Abstract
Data on the association between the SLC6A4 5-HTTLPR polymorphism and migraine are conflicting. We performed a systematic review and meta-analysis among studies published until September 2009. For each study with genotype information we calculated odds ratios (OR) and 95% confidence intervals (CI) assuming additive, dominant, and recessive genetic models. We then calculated pooled ORs and 95% CIs. Among the ten studies identified there was no overall association between the polymorphism and any migraine for Europeans or Asians. However, European women carrying the S allele had an increased risk for any migraine (dominant model: pooled OR=2.02; 95%CI 1.24–3.28). Results among Europeans further suggested an increased risk for migraine with aura among carriers of the S/S genotype (recessive model: pooled OR=1.41; 95%CI 0.83–2.40). While our results indicate no overall association between the SLC6A4 5-HTTLPR polymorphism and migraine among Europeans and Asians, gender and migraine aura status may have modifying roles among Europeans.
Keywords: migraine, serotonin transporter gene, SLC6A4, 5-HTTLPR, meta-analysis
Introduction
Migraine is a common, chronic disorder affecting 10–20% of the population; women 3–4 times more often than men (1). Up to one third of migraine patients experience an aura prior to or during the migraine headache.
Despite many advances in migraine research over the last decades, migraine pathophysiology is still incompletely understood. Current concepts view migraine as an inherited brain disorder, characterized by neurotransmitter imbalances that lead to neuronal dysfunctions in the form of recurrent headache attacks and combinations of gastrointestinal and autonomic nervous system symptoms (1, 2). The serotonergic system appears to play a pivotal role and alterations in serotonin metabolism and in the processing of central serotonin-mediated responses have been describes as typical for migraineurs (3).
Following axonal release serotonin (5-hydroxytryptamine; 5-HT) action is rapidly terminated by presynaptic reuptake (4). This is performed by the serotonin transporter SLC6A4 (5-HTT) (5), which is mostly expressed in the brainstem and midbrain (6), areas implicated in migraine pathophysiology. A 44-bp insertion/deletion polymorphism in the promoter region of the SLC6A4 gene, termed 5-HTTLPR (5-HTT Linked Polymorphic Region), has been described (7, 8). This 5-HTTLPR polymorphism translates into a long (L) and short (S) allele, which show functional differences. The S allele is associated with half the number of serotonin transporters compared with carriers of the L/L genotype (9). This results in a slower synaptic clearing of serotonin in carriers of the S allele. Subsequently it was speculated that the SLC6A4 5-HTTLPR polymorphism accounts for altered serotonin levels in migraineurs and may thus be associated with migraine.
However, results from studies looking at the association between the SLC6A4 5-HTTLPR polymorphism and migraine are conflicting. Some have reported an association with any migraine (10, 11), some only with migraine with aura (12, 13), and others did not find an association (14–20). This may in part be due to differences in genotype and allele frequencies between ethnic groups (21, 22), sample size, and study characteristics.
We sought to summarize the current evidence on the association between the SLC6A4 5-HTTLPR polymorphism and migraine including migraine with aura (MA) and migraine without aura (MO) by systematically reviewing the literature and performing a meta-analysis.
Methods
Selection of studies
We followed the guidelines for systematic reviews of genetic association studies (23). Two investigators (M.S., P.M.R.) independently searched MEDLINE, EMBASE, and Science Citation Index from inception to September 2009 combining text words and MESH terms, where appropriate, for serotonin and serotonin transporter (“Serotonin Plasma Membrane Transport Proteins” or “serotonin transporter” or “serotonin” or “SLC6A4”) with terms for genetic variations (“gene” or “polymorphism” or “genetic variation”) and terms for headache and migraine (“headache” or “headache disorders” or “migraine” or “migraine disorders”). The search terms were combined with the “explode” feature where applicable. We considered full articles without language restrictions. In addition, we manually searched the reference list of all primary articles and review articles.
A priori, we defined the following criteria for inclusion:
Studies must have a cross-sectional, case-control or cohort design and must be published as full papers.
Authors must investigate patients with migraine, diagnosed according to the criteria of the International Headache Society (IHS) (24, 25) and healthy control subjects.
Authors must provide information on genotype frequencies of the SLC6A4 5-HTTLPR polymorphism or sufficient data to calculate these.
In studies with overlapping cases and/or controls the largest study with extractable data was included.
In a first step, two investigators (M.S., T.K.) by consensus identified all studies not meeting any of the pre-specified criteria by screening the title and abstracts. These studies were excluded. In a second step, the same investigators evaluated the remaining studies in their entirety. Studies were excluded if they did not meet all criteria.
Data extraction
Two investigators (M.S., P.M.R.) independently extracted data from the published studies and entered them in a customized database. Disagreements were resolved by consensus. The extracted data included authors and title of study, year of publication, country of origin, ethnicity of population investigated, setting (clinic vs. population), study design, genotyping method, migraine status (any migraine, MA, MO), age range and gender of study individuals, study size, allele and genotype frequencies, and information on additional genetic variants as well as gene-gene and gene-environment interactions, if investigated. If not given, genotype frequencies were calculated where possible. If allele or genotype frequencies given did not match with the number of patients reported, the respective subgroup was excluded. We did not contact the authors to collect further information.
Statistical analysis
We first used logistic regression to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for the association between the SLC6A4 5-HTTLPR polymorphism and migraine assuming additive, dominant, and recessive genetic models. The additive model assumes that the risk for migraine among carriers of the heterozygous genotype (L/S) is half way between carriers of the homozygous genotypes (L/L, S/S). While the dominant model assumes that carriers of the heterozygous (L/S) and homozygous mutant genotypes (S/S) have the same risk of developing migraine compared with carriers of the homozygous wild-type genotype (L/L), a recessive model assumes that carrying the homozygous mutant genotype (S/S) is necessary to alter the risk for migraine compared to carriers of the heterozygous (L/S) and homozygous wild-type genotype (L/L). We also determined Hardy-Weinberg Equilibrium (HWE) for each study. The Hardy-Weinberg principle states that allele and genotype frequencies in a population remain constant (are in equilibrium) from generation to generation. Deviations from HWE might for example indicate genotyping error, limited population size, population substructure, or newly occurred mutations. A p-value ≥0.05 from a chi-square test used to test for HWE in the control population makes such errors/conditions unlikely (no deviation from HWE) and is considered a quality measure of a study. We investigated any migraine, MA, and MO.
We a priori decided to perform separate analyses among European and Asian populations since the genotype and allele frequencies differ between ethnic groups (21, 22). We then pooled results from studies stratified by country of origin. We grouped the Turkish study (20) with the other studies in European populations based on the similarity of allele and genotype frequencies in the controls (Table 2).
Table 2.
Allele and genotype frequencies for the SLC6A4 5-HTTLPR polymorphism
| Author | Disease status | Study size | Allele frequencies, n (%) | Genotype frequencies, n (%) | |||
|---|---|---|---|---|---|---|---|
| L | S | L/L | L/S | S/S | |||
| Yilmaz, 2001 (20) | controls | 80 | 83 (51.9) | 77 (48.1) | 25 (31.3) | 33 (41.3) | 22 (27.5) |
| any migraine | 52 | 53 (51.0) | 51 (49.0) | 16 (30.8) | 21 (40.4) | 15 (28.8) | |
| Kotani, 2002 (15) | controls | 190 | 87 (22.9) | 293 (77.1) | 11 (6.0) | 65 (34.0) | 114 (60.0) |
| any migraine | 151 | 66 (21.9) | 236 (78.1) | 7 (5.0) | 52 (34.0) | 92 (61.0) | |
| Juhasz, 2003 (11) | controls | 101 | 122 (60.4) | 80 (39.6) | 38 (37.6) | 46 (45.5) | 17 (16.9) |
| any migraine | 126 | 129 (51.2) | 123 (48.8) | 32 (25.4) | 65 (51.6) | 29 (23.0) | |
| MA | 10 | 10 (50.0) | 10 (50.0) | 3 (30.0) | 4 (40.0) | 3 (30.0) | |
| MO | 116 | 119 (51.3) | 113 (48.7) | 29 (25.0) | 61 (52.6) | 26 (22.4) | |
| Marziniak, 2005 (13) | controls | 115 | 132 (57.4) | 98 (42.6) | 38 (33.0) | 56 (48.7) | 21 (18.3) |
| any migraine | 197 | 211 (53.6) | 183 (46.4) | 61 (31.0) | 89 (45.2) | 47 (23.8) | |
| MA | 96 | 84 (43.7) | 108 (56.3) | 20 (20.8) | 44 (45.8) | 32 (33.4) | |
| MO | 101 | 127 (62.9) | 75 (37.1) | 41 (40.6) | 45 (44.5) | 15 (14.9) | |
| Borroni, 2005 (12) | controls | 105 | 118 (56.2) | 92 (43.8) | 32 (30.7) | 54 (51.3) | 19 (18.0) |
| any migraine | 144 | 162 (56.2) | 126 (43.8) | 52 (36.1) | 58 (40.3) | 34 (23.6) | |
| MA | 52 | 48 (46.2) | 56 (53.8) | 13 (25.0) | 22 (42.3) | 17 (32.7) | |
| MO | 92 | 114 (62.0) | 70 (38.0) | 39 (42.4) | 36 (39.1) | 17 (18.5) | |
| Kim, 2005 (14) | controls | 170 | 83 (24.4) | 257 (75.6) | 9 (5.3) | 65 (38.2) | 96 (56.5) |
| any migraine | 52 | 20 (19.2) | 84 (80.8) | 2 (3.8) | 16 (30.8) | 34 (65.4) | |
| Park, 2006 (16) | controls | 100 | 43 (22.0) | 157 (78.0) | 2 (2.0) | 39 (39.0) | 59 (59.0) |
| MO | 97 | 43 (22.0) | 151 (78.0) | 4 (4.0) | 35 (36.0) | 58 (60.0) | |
| Todt, 2006 (18) | controls | 506 | 617 (61.0) | 395 (39.0) | 188 (37.2) | 241 (47.6) | 77 (15.2) |
| MA | 472 | 599 (63.5) | 345 (36.5) | 188 (39.8) | 223 (47.2) | 61 (12.9) | |
| Szilagyi, 2006 (17) | controls | 464 | 540 (58.2) | 388 (41.8) | 167 (36.0) | 206 (44.4) | 91 (19.6) |
| any migraine | 87 | 107 (61.5) | 67 (38.5) | 34 (39.1) | 39 (44.8) | 14 (16.1) | |
| MA | 38 | 47 (61.8) | 29 (38.2) | 16 (42.1) | 15 (39.5) | 7 (18.4) | |
| MO | 49 | 60 (61.2) | 38 (38.8) | 18 (36.7) | 24 (49.0) | 7 (14.3) | |
| Gonda, 2007 (10) | controls | 52 | 66 (63.46) | 38 (36.54) | 20 (38.46) | 26 (50.0) | 6 (11.54) |
| any migraine | 45 | 41 (45.56) | 49 (54.44) | 8 (17.77) | 25 (55.56) | 12 (26.67) | |
MA: migraine with aura; MO: migraine without aura
We weighted the log of the ORs by the inverse of their variance to obtain pooled estimates. We ran random-effects models, which include assumptions about the variability between studies and provide more conservative estimates. We performed the DerSimonian and Laird Q test for heterogeneity and also calculated the I2 statistic for each analysis (26). This statistic describes the percentage of total variation across studies that is due to heterogeneity rather than chance (25%: low, 50%: medium, 75%: high heterogeneity). We used Galbraith plots to visually examine the impact of individual studies on the overall homogeneity test statistic. We evaluated potential publication bias visually by examining for possible skewness in funnel plots (27) and statistically with the methods described by Begg and Mazumdar (27) and Egger (28). The former method uses adjusted rank correlations, the latter method a weighted regression approach to investigate the association between outcome effects (log odds ratio) and its standard error in each study. P-values <0.05 may be regarded as an indication for publication bias.
All analyses were performed using SAS version 9.1 (SAS Institute Inc, Cary, NC) and STATA 10.1 (Stata, College Station, Texas, USA).
Since we only utilized previously published data, we did not obtain approval of an ethics committee or written informed consent.
Results
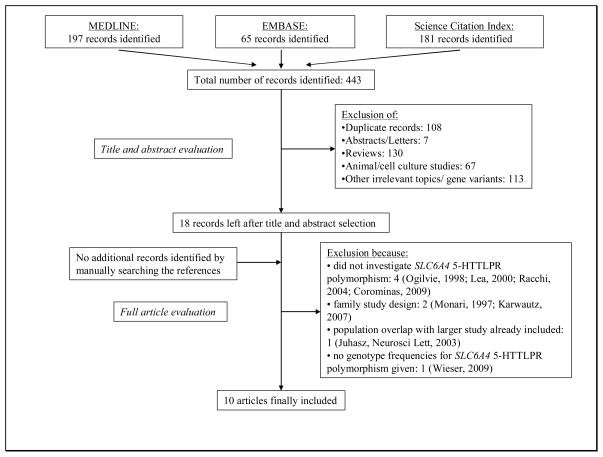
Figure 1 summarizes the process of identifying eligible studies. After title and abstract evaluation we were left with 18 studies (10–20, 29–35). We excluded eight more studies (19, 29–35) after evaluating the full paper versions of the remaining articles and were left with ten studies for this analysis (10, 12–18, 20, 30).
Figure 1.
Process of identifying studies
Study characteristics
Table 1 summarizes the characteristics of the ten included studies. Six studies were performed in European populations (10–13, 17, 18), one in a Turkish population (20), and three in Asian populations (14–16). Seven studies investigated only mixed female and male populations (12–15, 17, 18, 20) and three only women (10, 11, 16). Eight studies presented results for any migraine (10–15, 17, 20), five for MA (11–13, 17, 18), and five for MO (11–13, 16, 17). All studies had a case-control design and used standard polymerase chain reaction genotyping methods.
Table 1.
Characteristics of included studies investigating the association between the SLC6A4 5-HTTLPR polymorphism and migraine
| Author and year | Country | Setting | Population | Study size with genotypic information | Comment | |||
|---|---|---|---|---|---|---|---|---|
| Controls | Any migraine | MA | MO | |||||
| Yilmaz, 2001 (20) | Turkey | NS | mixed | 80 | 52 | ---- | ---- | Other polymorphisms investigated: SLC6A4 VNTR in intron 2. |
| Kotani, 2002 (15) | Japan | NS | mixed | 190 | 151 | ---- | ---- | Migraine attacks were more frequent for carriers of S/S genotype compared with L/S+L/L genotypes. |
| Juhasz, 2003 (11) | Hungary | clinic | women | 101 | 126 | 10 | 116 | Other polymorphisms investigated: 5HTR2A 102 T>C (rs6313) |
| Marziniak, 2005 (13) | Germany | clinic | mixed | 115 | 197 | 96 | 101 | |
| Borroni, 2005 (12) | Italy | clinic | mixed | 105 | 144 | 52 | 92 | |
| Kim, 2005 (14) | Korea | clinic | mixed | 170 | 52 | ---- | ---- | No association of any genotype with intensity or frequency of migraine or aura. |
| Park, 2006 (16) | Korea | clinic | women | 100 | ---- | ---- | 97 | Other polymorphisms investigated: SLC6A4 VNTR in intron 2. |
| Todt, 2006 (18) | Germany | NS | mixed | 506 | ---- | 472 | ---- | |
| Szilagyi, 2006 (17) | Hungary | clinic | mixed | 464 | 87 | 38 | 49 |
Other polymorphisms investigated: SLC6A4 VNTR in intron 2. Cases were children and adolescents, controls high school and university students. |
| Gonda, 2007 (10) | Hungary | clinic | women | 52 | 45 | ---- | ---- | |
| Total number of subjects | 1,883 | 854 | 668 | 455 | ||||
MA: migraine with aura; MO: migraine without aura; NS: not specified
The allele and genotype frequencies for the SLC6A4 5-HTTLPR polymorphism for migraineurs and controls in each of the studies are summarized in Table 2.
Table 3 summarizes for each of the studies the p-value for the HWE in the controls as well as ORs (95% CI) for the association between the SLC6A4 5-HTTLPR polymorphism and migraine assuming additive, dominant, and recessive genetic models. Genotype distributions in all control populations were in HWE. Table 4 summarizes the pooled ORs (95% CI), measures of heterogeneity, and publication bias.
Table 3.
Hardy-Weinberg Equilibrium and odds ratios (95% confidence intervals) for the association between the SLC6A4 5-HTTLPR polymorphism and migraine
| Author | Disease status | Study size | HWE* | Additive Model OR (95% CI) | p value | Dominant Model OR (95% CI) | p value | Recessive Model OR (95% CI) | p value |
|---|---|---|---|---|---|---|---|---|---|
| Yilmaz, 2001 (20) | controls | 80 | 0.12 | Referent | Referent | Referent | |||
| any migraine | 52 | ---- | 1.03 (0.66–1.63) | 0.89 | 1.02 (0.48–2.18) | 0.95 | 1.07 (0.49–2.32) | 0.87 | |
| Kotani, 2002 (15) | controls | 190 | 0.68 | Referent | Referent | Referent | |||
| any migraine | 151 | ---- | 1.06 (0.74–1.52) | 0.75 | 1.26 (0.48–3.34) | 0.64 | 1.04 (0.67–1.61) | 0.86 | |
| Juhasz, 2003 (11) | controls | 101 | 0.67 | Referent | Referent | Referent | |||
| any migraine | 126 | ---- | 1.45 (1.00–2.12) | 0.05 | 1.77 (1.00–3.13) | 0.05 | 1.48 (0.76–2.88) | 0.25 | |
| MA | 10 | ---- | 1.49 (0.61–3.64) | 0.39 | 1.41 (0.34–5.77) | 0.64 | 2.12 (0.50–9.02) | 0.31 | |
| MO | 116 | ---- | 1.45 (1.00–2.14) | 0.06 | 1.81 (1.01–3.24) | 0.05 | 1.43 (0.72–2.82) | 0.30 | |
| Marziniak, 2005 (13) | controls | 115 | 1 | Referent | Referent | Referent | |||
| any migraine | 197 | ---- | 1.16 (0.84–1.60) | 0.37 | 1.10 (0.67–1.80) | 0.70 | 1.40 (0.79–2.49) | 0.25 | |
| MA | 96 | ---- | 1.70 (1.16–2.50) | 0.01 | 1.88 (1.00–3.51) | 0.05 | 2.24 (1.19–4.23) | 0.01 | |
| MO | 101 | ---- | 0.80 (0.55–1.17) | 0.25 | 0.72 (0.41–1.26) | 0.25 | 0.78 (0.38–1.61) | 0.50 | |
| Borroni, 2005 (12) | controls | 105 | 0.70 | Referent | Referent | Referent | |||
| any migraine | 144 | ---- | 1.00 (0.71–1.41) | 0.99 | 0.78 (0.45–1.33) | 0.35 | 1.40 (0.75–2.62) | 0.29 | |
| MA | 52 | ---- | 1.49 (0.93–2.38) | 0.10 | 1.32 (0.62–2.79) | 0.48 | 2.20 (1.03–4.72) | 0.04 | |
| MO | 92 | ---- | 0.80 (0.54–1.18) | 0.26 | 0.60 (0.33–1.07) | 0.08 | 1.03 (0.50–2.12) | 0.94 | |
| Kim, 2005 (14) | controls | 170 | 0.83 | Referent | Referent | Referent | |||
| any migraine | 52 | ---- | 1.37 (0.79–2.38) | 0.27 | 1.40 (0.29–6.68) | 0.68 | 1.46 (0.76–2.78) | 0.26 | |
| Park, 2006 (16) | controls | 100 | 0.23 | Referent | Referent | Referent | |||
| MO | 97 | ---- | 0.96 (0.58–1.59) | 0.87 | 0.48 (0.09–2.65) | 0.40 | 1.03 (0.59–1.83) | 0.91 | |
| Todt, 2006 (18) | controls | 506 | 1 | Referent | Referent | Referent | |||
| MA | 472 | ---- | 0.90 (0.75–1.08) | 0.26 | 0.89 (0.69–1.16) | 0.39 | 0.83 (0.58–1.19) | 0.30 | |
| Szilagyi, 2006 (17) | controls | 464 | 0.06 | Referent | Referent | Referent | |||
| any migraine | 87 | ---- | 0.88 (0.64–1.21) | 0.44 | 0.88 (0.55–1.40) | 0.58 | 0.79 (0.43–1.46) | 0.44 | |
| MA | 38 | ---- | 0.87 (0.55–1.38) | 0.55 | 0.77 (0.40–1.51) | 0.45 | 0.93 (0.40–2.17) | 0.86 | |
| MO | 49 | ---- | 0.90 (0.59–1.34) | 0.58 | 0.97 (0.53–1.78) | 0.92 | 0.68 (0.30–1.57) | 0.37 | |
| Gonda, 2007 (10) | controls | 52 | 0.77 | Referent | Referent | Referent | |||
| any migraine | 45 | ---- | 2.25 (1.20–4.25) | 0.01 | 2.89 (1.12–7.45) | 0.03 | 2.79 (0.95–8.19) | 0.06 | |
MA: migraine with aura; MO: migraine without aura
p-value for Hardy-Weinberg Equilibrium.
Table 4.
Association between the SLC6A4 5-HTTLPR polymorphisms and migraine, heterogeneity, and publication bias
| Any migraine | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Genetic model | Population | No of studies | Odds ratio (95% CI) | Heterogeneity | Publication Bias | ||||
| Q | df | p-value | I2 in % | p-value Begg | p-value Egger | ||||
| additive | Europeans (10–13, 17, 20) | 6 | 1.16 (0.93–1.43) | 9.3 | 5 | 0.10 | 46.1 | 0.19 | 0.11 |
| European women (10, 11) | 2 | 1.68 (1.12–2.51) | 1.4 | 1 | 0.24 | 26.5 | 0.32 | --- | |
| Asians (14, 15) | 2 | 1.14 (0.85–1.55) | 0.6 | 1 | 0.45 | 0 | 0.32 | --- | |
| dominant | Europeans (10–13, 17, 20) | 6 | 1.16 (0.83–1.61) | 9.2 | 5 | 0.10 | 45.9 | 0.19 | 0.18 |
| European women (10, 11) | 2 | 2.02 (1.24–3.28) | 0.8 | 1 | 0.39 | 0 | 0.32 | --- | |
| Asians (14, 15) | 2 | 1.30 (0.57–2.97) | 0.01 | 1 | 0.92 | 0 | 0.32 | --- | |
| recessive | Europeans (10–13, 17, 20) | 6 | 1.27 (0.96–1.68) | 5.0 | 5 | 0.42 | 0 | 0.35 | 0.27 |
| European women (10, 11) | 2 | 1.76 (1.00–3.10) | 1.0 | 1 | 0.33 | 0 | 0.32 | --- | |
| Asians (14, 15) | 2 | 1.16 (0.81–1.66) | 0.7 | 1 | 0.40 | 0 | 0.32 | --- | |
| Migraine with aura* | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Genetic model | Population | No of studies | Odds ratio (95% CI) | Heterogeneity | Publication Bias | ||||
| Q | df | p-value | I2 in % | p-value Begg | p-value Egger | ||||
| additive | Europeans (11–13, 17, 18) | 5 | 1.19 (0.86–1.63) | 12.0 | 4 | 0.02 | 66.6 | 0.62 | 0.25 |
| dominant | Europeans (11–13, 17, 18) | 5 | 1.08 (0.78–1.50) | 5.9 | 4 | 0.21 | 32.1 | 0.62 | 0.33 |
| recessive | Europeans (11–13, 17, 18) | 5 | 1.41 (0.83–2.40) | 11.1 | 4 | 0.03 | 63.9 | 1.00 | 0.24 |
| Migraine without aura* | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Genetic model | Population | No of studies | Odds ratio (95% CI) | Heterogeneity | Publication Bias | ||||
| Q | df | p-value | I2 in % | p-value Begg | p-value Egger | ||||
| additive | Europeans (11–13, 17) | 4 | 0.95 (0.72–1.27) | 6.3 | 3 | 0.10 | 52.1 | 0.50 | 0.74 |
| Asians (16) | 1 | 0.96 (0.58–1.59) | --- | --- | --- | --- | --- | --- | |
| dominant | Europeans (11–13, 17) | 4 | 0.93 (0.58–1.50) | 8.0 | 3 | 0.05 | 62.7 | 1.00 | 0.82 |
| Asians (16) | 1 | 0.48 (0.09–2.65) | --- | --- | --- | --- | --- | --- | |
| recessive | Europeans (11–13, 17) | 4 | 0.97 (0.67–1.41) | 2.3 | 3 | 0.52 | 0 | 0.04 | 0.17 |
| Asians (16) | 1 | 1.03 (0.59–1.83) | --- | --- | --- | --- | --- | --- | |
There is no study available looking at the association between the SLC6A4 5-HTTLPR polymorphism and migraine with aura among Asian populations.
Association between the SLC6A4 5-HTTLPR polymorphism and migraine
Four of the ten studies suggested an increased risk for migraine among carriers of the S allele (Table 3) (10–13). In two studies among European women this occurred for any migraine and appeared most pronounced under a dominant model (10, 11), while in the other two among mixed populations this appeared only for MA and seemed to follow a recessive model (12, 13).
Our overall pooled analyses do not suggest an association between the SLC6A4 5-HTTLPR polymorphism and any migraine (Table 4). The pooled OR (95% CI) from an additive model for any migraine was 1.16 (0.93–1.43) among Europeans (10–13, 17, 20) and 1.14 (0.85–1.55) among Asians (14, 15). This did not change when assuming dominant or recessive models of inheritance. When we analyzed the subgroup of European women, however, the risk for any migraine appeared to be significantly increased, which was most pronounced assuming a dominant model (dominant model: pooled OR=2.02; 95% CI 1.24–3.28).
The studies looking at MA were all performed among European populations (11–13, 17, 18). The pooled effect estimates suggested an increased risk for carriers of the S/S genotype, however, this did not become statistically significant (recessive model: pooled OR=1.41; 95% CI 0.83–2.40). There was medium heterogeneity among these studies (recessive mode: I2=63.9%).
For MO neither the studies among Europeans (11–13, 17) nor the study in an Asian population (16) suggested an association with the SLC6A4 5-HTTLPR polymorphism. Formal investigation using Begg’s test suggested some publication bias when assuming a recessive model among Europeans for MO (p=0.04), however, Egger’s test did not. Neither test suggested publication bias for any of the outcomes or for any of the other models.
Sensitivity analyses
When we repeated the analysis on the association between the SLC6A4 5-HTTLPR polymorphism and any migraine, excluding the study in the Turkish population (20), the results among the European populations were very similar to the overall results (additive model: pooled OR=1.19; 95% CI 0.92–1.53).
For some of our analyses, Galbraith plots identified individual studies as important sources of heterogeneity. Hence, we performed sensitivity analyses by excluding studies that fell outside the margin set by the z score ±2 standard deviations.
For all analyses the effect estimates were lower and none indicated a statistically significant association. For example, for the association with any migraine the pooled OR (95% CI) from the additive model was 1.08 (0.91–1.27) after excluding one study (10). Likewise, for the association with MA among Europeans the pooled OR (95% CI) from the recessive model was 1.20 (0.70–2.07) after excluding one study (13).
Discussion
The overall results from this meta-analysis do not indicate an association between the SLC6A4 5-HTTLPR polymorphism and migraine among European and Asian populations. Studies among European women, however, indicate a twofold increased risk for any migraine for carriers of the S allele. The association with MA has only been investigated in European populations. While the pooled results suggested a 40% increased risk for MA among carriers of the S/S genotype compared to the other genotypes, these results were not statistically significant and there was remaining heterogeneity among studies.
Family and twin studies have clearly established a genetic component in migraine with a heritability of up to 50% (36). While mutations in individual genes have clearly been implicated in rare migraine forms such as familial hemiplegic migraine, it has been notoriously difficult to identify genetic markers for common forms of migraine (37).
The importance of serotonin in migraine has been acknowledged for some decades (3) and a dysfunction in the serotonergic system agrees with both pathophysiological models of a cortical dysbalance (38) as well as treatment success of triptans, which are serotonin receptor agonists (39). Among the main features in migraineurs are alterations in serotonin metabolism and in the processing of central serotonin-mediated responses compared to non-migraineurs (3). Since the SLC6A4 5-HTTLPR polymorphism determines serotonin levels with carriers of the S allele having impaired transporter activities (9), it was hypothesized that this variant may also be a marker for migraine. Despite biological plausibility, however, individual studies on the association between the SLC6A4 5-HTTLPR polymorphism and migraine came to conflicting results and our meta-analysis does not support an association. Results from our overall analysis are in agreement with two family-based studies. One used linkage analysis and did not find significant linkage of markers in the SLC6A4 gene region with migraine (33). The other employed the transmission disequilibrium test and did not find association of any of the 5-HTTLPR genotypes with overall migraine, MA, or MO (31). There are two possible explanations for the negative results. Either there may be truly no association and the positive studies may have occurred by chance or the pattern of association may be more complex and may depend on additional factors. Our results support the latter notion.
In this respect the following limitations of our meta-analysis need to be considered. First, migraine is a complex, heterogeneous disorder with a wide clinical spectrum (37). Hence, patients with different clinical phenotypes are summarized as having migraine and even strict classification according to IHS criteria (24, 25) may not sufficiently capture this variability and may be a source of misclassification in all studies. For example, the SLC6A4 5-HTTLPR polymorphism may not simply be associated with any migraine, but with attack frequency, as suggested by one study (15). Further, the association may be different for MA and MO. There is some indication for this from our pooled analyses, since the risk for MA may be increased by 40% for carriers of the S/S genotype. However, this was not statistically significant, possibly due to low sample size, and there was medium heterogeneity (I2=63.9%) leaving residual uncertainties. Moreover, the SLC6A4 5-HTTLPR polymorphism may be associated with early age of migraine onset. In one of the studies migraineurs were children and adolescents (17), however, this study did not find an association. Second, in addition to the 5-HTTLPR polymorphism another common polymorphism in the SLC6A4 gene, a variable number tandem repeat in intron 2 (termed STin2 VNTR), has been investigated with regard to its association with migraine, the results being likewise controversial (32, 34). The structural organization of the STin2 VNTR polymorphism is more complex than that of the 5-HTTLPR polymorphism, since it consists of three alleles and five genotypes. This does not easily allow employing genetic models (additive, dominant, recessive) based on one degree of freedom tests; hence, we did not to include it in our present analysis. Third, SLC6A4 is a large polymorphic gene comprising 37,810 bp organized in 15 exons and 14 introns with 380 single nucleotide polymorphisms (SNPs) reported (40, 41), which in addition to the SLC6A4 5-HTTLPR and STin2 VNTR polymorphisms may affect function of the transporter. Fourth, the traditional concept of the SLC6A4 5-HTTLPR polymorphism as being biallelic, with the S allele being associated with a lower number of serotonin transporters compared with carriers of the L/L genotype (9) has been challenged. Recent data suggest the existence of a low-frequency LG-allele variant, harboring an A>G SNP in its sequence, whose functional properties may be similar to the A allele (42, 43). However, this additional genotypic information was not available for any of the studies included in our analyses; hence, we could not investigate an association with migraine. Fifth, serotonin’s role in migraine is not just determined by transporter activity, but also by serotonin’s functional effect at the receptor level, which is likewise under genetic control and unaccounted for in all the studies. Sixth, although serotonin appears to be pivotal, other neurotransmitters like dopamine, orexin, and glutamate are also important in migraine pathophysiology (44). Hence, migraine susceptibility is likely a function of the interaction between various neurotransmitters, which again is determined by the interaction of various genetic variants. Seventh, migraine susceptibility is different between women and men, as indicated by the higher prevalence among women (45). Our results suggest that this may also be the case with regard to the SLC6A4 5-HTTLPR polymorphism, as European women carrying the S allele have a twofold increased risk for any migraine. Unfortunately, only three studies (10, 11, 16) were conducted in women and all other studies only reported results for mixed gender populations. Eighth, associations with migraine may differ by ethnicity, especially since the genotype distribution of the SLC6A4 5-HTTLPR polymorphism shows ethnic differences (21, 22). We have acknowledged this in our analysis by stratifying studies according to country of origin. However, studies are scarce among Asians and unavailable from other populations of non-European descent. Hence, in particular with regard to MA, a potential link with the SLC6A4 5-HTTLPR polymorphism remains unclear. Finally, we only used extractable data from the papers. However, only one paper did not provide genotypic information (19). The authors reported no association of the SLC6A4 5-HTTLPR polymorphism and migraine; hence, this would likely not have changed our results.
Additional research appears warranted to further delineate the association between the SLC6A4 5-HTTLPR polymorphism and migraine, in particular among women and in MA. Further, studies in non-European populations are scarce and may yield different results. Future studies need to be adequately powered, should use standardized migraine classification including aura status, and should also present results stratified by gender and migraine aura status. Further, gene-gene interactions should be investigated, even if individual gene variants do not suggest an association with migraine. Finally, other than migraine status, age at onset or markers of migraine severity including attack frequency and aura frequency may be better outcomes.
Acknowledgments
Funding and Support
There was no specific funding to conduct this study.
Footnotes
Full Disclosures for the last 2 years
Dr. Schürks has received an investigator-initiated research grant from the Migraine Research Foundation and honoraria from L.E.K. Consulting for telephone surveys. P. Rist has no disclosures.
Dr. Kurth has received investigator-initiated research funding from the French National Research Agency, the National Institutes of Health, Merck, and the Migraine Research Foundation. Further, he is a consultant to i3 Drug Safety and World Health Information Science Consultants, LLC; he has received honoraria from Genzyme, Merck, and Pfizer for educational lectures.
References
- 1.Haut SR, Bigal ME, Lipton RB. Chronic disorders with episodic manifestations: focus on epilepsy and migraine. Lancet Neurol. 2006;5:148–57. doi: 10.1016/S1474-4422(06)70348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pietrobon D, Striessnig J. Neurobiology of migraine. Nat Rev Neurosci. 2003;4:386–98. doi: 10.1038/nrn1102. [DOI] [PubMed] [Google Scholar]
- 3.Hamel E. Serotonin and migraine: biology and clinical implications. Cephalalgia. 2007;27:1293–300. doi: 10.1111/j.1468-2982.2007.01476.x. [DOI] [PubMed] [Google Scholar]
- 4.Rudnick G, Clark J. From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. Biochimica et Biophysica Acta. 1993;1144:249–63. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- 5.Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, et al. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:2542–6. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hornung JP. The human raphe nuclei and the serotonergic system. Journal of Chemical Neuroanatomy. 2003;26:331–43. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Heils A, Teufel A, Petri S, Seemann M, Bengel D, Balling U, et al. Functional promoter and polyadenylation site mapping of the human serotonin (5-HT) transporter gene. Journal of Neural Transmission - General Section. 1995;102:247–54. doi: 10.1007/BF01281159. [DOI] [PubMed] [Google Scholar]
- 8.Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. Journal of Neurochemistry. 1996;66:2621–4. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 9.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 10.Gonda X, Rihmer Z, Juhasz G, Zsombok T, Bagdy G. High anxiety and migraine are associated with the s allele of the 5HTTLPR gene polymorphism. Psychiatry Res. 2007;149:261–6. doi: 10.1016/j.psychres.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Juhasz G, Zsombok T, Laszik A, Gonda X, Sotonyi P, Faludi G, Bagdy G. Association analysis of 5-HTTLPR variants, 5-HT2a receptor gene 102T/C polymorphism and migraine. J Neurogenet. 2003;17:231–40. [PubMed] [Google Scholar]
- 12.Borroni B, Brambilla C, Liberini P, Rao R, Archetti S, Gipponi S, et al. Functional serotonin 5-HTTLPR polymorphism is a risk factor for migraine with aura. J Headache Pain. 2005;6:182–4. doi: 10.1007/s10194-005-0179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marziniak M, Mossner R, Schmitt A, Lesch KP, Sommer C. A functional serotonin transporter gene polymorphism is associated with migraine with aura. Neurology. 2005;64:157–9. doi: 10.1212/01.WNL.0000148597.52312.9E. [DOI] [PubMed] [Google Scholar]
- 14.Kim WK, Kim HS, Kim WJ, Lee KY, Park H, Kim CH, et al. Serotonin transporter gene polymorphism and migraine in the Korean population. Headache. 2005;45:1056–60. doi: 10.1111/j.1526-4610.2005.05187.x. [DOI] [PubMed] [Google Scholar]
- 15.Kotani K, Shimomura T, Shimomura F, Ikawa S, Nanba E. A polymorphism in the serotonin transporter gene regulatory region and frequency of migraine attacks. Headache. 2002;42:893–5. doi: 10.1046/j.1526-4610.2002.02209.x. [DOI] [PubMed] [Google Scholar]
- 16.Park JW, Han SR, Yang DW, Kim YI, Lee KS. Serotonin transporter protein polymorphism and harm avoidance personality in migraine without aura. Headache. 2006;46:991–6. doi: 10.1111/j.1526-4610.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- 17.Szilagyi A, Boor K, Orosz I, Szantai E, Szekely A, Kalasz H, et al. Contribution of serotonin transporter gene polymorphisms to pediatric migraine. Headache. 2006;46:478–85. doi: 10.1111/j.1526-4610.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- 18.Todt U, Freudenberg J, Goebel I, Heinze A, Heinze-Kuhn K, Rietschel M, et al. Variation of the serotonin transporter gene SLC6A4 in the susceptibility to migraine with aura. Neurology. 2006;67:1707–9. doi: 10.1212/01.wnl.0000242883.96822.93. [DOI] [PubMed] [Google Scholar]
- 19.Wieser T, Dresler K, Evers S, Gaul C, König D, Hölzl D, et al. No influence of 5-HTTLPR gene polymorphism on migraine symptomatology, comorbid depression, and chronification. Headache. 2009 doi: 10.1111/j.1526-4610.2009.01428.x. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz M, Erdal ME, Herken H, Cataloluk O, Barlas O, Bayazit YA. Significance of serotonin transporter gene polymorphism in migraine. J Neurol Sci. 2001;186:27–30. doi: 10.1016/s0022-510x(01)00491-9. [DOI] [PubMed] [Google Scholar]
- 21.Gelernter J, Cubells JF, Kidd JR, Pakstis AJ, Kidd KK. Population studies of polymorphisms of the serotonin transporter protein gene. American Journal of Medical Genetics. 1999;88:61–6. [PubMed] [Google Scholar]
- 22.Kunugi H, Hattori M, Kato T, Tatsumi M, Sakai T, Sasaki T, et al. Serotonin transporter gene polymorphisms: ethnic difference and possible association with bipolar affective disorder. Molecular Psychiatry. 1997;2:457–62. doi: 10.1038/sj.mp.4000334. [DOI] [PubMed] [Google Scholar]
- 23.Sagoo GS, Little J, Higgins JP. Systematic reviews of genetic association studies. PLoS Med. 2009;6:e28. doi: 10.1371/journal.pmed.1000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The International Classification of Headache Disorders. 2. Suppl 1. Vol. 24. Cephalalgia: 2004. pp. 9–160. [DOI] [PubMed] [Google Scholar]
- 25.Headache Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8 (Suppl 7):1–96. [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corominas R, Sobrido MJ, Ribasés M, Cuenca-León E, Blanco-Arias P, Narberhaus B, et al. Association study of the serotoninergic system in migraine in the spanish population. Am J Med Genet B Neuropsychiatr Genet. 2009 doi: 10.1002/ajmg.b.30972. [DOI] [PubMed] [Google Scholar]
- 30.Juhasz G, Zsombok T, Laszik A, Jakus R, Faludi G, Sotonyi P, Bagdy G. Despite the general correlation of the serotonin transporter gene regulatory region polymorphism (5-HTTLPR) and platelet serotonin concentration, lower platelet serotonin concentration in migraine patients is independent of the 5-HTTLPR variants. Neuroscience Letters. 2003;350:56–60. doi: 10.1016/s0304-3940(03)00834-6. [DOI] [PubMed] [Google Scholar]
- 31.Karwautz AF, Campos de Sousa S, Wober C, Wagner G, Li T, Konrad A, et al. Family-based analysis of serotonin transporter gene polymorphisms in migraine with and without aura. Cephalalgia. 2007;27:773–80. doi: 10.1111/j.1468-2982.2007.01344.x. [DOI] [PubMed] [Google Scholar]
- 32.Lea RA, Dohy A, Jordan K, Quinlan S, Brimage PJ, Griffiths LR. Evidence for allelic association of the dopamine beta-hydroxylase gene (DBH) with susceptibility to typical migraine. Neurogenetics. 2000;3:35–40. doi: 10.1007/pl00022977. [DOI] [PubMed] [Google Scholar]
- 33.Monari L, Mochi M, Valentino ML, Arnaldi C, Cortelli P, De Monte A, et al. Searching for migraine genes: exclusion of 290 cM out of the whole human genome. Italian Journal of Neurological Sciences. 1997;18:277–82. doi: 10.1007/BF02083304. [DOI] [PubMed] [Google Scholar]
- 34.Ogilvie AD, Russell MB, Dhall P, Battersby S, Ulrich V, Smith CA, et al. Altered allelic distributions of the serotonin transporter gene in migraine without aura and migraine with aura. Cephalalgia. 1998;18:23–6. doi: 10.1046/j.1468-2982.1998.1801023.x. [DOI] [PubMed] [Google Scholar]
- 35.Racchi M, Leone M, Porrello E, Rigamonti A, Govoni S, Sironi M, et al. Familial migraine with aura: association study with 5-HT1B/1D, 5-HT2C, and hSERT polymorphisms. Headache. 2004;44:311–7. doi: 10.1111/j.1526-4610.2004.04072.x. [DOI] [PubMed] [Google Scholar]
- 36.Kors EE, Vanmolkot KR, Haan J, Frants RR, van den Maagdenberg AM, Ferrari MD. Recent findings in headache genetics. Curr Opin Neurol. 2004;17:283–8. doi: 10.1097/00019052-200406000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Wessman M, Terwindt GM, Kaunisto MA, Palotie A, Ophoff RA. Migraine: a complex genetic disorder. Lancet Neurol. 2007;6:521–32. doi: 10.1016/S1474-4422(07)70126-6. [DOI] [PubMed] [Google Scholar]
- 38.Coppola G, Pierelli F, Schoenen J. Is the cerebral cortex hyperexcitable or hyperresponsive in migraine? Cephalalgia. 2007;27:1427–39. doi: 10.1111/j.1468-2982.2007.01500.x. [DOI] [PubMed] [Google Scholar]
- 39.Humphrey PP. The discovery of a new drug class for the acute treatment of migraine. Headache. 2007;47 (Suppl 1):S10–9. doi: 10.1111/j.1526-4610.2007.00672.x. [DOI] [PubMed] [Google Scholar]
- 40.http://genome.ucsc.edu/
- 41.http://www.ncbi.nlm.nih.gov/projects/SNP/
- 42.Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–26. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zalsman G, Huang YY, Oquendo MA, Burke AK, Hu XZ, Brent DA, et al. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry. 2006;163:1588–93. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]
- 44.Goadsby PJ, Charbit AR, Andreou AP, Akerman S, Holland PR. Neurobiology of migraine. Neuroscience. 2009;161:327–41. doi: 10.1016/j.neuroscience.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 45.Lipton RB, Bigal ME. The epidemiology of migraine. Am J Med. 2005;118 (Suppl 1):3S–10S. doi: 10.1016/j.amjmed.2005.01.014. [DOI] [PubMed] [Google Scholar]