Abstract
Transcriptional activation of p53 target genes, due to DNA damage, causes either apoptosis or survival by cell cycle arrest and DNA repair. However, the regulators of the choice between cell death and survival signaling have not been completely elucidated. Here we report that human adenocarcinoma cells (MCF-7) survive UV-induced DNA damage by heat shock protein 27 (Hsp27)-assisted Akt/p21 phosphorylation/translocation. Protein levels of the p53 target genes, such as p21, Bcl-2, p38 MAPK and Akt, showed a positive correlation to Hsp27 level during 48h post irradiation, whereas p53 expression increased initially but started decreasing after 12h. Hsp27 prevented the G1/S phase cell cycle arrest, observed after 8h of post UV irradiation, and PARP-1 cleavage was inhibited. Conversely, silencing Hsp27 enhanced G1/S arrest and cell death. Moreover, use of either Hsp27 or Akt siRNA reduced p21 phosphorylation and enhanced its retention in nuclei even after 48h post irradiation, resulting in enhanced cell death. Our results showed that Hsp27 expression and its direct chaperoning interaction increases Akt stability, and p21 phosphorylation and nuclear-to-cytoplasm translocation, both essential effects for survival of UV-induced DNA damaged cells. We conclude that the role of Hsp27 in cancer is not only for enhanced p53 proteolysis per se, rather it is also a critical determinant in p21 phosphorylation and translocation.
Keywords: Heat shock Proteins, UV radiation and DNA damage, Akt/p21 signaling, Apoptosis
Introduction
Stress-induced expression of heat shock proteins (Hsps, the molecular chaperones) represents an emerging paradigm for the coordinated multistep regulation of apoptotic signaling events to provide protection and to facilitate cellular recovery against various stress-induced injuries. Higher levels of Hsps are observed in many malignant tissues, including breast cancer, ovarian cancer, osteosarcoma, endometrial cancer, and leukemia, compared to non-transformed cells of the same tissue (1). Hsp27, a member of the small heat shock protein family (sHSP), is constitutively expressed at low levels in many cells and tissues. Its expression increases following heat shock or other types of stress and it enhances the cellular resistance (2–5). The molecular basis for over expression of Hsp27 in malignant tissues is not completely understood. In some poorly vascularized solid tumors Hsp27 accumulation is attributed to hypoxia, whereas oncogenic mutations could create an increased requirement for chaperone activity towards abnormally folded protein variants, leading to Hsps accumulation. Another possibility for increased Hsp27 protein level in malignant tissues could be the occurrence of functional mutations in transcription factors as observed in adenocarcinoma cell lines, where higher level of the heat shock transcription factor 1 (HSF-1) was attributed to an increased Hsp27 protein level (6).
Several functional consequences of the higher Hsp27 have been identified in malignant tissues, including drug resistance (1, 4, 7–9). It may contribute to tumorogenicity through various mechanisms, which include cytoprotective activity and molecular chaperone functions that could regulate cellular signaling to influence cell growth (10). Hsp27 also inhibits apoptosis in stressed cells, by regulating upstream signaling pathways such as Akt activation by forming a signaling complex with p38MAPK and MAPKAP-2, a kinase that phosphorylates Akt (11–14). The survival-promoting effects of Hsp27 have also been found to function through its ability to negatively regulate the Fas-mediated apoptotic pathway. Phosphorylated Hsp27 interacts directly with DAXX, preventing the association of DAXX with Fas and the protein kinase ASK1, to prevent cell death (15). Additionally, Hsp27 facilitates cell survival via enhanced degradation of proteins by the ubiquitin/proteosome pathway, which modulates the expression of death regulatory proteins to regulate apoptosis (16). For example, increasing the proteosome-mediated degradation of I-κBα, increases the intracellular content of the transcription factor, nuclear factor-κB (NF-κB) which enhances the expression of anti-apoptotic proteins (17).
Although, the origin and functional links of Hsp27 in malignant tissues have been extensively studied, functional consequences of transiently-induced Hsp27 during therapeutic regimens are not completely understood. For example, UV-C (principally 254 nm in wavelength) induces significant cellular damage, primarily producing DNA lesions such as thymidine dimers (18) and photoproducts (19, 20). Eukaryotic cells have developed a number of defense mechanisms to counteract this DNA damage, such as activation of p53 (19). On the other hand stress-inducible proteins, such as Hsp27, are also expected to be co-induced (21–23). However the interplay between p53 and the transiently co-induced Hsp27 due to DNA damage in cancer cells during therapeutic regimens, has not been studied, although many studies have found a correlation between the lethality due to DNA damage and Hsp27 expression levels in Hsp27 over expressed cells (23, 24). Heat shock proteins have been found to play a role in defense mechanisms as shown in heat shock pre-conditioned cells that became resistant to UV-B irradiation (25–29), possibly by functioning in nucleotide excision repair (30). This is evident by the difference in the expression of Hsp27 seen in UV-C resistant and sensitive human cells (31). However, the molecular mechanisms by which Hsp27 is involved in UV-C resistance have not been fully elucidated.
In an effort to understand the role of Hsp27 mediated cell survival after DNA damage, in the present study we used UV-induced DNA damage in human adenocarcinoma cells (MCF-7) as a model system. We have found that Hsp27, which is co-induced by activation of heat shock factor (HSF-1) along with p53 due to UV-induced DNA damage, plays an important role in protecting the MCF-7 cells in post UV irradiation. Indeed we found that UV-induced DNA damage activates HSF-1 and increases Hsp27 expression and facilitates the phosphorylation of p21 by Akt, followed by cell cycle arrest, DNA repair and mitosis. Although a role of Hsp27 as a negative regulator of p53 activity via modulation of p53 stability has already been reported in the literature, the present results demonstrate a new pathway of cancer cell survival, where late phase induction of Hsp27 enhances Akt stability and p21 phosphorylation in UV damaged cells. Subsequently the phospho p21 translocates from nucleus to cytoplasm, so that the cells could pass through the check points and proliferate; otherwise they exit to the apoptotic pathway.
Materials and Methods
Cell Culture and UV Treatment
MCF-7 cells (ATCC) were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (ATCC, USA) and antibiotics. Cells were irradiated with a germicidal lamp at a dose rate of one J/m2 per second for 15 seconds (15 J/m2 total) as measured by a Kettering model 65 radiometer (Cole Palmer Instrument, Co., Vernon Hill, IL). During irradiation, the cells were covered by a thin layer of phosphate buffered saline (PBS). Afterwards, the PBS was removed and fresh growth medium was added and the culture dish was returned to the incubator for the indicated times (i.e. post- irradiation time). Control cells were treated with PBS but were not irradiated.
Hsp27 and Akt siRNA transfection
MCF-7 cells (2 × 106/plate) were seeded in 100 mm plates and cultured for 24 h before transfection. After growing in regular growth medium for 24 h, cells were irradiated with UV and the medium was replaced with small interference RNA (siRNA) containing medium and maintained until they are used at different post irradiation times. Cells were transfected with either human Hsp27 siRNA (5′ GAUCACCAUCCCAGUCACC 3′) with two bases overhanging at the 3′ end of the antisense strand (Ambion Inc.) (32) or Akt siRNA and (signalsilence Akt siRNA II, Cat. # 6510). SiRNAs were mixed with DharmaFect 4 transfection agent (Ambion Inc.) in antibiotic containing serum-free medium for 20 min and then added to the cells at a final concentration of 2.5 nM for Hsp27 and 50 nM for Akt siRNA. A scrambled Hsp27 siRNA (5′AAAUCAAACUG UUGUCAGCGCUG3′) and signalsilence control siRNA (Cat. # 6568) were used as controls for Hsp27 and Akt respectively.
Clonogenic Assay
After UV-C irradiation and siRNA treatment, the colony-forming ability of the MCF-7 cells was determined by clonogenic assay as described previously (33).
Western Blot Analysis
At the indicated times of post UV irradiation, the cells were trypsinized, washed twice with ice cold PBS, lysed in RIPA lysis buffer supplemented with protease inhibitor cocktail. 60 μg of total protein was resolved by 4–12% SDS-PAGE, unless stated otherwise, and transferred to PVDF membrane. Western blot analyses were performed using primary antibodies for Hsp27 (stressgen), p-Hsp 27, Bax, Bcl-2, p21, p-p21, p53, p15, PARP-1, GAPDH, Akt, p-p38MAPK and p-MAPKAP-2 (Cell Signaling, Beverly, MA) and enhanced chemiluminescence reagent (Pierce Protein Research Products, Rockford, IL).
Antibody Conjugation and Coimmunoprecipitations
Primary antibody for Hsp27 was treated with protein G-agarose beads in PBS for over night at 4°C. The antibody conjugated beads were washed four times in RIPA lysis buffer and these antibody conjugated beads were used for immunoprecipitation. For each treatment, 1 mg of total protein was incubated with 8 μl of antibody-conjugated beads in a total volume of 300 μl of lysis buffer. Incubation was carried out for 3 h at 4°C; beads were then washed four times with lysis buffer before being resolved by 4–12% SDS-PAGE. Immunoblots were carried out as described above. The reciprocal pull down experiment was performed using the antibody against Akt.
Electrolytic Mobility Shift Assay (EMSA)
The cells were collected at different post irradiation time and nuclear proteins were extracted using a nuclear protein extraction kit (NE-PER) from Pierce. EMSA for HSF-1 was performed using an oligonucleotide probe corresponding to a double stranded heat shock element (HSE) consensus sequence, labeled with biotin in the 5′ end (5′-Biotin-CTAGAAGCTTCTAGAAGCTTCTAG-3′). For each sample, 15 μg of extracted nuclear protein was added with 20 fmol of HSE, 2.5% glycerol, 50 ng/μl of poly (dI-Dc) and 5mM MgCl2 provided in the EMSA kit (Pierce). This sample complex was incubated at 25°C for 20 min and loaded onto 6% pre-cast DNA retardation gel (Invitrogen) and transferred to a nylon membrane. The transferred DNA was cross-linked to the membrane with a hand held UV lamp for 5 min. Finally the biotin labeled DNA was detected by chemiluminescence as per the protocol in the EMSA kit (Pierce).
Analysis of p21 using Immunofluorescence Microimaging
MCF-7 cells were grown on cover slips, UV-C irradiated with 15 J/m2, at indicated times, fixed for 30 min with 4% paraformaldehyde in PBS and permeabilized for 10 min with 1% Triton X-100. Cells were then blocked with 5% BSA in PBS containing 0.1% Triton X-100 for 1 h at room temperature. Next, primary monoclonal p21 (1:100) antibody prepared in PBS containing 0.1% Tween 20 buffer was added, and incubated on the cover slips for 1 h at room temperature. After washing with PBS, fluorescent Texas Red tag conjugated goat anti mouse IgG (1:200) secondary antibody was added, and the slides were incubated at room temperature for 1 hr. Slides were further stained for nuclei with DAPI (Vector Laboratories, Burlingame, CA). Images were acquired at room temperature with a Nikon fluorescence microscope E80i (Nikon, Tokyo, Japan) fitted with filters for Texas Red and DAPI. The digital images were captured with a CCD camera and processed using SPOT software (Diagnostic Instruments, Sterling Heights, MI).
Cell Cycle Analysis
Control and UV-C irradiated MCF-7 cells were cultured, treated with or without Hsp27 and Akt siRNA, harvested at the indicated times, fixed in ice-cold ethanol (75%) and left at −20°C overnight. Then cells were stained with propidium iodide 10 μl (10 mg/ml) in the presence of 10μg/ml of RNase A, and analyzed using BD Calibur.
MTT assay
Cell viability against UV irradiation after Akt siRNA silencing and control siRNA was determined using (3,4,5-dimethylthiazol-2-yl)-25-diphenyltetrazolium bromide (MTT) in 96 well plate as described previously(34).
Results
Down regulation of Hsp27 enhances MCF-7 cells susceptibility to UV C –induced Apoptosis
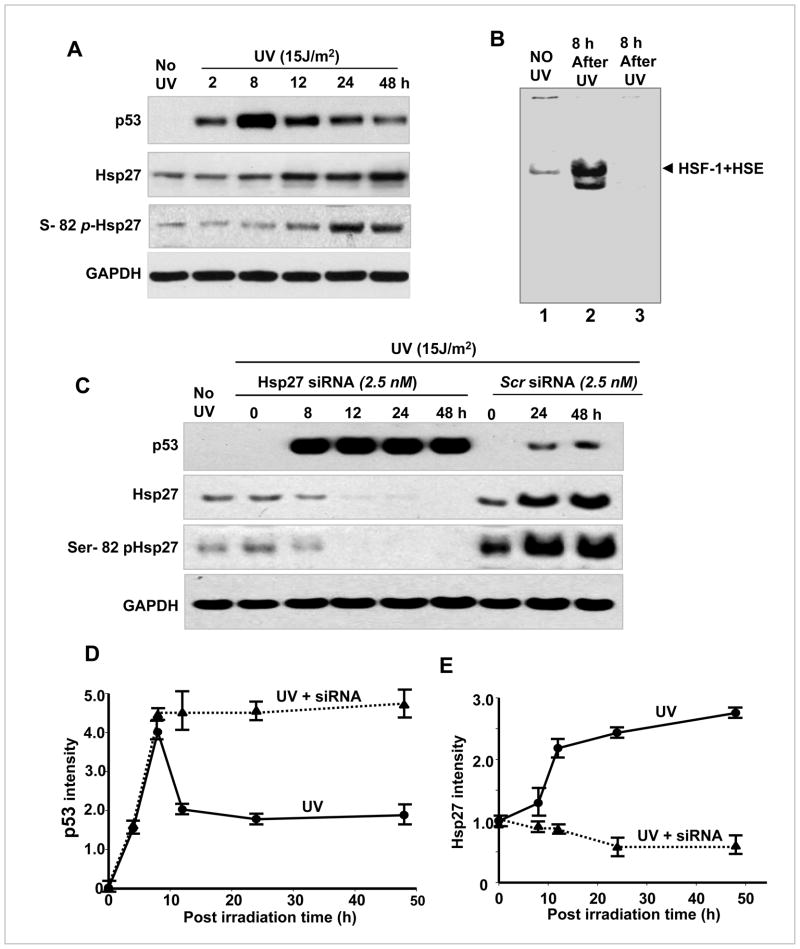
MCF-7 cells were irradiated with UV (15J/m2) in order to create DNA damage. The UV-irradiated cells were analyzed at various post irradiation time points, between 2–48 h. Western blots showed that p53 level peaked at 8 h post irradiation and reduced gradually from 12 to 48 h (Fig. 1A&D). The Hsp27 level increased (Fig. 1A&E) during this time showing a negative correlation between Hsp27 and p53 expression in UV-irradiated cells. The Ser-82 phospho Hsp27 (Fig. 1A) was also observed to increase with post irradiation time indicating that MAPKAP-2 activity is higher in UV irradiated cells, as it is known to phosphorylate the Ser-82 of Hsp27 (35). Hsp27 expression is rapidly up regulated to provide an endogenous defense mechanism to cells. The elevated synthesis of Hsp27 is facilitated by the stress-induced activation of HSF-1, a transcription factor of inducible Hsp27. Electro mobility shift assay (EMSA) confirmed that HSF-1 is activated upon treating with UV and its DNA binding was increased (Fig. 1B). The nuclear extracts of 8 h post UV treated MCF-7 cells showed a strong increase in HSF-1–HSE complex (36), confirming UV irradiation to be a potent inducer of HSF-1 binding to DNA, leading to transcriptional activation of the inducible Hsp27 gene.
Figure 1. Hsp27 depletion by siRNA reduces cell viability and sensitizes MCF 7 cells to UV-C irradiation.
MCF-7 cells (5×104, seeded onto 100 mm dishes) were irradiated with UV-C and changed to normal medium or medium containing either Hsp27 siRNA or scrambled siRNA for a desired post irradiation time as indicated in the figure. A. Western blots of p53, Hsp27 and Ser-82 phospho Hsp27 in UV irradiated cells, determined at various post irradiation times as mentioned. B. Electrolytic mobility shift assay (EMSA) of HSF-1 binding to biotin labeled HSE consensus sequence. Higher HSF-1 binding was observed in UV-C irradiated MCF-7 nuclear extracts (column 2) compared to control (column 1). Prior incubation of UV irradiated sample with cold HSE (no biotin label) did not give any band, confirming the competitive binding (column 3). C. Same as A, but the cells were switched to siRNA or scrambled siRNA containing medium and maintained for a desired post irradiation time. D&E. Quantitative plots of p53 and Hsp27 in UV irradiated and UV+Hsp27 silenced cells (n =3).
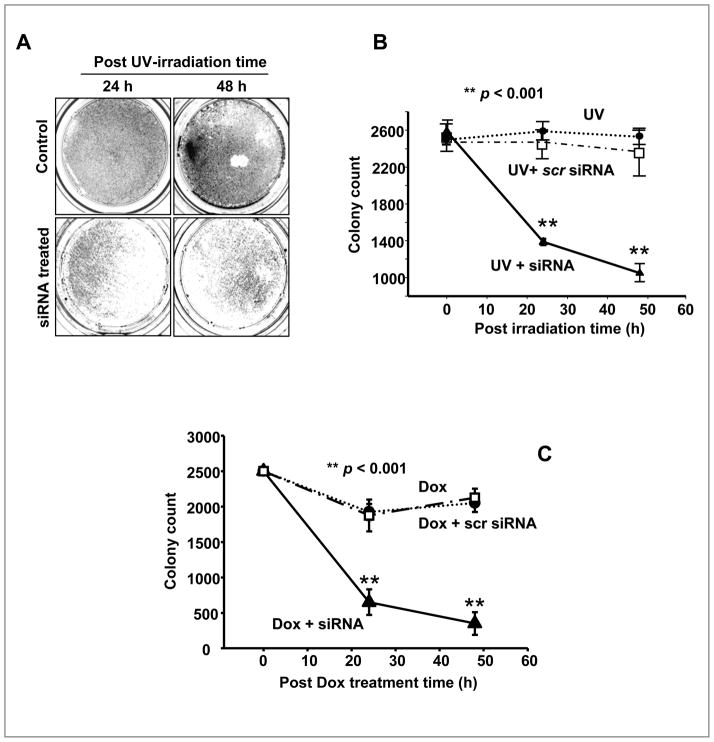
The negative correlation of Hsp27 and p53 implies that Hsp27 potentiates the degradation of p53 after 8 h of post irradiation. It is known that Hsp27 affects MDM-2 to accelerate proteolysis of p53 in drug treated cancer cells (37). To confirm the role of Hsp27 in p53 degradation in UV treated cells, Hsp27 was silenced with siRNA after the UV-irradiation. In siRNA treated cells, the p53 continued to accumulate up to 48 h of post irradiation (Fig. 1C& D), while obviously there was no significant accumulation of Hsp27 observed during this time (Fig. 1C&E). However, in scrambled Hsp27 siRNA treated cells, the Hsp27 and p53 expression patterns were similar to Fig. 1A, D and E (Fig. 1C). These results confirm that transiently expressed Hsp27, in response to UV induced DNA damage, does accelerate the degradation of p53. Fig. 2A shows colony density in 10 cm2 plates, stained with crystalline violet after 24 and 48 h of post irradiation in UV irradiated and in UV irradiated/Hsp27 siRNA treated cells. As seen in the Fig. 2A, in the UV irradiated case, the cells repopulated (almost 100%). But, the Hsp27-silenced MCF-7 showed enhanced cell death in response to irradiation. The quantitative estimation (Fig. 2B) shows that the UV treated cells repopulated almost an equivalent number of colonies, compared to pre-irradiation, due to survival of these cells from UV-induced DNA damage and recovering the active proliferation. However, in Hsp27 silenced cells, less than 40% colony forming activity was reestablished, whereas in scrambled Hsp27 siRNA treated cells almost 100% survival was obtained (Fig. 2B). In order to confirm whether similar results could be obtained with the use of chemotherapeutic agents, normal and Hsp27 silenced MCF-7 cells were treated with Doxorubicin and the colony density was determined. As shown in Fig. 2C, the results are similar to UV irradiation, illustrating that the fundamental mechanism is same in both UV induced DNA damage or anti-neoplastic agents induced DNA damage. These results show that silencing the transiently expressed Hsp27 potentiates cell death by UV-irradiation in MCF-7 cell, through a mechanism involving Hsp27 and p53.
Figure 2. Effect of Hsp27 silencing in clonogenic ability of UV treated MCF-7 cells.
A. Images of MCF-7 colonies, stained with crystal violet, after 24 and 48 h of post UV-C irradiation with and without Hsp27 siRNA. B. Quantitative plots of colony count (Col-Count®) at different post irradiation times (n = 3). In normal UV treated cells almost 100 % equivalent to un-irradiated cells were obtained after 48 h of post UV irradiation. In Hsp27 silenced cells, the confluence was less than 40%. C. Effect of 0.5μM Doxorubicin (Dox) on normal and Hsp27 siRNA or scrambled Hsp27 siRNA treated cells. Cells were treated Dox (for 3h) and maintained in a medium containing siRNA for 24 or 48h.
Hsp27-dependent p53 target genes and G1/S cell cycle arrest
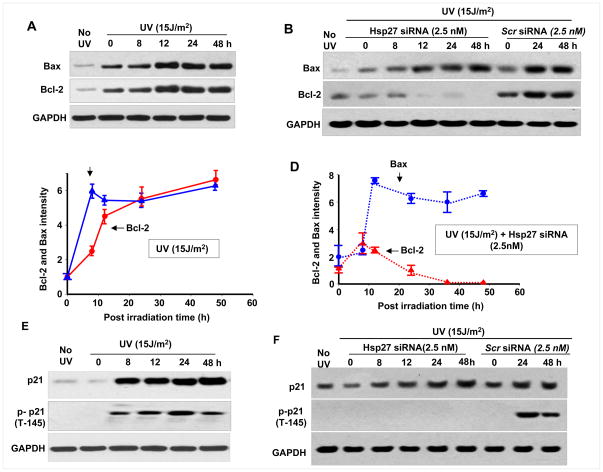
Since the stability of p53 after UV irradiation is dependent on Hsp27, the level and dependence on Hsp27 of p53 target proteins, such as Bax, Bcl-2 and p21 were determined. As shown in Fig. 3A, the levels of Bax and Bcl-2 increased with time, whereas in Hsp27 depleted cells Bcl-2 completely disappeared 36 h post UV irradiation (Fig. 3B). However in scrambled Hsp27 siRNA maintained cells, there was no such loss of Bcl-2 even after 48 h post irradiation. Quantitative determination showed that Bax and Bcl-2 ratio remained almost the same in UV irradiated cells (Fig. 3C). However, compared to Bcl-2, a very high level of Bax (~5 fold increase) was observed in UV irradiated/Hsp27 siRNA treated cells (Fig. 3D). The p21 level increased from 8 h to 48 h post irradiation in UV irradiated/Hsp27 siRNA treated cells (Fig. 3E&F). Although an increase in p21 was observed in both cells, no phosphorylated p21 could be detected in Hsp27 silenced cells (Fig. 3F). These results indicate that Bax and Bcl-2 induction occurs as an immediate response to UV-C irradiation and the pro-apoptotic protein Bax increases continuously with time after UV irradiation, both in UV irradiated and UV irradiated/Hsp27 siRNA treated cells. However, in only UV irradiated cells, such an increase in Bax is compensated by an increase in Bcl-2 (Fig. 3A), and the observed down regulation in Bcl-2 in Hsp27 silenced cells (Fig. 3B) increases apoptosis.
Figure 3. Hsp27 knockdown enhances apoptosis after UV irradiation of MCF-7 cells.
Western blots of Bax, BCl-2 and p21 in UV treated cells (A) and in UV and Hsp27 siRNA or scrambled siRNA treated cells (B), determined at different post irradiation times as indicated. In Hsp27 knock down cells, the Bax increased but BCl-2 is down regulated with post irradiation time. (C&D). Quantitative plots of the Bax and Bcl-2, obtained in three independent experiments. GAPDH intensity was used to normalize the protein loading. E&F. Western blots of p21 and T 145 phosphorylated p21 in UV irradiated and UV and siRNA treated cells respectively. The p21 increased up to 48 h of post irradiation. But T145 phosphorylated p21 was down regulated in Hsp27 siRNA treated cells.
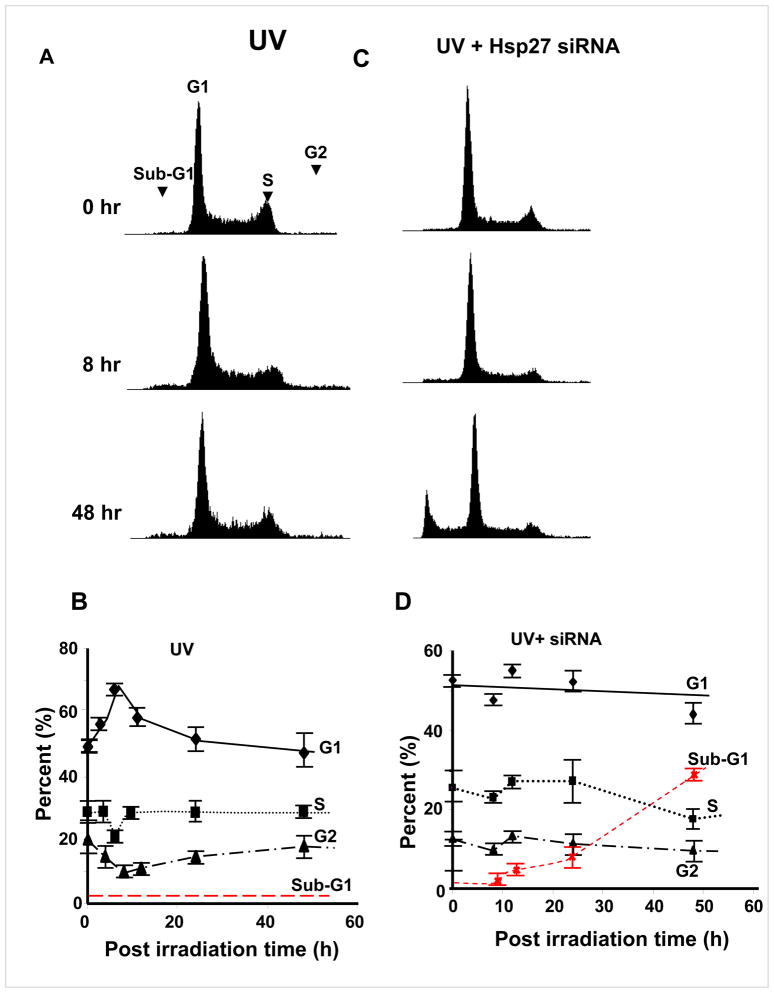
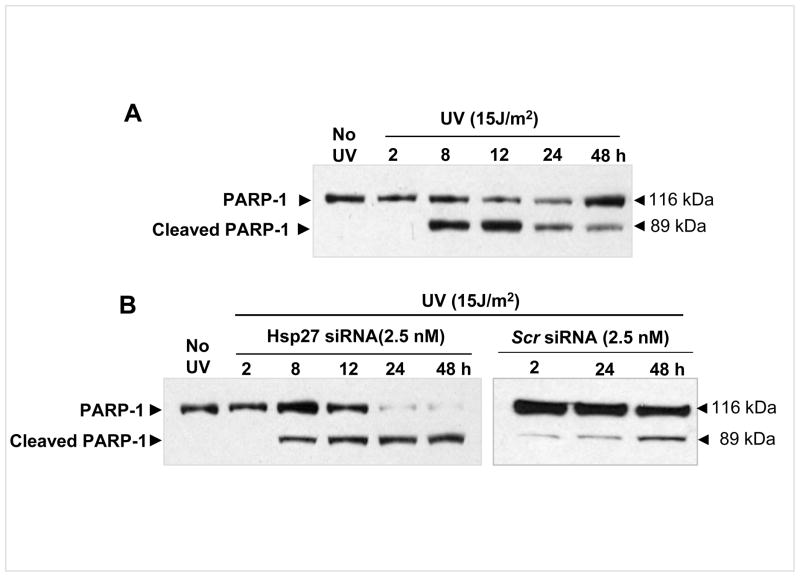
Since large differences in the phosphorylation of cell cycle related proteins such as p21 were noticed (among UV alone treated and UV irradiated/Hsp27 siRNA treated cells), cell cycle analysis after UV-C irradiation was carried out using fluorescence-activated cell sorting (FACS). In UV irradiated cells, G1/S phase cell cycle arrest was observed (Fig. 4A) at 8 h after exposure to UV irradiation. However, the G1/S phase accumulation was depleted 24 h post irradiation. Quantitative analysis (Fig. 4B) showed that cells in G1 increased from 50% to 70 % by 8 h post irradiation and fell back to 50% by 24 h. The G2 and S phase populations decreased from 30 to 20% and 20 to 10% respectively and increased back. No apoptotic cell population (sub-G1 phase) was accumulated after 24 and 48 h post UV-C irradiation (Fig. 4A&B). In Hsp27 silenced cells, however, there was no obvious cell cycle arrest (Fig. 4C&D). But the apoptotic cell population (sub-G1, apoptotic cells) gradually accumulated with increasing post irradiation time (increased from 2% in un-irradiated cells to 6 and 29% after 24 and 48 h of post UV-C irradiation respectively, Fig. 4D), whereas the G2/M peak dropped after onset of apoptosis (Fig. 4C&D). Increased cell death (Fig. 2) and cell cycle arrest upon treating with Hsp27 siRNA indicate that these cells exit to an apoptotic pathway rather than DNA repair or survival. To further substantiate that the cells undergo increased apoptosis upon silencing Hsp27, PARP-1 integrity was determined at various post irradiation times. It has been very well established that caspase-3 activation leads to cleavage of PARP-1, which can be detected by western blot by the appearance of a distinct band at 89 kDa indicating cleaved PARP-1 (38). The UV treated MCF-7 showed this cleaved band, but its intensity decreased with time. Indeed at 24 and 48 h of post irradiation, the un-cleaved PARP-1 was higher than the cleaved band intensity (Fig. 5A). Conversely, in Hsp27 silenced cells, the intensity of the cleaved PARP-1 band increased through 48 h of post UV irradiation (Fig. 5B), and scrambled siRNA treated cells showed no change at 24 or 48 h of post irradiation. Taken together, these results suggest that the loss of Hsp27 enhances cellular susceptibility to UV-C induced apoptosis. Therefore, Hsp27 is a critical determinant in cell susceptibility to apoptosis induced by UV-C induced DNA damage.
Figure 4. Cell cycle arrest analysis by fluorescence-activated cell sorting (FACS) in UV irradiated MCF-7 cells.
Cells were seeded and treated with UV and switched to siRNA containing medium as in Fig. 1. The cells were then fixed and stained with propidium iodide. Histograms of cell distribution in UV irradiated (C) and UV+ Hsp27 siRNA treated (B) cells that were analyzed at different times of post UV-C irradiation as indicated. Only in siRNA treated cells, significant sub-G1 phase population was observed at 48 h of post UV irradiation. (B&D). Quantitative plots of different phases (sub-G1, G1, S and G2) of cell cycle for UV irradiated and UV+Hsp27 siRNA treated cells respectively.
Figure 5. PARP-1 cleavage upon treating with UV-C.
MCF-7 cells were cultured and treated as described in Fig. 1. Western blots of intact PARP-1 and cleaved PARP-1 in UV treated and UV + siRNA treated cells. PARP-1 fragment, observed at 89 kDA due to caspase 3 activation, was high at 8 h of post irradiation time, but continued to decrease with post irradiation time. However in siRNA treated cells, the intensity of this fragment was increased with time up to 48 h.
Hsp27 dependent Akt stabilization and p21 phosphorylation
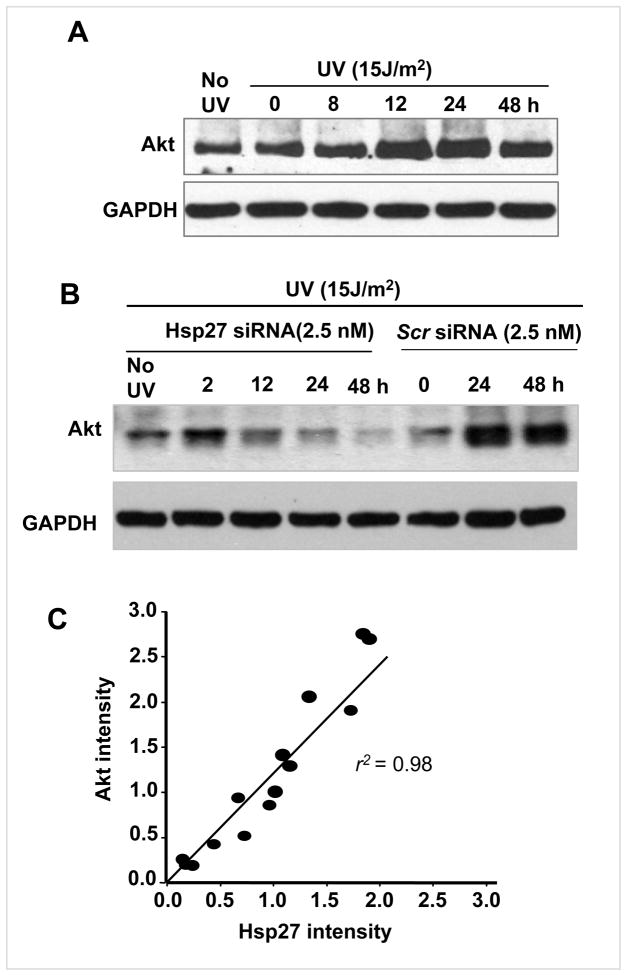
As shown in Fig. 6A, the Akt level increased between 8 and 48 h post UV irradiation. Interestingly, during this time Hsp27 was observed to be high (Fig. 1A). However, there was no such increase in Akt level (Fig. 6B) in Hsp27 silenced cells, suggesting that Hsp27 is required for Akt stabilization in DNA damaged cells, as an integral part of the DNA repair process in UV irradiated cells. Moreover a correlation plot between Hsp27 and Akt blot intensities (pooled together from normal and Hsp27 silenced cells after UV irradiation) shows that there is more or less linear correlation (r2 =0.98), confirming that Akt stabilization is proportionate to the Hsp27 expression (Fig. 6C). These observations suggest that Hsp27 is pivotal in governing cellular sensitivity to genotoxic stress through orchestrating Akt activity.
Figure 6. Disruption of Hsp27 level and loss of Akt stabilization.
Western blots of Akt in UV treated (A) and UV + Hsp27 siRNA treated (B) cells as function of post irradiation time. Akt intensity in UV irradiated cells showed an increase with post irradiation time, whereas Akt was depleted in UV+ Hsp27 siRNA treated cells. In scrambled siRNA maintained cells, Akt was retained. C. Correlation plot of Hsp27-Akt, plotted together from the blot intensities obtained from normal and siRNA treated cells. A fair linear correlation was obtained (r2=0.98)
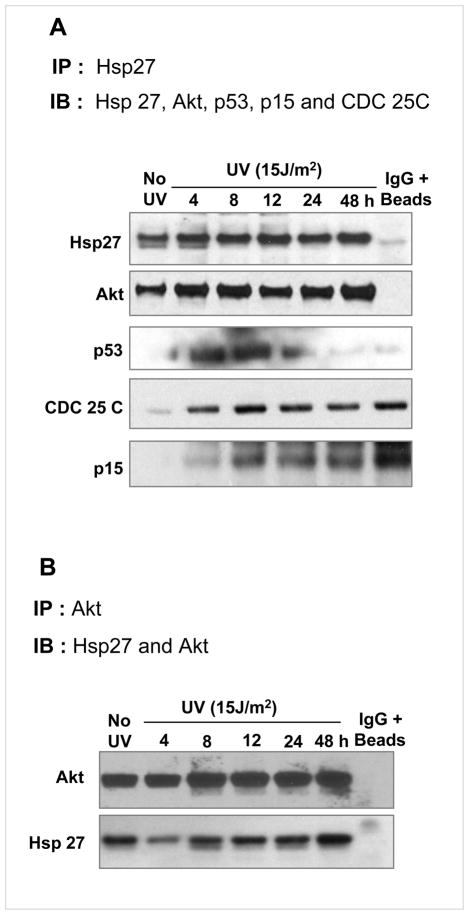
To investigate whether there is any direct interaction between Hsp27 and Akt in DNA damaged cells, immunoprecipitation and immunoblotting experiments were carried out. In UV treated cell lysates, Hsp27 was immunoprecipitated and immunoblotted with Hsp27 and Akt antisera. In the immunoprecipitate of Hsp27, the Akt was detected and vice versa, showing that they co-immunoprecipitate each other (Fig. 7A&B) due to a protein: protein interaction between them. The Hsp27/Akt interaction increased with increasing Hsp27 level after post irradiation (Fig. 7A). A positive interaction between Hsp27 and p53 was also observed (Fig. 7A). Such an interaction may lead to proteolytic degradation of p53 as previously established (37). Obviously, there was no such an Hsp27/Akt association, detected in Hsp27 silenced cells (data not given). A similar interaction was observed with other cell cycle regulators including CDC-25C and p15, and there was systematic increase in p15 level but not in CDC 25C with time, as observed for Akt (Fig. 7A).
Figure 7. Interaction of Hsp27 with Akt.
Immunoblots of Hsp27 associated protein were obtained by immunoprecipitation of Hsp27 with monoclonal antibody. Immunoprecipitates of Hsp27 from the samples of different post irradiation times (1 mg of total lysate protein) were probed for co-immunoprecipitated proteins as shown in the figure. Significantly increased association of Akt was observed after 24 and 48 h of post irradiation, indicating higher interaction at these time points. Other proteins namely CDC 25C, but p15, did not show any significant trend.
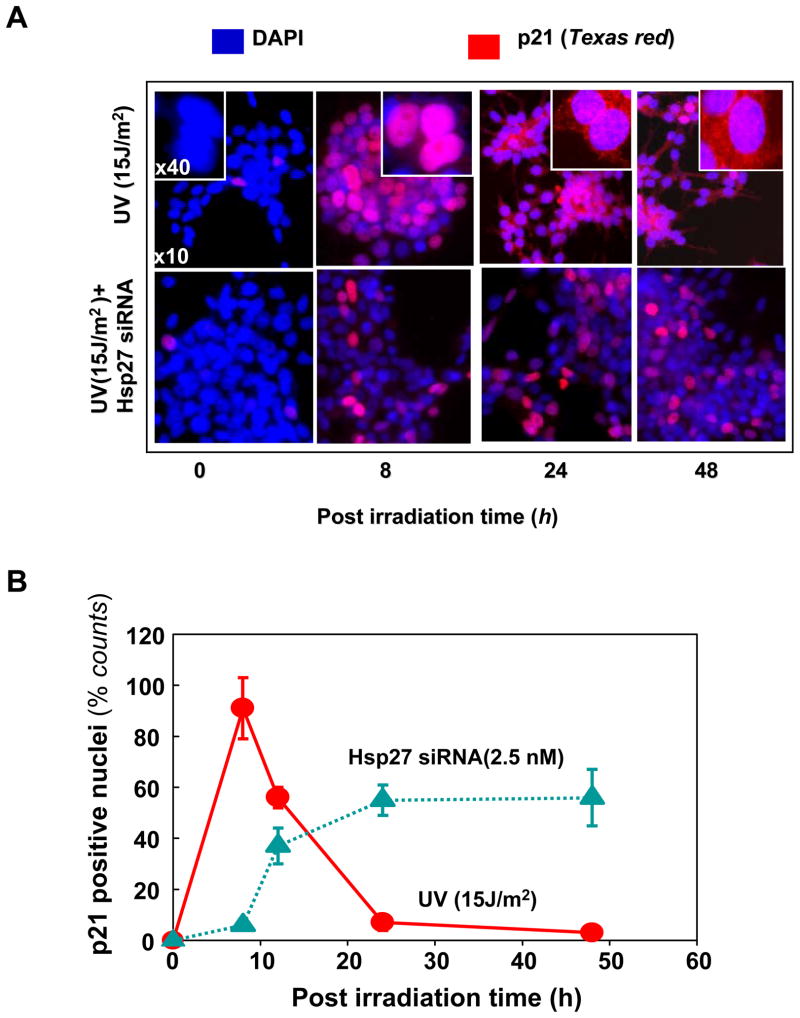
Cell-cycle progression is tightly regulated by the family of cyclin-dependent kinase (CDK) inhibitors and its expression is induced by activation of wild-type p53 (39, 40). The cell-growth-inhibiting activity of p21 is strongly correlated with its nuclear localization (39–41). Phosphorylation of p21 at T145 by Akt is known to initiate translocation from nucleus to cytoplasm and thereby regulating the growth-regulating activity of p21. Since G1/S phase cell cycle arrest was observed in UV-C irradiated cells (Fig. 4), we determined the localization of p21 at different post irradiation times using fluorescent microscopic imaging. Fig. 8A shows the microscopic images of dual stained (DAPI and Texas Red tagged IgG against the p21) for both UV irradiated and UV irradiated/Hsp27 siRNA cells (presented images are superimposed red and blue fluorescence). In UV irradiated cells, the p21 was observed in the nuclei for the first 8 h post irradiation, but disappeared from the nuclei afterwards. But in the case of UV irradiated/Hsp27 siRNA treated cells, p21 continues to be present in nuclei even after 48 h post irradiation (Fig. 8B). These results indicate that in siRNA treated cells the possible T-145 phosphorylation of p21 did not occur and thus the translocation of p21 to the cytoplasmic region did not proceed to remove cell cycle arrest, as happened in Hsp27 un-silenced cells. Thus the cells are forced to undergo apoptosis when treated with Hsp27 siRNA. These results also indicate that Hsp27- dependent p21 phosphorylation at T145 by Akt is critical in determining its cellular localization.
Figure 8. Fluorescence microscopic imaging of p21 in UV irradiated MCF-7 cells.
UV irradiated cells were fixed and dual stained with DAPI (blue), p21 monoclonal antibody and Texas Red tagged IgG secondary antibody (red). Superimposed images are presented here. (A). In UV treated cells (top panel), after 8 h post irradiation time, ~ 90% of the red fluorescence (corresponding to p21) was detected in the nucleus. Such a p21 accumulation in nuclei leads to cell cycle arrest, as observed in FACS analysis (Fig. 4). At about 24 h onwards no significant p21 was detected in the nucleus suggesting that Hsp27 assisted phosphorylation of p21 by Akt, removes it from nucleus. In UV + siRNA treated cells (bottom panel), p21 was localized in nucleus even after 48 hr, suggesting that the absence of Hsp27 does not favor the p21 phosphorylation by Akt and the p21 resides in nucleus longer time, forcing the cells to exit to apoptotic pathway. (B). Quantitative analysis of p21 positive nuclei (by counting red nuclei to total, sampled >100 in each case).
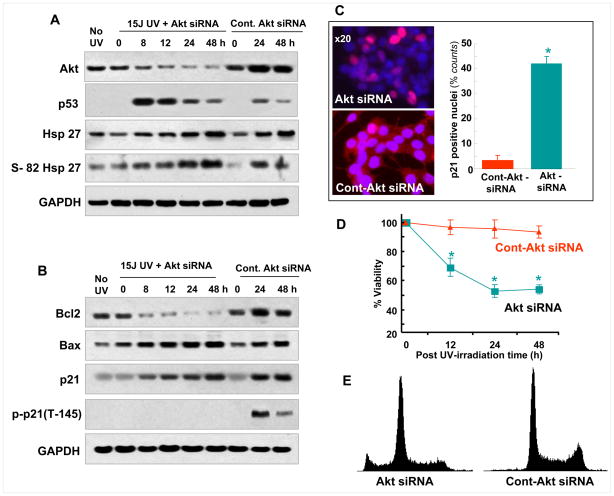
Addition of Akt siRNA further confirmed the results on p21 phosphorylation, described above. Akt was observed to decrease with post irradiation time; whereas it increased in control siRNA maintained cells (Fig. 9A). Also neither Hsp27 expression, nor Hsp27 phosphorylation pattern was affected by Akt silencing, suggesting that Hsp27 expression is independent of Akt in UV-treated MCF-7 cells. However, Bcl-2 expression was found to be affected by silencing Akt. As seen in Fig. 9B, Bcl-2 decreased with increasing post irradiation time, while control Akt siRNA (Fig. 9B) or no siRNA treatment showed (Fig. 3A) increased Akt with increasing post irradiation time. However, total Bax and p21 levels were unaffected by Akt siRNA (Fig. 9B). Interestingly, p21 phosphorylation is completely inhibited by Akt silencing (Fig. 9B), whereas there was no change in the pattern of its expression in control siRNA treated cells (F.9B) and no siRNA treated cells (Fig. 3E). These results, in combination with the data from Hsp27 silenced cells (Fig. 3F) show that Hsp27 is required for protecting from degradation/denaturing of Akt and maintaining the phosphorylation of p21. Furthermore the p21/DAPI immunofluorescence confirmed that the Akt siRNA treated cells retained p21 in nuclei (Fig. 9C). The MTT assay summarized in Fig. 9D showed increased loss of cells in Akt siRNA treated cells. Finally, more than 10% of the cell population was found to be apoptotic as detected by flowcytometry analysis in Akt siRNA treated cells (Fig. 9E). Overall, results in Fig. 9 establish that Hsp27-assisted Akt phosphorylation of p21 is required for survival after UV irradiation.
Figure 9. Effect of Akt siRNA on the MCF-7 cell survival after UV-C irradiation.
(A). Western blots of Akt, Hsp27 and s-82 phospho Hsp27 in Akt siRNA or control Akt siRNA (50nM) treated cells, obtained at different post irradiation time. Decrease in Akt was observed with siRNA treated cells, whereas in the control siRNA treated cells it increased with post irradiation time. (B) Western blots of Bcl-2, Bax, p21 and T145 phospho-p21. p21 phosphorylation was completely inhibited in Akt siRNA treated cells. (C). Fluorescent microscopic imaging of p21 (red) and DAPI dual stained (as described in Fig. 8) cells that were maintained in either Akt siRNA or control Akt-siRNA for 48 h of post irradiation. Increased amount of p21 positive cells are seen in Akt siRNA maintained cells as shown in the quantitative plot. (D). Cell viability measurements, by MTT assay, in Akt-siRNA or control siRNA maintained cells at different post irradiation times. In Akt siRNA maintained cells, increased cell death was observed compared with control siRNA. (E). Cell cycle analysis by flow cytometry at 48 h of post irradiation time, in Akt siRNA or control siRNA maintained cells. More than 10% apoptotic population was detected in Akt siRNA maintained cells.
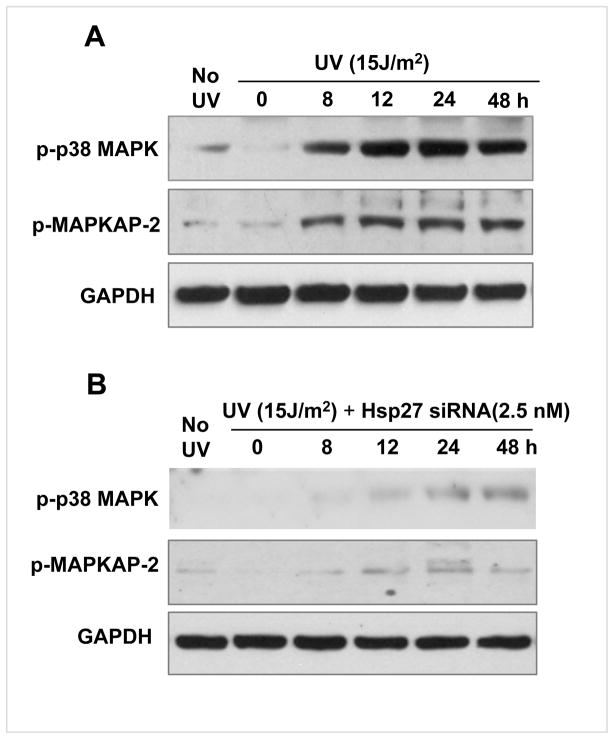
The levels of protein kinases that can phosphorylate Hsp27, namely p-p38MAPK and its down stream p-MAPKAP-2, were also determined in UV irradiated cells, as summarized in Fig. 10. The p-p38MAPK started to appear at 8 h post irradiation and increased with time (Fig. 10A). This increase was in parallel with the Hsp27 expression (Fig 1A & B). However, in Hsp27 siRNA treated cells, p-p38MAPK was not increased at any of the time points studied (Fig. 10B). Similarly, the p-MAPKAP-2 was also observed to be high in 8 h post irradiation, whereas in siRNA treated cells there was no UV-irradiation induced change in p-MAPKAP-2 (Fig. 10A&B).
Figure 10. p-p38MAPK and p-MAPKAP-2 in UV irradiated MCF-7 cells.
A. Western blots of p-p38MAPK and p-MAPKAP-2 in UV irradiated and UV + Hsp27 siRNA treated cells. In UV treated cells, p38MAPK is increasingly phosphorylated up to 48 h of post irradiation. Also p-MAPKAP-2 increased with post irradiation time. B. Same as A, but maintained in Hsp27 siRNA medium. Very low p-p38MAPK and no p-MAPKAP-2 were noticed.
Discussion
The primary finding of the present work is that the survival of MCF-7 cancer cells, through p53 activation after UV induced DNA damage, depends upon Hsp27 level and the Hsp27-assisted p21 phosphorylation by Akt. MCF-7 cells treated with Hsp27 siRNA showed higher sensitivity to UV-C lethality compared to cells treated with scrambled siRNA or no siRNA. Therefore Hsp27 is likely involved in the resistance of human cancer cells to DNA damaging anti-neoplastic agents, at least in MCF-7 cells. Human cancer cells can survive after DNA damage by UV irradiation at low dosage, via DNA repair and other protective mechanisms, whereas severe damage due to high dosages of UV, would induce cell death through the activation of apoptosis (42). Although Hsp27 has been known to protect cells from various stresses, in this work we have identified a new pathway of human cancer cell survival involving Hsp27/Akt and interference in cell cycle activity through modulation of p21 activity. Many cancer cell lines and tumor biopsies show high levels of Hsp27 when treated with DNA damaging agents, suggesting that up-regulation of Hsp27 has a protective role against the genotoxicity of drugs. Recently it was shown that over expression of Hsp27 suppressed p21 accumulation in human epithelial cells after treating with Doxorubicin and reduced the cell senescence (37).
The involvement of HSPs in cellular resistance to the deleterious effects of UV irradiation is somewhat controversial. Trautinger et al (27, 28) reported that over expression of Hsp27 did not confer resistance to UV-A and UV-B induced cell death in squamous cell carcinoma. On the other hand, another study demonstrated that human cells tolerate damage caused by UV-B irradiation if they are pretreated with heat (26, 43, 44). Trautinger et al (27) did show that inducible HSPs play roles in cellular responses after UV irradiation.
p53 accumulation and expression of the mRNAs of matrix metalloproteinase and collagenase were reported to be induced following UV irradiation in human cells (21, 45). In the present work, we observed that p53 and Hsp27 are co-induced upon treating with UV-C (Fig. 1A). While the Hsp27 level is increased after 8 h of post UV irradiation, induced p53 (Fig. 1A) degraded during this period of time. Hsp27 assisted proteosomal degradation of p53 has been reported in many previous studies; most of these studies used Hsp27 over-expressing cells. Similarly, silencing Hsp27 using siRNA has been found to increase the stability of p53 and to accelerate cancer cell death (37). However the exact mechanism of the transient induction of Hsp27 in response to DNA damage such as UV irradiation, and its interaction with p53 has not been studied before. Recently, it was shown that Hsp27 inhibited radiation induced apoptosis by inhibiting PKC mediated reactive oxygen species (46). Other studies have reported that Hsp27 is induced by p53 and as such Hsp27 is one of the p53 target genes (47). Contrarily, Hsp27 has been found to be independently induced and to play an important role in transactivating p53 (48). In the present work we provide two evidences that Hsp27 is independently induced and indeed that Hsp27 plays an important role in p53 degradation after UV irradiation. First, we observed that HSF-1, the transcription factor that is responsible for Hsp27 induction, is activated (Fig. 1B). Second we found that silencing Hsp27 preserved the p53 protein level (Fig. 1C). We observed a negative correlation between the Hsp27 and p53 protein levels in the present work (Fig. 1A). It was also found that the p21 phosphorylation by Akt is dependent on Hsp27 level (Fig. 3E&F). Thus, it appears that the transient increase in Hsp27 in cancer cells not only serves to transactivate p53 and subsequently suppress apoptosis but also regulates the activities of p53 target genes such as p21. Inspite of the fact that in Hsp27 silenced cells, the p53 is stabilized (Fig. 1C), the Akt level is depleted (Fig. 6C).
Another important observation made in the present study is the differential response of the anti-apoptotic protein Bcl-2 expression and cell cycle arrest, depending upon the level of Hsp27 in UV irradiated MCF-7 adenocarcinoma cells (Fig. 4A&C). The p53 target proteins such as p21, Bax, Bcl-2 are indirectly dependent on the level of Hsp27 (Fig. 3). Since the life span of p53 is influenced by Hsp27, the Bcl-2 level is decreased in siRNA treated cells, an indication that p53 transcriptional activity is modulated by Hsp27. Although p21 expression is unaffected upon silencing Hsp27, the T145 phosphorylated p21 level is reduced in these Hsp27 silenced cells (Fig. 3F). This is once again inspite of the fact that p53 is stabilized even after 24 h post irradiation in Hsp27 silenced cells. Thus it is clear that Hsp27 is essential for Akt activity in DNA damaged cells leading to the phosphorylation of p21 as a survival pathway. Furthermore, Hsp27 is found to co-immunoprecipitate with Akt (Fig. 7A). Indeed the results indicate that the protein level of Hsp27 correlates with the extent of the cell survival after UV irradiation. Hsp27 had been observed to protect Akt and enhance its phosphorylation of p21 (Fig. 3A&B). Silencing Hsp27 depleted Akt and increased apoptosis (Fig. 4A&C). Recently it was shown that phosphorylation of Akt by MAPKAP-2 was enhanced by the Hsp27 association (13, 14, 49). Prior dissociation of Hsp27 before the phosphorylation was shown to induce apoptosis. Also it was shown that cisplatin resistant L929 cells survived by over expressing Hsp27 through Akt activation (50). In the present study we show that the Hsp27 interactions with Akt, is a critical determinant of Akt stabilization, as removal of Hsp27 prevent Akt stabilization and results in apoptosis in UV-C irradiated MCF-7 cells (Fig. 4A&C).
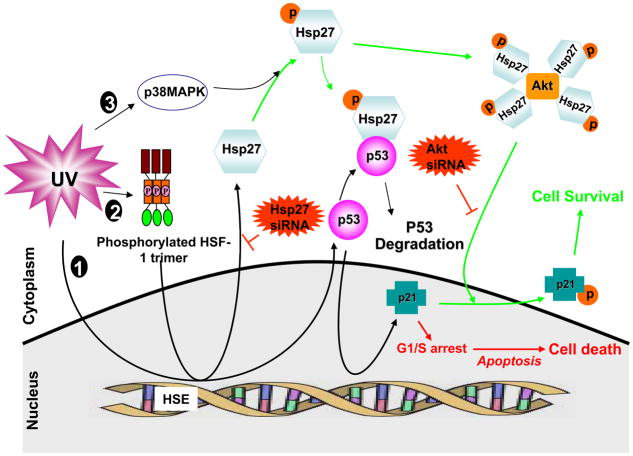
The cellular localization of p21 was recently proposed to regulate p21 function (51). The mechanism of the cellular translocalization of p21 was demonstrated by Zhou et al (39), where phosphorylation of p21 by Akt at T145 was found to relocate the p21 to the cytoplasm and suppress growth-inhibitory activity. Cytoplasmic p21 has been shown to form a complex with ASK1 to inhibit the stress-induced MAP kinase cascade, which results in resistance to apoptosis induced by many stimuli (52). Thus, our results, together with the previous findings (52), support this mechanism for the Hsp27-assisted anti-apoptotic effect of T145 phosphorylated p21. Based on our results, we propose a novel mechanism of survival of MCF-7 adenocarcinoma cells after UV induced DNA damage, orchestrated by Hsp27, as shown in Fig. 11.
Figure 11. Schematic illustration of Hsp27 acting as a switch between cell death and survival after UV-C irradiation.
UV irradiation triggers three major signaling that are relevant to present work: 1. It causes DNA damage and p53 activation; 2. HSF-1 activation and transcription of heat shock proteins such as Hsp27; 3. activation of MAP kinases. The UV-induced p53 is transactivated to induce its target genes such as Bax, Bcl-2, p21 etc. The p21 involves cell cycle arrest at G1/S phase and leads to apoptosis (cell death pathway). On the other hand the UV-induced Hsp27 is phosphorylated by p38MAPK/MAPKAP-2 (also induced by UV). The p-Hsp27 plays multiple roles. It can negatively regulate the p53. Also the p-Hsp27 chaperones Akt and stabilizes. The Hsp27 stabilized Akt phosphorylates p21 and translocates it to cytoplasm, removing the cell cycle arrest so that the cells can actively go through the check points (cell survival pathway). Silencing Hsp27 makes the p53 to survive longer and continued presence of p21 in the nucleus for longer time, arresting cell cycle and leading to cell death. Thus Hsp27 is acting as switch between cell death and survival and indeed removal of transiently induced-Hsp27 could be an effective approach in UV-C therapy.
In summary, the results of the present study illustrate that transcriptional activity of UV induced p53 is regulated by co-induced Hsp27, so that DNA damage can be fixed and cells can survive. Hsp27, appearing after depletion of p53, enhances Akt stabilization and phosphorylation of p21. Such a phosphorylation results in the translocation of p21 from nuclei to cytoplasm, removing the cell cycle arrest, so that the cells proliferate normally (Fig. 11). Depletion of Hsp27 enhances the cell death due to inadequate phosphorylation of p21 and 4its inability to translocate to cytoplasm in order to facilitate active proliferation, and hence the cells exit to apoptosis. Thus, Hsp27 acts as a switch between the apoptosis and survival by modulating Akt stability. Limitation of the present work, however, is that knockdown of Hsp27 affects a broad spectrum of proteins in terms of stability/folding. This study illustrates a mechanism concerning Hsp27/Akt, while the other possibilities cannot be ruled out.
Acknowledgments
We acknowledge the financial support from NIH Grants R21 EB004658 and American Heart Association Grant BGIA 0365203B to GI. The services of DHLRI core facilities namely Proteomics, Flow cytometry and Microscopy, are gratefully acknowledged.
References
- 1.Garrido C, Ottavi P, Fromentin A, Hammann A, Arrigo AP, Chauffert B, Mehlen P. HSP27 as a mediator of confluence-dependent resistance to cell death induced by anticancer drugs. Cancer Res. 1997;57:2661–2667. [PubMed] [Google Scholar]
- 2.Arrigo AP. sHsp as novel regulators of programmed cell death and tumorigenicity. Pathol Biol (Paris) 2000;48:280–288. [PubMed] [Google Scholar]
- 3.Huot J, Roy G, Lambert H, Chretien P, Landry J. Increased survival after treatments with anticancer agents of Chinese hamster cells expressing the human Mr 27,000 heat shock protein. Cancer Res. 1991;51:5245–5252. [PubMed] [Google Scholar]
- 4.Oesterreich S, Weng CN, Qiu M, Hilsenbeck SG, Osborne CK, Fuqua SA. The small heat shock protein hsp27 is correlated with growth and drug resistance in human breast cancer cell lines. Cancer Res. 1993;53:4443–4448. [PubMed] [Google Scholar]
- 5.Jiao W, Hong W, Li P, Sun S, Ma J, Qian M, Hu M, Chang Z. The dramatically increased chaperone activity of small heat-shock protein IbpB is retained for an extended period of time after the stress condition is removed. Biochem J. 2008;410:63–70. doi: 10.1042/BJ20071120. [DOI] [PubMed] [Google Scholar]
- 6.Hoang AT, Huang J, Rudra-Ganguly N, Zheng J, Powell WC, Rabindran SK, Wu C, Roy-Burman P. A novel association between the human heat shock transcription factor 1 (HSF1) and prostate adenocarcinoma. Am J Pathol. 2000;156:857–864. doi: 10.1016/S0002-9440(10)64954-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrido C, Mehlen P, Fromentin A, Hammann A, Assem M, Arrigo AP, Chauffert B. Inconstant association between 27-kDa heat-shock protein (Hsp27) content and doxorubicin resistance in human colon cancer cells. The doxorubicin-protecting effect of Hsp27. Eur J Biochem. 1996;237:653–659. doi: 10.1111/j.1432-1033.1996.0653p.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Sun D, Cho KJ, Kim MS, Hong MH, Kim IK, Lee JS, Lee JH. Overexpression of human 27 kDa heat shock protein in laryngeal cancer cells confers chemoresistance associated with cell growth delay. J Cancer Res Clin Oncol. 2007;133:37–46. doi: 10.1007/s00432-006-0143-3. [DOI] [PubMed] [Google Scholar]
- 9.Richards EH, Hickey E, Weber L, Master JR. Effect of overexpression of the small heat shock protein HSP27 on the heat and drug sensitivities of human testis tumor cells. Cancer Res. 1996;56:2446–2451. [PubMed] [Google Scholar]
- 10.Garrido C, Fromentin A, Bonnotte B, Favre N, Moutet M, Arrigo AP, Mehlen P, Solary E. Heat shock protein 27 enhances the tumorigenicity of immunogenic rat colon carcinoma cell clones. Cancer Res. 1998;58:5495–5499. [PubMed] [Google Scholar]
- 11.Rane MJ, Coxon PY, Powell DW, Webster R, Klein JB, Pierce W, Ping P, McLeish KR. p38 Kinase-dependent MAPKAPK-2 activation functions as 3-phosphoinositide-dependent kinase-2 for Akt in human neutrophils. J Biol Chem. 2001;276:3517–3523. doi: 10.1074/jbc.M005953200. [DOI] [PubMed] [Google Scholar]
- 12.Rane MJ, Pan Y, Singh S, Powell DW, Wu R, Cummins T, Chen Q, McLeish KR, Klein JB. Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem. 2003;278:27828–27835. doi: 10.1074/jbc.M303417200. [DOI] [PubMed] [Google Scholar]
- 13.Matsui Y, Hadaschik BA, Fazli L, Andersen RJ, Gleave ME, So AI. Intravesical combination treatment with antisense oligonucleotides targeting heat shock protein-27 and HTI-286 as a novel strategy for high-grade bladder cancer. Mol Cancer Ther. 2009;8:2402–2411. doi: 10.1158/1535-7163.MCT-09-0148. [DOI] [PubMed] [Google Scholar]
- 14.Hadchity E, Aloy MT, Paulin C, Armandy E, Watkin E, Rousson R, Gleave M, Chapet O, Rodriguez-Lafrasse C. Heat shock protein 27 as a new therapeutic target for radiation sensitization of head and neck squamous cell carcinoma. Mol Ther. 2009;17:1387–1394. doi: 10.1038/mt.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charette SJ, Lavoie JN, Lambert H, Landry J. Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol. 2000;20:7602–7612. doi: 10.1128/mcb.20.20.7602-7612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrido C. Size matters: of the small HSP27 and its large oligomers. Cell Death Differ. 2002;9:483–485. doi: 10.1038/sj.cdd.4401005. [DOI] [PubMed] [Google Scholar]
- 17.Parcellier A, Schmitt E, Gurbuxani S, Seigneurin-Berny D, Pance A, Chantome A, Plenchette S, Khochbin S, Solary E, Garrido C. HSP27 is a ubiquitin-binding protein involved in I-kappaBalpha proteasomal degradation. Mol Cell Biol. 2003;23:5790–5802. doi: 10.1128/MCB.23.16.5790-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trautinger F. Mechanisms of photodamage of the skin and its functional consequences for skin ageing. Clin Exp Dermatol. 2001;26:573–577. doi: 10.1046/j.1365-2230.2001.00893.x. [DOI] [PubMed] [Google Scholar]
- 19.de Gruijl FR, van Kranen HJ, Mullenders LH. UV-induced DNA damage, repair, mutations and oncogenic pathways in skin cancer. J Photochem Photobiol B. 2001;63:19–27. doi: 10.1016/s1011-1344(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 20.Sinha RP, Hader DP. UV-induced DNA damage and repair: a review. Photochem Photobiol Sci. 2002;1:225–236. doi: 10.1039/b201230h. [DOI] [PubMed] [Google Scholar]
- 21.Campbell C, Quinn AG, Angus B, Farr PM, Rees JL. Wavelength specific patterns of p53 induction in human skin following exposure to UV radiation. Cancer Res. 1993;53:2697–2699. [PubMed] [Google Scholar]
- 22.Tyrrell RM. UV activation of mammalian stress proteins. Exs. 1996;77:255–271. [PubMed] [Google Scholar]
- 23.Wano C, Kita K, Takahashi S, Sugaya S, Hino M, Hosoya H, Suzuki N. Protective role of HSP27 against UVC-induced cell death in human cells. Exp Cell Res. 2004;298:584–592. doi: 10.1016/j.yexcr.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 24.Trautinger F, Kokesch C, Herbacek I, Knobler RM, Kindas-Mugge I. Overexpression of the small heat shock protein, hsp27, confers resistance to hyperthermia, but not to oxidative stress and UV-induced cell death, in a stably transfected squamous cell carcinoma cell line. J Photochem Photobiol B. 1997;39:90–95. doi: 10.1016/s1011-1344(96)00010-3. [DOI] [PubMed] [Google Scholar]
- 25.Kane KS, Maytin EV. Ultraviolet B-induced apoptosis of keratinocytes in murine skin is reduced by mild local hyperthermia. J Invest Dermatol. 1995;104:62–67. doi: 10.1111/1523-1747.ep12613497. [DOI] [PubMed] [Google Scholar]
- 26.Maytin EV, Murphy LA, Merrill MA. Hyperthermia induces resistance to ultraviolet light B in primary and immortalized epidermal keratinocytes. Cancer Res. 1993;53:4952–4959. [PubMed] [Google Scholar]
- 27.Trautinger F, Kindas-Mugge I, Dekrout B, Knobler RM, Metze D. Expression of the 27-kDa heat shock protein in human epidermis and in epidermal neoplasms: an immunohistological study. Br J Dermatol. 1995;133:194–202. doi: 10.1111/j.1365-2133.1995.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 28.Trautinger F, Kindas-Mugge I, Knobler RM, Honigsmann H. Stress proteins in the cellular response to ultraviolet radiation. J Photochem Photobiol B. 1996;35:141–148. doi: 10.1016/s1011-1344(96)07344-7. [DOI] [PubMed] [Google Scholar]
- 29.Trautinger F, Knobler RM, Honigsmann H, Mayr W, Kindas-Mugge I. Increased expression of the 72-kDa heat shock protein and reduced sunburn cell formation in human skin after local hyperthermia. J Invest Dermatol. 1996;107:442–443. doi: 10.1111/1523-1747.ep12365498. [DOI] [PubMed] [Google Scholar]
- 30.Mendez F, Sandigursky M, Franklin WA, Kenny MK, Kureekattil R, Bases R. Heat-shock proteins associated with base excision repair enzymes in HeLa cells. Radiat Res. 2000;153:186–195. doi: 10.1667/0033-7587(2000)153[0186:hspawb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 31.Higuchi Y, Kita K, Nakanishi H, Wang XL, Sugaya S, Tanzawa H, Yamamori H, Sugita K, Yamaura A, Suzuki N. Search for genes involved in UV-resistance in human cells by mRNA differential display: increased transcriptional expression of nucleophosmin and T-plastin genes in association with the resistance. Biochem Biophys Res Commun. 1998;248:597–602. doi: 10.1006/bbrc.1998.8978. [DOI] [PubMed] [Google Scholar]
- 32.Rocchi P, Jugpal P, So A, Sinneman S, Ettinger S, Fazli L, Nelson C, Gleave M. Small interference RNA targeting heat-shock protein 27 inhibits the growth of prostatic cell lines and induces apoptosis via caspase-3 activation in vitro. BJU Int. 2006;98:1082–1089. doi: 10.1111/j.1464-410X.2006.06425.x. [DOI] [PubMed] [Google Scholar]
- 33.Ilangovan G, Bratasz A, Li H, Schmalbrock P, Zweier JL, Kuppusamy P. In vivo measurement and imaging of tumor oxygenation using coembedded paramagnetic particulates. Magn Reson Med. 2004;52:650–657. doi: 10.1002/mrm.20188. [DOI] [PubMed] [Google Scholar]
- 34.Turakhia S, Venkatakrishnan CD, Dunsmore K, Wong H, Kuppusamy P, Zweier JL, Ilangovan G. Doxorubicin-induced cardiotoxicity: direct correlation of cardiac fibroblast and H9c2 cell survival and aconitase activity with heat shock protein 27. Am J Physiol Heart Circ Physiol. 2007;293:H3111–3121. doi: 10.1152/ajpheart.00328.2007. [DOI] [PubMed] [Google Scholar]
- 35.Stokoe D, Engel K, Campbell DG, Cohen P, Gaestel M. Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Lett. 1992;313:307–313. doi: 10.1016/0014-5793(92)81216-9. [DOI] [PubMed] [Google Scholar]
- 36.Singh IS, Gupta A, Nagarsekar A, Cooper Z, Manka C, Hester L, Benjamin IJ, He JR, Hasday JD. Heat shock co-activates interleukin-8 transcription. Am J Respir Cell Mol Biol. 2008;39:235–242. doi: 10.1165/rcmb.2007-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Callaghan-Sunol C, Gabai VL, Sherman MY. Hsp27 modulates p53 signaling and suppresses cellular senescence. Cancer Res. 2007;67:11779–11788. doi: 10.1158/0008-5472.CAN-07-2441. [DOI] [PubMed] [Google Scholar]
- 38.Putt KS, Beilman GJ, Hergenrother PJ. Direct quantitation of poly(ADP-ribose) polymerase (PARP) activity as a means to distinguish necrotic and apoptotic death in cell and tissue samples. Chembiochem. 2005;6:53–55. doi: 10.1002/cbic.200400330. [DOI] [PubMed] [Google Scholar]
- 39.Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol. 2001;3:245–252. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- 40.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 41.Goubin F, Ducommun B. Identification of binding domains on the p21Cip1 cyclin-dependent kinase inhibitor. Oncogene. 1995;10:2281–2287. [PubMed] [Google Scholar]
- 42.Iyer NG, Chin SF, Ozdag H, Daigo Y, Hu DE, Cariati M, Brindle K, Aparicio S, Caldas C. p300 regulates p53-dependent apoptosis after DNA damage in colorectal cancer cells by modulation of PUMA/p21 levels. Proc Natl Acad Sci U S A. 2004;101:7386–7391. doi: 10.1073/pnas.0401002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maytin EV. Heat shock proteins and molecular chaperones: implications for adaptive responses in the skin. J Invest Dermatol. 1995;104:448–455. doi: 10.1111/1523-1747.ep12605702. [DOI] [PubMed] [Google Scholar]
- 44.Maytin EV, Wimberly JM, Kane KS. Heat shock modulates UVB-induced cell death in human epidermal keratinocytes: evidence for a hyperthermia-inducible protective response. J Invest Dermatol. 1994;103:547–553. doi: 10.1111/1523-1747.ep12396274. [DOI] [PubMed] [Google Scholar]
- 45.Hermann G, Wlaschek M, Lange TS, Prenzel K, GG, Scharffetter-Kochanek K. UVA irradiation stimulates the synthesis of various matrix-metalloproteinases (MMPs) in cultured human fibroblasts. Exp Dermatol. 1993;2:92–97. doi: 10.1111/j.1600-0625.1993.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 46.Kim EH. Inhibition of Heat Shock Protein 27–Mediated Resistance to DNA Damaging Agents by a Novel PKC-V5 Heptapeptide. Cancer Research. 2007;67:6333–6341. doi: 10.1158/0008-5472.CAN-06-4344. [DOI] [PubMed] [Google Scholar]
- 47.Gao C, Zou Z, Xu L, Moul J, Seth P, Srivastava S. p53-dependent induction of heat shock protein 27 (HSP27) expression. Int J Cancer. 2000;88:191–194. [PubMed] [Google Scholar]
- 48.Venkatakrishnan CD, Dunsmore K, Wong H, Roy S, Sen CK, Wani A, Zweier JL, Ilangovan G. HSP27 regulates p53 transcriptional activity in doxorubicin-treated fibroblasts and cardiac H9c2 cells: p21 upregulation and G2/M phase cell cycle arrest. Am J Physiol Heart Circ Physiol. 2008;294:H1736–1744. doi: 10.1152/ajpheart.91507.2007. [DOI] [PubMed] [Google Scholar]
- 49.Wu R, Kausar H, Johnson P, Montoya-Durango DE, Merchant M, Rane MJ. Hsp27 regulates Akt activation and polymorphonuclear leukocyte apoptosis by scaffolding MK2 to Akt signal complex. J Biol Chem. 2007;282:21598–21608. doi: 10.1074/jbc.M611316200. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Shen X. Heat shock protein 27 protects L929 cells from cisplatin-induced apoptosis by enhancing Akt activation and abating suppression of thioredoxin reductase activity. Clin Cancer Res. 2007;13:2855–2864. doi: 10.1158/1078-0432.CCR-06-2090. [DOI] [PubMed] [Google Scholar]
- 51.Porter AG. Protein translocation in apoptosis. Trends Cell Biol. 1999;9:394–401. doi: 10.1016/s0962-8924(99)01624-4. [DOI] [PubMed] [Google Scholar]
- 52.Asada M, Yamada T, Ichijo H, Delia D, Miyazono K, Fukumuro K, Mizutani S. Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation. Embo J. 1999;18:1223–1234. doi: 10.1093/emboj/18.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]