In the current issue of the Journal, Bettina Salle and coworkers describe infection of female macaques following atraumatic instillation of SIV-infected cells into the vaginal cavity [1]. This new SIV infection model represents a significant advance for HIV transmission and prevention research. Whereas HIV-infected cells in genital secretions (“Trojan Horse leukocytes”) may play an important role in the sexual transmission of HIV [2], they have been largely overlooked in recent studies on mechanisms of HIV transmission and in the design and testing of HIV vaccine and microbicide candidates. Current preclinical assays for the development of HIV prevention drugs and vaccines predominately use cell-free viral stocks, and the most popular macaque vaginal SIV transmission models used for vaccine and microbicide preclinical efficacy trials require super-physiological doses of cell-free virus and treatment with high doses of progestins to achieve high infection rates [3]. Since the molecular events underlying cell-associated transmission differ from those involved in cell-free virus transmission, many of the current vaccine and microbicide candidates shown to be effective against cell-free virus may not protect against cell-associated viral transmission. The failure of several recent vaccine and microbicide clinical trials to prevent HIV transmission [4] may be due, in part, to this oversight.
Cell-associated HIV transmission is an attractive theory because infected cells can transport virus across the mucosal epithelium while avoiding adverse effects of antiviral defense molecules in the genital environment, and because cell-to-cell HIV transfer through viral synapses is highly efficient. Several studies have conclusively shown that infected cells are much more effective than cell-free virus at infecting subepithelial target cells in polarized epithelial monolayer cultures [5-8], and effective cell-associated mucosal transmission has also been demonstrated in small animal models such as the Feline Immunodeficiency Virus [9, 10] and hu-SCID mouse HIV infection models [11]. Furthermore, recent clinical studies provide evidence that human cell-associated HIV transmission may occur. In one study, unprotected heterosexual intercourse was associated with alloimmunization to partner's HLA antigens [12], indicating that seminal leukocytes commonly infiltrate the vaginal epithelium after intercourse. In another study, genetic sequencing of HIV in blood from acutely infected individuals showed that the genotype of the infecting virus in 3 out of 5 cases more closely matched that of HIV in semen cells than in free virus [13].
Only four nonhuman primate studies on cell-associated SIV/HIV transmission have been published to date. In 1998, one group reported that chimpanzees could be infected with HIV following placement of either cell-free virus or infected PBMCs near the cervical os [14], whereas another group did not detect systemic infection in rhesus macaques following vaginal exposure to cryopreserved SIV-infected PBMCs [15]. More recently, scientists at the Primate Center in Madison, Wisconsin demonstrated transvaginal infection of rhesus macaques following multiple low dose exposures to fresh SIV-infected PBMCs in animals with chemically-induced vaginal ulcers as well as untreated intact animals that were used as controls [16, 17]. These preliminary studies suggest that HIV/SIV-infected leukocytes can be infectious when delivered vaginally to nonhuman primates, but that results depend on characteristics of the viral stock and the dosing protocol.
Why has cell-associated HIV transmission been largely overlooked in research on HIV transmission mechanisms and preclinical drug development? Probably because convenient commercial assays to quantify cell-free viral titers in blood and genital secretions, as well as well-characterized cell-free viral stocks, are readily available. Salle et al. questioned whether viruses produced in vitro represent replicating populations in vivo, and for their current study they harvested infected cells from spleens of SIVmac251-infected rhesus macaques at the peak of viremia (12 days post infection). Infected leukocytes were enriched on Ficoll-hypaque and frozen in DMSO at 107 cells/ampoule. A representative batch of infected spleen cells from one donor contained 4.2 × 105 viral DNA copies/106 cells and a TCID50 of 5,576 cells. Central memory T cells, comprising 25% of the total spleen cell population, contained 2.17 × 105 viral DNA copies and 7.92 × 106 viral mRNA copies/106 cells. The monocyte/macrophage population comprised only 2% of the population, and contained 5.37 × 103 viral DNA copies and 1.64 × 106 viral mRNA copies/106 cells.
To document transepithelial penetration of infected spleen leukocytes, cells were tracked at 21 and 41 hours post vaginal application. Fluorescein-labelled spleen cells were detected in draining lymph nodes and peripheral blood, and SIV-infected cells were detected by in situ hybridization in the lamina propria of the vaginal epithelium and T cell areas of distal lymph nodes at 21 and 41 hours post exposure. Four of five female animals that received single intravaginal doses of 107 spleen cells became systemically infected with SIV. The dose needed to infect 50% of females was determined to be 6.69 × 105 viral DNA copies, which corresponds to the number of HIV DNA proviral copies detected in semen cells from some HIV infected men [2].
The new macaque model for cell-associated SIV transmission presented by Salle et al. is promising, but several questions remain. One advantage to using spleen cells from infected animals is the availability of differentiated macrophages, a cell type normally present in semen that may be especially efficient at SIV/HIV transmission. However, the viral stocks in this study contained few mature macrophages, possibly because they were not retained on the Ficoll-hypaque gradients. Future studies should be conducted to determine the relative efficiency of SIV-infected T cells vs. macrophages in cell-associated HIV/SIV transmission. Other questions pertain to the physiological relevance of the model. Is progestin treatment required to achieve reliable cell-associated SIV transepithelial transmission? It would be preferable to avoid high dose progestin treatment because it is immunosuppressive and blocks ovarian estrogen production, resulting in an artificially thinned vaginal epithelium. Does systemic infection occur following multiple low dose vaginal exposures? A low dose multiple exposure model could more closely represent natural conditions where HIV infection occurs at a low frequency in healthy women.
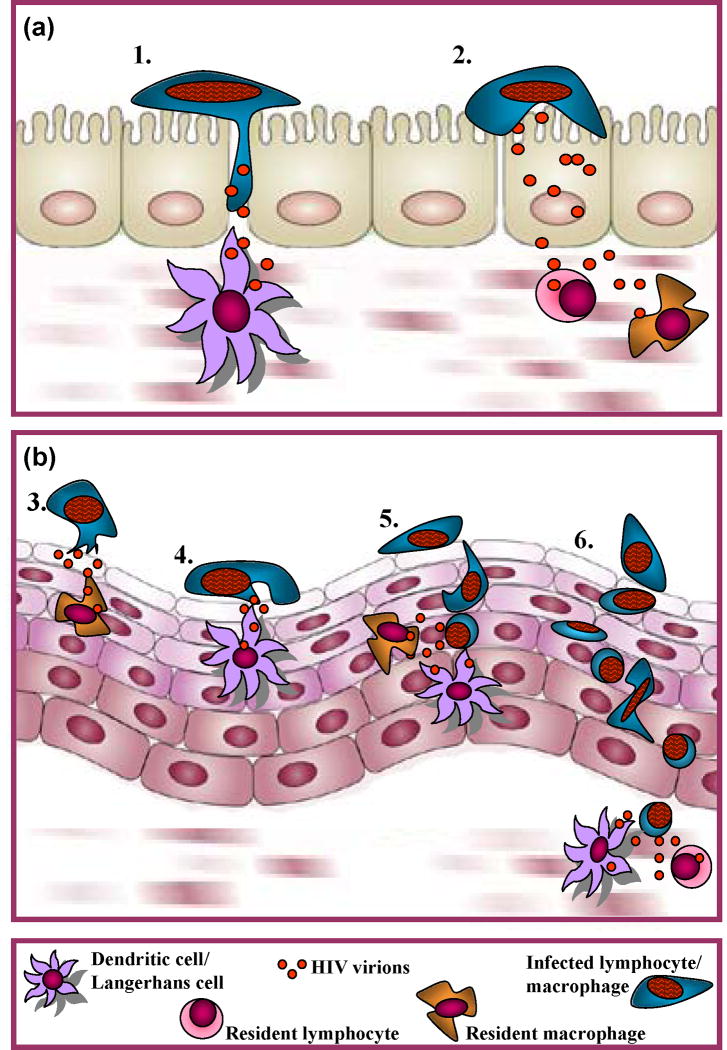
This model could also be used to evaluate the contribution of risk factors such as specific STIs to cell-associated HIV/SIV transmission, and to further define molecular mechanisms underlying this mode of transmission. Based on limited experimental evidence, we recently proposed three potential cell-associated HIV transmission pathways [2]: 1) HIV-infected leukocytes attach to the apical surface of epithelial cells and shed nascent virions towards the epithelial cell plasma membrane. These highly infectious viral particles may be sequestered by epithelial cells for subsequent transfer to HIV-susceptible host cells within the epithelium, or transferred through epithelial cell layers by transcytosis to target cells in the lamina propria. 2) HIV is directly transferred from infected leukocytes to target cells within the epithelium, possibly through the formation of an infectious synapse; it is possible that target cells are attracted to infected leukocytes by chemokines released either by the infected cell or by epithelial cells that are activated by contact with infected cells. 3) Infected leukocytes may migrate through the epithelium to infect target cells in the lamina propria or draining lymph nodes (Figure 1). Each of these pathways entails molecular interactions that could be targeted and tested in an authentic macaque model of cell-associated HIV/SIV transmission. If cell-associated HIV/SIV transmission proves to be a major infection mechanism, such research could very well lead to new HIV prevention strategies.
Figure 1. Potential mechanisms underlying cell-associated HIV transmission.
-
Columnar epithelium:1) Infected cell migrates between epithelial cells to infect susceptible host cells in the lamina propria or draining lymph nodes.2) HIV trancytosis through epithelial cells to infect susceptible target cells in lamina propria.
-
Stratified squamous epithelium:3) Transfer of HIV from infected leukocyte to epithelial cell, which transfers virus to intraepithelial or subepithelial target cells through (a) transcytosis or (b) attraction via release of chemokines.4) Direct cell-to-cell transfer of HIV from infected leukocyte to intraepithelial target cell via viral synapses.5) Transepithelial migration of infected leukocyte to infect intraepithelial target cells within the epithelium.6) Transepithelial migration of infected cell to infect target cells in the subepithelium or draining lymph nodes.Originally published in [2].
Acknowledgments
Supported by NIH grant R33 AI076996 (DJA).
Footnotes
There are no conflicts of interest.
References
- 1.Salle B, Brouchard P, Bourry O, Abdelkrim M, Andrieu T, Prevot S, Dejucq-Rainsford N, Dereuddre-Bosquet N, LeGrand R. Journal of Infectious Diseases. 2010 doi: 10.1086/653619. In Press. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DJ, Politch JA, Nadolski AM, Blaskewicz CD, Pudney J, Mayer KH. Targeting Trojan Horse leukocytes for HIV prevention. AIDS. 2010;24:163–87. doi: 10.1097/QAD.0b013e32833424c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shedlock DJ, Silvestri G, Weiner DB. Monkeying around with HIV vaccines: using rhesus macaques to define ‘gatekeepers’ for clinical trials. Nat Rev Immunol. 2009;9:717–28. doi: 10.1038/nri2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas C. Roadblocks in HIV research: five questions. Nat Med. 2009;15:855–9. doi: 10.1038/nm0809-855. [DOI] [PubMed] [Google Scholar]
- 5.Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med. 1997;3:42–7. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- 6.Chancey CJ, Khanna KV, Seegers JF, et al. Lactobacilli-expressed single-chain variable fragment (scFv) specific for intercellular adhesion molecule 1 (ICAM-1) blocks cell-associated HIV-1 transmission across a cervical epithelial monolayer. J Immunol. 2006;176:5627–36. doi: 10.4049/jimmunol.176.9.5627. [DOI] [PubMed] [Google Scholar]
- 7.Tan X, Pearce-Pratt R, Phillips DM. Productive infection of a cervical epithelial cell line with human immunodeficiency virus: implications for sexual transmission. J Virol. 1993;67:6447–52. doi: 10.1128/jvi.67.11.6447-6452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Herrewege Y, Michiels J, Waeytens A, et al. A dual chamber model of female cervical mucosa for the study of HIV transmission and for the evaluation of candidate HIV microbicides. Antiviral Res. 2007;74:111–24. doi: 10.1016/j.antiviral.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Bishop SA, Stokes CR, Gruffydd-Jones TJ, Whiting CV, Harbour DA. Vaginal and rectal infection of cats with feline immunodeficiency virus. Vet Microbiol. 1996;51:217–27. doi: 10.1016/0378-1135(96)00038-7. [DOI] [PubMed] [Google Scholar]
- 10.Moench TR, Whaley KJ, Mandrell TD, Bishop BD, Witt CJ, Cone RA. The cat/feline immunodeficiency virus model for transmucosal transmission of AIDS: nonoxynol-9 contraceptive jelly blocks transmission by an infected cell inoculum. AIDS. 1993;7:797–802. doi: 10.1097/00002030-199306000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Khanna KV, Whaley KJ, Zeitlin L, et al. Vaginal transmission of cell-associated HIV-1 in the mouse is blocked by a topical, membrane-modifying agent. J Clin Invest. 2002;109:205–11. doi: 10.1172/JCI13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters B, Whittall T, Babaahmady K, Gray K, Vaughan R, Lehner T. Effect of heterosexual intercourse on mucosal alloimmunisation and resistance to HIV-1 infection. Lancet. 2004;363:518–24. doi: 10.1016/S0140-6736(04)15538-4. [DOI] [PubMed] [Google Scholar]
- 13.Zhu T, Wang N, Carr A, et al. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J Virol. 1996;70:3098–107. doi: 10.1128/jvi.70.5.3098-3107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girard M, Mahoney J, Wei Q, et al. Genital infection of female chimpanzees with human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1998;14:1357–67. doi: 10.1089/aid.1998.14.1357. [DOI] [PubMed] [Google Scholar]
- 15.Sodora DL, Gettie A, Miller CJ, Marx PA. Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S119–23. [PubMed] [Google Scholar]
- 16.Kaizu M, Weiler AM, Weisgrau KL, et al. Repeated intravaginal inoculation with cell-associated simian immunodeficiency virus results in persistent infection of nonhuman primates. J Infect Dis. 2006;194:912–6. doi: 10.1086/507308. [DOI] [PubMed] [Google Scholar]
- 17.Weiler AM, Li Q, Duan L, et al. Genital ulcers facilitate rapid viral entry and dissemination following intravaginal inoculation with cell-associated simian immunodeficiency virus SIVmac239. J Virol. 2008;82:4154–8. doi: 10.1128/JVI.01947-07. [DOI] [PMC free article] [PubMed] [Google Scholar]