation at the DN3 to DN4 transition. To test this we compared apoptosis in DN3 and DN4 populations by Annexin-V staining in the E15.5 Gli1−/− and WT littermate thymus (Table 1). We found no significant differences in the percentage of apoptotic cells between Gli1−/− and WT, indicating that the decrease in the DN4 population in the Gli1−/− thymus was not the result of a requirement for Gli1 for survival at that developmental stage. Therefore the enrichment of the DN3 population suggested that differentiation from DN3 to DN4 was partially arrested in the Gli1−/− thymus. As pre-TCR signaling drives differentiation from DN3 to DP, the partial arrest in differentiation of DN3 cells could be the result of reduced TCRβ gene locus rearrangement, and expression of a functional pre-TCR. Therefore, to assess TCRβ protein expression, we measured intra-cellular anti-TCRβ staining by flow cytometry. Both Gli1−/− E15.5 DN3 cells and their WT littermate counterparts expressed similar levels of icTCRβ (∼12%) and there was no significant difference in the proportion of icTCRβ+ cells between WT and Gli1−/− (Fig. 2D). Thus, as seen in Smoothened-, Gli2-, Gli3- and Ihh-deficient thymi,13,15,17,21 we found no evidence for an influence of Hh signaling on TCRβ chain gene rearrangement, but rather Gli1-deficiency seemed to lead to a reduction in differentiation independent of TCRβ expression. This is reminiscent of the Ihh−/− thymus, in which Ihh-deficiency reduces differentiation prior to pre-TCR signal transduction,17 and demonstrates a non-redundant function for Gli1. This requirement for Gli1 for normal differentiation of DN3 cells is consistent with the fact that Gli1 is most highly expressed in the DN3 population of thymocytes, and is in contrast to the phenotype of the Gli2−/− E15.5 thymus, which shows no enrichment of DN3 cells or significant difference in cell number compared to WT.21
Figure 2.
Gli1−/− thymus on E15.5. (A) Relative cell number in E15.5 Gli1+/+ and Gli1−/− thymi, (calculated as in Fig. 1C). The E15.5 Gli1−/− thymus was significantly smaller than WT (p = 0.015). (B) CD25 and CD44 expression in E15.5 thymocytes from Gli1+/+ and Gli1−/− littermates. Percentage of cells in each quadrant is given. Live cells were gated by FSC/SSC profile. (C) The relative percentage of DN3 cells (left-hand bar chart) and the relative percentage of DN4 cells (right-hand bar chart) in E15.5 Gli1+/+ and Gli1−/− thymus. To allow comparison between litters the relative percentages were calculated for each litter, as in Figure 1B. The mean relative percentage of DN3 cells was significantly greater in the Gli1−/− compared to WT (p = 0.05). The mean relative percentage of DN4 cells was significantly smaller in the Gli1−/− compared to WT (p = 0.001). (D) Intracellular (ic) anti-TCRβ staining in DN3 cells from Gli1+/+ and Gli1−/− E15.5 thymus. Cells were gated by FSC/SSC profile, and cells that stained positive with antibodies, CD4+, CD8+, CD44+ and CD3+ cells were excluded. Cells were then gated to be DN3 (CD25+) and DN4 (CD25−). The percentage of cells falling in the marker is given. There were no significant differences between the WT and Gli1−/−.
Table 1.
Table shows the percentage of Annexin-V positive cells in the DN3 and DN4 populations in the E15.5 and E16.5 Gli1+/+ and Gli1−/− thymus
| E15.5 | E16.5 | ||||
| Percentage annexin-V positive | Standard deviation | Percentage annexin-V positive | Standard deviation | ||
| DN3 | DN3 | ||||
| Gli1+/+ | 2.53 | ±0.757 | Gli1+/+ | 0.9466 | ±0.589 |
| Gli1−/− | 2.97 | ±0.672 | Gli1−/− | 0.82 | ±0.099 |
| DN4 | DN4 | ||||
| Gli1+/+ | 2.36 | ±0.970 | Gli1+/+ | 1.93 | ±1.774 |
| Gli1−/− | 3.3 | ±1.14 | Gli1+/+ | 1.699 | ±0.622 |
Cells were stained with antibodies directed against CD4, CD8, CD3, CD44 and with Annexin-V. Cells were gated by FSC/SSC profile and cells that stained positive with antibodies against CD4, CD8, CD3 or CD44 were excluded. DN3 and DN4 populations were gated to be CD25+ (DN3) and negative (DN4).
The presence of Gli1 decreases differentiation from DN to DP cell.
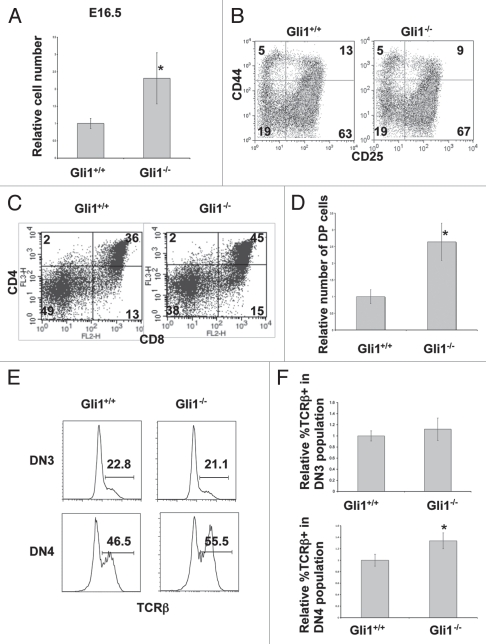
To investigate the function of Gli1 at the transition from DN to DP cell, we studied the E16.5 thymus, as DP cells first emerge on E16.5. In contrast to the E15.5 thymus, on E16.5 the Gli1−/− thymus contained more thymocytes than WT (Fig. 3A), consistent with Hh pathway activation negatively regulating pre-TCR induced differentiation to DP cell. The relative proportion of DN populations was not significantly different (Fig. 3B), but there was an increase in the proportion and number of DP cells (Fig. 3C and D), demonstrating a non-redundant role for Gli1 in the negative regulation of differentiation from DN to DP cell. There was no difference in expression of intra-cellular (ic) TCRβ in the DN3 population between Gli1−/− and WT, indicating that Gli1 expression is not necessary for TCRβ rearrangement (Fig. 3E and F). In the E16.5 foetal thymus, approximately half of DN4 cells do not express TCRβ and differentiation from DN3 to DN4 is therefore uncoupled from pre-TCR expression,43 although pre-TCR expression drives expansion of those cells that have successfully rearranged and expressed TCRβ.4,43 Interestingly, the proportion of icTCRβ+ cells was increased in the Gli1−/− DN4 population, compared to WT (Fig. 3E and F). This increase in icTCRβ+ DN4 cells is therefore consistent with Gli1-dependent loss of Hh-induced negative regulation of pre-TCR signal transduction, as pre-TCR signaling induces expansion of icTCRβ+ DN4 cells.
Figure 3.
Gli1−/− thymus on E16.5. (A) Relative cell number in E16.5 Gli1+/+ and Gli1−/− thymi, calculated as in Figure 1C. The E16.5 Gli1−/− thymus was significantly greater than WT (p = 0.018). (B) CD25 and CD44 expression in E16.5 DN thymocytes from Gli1+/+ and Gli1−/− littermates. Percentage of cells in each quadrant is given. Live cells were gated by FSC/SSC profile. Cells were stained with antibodies against CD4, CD8, CD3, CD25 and CD44. Cells that stained positive for anti-CD3, anti-CD4 and anti-CD8 were excluded. (C) CD4 and CD8 expression in thymocytes from E16.5 Gli1+/+ and Gli1−/− littermates. The percentage of cells in each quadrant is given. Live cells were gated by FSC/SSC profile. (D) The relative number of DP cells in E16.5 Gli1+/+ and Gli1−/− thymi. Relative cell number was calculated as described for Figure 1C to allow comparison between litters. The increase in relative number of DP cells in the Gli1−/− thymus was statistically significant (p = 0.001). (E) Intracellular TCRβ staining in DN3 and DN4 populations from E16.5 Gli1+/+ and Gli1−/− littermate thymi. Live cells were gated by FSC/SSC profile and cells that stained positive with antibodies directed against CD44, CD3, CD4 and CD8 were excluded. DN3 and DN4 populations were then gated by staining positively or negatively respectively with anti-CD25. (F) Bar charts show the mean relative percentage of icTCRβ positive cells in the E16.5 DN3 (upper chart) and DN4 (lower chart) Gli1−/− and WT thymus. Relative percentage was calculated as described in Figure 1B, to allow comparison between litters. There was no significant difference between genotypes in the DN3 population, but the relative mean % icTCRβ+ was significantly greater in the Gli1−/− DN4 population compared to WT (p = 0.0017).
Gli1 function in the adult thymus.
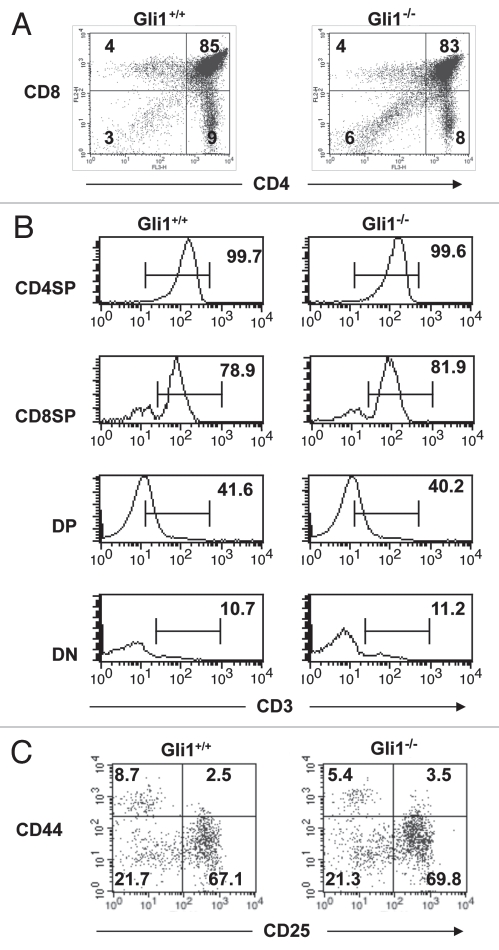
Given that Gli1 has a non-redundant role in Hh-dependent negative regulation of differentiation from DN to DP cell in the foetal thymus, we also assessed its function in the adult. There were no significant differences in the proportion of DN, DP or mature CD4 and CD8SP populations between Gli1−/− and WT (Fig. 4A) and CD3 expression appeared normal in these populations (Fig. 4B). Likewise, we did not detect significant differences in the composition of the DN populations between Gli1−/− and WT (Fig. 4C), and the Gli1−/− thymus seemed grossly normal and indistinguishable from WT. We therefore introduced a transgenic TCR to ask if Gli1 deficiency influenced its selection.
Figure 4.
Thymocyte populations in adult Gli1−/− thymus. (A) CD4 and CD8 expression in thymocytes from adult Gli1+/+ and Gli1−/− littermate mice. The percentage of cells in each quadrant is given. Thymocyte counts were 1.99 × 108 (WT); 2.06 × 108 (Gli1−/−). Differences between proportion of DN, DP, CD4SP and CD8SP were not statistically significant. (B) CD3 expression in CD4SP, CD8SP, DP and DN populations. Live cells (gated by FSC/SSC) were gated according to CD4 and CD8 profile, as shown in the quadrants in (A), and CD3 profile is given for each population. The percentage of cells falling within the marker is given. (C) Double negative populations in Gli1−/− and WT adult thymus. Live cells were gated by FSC/SSC profile, and cells that stained positive for CD4, CD8 and CD3 were excluded. CD44 and CD25 profiles are shown and the percentage of cells falling in each quadrant is given. Cell counts were for DN3: 2.67 × 106 (WT); 2.88 × 106 (Gli1−/−); for DN4: 8.64 × 105 (WT); 8.78 × 105 (Gli1−/−).
Non-redundant function for Gli1 in positive selection of HY-TCR.
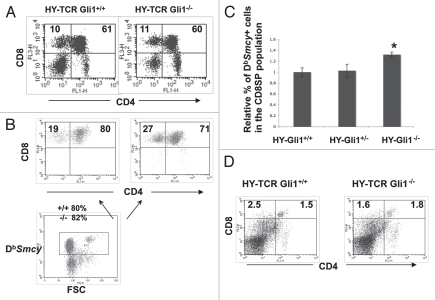
We have previously shown that constitutive activation of the Hedgehog pathway in thymocytes, by overexpression of a transgenic truncated form of Gli2, reduced positive and negative selection of the transgenic Class I-restricted HY-TCR, whereas positive selection of the HY-TCR was increased in the Shh−/− foetal thymus.19 Therefore, to test if Gli1 has a non-redundant function down-stream of Shh in modulating TCR repertoire selection in thymocytes, we introduced the transgenic HY-TCR,44 and compared positive selection of the Db restricted HY-TCR (reactive with Db + Smcy peptide)45 to the CD8 lineage in female Gli1−/− and WT litter-mate mice. The proportions of CD8SP and DP cells were not significantly different between the Gli1−/− and WT female littermate thymus (Fig. 5A). However, when we stained with DbSmcy-tetramer, and gated on tetramer+ cells, we found that positive selection of the tetramer+ (HY-TCR expressing) cells was increased in the Gli1−/− thymus compared to WT (Fig. 5B). In the Gli1−/− thymus, 27% of tetramer+ cells were CD8SP and 71% DP (ratio of DP:CD8SP = 1:2.63); compared to 19% CD8SP and 80% DP (ratio of DP:CD8SP = 1:4.21) in the WT (Fig. 5B). In addition, the percentage of CD8SP cells that stained positive with the DbSmcy tetramer was significantly increased in the Gli1−/− compared to WT and Gli1+/− littermates (Fig. 5C). Thus, Gli1 has a non-redundant function in Hh signaling for modulation of TCR repertoire selection in thymocytes.
Figure 5.
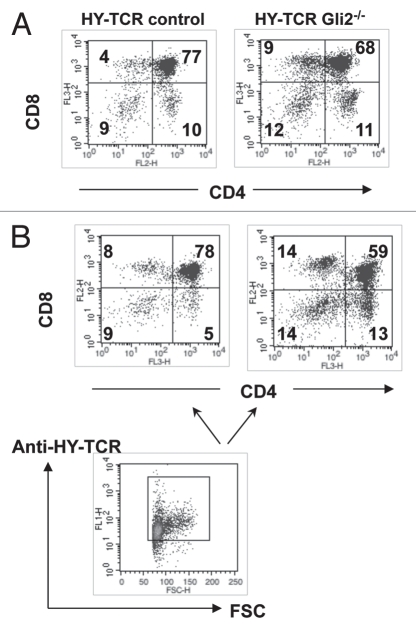
Selection of the transgenic HY-TCR in the Gli1−/− adult thymus. (A) CD4 and CD8 expression in female HY-TCRGli1+/+ and HYTCRGli1−/− thymus. The percentage of CD8SP and DP cells are given. Live cells were gated by FSC/SSC profile. Thymocyte numbers: 6.54 × 107 (HY-TCRGli1+/+); 4.36 × 107 (HY-TCRGli1−/−). (B) CD4 and CD8 expression in cells that stain positive with DbSmyc-tetramer in female HY-TCRGli1+/+ and HY-TCRGli1−/− thymus (upper part). The percentage of DbSmyc-tetramer+ CD8SP and DP cells is given. The lower dot-plot shows the gate used to select DbSmyc-tetramer+ cells that are shown in the upper part, and the percentage of cells that fall in this gate for Gli1+/+ and Gli1−/− is given. (C) The relative percentage of DbSmyc-tetramer+ cells in the CD8SP population in female HY-TCRGli1+/+, HY-TCRGli1+/− and HY-TCRGli1−/− thymus. The difference in the mean relative percentage between Gli1−/− and Gli1+/+ was statistically significant by student's t-test, (p = 0.0305), and the difference between Gli1+/− and Gli1+/+ was not significant (p = 0.0813). (D) CD4 and CD8 expression in male HY-TCRGli1+/+ and HY-TCRGli1−/− thymus. The percentage of CD8SP and DP cells is given. Live cells were gated by FSC/SSC profile. Mean thymocyte numbers: 1.3 × 107 (HY-TCRGli1−/−); 1.53 × 107 (HY-TCR control littermate).
In male HY-TCR transgenic mice, HY-TCR+ thymocytes are negatively selected as a result of interaction with the HY protein (Smcy peptide).44,45 Given that clonal deletion is very efficient in this model and HY-TCR+ CD8SP cells do not persist in the WT male thymus,44 it was unlikely that absence of Gli1 would influence repertoire selection in the male. Indeed, we found no statistically significant differences between male HY-TCR Gli1+/+ and HY-TCR Gli1−/− thymi (Fig. 5D).
Non-redundant function for Gli2 in positive selection of HY-TCR.
Both Gli1 and Gli2 function as activators of transcription down-stream of Hh pathway activation. As Gli1 has a nonredundant function in TCR repertoire selection at the transition from DP to SP cell, we asked if Gli2 also influences this process. Gli2−/− embryos die before birth,32 so we analysed repertoire selection of the transgenic HY-TCR in foetal thymus organ culture (FTOC) from female E15.5 HY-TCR Gli2−/− and Gli2+/+ embryos, cultured for 10 days. In the Gli2−/− FTOC, positive selection to the CD8 lineage was increased and the proportion of DP cells was decreased compared to WT (Fig. 6A). When we gated on cells expressing the HY-TCR (T3.70+ cells), we again found increased positive selection to the CD8 lineage in the Gli2−/− FTOC, with 14% CD8SP and 59% DP (ratio of DP:CD8Sp = 1:4.21), compared to 8% CD8SP and 78% DP (ratio of DP:CD8Sp = 1:9.75) in WT (Fig. 5B). Interestingly, the proportion of CD4SP cells was also increased in the Gli2−/− FTOC, consistent with the increased differentiation to both SP lineages observed in the Shh−/− FTOC.19
Figure 6.
Selection of the transgenic HY-TCR in Gli2−/− FTOC. (A) CD4 and CD8 expression in female HY-TCRGli2−/− and control littermate HY-TCR E15.5 FTOC after 10 days of culture. Mean cell recoveries were 4.1 × 104 (Gli2−/−) and 5.8 × 104 (control), and of CD8SPs were 3.69 × 103 (Gli2−/−) and 2.32 × 103 (Gli2+/+). The percentage of cells in each quadrant is given. Live cells were gated by FSC/SSC profile. (B) CD4 and CD8 expression in cells that stain positive with anti-HY-TCR antibody (T3.70) in female HY-TCRGli2+/+ and HY-TCRGli2−/− FTOC cultured as in (A) (upper part). The percentage of cells in each quadrant is given. The lower dot-plot shows the gate used to select anti-HY-TCR+ cells.
Discussion
Here we showed that Gli1 has non-redundant functions in thymocyte development, in the differentiation of DN thymocytes, the transition from DN to DP cell, and in TCR repertoire selection at the transition from DP to CD8SP cell. It has previously been difficult to define non-redundant functions for Gli1 in mouse development and adult tissue homeostasis,28,37 although recently a non-redundant function for Gli1 has been demonstrated in the regulation of proliferation of haematopoietic stem cells and myeloid progenitors in adult mice.46 Our data show that during thymocyte development in both the adult and the foetal thymus, Gli2A activity alone is insufficient to achieve physiological Hh pathway activity for normal control of T-cell development and TCR repertoire selection.
Comparison of thymocyte development in the Gli2−/− and Gli1−/− foetal thymus is consistent with the expression patterns of Gli genes in the DN subsets and with Gli1, itself a transcriptional target of Hh signaling, functioning at later time points after initiation of Hh pathway activation than Gli2.13,16,19,21 Gli2 transcription is highest in DN1 and DN2, whereas Gli1 transcription is repressed by Gli3 in DN1 cells, but peaks in DN3, presumably after Gli2-dependent initiation of Hh pathway activation. Gli2-deficiency first causes a partial arrest in differentiation at the transition from DN1 to DN2, when Gli2 transcription is highest in WT,21 whereas Gli1-deficiency first influences thymocyte development at the DN3 stage, when Gli1 transcription is highest in WT (illustrated in Fig. 7).
Figure 7.
Summary of Gli gene expression patterns and non-redundant functions in early thymocyte development. The Figure illustrates at which stages of early thymocyte development deficiency in Gli1, Gli2 or Gli3 either partially arrests or accelerates thymocyte differentiation, indicating at which stages each gene has a non-redundant function. Below this, the expression patterns of each gene are shown, to illustrate how expression correlates to non-redundant function.
Both Gli1 and Gli2 are required for physiological negative regulation of differentiation from DN to DP, so deficiency in either gene results in accelerated production of DP cells. The E16.5 Gli1−/− thymus is larger and contains more DP cells than WT littermates, but the E16.5 Gli2−/− thymus is smaller than WT and does not overtake its WT counterparts in DP production and cell number until E17.5.21 Rather than indicating that Gli1 has an independent role at this transition prior to the requirement for Gli2, this difference most likely reflects a more profound impact of Gli2-deficiency on the DN populations, delaying the differentiation to DP. This is in contrast to the weaker impact of Gli1-deficiency on the DN3 population, allowing differentiation to DP to exceed WT already on E16.5. This hypothesis is also consistent with gene transcription analysis showing increased expression of Gli2 at the DN4 population, in addition to DN1 and DN2 populations, while Gli1 expression is reduced following the DN3 stage and its level remains low until the DP stage.
In the Gli1−/− adult thymus, we did not detect differences in the DN populations or in the rate of differentiation to DP cell. However, it is easier to detect changes in the rate of transition between these populations in the foetal thymus, where thymocyte development is initially much more synchronised than in the adult steady-state thymus.43 Gli1-deficiency, however, did increase positive selection in the adult thymus. We found that Gli1 and Gli2 are non-redundant in Shh-mediated modulation of positive selection of a transgenic Class I-restricted TCR at the transition from DP to CD8SP cell. Deficiency in either transcription factor alone is sufficient to increase differentiation. This finding has implications for the study of autoimmunity and TCR repertoire, as it suggests that changes in Gli expression or activity, which cause no apparent developmental phenotype (such as Gli1 deficiency), could nevertheless influence the TCR repertoire.
In summary, we show that Gli1 is necessary for the normal regulation of T-cell development in both the foetal thymus and the adult thymus.
Materials and Methods
Mice.
C57BL/6 [purchased from B & K Universal Ltd., (UK)], C57BL/6Gli3+/− (purchased from Jackson laboratories, USA) Gli1+/− mice (a kind gift from A. Joyner),28 Gli2+/− mice (a kind gift from C. Hui),32 and HY-TCR transgenic mice44 were bred and maintained at University College London according to UK Home Office regulations. Gli1+/− and Gli2+/− mice were back-crossed onto C57BL/6 for at least six generations. Adult mice were 6–8 weeks old. Timed mates were performed by mating a male with two females overnight and monitoring the females for plugs. The day the plug was found was counted as E 0.5.
Flow cytometry and antibodies.
Thymocyte suspensions were prepared by crushing thymi between two pieces of ground glass. Cells were stained using combinations of directly conjugated antibodies obtained from BD Pharmingen and EBioscience. Cell suspensions were stained with the antibodies for 30 minutes on ice in 50 µl of Dulbeccos modified medium (Life Technologies), supplemented with 5% FCS and 0.01% sodium azide. Cells were washed in this medium between incubations and prior to analysis on the FACScan (Becton Dickinson). Events were collected in list mode using CellQuest software and data analyzed using CellQuest Pro and FlowJo software. Live cells were gated according to their FSC and SSC profiles. Intracellular staining for TCRβ was performed on cells stained for surface markers as above following fixation and permeabilisation with the Cytofix/Cytoperm™ solutions (BD biosciences) according to the manufacturer's instructions.
DbSmycPE-tetramer (ProImmune Oxford, UK) staining was carried out in PBS for 15 minutes at 25°C in the dark, prior to other antibody staining.
Data are representative of at least three experiments. Student's t-test was used to calculate statistical significance of difference between mean values between samples.
Cell sorting.
C57BL/6J and Gli3−/− foetal thymocytes were sorted on a Modular Flow Cytometer (MoFlo, Cytomation, Inc., Fort Collins, CO) at the Cancer Research UK FACS laboratory. For purification of the DN1 population, cells falling within the FSC/SSC live-gate, were sorted using antibodies directed against CD25, CD45.2 and CD44, for CD45.2+CD44+CD25−.
Foetal thymic organ cultures (FTOC).
E15.5 foetal thymi were cultured on 8 µm pore size Millipore filters (Millipore) in AIM-V serum free medium (Life Technologies) at 37°C and 5% CO2 as previously described.16
Quantitative RT-PCR analysis.
RNA was extracted from the sorted thymocytes with the Absolutely RNA miniprep kit (Stratagene) and cDNA was synthesized with Superscript III (Invitrogen, Carlsbad, CA). The cDNA samples were analyzed in triplicate by real time PCR on an iCycler (Bio-Rad Laboratories Lmt.) using the iQ™SYBR® Green Supermix (Bio-Rad) according to the manufacturer's instructions and as previously described.13 At least one primer for each amplification pair was designed to span exon-exon boundaries to avoid amplification of genomic DNA. For the amplification of Gli1 transcripts we used primers Gli1 Forward: AAA CCT CAA GAC GCA CCT TCG G and Gli1 Reverse: CGT ATG GCT TCT CAT TGG AGT G. For each sample, Gli1 transcript levels were normalized to Actβ levels prior to calculation of relative fold difference in transcription levels comparing to DN1 WT thymocytes. For the amplification of Actβ transcripts we used primers Actβ Forward: CCA ACC GTG AAA AGA TGA CC; Actβ Reverse: GGC ATA CAG GGA CAG CAC AG.
Acknowledgements
This work was supported by grants from the Wellcome Trust, MRC and Leukaemia Lymphoma Research. N.J.R. is supported by a Foulkes Foundation Fellowship. A.L.F. is supported by Great Ormond Street Hospital/UCL Institute of Child Health Biomedical Research Centre, which received funding from the Department of Health's NIHR Biomedical Research Centres funding scheme. We thank Derek Davies (Cancer Research UK) for cell sorting.
Abbreviations
- DN
double negative
- DP
double positive
- SP
single positive
- FTOC
foetal thymic organ culture
- Hh
hedgehog
- Shh
sonic hedgehog
- Ihh
indian hedgehog
- E
embryonic day
- WT
wild type
- R
repressor
- A
activator
- ic
intracellular
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/13453
References
- 1.Petrie HT, Zuniga-Pflucker JC. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu Rev Immunol. 2007;25:649–679. doi: 10.1146/annurev.immunol.23.021704.115715. [DOI] [PubMed] [Google Scholar]
- 2.Ciofani M, Zuniga-Pflucker JC. The thymus as an inductive site for T lymphopoiesis. Annu Rev Cell Dev Biol. 2007;23:463–493. doi: 10.1146/annurev.cellbio.23.090506.123547. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3−CD4−CD8− triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- 4.Michie AM, Zuniga-Pflucker JC. Regulation of thymocyte differentiation: pre-TCR signals and beta-selection. Semin Immunol. 2002;14:311–323. doi: 10.1016/s1044-5323(02)00064-7. [DOI] [PubMed] [Google Scholar]
- 5.Germain RN. T-cell development and the CD4−CD8 lineage decision. Nat Rev Immunol. 2002;2:309–322. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- 6.von Boehmer H, Kisielow P. Negative selection of the T-cell repertoire: where and when does it occur? Immunol Rev. 2006;209:284–289. doi: 10.1111/j.0105-2896.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 7.Varas A, Hager-Theodorides AL, Sacedon R, Vicente A, Zapata AG, Crompton T. The role of morphogens in T-cell development. Trends Immunol. 2003;24:197–206. doi: 10.1016/s1471-4906(03)00033-4. [DOI] [PubMed] [Google Scholar]
- 8.Varas A, Hernandez-Lopez C, Valencia J, Mattavelli S, Martinez VG, Hidalgo L, et al. Survival and function of human thymic dendritic cells are dependent on autocrine Hedgehog signaling. J Leukoc Biol. 2008;83:1476–1483. doi: 10.1189/jlb.1107792. [DOI] [PubMed] [Google Scholar]
- 9.Varas A, Sacedon R, Hidalgo L, Martinez VG, Valencia J, Cejalvo T, et al. Interplay between BMP4 and IL-7 in human intrathymic precursor cells. Cell Cycle. 2009;8:4119–4126. doi: 10.4161/cc.8.24.10149. [DOI] [PubMed] [Google Scholar]
- 10.Shah DK, Hager-Theodorides AL, Outram SV, Ross SE, Varas A, Crompton T. Reduced thymocyte development in sonic hedgehog knockout embryos. J Immunol. 2004;172:2296–2306. doi: 10.4049/jimmunol.172.4.2296. [DOI] [PubMed] [Google Scholar]
- 11.Sacedon R, Varas A, Hernandez-Lopez C, Gutierrez-deFrias C, Crompton T, Zapata AG, et al. Expression of hedgehog proteins in the human thymus. J Histochem Cytochem. 2003;51:1557–1566. doi: 10.1177/002215540305101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutierrez-Frias C, Sacedon R, Hernandez-Lopez C, Cejalvo T, Crompton T, Zapata AG, et al. Sonic hedgehog regulates early human thymocyte differentiation by counteracting the IL-7-induced development of CD34+ precursor cells. J Immunol. 2004;173:5046–5053. doi: 10.4049/jimmunol.173.8.5046. [DOI] [PubMed] [Google Scholar]
- 13.Hager-Theodorides AL, Dessens JT, Outram SV, Crompton T. The transcription factor Gli3 regulates differentiation of fetal CD4−CD8− double-negative thymocytes. Blood. 2005;106:1296–1304. doi: 10.1182/blood-2005-03-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hager-Theodorides AL, Furmanski AL, Ross SE, Outram SV, Rowbotham NJ, Crompton T. The Gli3 transcription factor expressed in the thymus stroma controls thymocyte negative selection via Hedgehog-dependent and -independent mechanisms. J Immunol. 2009;183:3023–3032. doi: 10.4049/jimmunol.0900152. [DOI] [PubMed] [Google Scholar]
- 15.Andaloussi AE, Graves S, Meng F, Mandal M, Mashayekhi M, Aifantis I. Hedgehog signaling controls thymocyte progenitor homeostasis and differentiation in the thymus. Nat Immunol. 2006;7:418–426. doi: 10.1038/ni1313. [DOI] [PubMed] [Google Scholar]
- 16.Outram SV, Varas A, Pepicelli CV, Crompton T. Hedgehog signaling regulates differentiation from double-negative to double-positive thymocyte. Immunity. 2000;13:187–197. doi: 10.1016/s1074-7613(00)00019-4. [DOI] [PubMed] [Google Scholar]
- 17.Outram SV, Hager-Theodorides AL, Shah DK, Rowbotham NJ, Drakopoulou E, Ross SE, et al. Indian hedgehog (Ihh) both promotes and restricts thymocyte differentiation. Blood. 2009;113:2217–2228. doi: 10.1182/blood-2008-03-144840. [DOI] [PubMed] [Google Scholar]
- 18.Rowbotham NJ, Furmanski AL, Hager-Theodorides AL, Ross SE, Drakopoulou E, Koufaris C, et al. Repression of hedgehog signal transduction in T-lineage cells increases TCR-induced activation and proliferation. Cell Cycle. 2008;7:904–908. doi: 10.4161/cc.7.7.5628. [DOI] [PubMed] [Google Scholar]
- 19.Rowbotham NJ, Hager-Theodorides AL, Cebecauer M, Shah DK, Drakopoulou E, Dyson J, et al. Activation of the Hedgehog signaling pathway in T-lineage cells inhibits TCR repertoire selection in the thymus and peripheral T-cell activation. Blood. 2007;109:3757–3766. doi: 10.1182/blood-2006-07-037655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowbotham NJ, Hager-Theodorides AL, Furmanski AL, Crompton T. A novel role for Hedgehog in T-cell receptor signaling: implications for development and immunity. Cell Cycle. 2007:2138–2142. doi: 10.4161/cc.6.17.4644. [DOI] [PubMed] [Google Scholar]
- 21.Rowbotham NJ, Hager-Theodorides AL, Furmanski AL, Ross SE, Outram SV, Dessens JT, et al. Sonic hedgehog negatively regulates pre-TCR-induced differentiation by a Gli2-dependent mechanism. Blood. 2009;113:5144–5156. doi: 10.1182/blood-2008-10-185751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riobo NA, Lu K, Emerson CP. Hedgehog signal transduction: signal integration and cross talk in development and cancer. Cell Cycle. 2006;5:1612–1615. doi: 10.4161/cc.5.15.3130. [DOI] [PubMed] [Google Scholar]
- 23.Ingham PW, Placzek M. Orchestrating ontogenesis: variations on a theme by sonic hedgehog. Nat Rev Genet. 2006;7:841–850. doi: 10.1038/nrg1969. [DOI] [PubMed] [Google Scholar]
- 24.Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–434. doi: 10.1016/s0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 25.Pan Y, Bai CB, Joyner AL, Wang B. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol. 2006;26:3365–3377. doi: 10.1128/MCB.26.9.3365-3377.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development. 1999;126:3915–3924. doi: 10.1242/dev.126.17.3915. [DOI] [PubMed] [Google Scholar]
- 27.Aza-Blanc P, Lin HY, Altaba AR, Kornberg TB. Expression of the vertebrate Gli proteins in Drosophila reveals a distribution of activator and repressor activities. Development. 2000;127:4293–4301. doi: 10.1242/dev.127.19.4293. [DOI] [PubMed] [Google Scholar]
- 28.Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–4761. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- 29.Koebernick K, Pieler T. Gli-type zinc finger proteins as bipotential transducers of Hedgehog signaling. Differentiation. 2002;70:69–76. doi: 10.1046/j.1432-0436.2002.700201.x. [DOI] [PubMed] [Google Scholar]
- 30.te Welscher P, Fernandez-Teran M, Ros MA, Zeller R. Mutual genetic antagonism involving GLI3 and dHAND prepatterns the vertebrate limb bud mesenchyme prior to SHH signaling. Genes Dev. 2002;16:421–426. doi: 10.1101/gad.219202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.te Welscher P, Zuniga SA, Kuijper T, Drenth HJ, Goedemans F, Meijlink, et al. Progression of vertebrate limb development through SHH-mediated counteraction of GLI3. Science. 2002;298:827–830. doi: 10.1126/science.1075620. [DOI] [PubMed] [Google Scholar]
- 32.Mo R, Freer AM, Zinyk DL, Crackower MA, Michaud J, Heng HH, et al. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development. 1997;124:113–123. doi: 10.1242/dev.124.1.113. [DOI] [PubMed] [Google Scholar]
- 33.Matise MP, Epstein DJ, Park HL, Platt KA, Joyner AL. Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development. 1998;125:2759–2770. doi: 10.1242/dev.125.15.2759. [DOI] [PubMed] [Google Scholar]
- 34.Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell. 2004;6:103–115. doi: 10.1016/s1534-5807(03)00394-0. [DOI] [PubMed] [Google Scholar]
- 35.Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, et al. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development. 2000;127:1593–1605. doi: 10.1242/dev.127.8.1593. [DOI] [PubMed] [Google Scholar]
- 36.Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku M, Ishii S. Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J Biol Chem. 1999;274:8143–8152. doi: 10.1074/jbc.274.12.8143. [DOI] [PubMed] [Google Scholar]
- 37.Bai CB, Joyner AL. Gli1 can rescue the in vivo function of Gli2. Development. 2001;128:5161–5172. doi: 10.1242/dev.128.24.5161. [DOI] [PubMed] [Google Scholar]
- 38.Neumann CJ. Hedgehogs as negative regulators of the cell cycle. Cell Cycle. 2005;4:1139–1140. doi: 10.4161/cc.4.9.1999. [DOI] [PubMed] [Google Scholar]
- 39.Agathocleous M, Locker M, Harris WA, Perron M. A general role of hedgehog in the regulation of proliferation. Cell Cycle. 2007;6:156–159. doi: 10.4161/cc.6.2.3745. [DOI] [PubMed] [Google Scholar]
- 40.Ashe HL, Briscoe J. The interpretation of morphogen gradients. Development. 2006;133:385–394. doi: 10.1242/dev.02238. [DOI] [PubMed] [Google Scholar]
- 41.Knoepfler PS, Kenney AM. Neural precursor cycling at sonic speed: N-Myc pedals, GSK-3 brakes. Cell Cycle. 2006;5:47–52. doi: 10.4161/cc.5.1.2292. [DOI] [PubMed] [Google Scholar]
- 42.Crompton T, Outram SV, Hager-Theodorides AL. Sonic hedgehog signalling in T-cell development and activation. Nat Rev Immunol. 2007;7:726–735. doi: 10.1038/nri2151. [DOI] [PubMed] [Google Scholar]
- 43.Hager-Theodorides AL, Rowbotham NJ, Outram SV, Dessens JT, Crompton T. Beta-selection: abundance of TCRbeta−/gammadelta− CD44− CD25− (DN4) cells in the foetal thymus. Eur J Immunol. 2007;37:487–500. doi: 10.1002/eji.200636503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+ 8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 45.Markiewicz MA, Girao C, Opferman JT, Sun J, Hu Q, Agulnik AA, et al. Long-term T cell memory requires the surface expression of self-peptide/major histocompatibility complex molecules. Proc Natl Acad Sci USA. 1998;95:3065–3070. doi: 10.1073/pnas.95.6.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merchant A, Joseph G, Wang Q, Brennan S, Matsui W. Gli1 regulates the proliferation and differentiation of HSCs and myeloid progenitors. Blood. 115:2391–2396. doi: 10.1182/blood-2009-09-241703. [DOI] [PMC free article] [PubMed] [Google Scholar]