Abstract
Background
Growth hormone replacement therapy is more effective the earlier it is begun. This article adresses the question whether children with growth hormone deficiency (GHD) were able to begin treatment earlier through the use of the CrescNet system in the Department of Pediatrics at the Leipzig University Hospital. CrescNet is a network of pediatricians and endocrinological treatment centers, established in Leipzig in 1998, whose aim is to improve the early detection of growth disorders.
Methods
Pediatricians participating in CrescNet provided anonymized data on their patients’ height and weight to the CrescNet database. Each participating pediatrician received a quarterly screening report with recommendations for the work-up of children with abnormal growth. Some patients with GHD who were treated in the Leipzig treatment center were referred in response to these recommendations, while others came spontaneously from the practices of pediatricians participating or not participating in CrescNet. We determined the age at the onset of treatment for the 139 patients treated for GHD in the University Children’s Hospital Leipzig from 1999 to 2005 and compared the findings with data from other treatment centers in Germany.
Results
Patients from CrescNet practices began treatment at a younger age than those from non-CrescNet practices (8.77 ± 3.40 versus 9.50 ± 3.78 years, p = 0.26). Patients from CrescNet practices whose GHD was detected by screening began treatment earlier than those for whom no data had been sent (7.67 ± 3.21 versus 9.28 ± 3.39 years, p = 0.031). In the center in Leipzig, but not in other German GHD treatment centers, the age at onset of treatment dropped significantly over the period of the study and then remained steady till 2009 in the range of 7.61 ± 3.0 years.
Conclusion
These descriptive results imply that the linking of pediatricians’ practices with the CrescNet system resulted in earlier treatment of children with GHD.
In all practices that care for children (pediatricians, general practitioners, and others) height and weight are regularly measured. From a statistical point of view, 3% of children are short statured and 3% are tall statured, that is, they are auxologically abnormal. This includes children with classic growth hormone deficiency (GHD). The prevalence is 1:6000 children, meaning that GHD is relatively rare (1). Unfortunately, any additional diagnostic steps and any necessary therapeutic steps for auxologically abnormal children are delayed or are not taken at all. This is all the more alarming because the period in which pediatric growth disorders can be treated is limited. It is also well documented in the literature that treatment of children with growth hormone deficiency is more successful the earlier the substitution therapy with human growth hormone (hGH) is started (2– 7). It is therefore essential that patients requiring treatment are detected and therapy initiated as soon as possible. The CrescNet system was introduced in 1998 in the University Children’s Hospital Leipzig after several years of development with the aim of early detection of disorders of growth and weight development. The background was a resolution passed by the State Medical Association of Saxony in 1992 in which it was recommended that chronically ill children receive joint medical care from pediatricians in private practice or family physicians and a specialist in a treatment center. The structure and function of CrescNet have since been described several times from different aspects (8– 16). It is apparent from this that CrescNet carries weight across a large group of doctors and allows a diverse range of clinical questions to be examined. In 2006, the project was converted to a not-for-profit limited liability company (CrescNet gGmbH) based in Leipzig. The CrescNet network currently (March 2010) includes 323 medical practices and 22 pediatric endocrinology treatment centers from across Germany. It is, therefore, a very large association of doctors.
The following descriptive analysis concentrates on the question of whether this system has achieved its goal of initiating therapy for patients with GHD earlier than in the past. Anthropometric data are not considered because they are not relevant for answering this question.
Patients and methods
Partners in CrescNet can be any pediatrician and any family physician who provides basic care for children and adolescents. Measurements of height and weight are done by the participating doctors under standardized conditions. They are provided with uniform precision measuring instruments for height (17), funded by the project, and are trained in the measuring technique. The collected data are pseudonymized and sent for central analysis to the database which is maintained and administered by CrescNet gGmbH. All measurements are subject to plausibility checks.
Each CrescNet practice receives a screening report prepared by the responsible endocrinologists at about 3-monthly intervals from the start of their involvement to the present. This enables detection of any abnormalities in growth and weight development of the children and recommendations to be made on further actions to the pediatricians providing care. The pediatrician is either requested to provide any missing data, to take subsequent measurements at shorter intervals or, in cases of serious abnormalities, to immediately refer the child to a pediatric endocrinologist in a treatment center for an assessment. Patients were also referred from CrescNet practices if their growth and development data had not previously been sent to the CrescNet database. Patients also came from practices that are not associated with CrescNet.
The descriptive analysis covers the period from 1 January 1999 to 31 December 2005. The data of all children presenting to the University Children’s Hospital Leipzig with suspected growth disorders were retrospectively analyzed. Only those patients with a verified growth hormone deficiency were then selected for the present study. The inclusion criterion was a growth hormone peak of <10 µg/mL in two stimulation tests.
In the Leipzig center 139 patients with GHD who had been newly placed on therapy could be included in the study over the course of the study. The following information was collected from the patients for the analysis: firstly, the age at the start of therapy, secondly, whether the referring practice was a partner in the CrescNet system or not, and thirdly, whether GHD in a patient in a CrescNet practice was detected primarily by screening of the CrescNet data or not.
Three infants were not included in the study whose growth hormone deficiency was verified and therapy initiated in the first year of life (mean age 0.59 ± 0.21 years) not due to a manifest growth disorder but to either hypoglycemia or indications of a familial genetic disorder. Nine patients with brain tumors (craniopharyngioma, medulloblastoma, neurofibromatosis) were also not included because most of them had been referred either only postoperatively from oncological treatment sites or for a second assessment by other pediatric endocrinologists (mean age: 10.92 ± 3.62 years).
To examine the question of whether any reduction in the age at the start of treatment could otherwise be observed over this period, the authors collected corresponding data from other treatment centers in Germany which have had a supraregional catchment area for many years and are specialist pediatric endocrinology centers and which were prepared to make their patient data available for comparisons. Centers in the former West German states and the former East German states were differentiated.
For the statistical tests we used the two-tailed t test, chi-square test (Tables 1 and 3) and linear regression (least squares method) (Figures 1 and 2). p values below 0.05 were considered statistically significant. A check of multiple errors was not planned because this is a descriptive study. SD is the standard deviation from the mean. Deviations from age- and sex-specific reference values were expressed as the SDS (standard deviation score, z-score).
Table 1. Mean age at the start of therapy ± SD (in brackets: number of patients).
| GHD total (139) | |
| Not a CrescNet partner | 9.50 ± 3.78 (44) |
| p = 0.26 | |
| CrescNet partner | 8.77 ± 3.40 (95) |
| Patients without screening | 9.28 ± 3.39 (65) |
| p = 0.031 | |
| Patients after screening | 7.67 ± 3.21 (30) |
The patients from non-CrescNet partners began therapy later than those from CrescNet partners. The age at which therapy was started for patients detected by screening from practices of CrescNet partners was significantly lower than that of patients not detected by screening. SD, standard deviation; GHD, growth hormone deficiency
Table 3. Comparison of mean age at the start of therapy ± SD in the various treatment centers.
| Center | n | Therapy age (years) | p (t test) | |
| for 1a | for 1b | |||
| 1a former East German states, Leipzig total | 139 | 9.04 ± 3.51 | — | — |
| 1b former East German states, Leipzig after screening | 30 | 7.67 ± 3.21 | — | — |
| 2 former East German states | 72 | 10.63 ± 4.18 | 0.004 | <0.001 |
| 3 former East German states | 59 | 10.36 ± 4.01 | 0.023 | 0.002 |
| 4 former East German states | 16 | 10.17 ± 3.14 | 0.22 | 0.015 |
| 5 former East German states (2001–2005) | 20 | 8.84 ± 4.60 | 0.82 | 0.29 |
| 6 former West German states | 449 | 10.67 ± 2.99 | 0 | 0 |
| 7 former West German states | 246 | 7.69 ± 3.67 | <0.001 | 0.97 |
| 8 former West German states | 43 | 10.55 ± 3.27 | 0.013 | < 0.001 |
| 9 former West German states (1999–2004) | 14 | 7.27 ± 3.79 | 0.08 | 0.72 |
SD, standard deviation
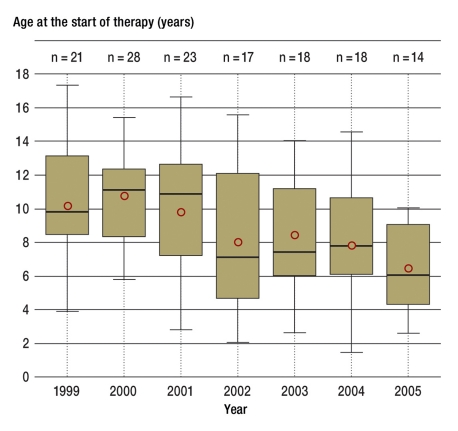
Figure 1.
The box plots show the median values of the age at the start of therapy according to the standards between 1999 and 2005 with the 1st and 3rd quartiles and the highest and lowest individual value; the circle indicates the arithmetic mean. The drop in the median value in the patients with growth hormone deficiency between 1999 and 2005 in the Leipzig center is clear
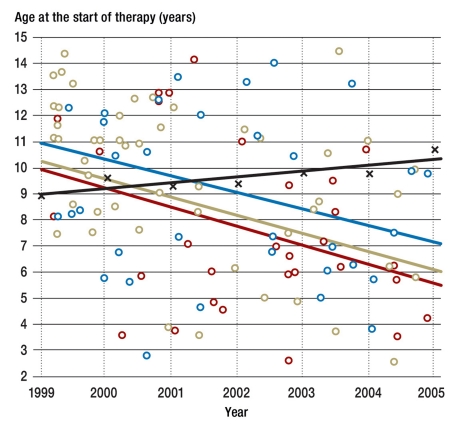
Figure 2.
Drop in the age at the start of therapy in the Leipzig patients o not a CrescNet partner; o CrescNet partner without screening; o CrescNet partner with screening; x weighted mean of the other treatment centers for comparison
Results
Table 1 shows that the patients from CrescNet practices began therapy earlier than those from non-CrescNet practices, although without significant differences (8.77 ± 3.40 versus 9.50 ± 3.78 years; p = 0.26). The differences between patients from CrescNet practices with and without previous screening on the other hand were significant (7.67 ± 3.21 versus 9.28 ± 3.39 years; p = 0.031). The difference between the patients from CrescNet practices with previous screening and patients from non-CrescNet practices was also significant (7.67 ± 3.21 versus 9.50 ± 3.78 years; p = 0.033) This means that these patients began treatment after preceding screening 1.8 years earlier on average.
The box plots in Figure 1 show the mean values with the 1st and 3rd quartile and the highest and lowest individual value of the age at the start of therapy according to the standards from 1999 to 2005. The circle indicates the mean value. The mean age at the start of therapy was initially (1999/2000) approximately 10.4 years and fell to 6.5 years by 2005. This drop in the age at the start of therapy is significant (p<0.001). Figure 2 shows that this trend affected patients from non-CrescNet practices (p = 0.012), patients from CrescNet practices (p<0.001), and patients after previous screening (p = 0.056) to a comparable degree. The black line in Figure 2 depicts the weighted mean of centers 2 to 9. The slight increase is not significant. A drop in the age at which therapy started was not recorded in the centers 2 to 9 in the former West and East German states (Table 2).
Table 2. Mean age at the start of therapy from 1999 to 2005 in comparable centers in which no drop was recorded (in brackets: number of patients; — no patients placed on therapy).
| Center | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 |
| 1a former East German states, Leipzig total | 10.11 (21) | 10.72 (28) | 9.76 (23) | 8.51 (17) | 8.47 (18) | 7.81 (18) | 6.50 (14) |
| 1b former East German states, Leipzig after screening | — | 10.21 (3) | 9.57 (5) | 6.77 (6) | 6.97 (7) | 8.45 (5) | 5.02 (4) |
| 2 former East German states | 7.02 (4) | 10.16 (8) | 9.63 (10) | 12.21 (14) | 13.11 (10) | 10.9 (16) | 8.32 (10) |
| 3 former East German states | 11.22 (17) | 9.75 (12) | 13.86 (10) | 10.38 (6) | 10.59 (4) | 6.11 (8) | 7.02 (3) |
| 4 former East German states | 8.04 (1) | 6.89 (3) | 9.67 (3) | — | 10.37 (3) | 11.3 (3) | 13.24 (3) |
| 5 former East German states (2001–2005) | — | — | 3.75 (4) | 13.17 (1) | 11.33 (4) | 8.16 (7) | 11.54 (4) |
| 6 former West German states | 9.04 (13) | 12.1 (21) | 10.05 (56) | 10.05 (64) | 10.87 (77) | 10.85 (102) | 11.72 (116) |
| 7 former West German states | 8.20 (50) | 8.48 (59) | 7.57 (38) | 7.47 (35) | 6.82 (22) | 6.01 (20) | 7.34 (22) |
| 8 former West German states | 10.40 (10) | 9.86 (2) | 9.64 (8) | 8.76 (7) | 12.58 (3) | 11.14 (4) | 10.02 (9) |
| 9 former West German states (1999–2004) | 6.10 (2) | 6.70 (1) | 4.99 (1) | 4.26 (3) | 12.64 (3) | 6.76 (4) | — |
The mean age of all patients at the start of therapy was significantly higher in most centers than that in Leipzig (Table 3). Only in center 7 of the former West German states was the age at the start of therapy significantly lower compared to group 1a. In group 1b (patients after screening) the age at the start of therapy corresponded to the age in center 7 of the former West German states, although the difference was not significant.
Update
After separation of the CrescNet system from the University Hospital and its change to CrescNet gGmbH, the trend from 2006 to 2009 was as follows: In the Leipzig treatment centers for GHD, a total of 81 patients were newly placed on therapy. The mean age at the start of therapy exhibited slight fluctuations: 2006 = 8.23 ± 2.75 years (n = 21); 2007 = 7.26 ± 2.64 years (n = 22); 2008 = 8.29 ± 3.9 years (n = 10); 2009 = 7.18 ± 3.14 years (n = 28). This amounts to a total of 7.61 ± 3.0 years for this period with a median value of 6.64 years.
Discussion
That the success of substitution therapy with growth hormone is greater the earlier therapy is begun has been demonstrated in a number of publications (2– 7). Blethen et al. take the clearest position on this issue (2). The other authors repeatedly identify the age at the start of therapy as one of the most important predictors of final adult height (3– 7). Despite all the advances over the last two decades in terms of therapy safety and treatment modalities, even patients with growth hormone deficiency, that is, patients with the ’classic’ therapy indication, lag behind the expected target height (7). During the attempt to further improve treatment procedures, prediction models for the response to human growth hormone (hGH) therapy were developed. Using comprehensive patient follow-up observations, it could be demonstrated that only a few clinical parameters are clearly correlated with the treatment outcome such as the severity of the growth hormone (GH) deficiency (measured using the maximal GH values that are reached in a GH stimulation test), the age at the start of the GH therapy, the duration of the hGH substitution, and the sex of the patients (4, 7). Interestingly, in most studies no correlation was found between the adult height and the level of the hGH substitution dose. There was also no correlation between the level of catch-up growth, which is empirically greatest after the start of therapy, and the age at the start of therapy. In total there is a height gain of + 0.2 SDS per year of treatment on average; this is approximately 1.2 cm per year. It is clear from this that the only important clinical parameter that can be influenced by the therapists and improved is the age at the start of therapy.
In the efforts to lower the age at the start of therapy in the CrescNet system, successes could be achieved in the partnerships with the contractually bound medical practices, particularly after previous screening (Table 1). Between 1999 and 2005 the age at the start of therapy dropped significantly by about four years (Figure 1, Table 2). The previously mentioned average gain in height per year of treatment yields an additional gain in final height of about 5 cm. A decrease in the age at the start of therapy was not observed in this period in the treatment centers enlisted for comparison (Figure 2, Table 2).
Overall, the awareness of pediatricians for the problems associated with short stature has certainly increased as a result of their participation in the CrescNet program because the mean age at the start of therapy in the whole group and specifically in the group of patients after screening was significantly below that of most other treatment centers (Table 3). Treatment center 7 in the former West German states has the longest history of treating short-statured children of all the centers, and the center receives predominantly problematic cases that are discovered early from across a very large catchment area. Treatment center 5 of the former East German states with more recent data (2001 to 2005) is situated in Saxony and thanks to CrescNet activities already benefits from the increased awareness of pediatricians to the problem of short stature.
The question of whether the trend towards earlier treatment described also has a favorable cost-benefit effect and a positive effect on final height cannot yet be determined because most patients in this study have not yet reached their final height.
Growth hormone deficiency served as a model disease in this study. Due to its rarity the case numbers were not very large. Additional currently recognized indications for growth hormone therapy include Ullrich-Turner syndrome, previously small for gestational age neonates (SGA) whose growth does not catch up, Prader-Willi syndrome, SHOX gene defects, and chronic renal failure. The CrescNet system is of course also suitable for early detection of other chronic diseases that are associated with disorders of growth and weight development and that do not become apparent predominantly due to other symptoms (such as disorders of pubertal development [18]). Finally, the clinical questions and objectives discussed here are also interesting for doctors in other specialties such as endocrinologists and andrologists.
Summary for practical application
Using the example of classic growth hormone deficiency, the descriptive analysis of data from different points of view indicated that the CrescNet system in the Leipzig treatment center enabled earlier detection of growth disorders than previously. The age at the start of therapy could be significantly decreased. By incorporating additional practices into the CrescNet system this effect should become apparent in all regions of Germany.
Key Messages.
Using the example of growth hormone deficiency, the descriptive analysis shows that the CrescNet system
-allows screening for growth disorders
ensures growth disorders and their development are detected earlier
decreases the age at diagnosis
enables earlier treatment
and an additional gain in final height of approximately 5 cm can be expected.
Acknowledgments
Translated from the original German by language & letters.
The authors are much obliged to the following colleagues for providing patient data: Prof. Jürgen Brämswig, Münster; Dr. Klaus Hartmann, Frankfurt/Main; Prof. Angela Hübner, Dresden; Dr. Alexandra Keller, Leipzig; PD Dr. Klaus Mohnike, Magdeburg; Prof. Michael Ranke and Dr. Roland Schweizer, Tübingen; Dr. Claudia Vilser, Jena; Dr. Christian Vogel, Chemnitz; Prof. Peter Willig, Hamburg.
We thank Ms Mandy Vogel for her help with the statistical calculations.
The not-for-profit organization CrescNet is supported by the following companies with donations in line with its charitable aims: Ferring Arzneimittel GmbH, Ipsen Pharma GmbH, Lilly Deutschland GmbH, Merck Serono GmbH, Novo Nordisk Pharma GmbH, Pfizer Pharma GmbH, Sandoz Pharmaceuticals GmbH.
Special thanks is due to the Leipzig University Hospital for maintaining and securing the CrescNet server in its data center.
Footnotes
Conflict of interest statement
The donor funds are administered according to the not-for-profit status of CrescNet gGmbH. CrescNet gGmbH’s employees declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors. Professor Pfäffle received research funding from Eli Lilly, Ferring, and Pfizer and remuneration for lectures from Eli Lilly, Ferring, NovoNordisk, and Pfizer. He is a consultant to Eli Lilly.
References
- 1.Thomas M, Massa G, Craen M, et al. Prevalance and demographic features of childhood growth hormone deficiency in Belgium during the period 1986-2001. Eur J Endocrinol. 2004;151:67–72. doi: 10.1530/eje.0.1510067. [DOI] [PubMed] [Google Scholar]
- 2.Blethen SL, Baptista J, Kuntze J, Foley T, LaFranchi S, Johanson A. Adult height in growth hormone (GH)-deficient children treated with biosynthetic GH. The Genentech Growth Study Group. J Clin Endocrinol Metab. 1997;82:418–420. doi: 10.1210/jcem.82.2.3734. [DOI] [PubMed] [Google Scholar]
- 3.Cacciari E, Cicognani A, Pirazzoli P, et al. Final height of patients treated for isolated GH deficiency: Examination of 83 patients. Eur J Endocrinol. 1997;137:53–60. doi: 10.1530/eje.0.1370053. [DOI] [PubMed] [Google Scholar]
- 4.Ranke MB, Lindberg A, Chatelain P, et al. Derivation and validation of a mathematical model for predicting the response to exogenous recombinant human growth hormone (GH) in prepubertal children with idiopathic GH deficiency. J Clin Endocrinol Metab. 1999;84:1174–1183. doi: 10.1210/jcem.84.4.5634. [DOI] [PubMed] [Google Scholar]
- 5.Ranke MB, Lindberg A, Cowell CT, et al. Prediction of response to growth hormone treatment in short children born small for gestational age: Analysis of data from KIGS (Pharmacia International Growth Database) J Clin Endocrinol Metab. 2003;88:125–131. doi: 10.1210/jc.2002-020867. [DOI] [PubMed] [Google Scholar]
- 6.Ranke MB, Price DA, Albertsson-Wikland K, Maes M, Lindberg A. Factors determining pubertal growth and final height in growth hormone treatment of idiopathic growth hormone deficiency. Analysis of 195 patients of the Kabi Pharmacia International Growth Study. Horm Res. 1997;48:62–71. doi: 10.1159/000185487. [DOI] [PubMed] [Google Scholar]
- 7.Westphal O, Lindberg A. Final height in Swedish children with idiopathic growth hormone deficiency enrolled in KIGS treated optimally with growth hormone. Acta Paediatr. 2008;97:1698–1706. doi: 10.1111/j.1651-2227.2008.01053.x. [DOI] [PubMed] [Google Scholar]
- 8.Keller E, Burmeister J, Gausche R, Keller A, Hermanussen M, Kiess W. Modellprogramm zur Früherkennung und optimalen Behandlung von Störungen des Wachstums und der körperlichen Entwicklung mit Hilfe eines medizinischen Kompetenznetzwerkes. Z ärztl Fortbild Qual sich (ZaeFQ) 2000;94:695–698. [PubMed] [Google Scholar]
- 9.Kiess W, Gausche R, Keller A, Burmeister J, Willgerodt H, Keller E. Computer guided, population-based screening system for growth disorders (CrescNet) and on-line generation of normative data for growth and development. Horm Res. 2001;56(Suppl 1):59–66. doi: 10.1159/000048137. [DOI] [PubMed] [Google Scholar]
- 10.Keller E, Gausche R, Meigen C, Keller A, Burmeister J, Kiess W. Auxological computer based network for early detection of disorders of growth and weight attainment. J Pediatr Endocrinol Metab. 2002;15:149–156. doi: 10.1515/jpem.2002.15.2.149. [DOI] [PubMed] [Google Scholar]
- 11.Keller E, Gausche R, Hoepffner W, Burmeister J, Meigen C, Kiess W. System „CrescNet“. Erkennung von Störungen des Wachstums und der Gewichtsentwicklung sowie Gewinnung aktueller Entwicklungsdaten. Pädiat Prax. 2004;65:569–579. [Google Scholar]
- 12.Gausche R, Hoepffner W, Keller E. Grundlagen eines Programms zur Adipositasprävention bei Kindern und Jugendlichen. Psychomed. 2005;17:90–93. [Google Scholar]
- 13.Hoepffner W, Gausche R, Meigen C, Keller A, Keller E. Aufbau, klinische Relevanz und Projekte des Wachstumsnetzwerks CrescNet. Ärzteblatt Sachsen. 2007;18:461–463. [Google Scholar]
- 14.Heger S, Körner A, Meigen C, Gausche R, Keller A, Kiess W. Impact of weight status on the onset and parameters of puberty: Analysis of three representative cohorts from central Europe. J Pediatr Endocrinol Metab. 2008;21:865–877. doi: 10.1515/JPEM.2008.21.9.865. [DOI] [PubMed] [Google Scholar]
- 15.Meigen C, Keller A, Gausche R, et al. Secular trends in body mass index in German children and adolescents: A cross-sectional data analysis via CrescNet between 1999 and 2006. Metabolism. 2008;57:934–939. doi: 10.1016/j.metabol.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Keller A, Klossek A, Gausche R, Hoepffner W, Kiess W, Keller E. Gezielte primäre Adipositasprävention bei Kindern. Dtsch Med Wschr. 2009;134:13–18. doi: 10.1055/s-0028-1105883. [DOI] [PubMed] [Google Scholar]
- 17.Keller E. Screening auf Vorliegen einer Wachstumsstörung mit Hilfe des Präzisionsmessinstrumentes und Systems Dr. Keller. Anthrop Anz. 1994;52:321–326. [PubMed] [Google Scholar]
- 18.Brämswig J, Dübbers A. Disorders of pubertal development. Dtsch Arztebl Int. 2009;106:295–304. doi: 10.3238/arztebl.2009.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]