Abstract
We investigated the effect of age-related pseudocapillarization of the liver sinusoidal endothelium on the hepatic disposition of acetaminophen. The multiple indicator dilution technique assessed the hepatic disposition of tracer 14C-acetaminophen and reference markers in isolated perfused livers of young (n = 11) and old (n = 12) rats. Electron microscopy confirmed defenestration of the sinusoidal endothelium in old rats compared with young rats. Acetaminophen recovery following a single pass through the liver was significantly increased in old rats (0.64 ± 0.04, old; 0.59 ± 0.05, young; p < .05). In old age, there was significant reduction of the intercompartmental rate constant k1 (0.34 ± 0.10s-1, old; 0.61 ± 0.38s-1, young; p < .05) and the permeability-surface area product for the transfer of acetaminophen across the sinusoidal endothelium (0.034 ± 0.006 mL/s/g, old; 0.048 ± 0.014 mL/s/g, young; p < .005). There was no difference in k3, the measure of sequestration of acetaminophen that reflects enzyme activity. Age-related pseudocapillarization of the liver sinusoid resulted in increased acetaminophen recovery and decreased transfer of acetaminophen into the liver.
Keywords: Acetaminophen, Pseudocapillarization, Aging, Isolated liver perfusion, Extraction
IT has been proposed that changes in the liver sinusoidal endothelial cells (LSECs) will affect the clearance of protein-bound substrates (1). The LSECs are a specialized endothelium, lining the terminal blood vessels of the liver (the sinusoids), perforated with fenestrations approximately 100 nm in diameter arranged into clusters or “sieve plates” (1). Sieve plates act as a filter separating the sinusoidal blood from the hepatocytes (2). In young healthy livers the LSECs do not impede the passage of plasma or plasma proteins, lipoproteins, and free or protein-bound drugs into the space of Disse (1). However, changes in the LSECs due to aging or disease can impair the transfer of solutes across the endothelium. In cirrhosis, capillarization occurs, whereby defenestration and reduction in the porosity of the sinusoidal endothelium have been associated with impaired uptake of solute. This is consistent with a slower solute diffusivity and longer diffusion path length as a consequence of collagenization of the Disse space (3). In cirrhotic rats, the recovery of lidocaine (>80% protein bound) increases with increasing degrees of capillarization (1,4).
With aging, the endothelium of the liver sinusoid undergoes ultrastructural changes termed “pseudocapillarization” (5,6). These changes are similar to, but less severe than, the capillarization of the LSECs that occurs in liver cirrhosis (4,7) and include a reduction in the size and number of fenestrations, thickening of the endothelium by approximately 50%, inconsistent formation of basement membrane, and minor deposition of collagen into the space of Disse (1,5,6). The bridging fibrosis, nodular regeneration, periportal or pericentral fibrosis, and loss of hepatocyte microvilli seen in cirrhosis are not seen with normal aging (5). Defenestration is associated with impaired transfer of small chylomicrons from the sinusoidal blood to the space of Disse in old rats and may provide a novel mechanism for age-related hyperlipidemia (8).
It is likely that a direct consequence of pseudocapillarization is pharmacological (9). Combined with a reduction in liver size and blood flow (9), pseudocapillarization may have important consequences for hepatic drug disposition and clearance (1). It has been suggested that for capacity-limited (low extraction ratio) drugs like acetaminophen, the reduction in intrinsic clearance could be secondary to altered liver size, enzyme activity, or transfer (10). In rats, aging is associated with reduced Phase I enzyme activity (particularly for males) and reduced clearance of drugs metabolized by these enzymes (10). In both rats and humans, in vitro Phase II enzyme activity does not appear to change with age; however, a recent reanalysis of studies found acetaminophen clearance was reduced in old age (10). It has been proposed that defenestration will impede the clearance of protein-bound substrates and that the reduction in extraction of protein-bound drugs should be directly proportional to the degree of defenestration (1). In mice with hepatic steatosis, the reduced susceptibility to acetaminophen hepatotoxicity is a likely consequence of defenestration of the liver sinusoidal endothelium in addition to reduced expression of CYP2E1 (11).
Acetaminophen is the commonest cause of drug-induced liver injury and is used by approximately 50% of older people. The use of acetaminophen in older people is limited by concerns about the risk of hepatotoxicity. In studies involving old rats, the risk of acetaminophen hepatotoxicity appears to be reduced (12); however, the risk of hepatotoxicity of acetaminophen in older people at both therapeutic and supratherapeutic doses remains unclear. Older frail adults taking therapeutic acetaminophen have higher serum acetaminophen concentrations but do not appear to experience elevated alanine aminotransferase activity (a marker of hepatotoxicity) as younger people do (13). Changes in the hepatic disposition of acetaminophen in old age may partially explain the apparent reduction in hepatotoxicity.
We recently reported a reduction in the hepatic extraction of acetaminophen in rats treated with Poloxamer-407 (14), a surfactant which causes acute defenestration of the LSECs and can be used as an a model of age-related pseudocapillarization (15). However, it is still unknown how age-related pseudocapillarization alters the hepatic disposition of acetaminophen, which is <20% protein bound in plasma. Therefore, the aim of this study was to determine the effect of age-related defenestration of the liver sinusoid on the hepatic extraction of acetaminophen in old rats.
MATERIALS AND METHODS
Animals
Young mature adult (aged 4–6 months, weight 370–490 g) and old adult (aged 24–26 months, weight 310–430 g) Fisher 344 male rats were obtained from the National Institute of Aging (Bethesda, MD). The animals were allowed free access to water and commercial rat pellets ad libitum. This study was approved by the Royal North Shore Hospital Animal Care and Ethics Committee and the University of Sydney Animal Ethics Committee.
Materials
3H-Sucrose (0.1 mCi/mL) was obtained from American Radiolabeled Chemicals (Bioscientific, Sydney, Australia), and 14C-acetaminophen (0.1 mCi/mL) and bovine serum albumin were obtained from Sigma-Aldrich (Sydney, Australia). Carbogen gas (95% O2–5% CO2) was obtained from BOC Gases (North Ryde, Australia). Evans Blue (10 mg/mL) was obtained from APS (Crown Scientific, Sydney, Australia).
Liver Perfusion and Multiple Indicator Dilution Method
Liver perfusion and multiple indicator dilution experiments were performed as described previously (8). To minimize the effect of temporal variation in acetaminophen metabolism (16), liver perfusions were performed between 10 AM and 2 PM and the perfusions on old and young rats were performed within 1 hour of each other. In brief, rats were anesthetized with an intraperitoneal injection of ketamine (75 mg/kg; Parnell Laboratories Pty Ltd, Sydney, Australia) and xylazine (10 mg/kg; Troy Laboratories Pty Ltd, Sydney, Australia). The abdomen was opened with a midline incision. Heparin (300 U) was administered via the inferior vena cava. The portal vein was cannulated with an 18G intravenous catheter (BD, Sydney, Australia) and the thoracic portion of the inferior vena cava with a 12 cm length of PE240 tubing. The liver was perfused in situ with erythrocyte-free oxygenated Krebs-Henseleit bicarbonate buffer (95% O2–5% CO2, 37°C) containing 2% bovine serum albumin for 15 minutes prior to experimentation. The perfusate was delivered by a peristaltic pump (Gilson, Sydney, Australia) in a single-pass mode at 1–1.5 mL/min/g of liver. Liver viability was assessed by macroscopic appearance and portal vein pressure measured using a vertical manometer attached to the portal vein cannula.
The multiple indicator dilution method (17) was used to describe the disposition of acetaminophen in the liver. The injectate used in this study contained 3H-sucrose (0.2 μCi), 14C-acetaminophen (0.2 μCi), and Evans Blue (20 μL) made up to a total of 100 μL volume with Krebs-Henseleit bicarbonate buffer containing 2% bovine serum albumin. 3H-sucrose and Evans Blue are the nonextracted markers of the extracellular and vascular spaces, respectively (18). The injectate was administered as a bolus injection into the portal vein catheter. Outflow samples were collected using a universal fraction collector every 2.1 seconds for a total of 124 seconds. Outflow samples were analyzed for absorbance (Triad Plate Reader; Tecan, Clontarf, Australia) and for 14C- and 3H-specific activity (Tricarb 2900TR liquid scintillation analyzer; Perkin Elmer, Sydney, Australia).
Pharmacokinetic Modeling
The outflow activity for each reference marker and acetaminophen was expressed as a fraction of the injected activity per milliliter, and dose-normalized outflow time-activity curves were constructed. The mean transit time was calculated from the ratio of the area under the first moment of the curve (AUMC) and area under the curve (AUC). The AUMC and AUC were determined using the rectangular rule because the outflow was collected for the entire time period rather than at discrete time points (19). Recovery was estimated from the AUC and flow (Q). It should be noted that recovery is equal to (1 − EH), where EH is the hepatic extraction ratio, which is the fraction of a substrate that is eliminated by the liver. The apparent volume of distribution of markers (acetaminophen, Evans Blue, and sucrose) within the liver was calculated from the mean transit time corrected for time zero (T0) and the flow rate (Q, mL/s). T0 was estimated from the first appearance of 3H and 14C radioactivity above background and represents the transit time of solutes through the nonhepatic parts of the isolated perfused rat liver system (such as tubing) (17,20).
Goresky Model for Barrier Sequestration Exchange
The Goresky model of barrier sequestration exchange (21) was used to model the disposition of acetaminophen in the liver. Using Evans Blue as the reference marker, the intercompartmental rate constants (k1, k2, and k3) were estimated by regression of the acetaminophen outflow curve on the Evans Blue outflow curve (22). The rate constants k1 (per second) and k2 (per second) are the rate constants for influx and efflux of acetaminophen, respectively, whereas k3 (per second) is the rate constant for sequestration and is an indicator of the liver enzyme activity (23). Pharmacokinetic modeling was performed using QBasic (24). The permeability-surface area (PS) product that describes the influx (PSinflux) and efflux (PSefflux) of acetaminophen across the liver sinusoidal endothelium, respectively, were calculated according to the equations (25):
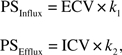 |
where ECV is the extracellular volume calculated as a product of the mean transit time of Evans Blue corrected for T0 and the measured flow rate (21), k1 is the rate constant for influx of acetaminophen, and k2 is the rate constant for efflux of acetaminophen (23). The intracellular volume (ICV) was calculated as 1 − ECV. The units for PSinflux and PSefflux are milliliters per second per gram of liver (mL/s/g).
Crone–Renkin Early Extraction
Outflow curves were also analyzed to determine the permeability-surface area product (PS) for the transfer of acetaminophen across the LSECs (26) using the early extraction method of Crone–Renkin (26–29). This model assumes that a deviation of the outflow curve of diffusible marker (acetaminophen) from that of the vascular marker (Evans Blue) at early time points is caused by the unidirectional transfer of the substrate into the tissue (30). The early extraction (E) ratio of acetaminophen was defined as the extraction of acetaminophen across the LSECs and was calculated using the formula:
where CEB is the fractional outflow concentration of Evans Blue (vascular space marker), and CAPAP is the fractional outflow concentration of acetaminophen (APAP) at each time point.
The early extraction (E) ratio of acetaminophen was obtained from the plateau of the extraction-time curve and was used to calculate the PS product (mL/s/g) for the transfer of acetaminophen across the LSECs according to the equation:
where Q is the flow rate of the perfusate. Note that early extraction (E) is a term that reflects the rate of transfer of a substrate across the endothelium, and this should not be confused with hepatic extraction (EH), which is the fraction of a substrate that is eliminated by the entire liver.
Scanning Electron Microscopy
After the multiple indicator dilution experiments were completed, the livers of five young and five old rats were fixed for scanning electron microscopy using 2% glutaraldehyde–3% paraformaldehyde in 0.1 M sodium cacodylate buffer (0.1 M sucrose, 2 mM CaCl2). Six randomly selected blocks from each animal were then dried and stained, mounted onto stubs, and sputter coated with platinum (31). A Jeol 6380 Scanning Electron Microscope (JEOL Ltd, Tokyo, Japan) at a resolution of 15,000× was used to examine fenestrations in the liver sinusoidal endothelium.
Statistical Analysis
All data analyses were performed using Microsoft Excel. Data are expressed as means ± SD. Comparisons between young and old groups were performed using a two tailed Student’s t test and were considered statistically significant when p < .05.
RESULTS
Recovery and Volume of Distribution
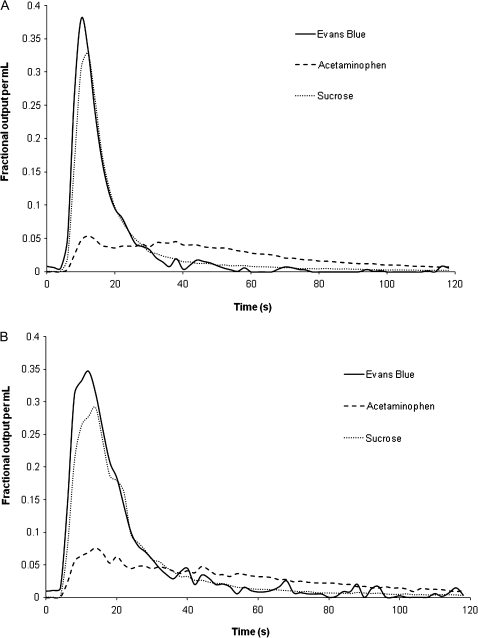
Representative dose-normalized outflow curves for the multiple indicator dilution experiments with young and old rats are shown in Figure 1A and B, respectively. The Evans Blue curve slightly precedes the sucrose curve, indicating that Evans Blue distributes into a smaller liver volume than sucrose, consistent with the albumin space. The curve for acetaminophen is delayed compared with the nonextracted markers indicating distribution of acetaminophen into the cellular space. The AUC for acetaminophen is significantly smaller in both young and old rats compared with the tracers Evans Blue and sucrose, indicating extraction of acetaminophen but not of Evans Blue or sucrose by the liver. The AUC for acetaminophen in old rats is significantly greater than that in young rats, indicating that the hepatic extraction of acetaminophen is reduced in old age.
Figure 1.
Representative dose-normalized outflow curves for acetaminophen, sucrose, and Evans Blue from multiple indicator dilution experiments in isolated perfused rat livers from a young (A) and old (B) rat livers perfused with 2% bovine serum albumin.
The recovery and volume of distribution (Vd) of acetaminophen and tracers are shown in Tables 1 and 2, respectively. The recovery of acetaminophen was significantly higher in old rats compared with young rats (0.64 ± 0.04, old; .0.59 ± 0.05, young; p < .05). The recoveries of sucrose and Evans Blue were not different. The ratio of recovery of acetaminophen to that of sucrose (the non-extracted extracellular marker) was significantly higher in old rats (0.75 ± 0.04, old; 0.70 ± 0.03, young; p < .05; Table 1).
Table 1.
The Effect of Age on the Recovery of Acetaminophen, Evans Blue, and Sucrose. Data Are Presented as Mean ± SD
| Young | Old | |
| n | 11 | 12 |
| Fractional recovery | ||
| Evans Blue | 0.96 ± 0.11 | 0.98 ± 0.11 |
| Sucrose | 0.85 ± 0.07 | 0.86 ± 0.03 |
| Acetaminophen | 0.59 ± 0.05 | 0.64 ± 0.04* |
| Ratio of recovery | ||
| Acetaminophen:sucrose | 0.70 ± 0.03 | 0.75 ± 0.04* |
| Evans Blue:sucrose | 1.13 ± 0.16 | 1.13 ± 0.14 |
| Evans Blue:acetaminophen | 0.63 ± 0.07 | 0.67 ± 0.09 |
p < .05 young versus old.
Table 2.
The Effect of Age on the Apparent Volume of Distribution of Acetaminophen, Evans Blue, and Sucrose. Data Are Presented as Mean ± SD
| Young | Old | |
| n | 11 | 12 |
| Apparent volume of distribution | ||
| Evans Blue (mL/g) | 0.20 ± 0.06 | 0.29 ± 0.08* |
| Sucrose (mL/g) | 0.31 ± 0.07 | 0.38 ± 0.07* |
| Acetaminophen (mL/g) | 0.76 ± 0.11 | 0.74 ± 0.10 |
| Ratio of apparent volume of distribution | ||
| Acetaminophen:sucrose | 2.51 ± 0.26 | 1.95 ± 0.17** |
| Evans Blue:sucrose | 0.65 ± 0.11 | 0.77 ± 0.14 |
| Evans Blue:acetaminophen | 4.00 ± 1.02 | 2.63 ± 0.51** |
p < .05 young versus old,
p < .005 young versus old.
The volume of distribution of acetaminophen was not different between old and young rats, although the Vd of both Evans Blue and sucrose was significantly higher in old rats than in young rats (Table 2). The ratio of the Vd of acetaminophen to sucrose and acetaminophen to Evans Blue was significantly reduced in old rats (1.95 ± 0.17, sucrose; 2.63 ± 0.51, Evans Blue) compared with young rats (2.51 ± 0.26, sucrose, p < .01; 4.00 ± 1.02, Evans Blue, p < .01). However, it should be noted that the Vd of acetaminophen will be reduced by changes in extraction as well as its distribution within the liver.
Goresky Model for Barrier Sequestration Exchange
Regression of the acetaminophen outflow curve was performed according to the Goresky model for barrier sequestration exchange using Evans Blue as the reference marker. The results are presented in Table 3. The intercompartmental rate constant k1 was significantly reduced in old rats compared with young rats (0.34 ± 0.10s-1, old; 0.61 ± 0.38s-1, young; p < .05). Importantly, there was no difference in k3, which is the measure of sequestration of acetaminophen and reflects enzyme activity. There was a trend toward a reduction in PSinflux in old rats (0.13 ± 0.02 mL/s/g, old; 0.18 ± 0.09 mL/s/g, young; p = 0.09). PSEfflux was similar for both old and young rats.
Table 3.
Permeability-Surface Area (PS) Product for the Influx and Efflux of Acetaminophen Across the Liver Sinusoidal Endothelium Calculated According to the Goresky Model of Barrier-Sequestration Exchange Using Evans Blue as the Reference Marker. Data Are Presented as Mean ± SD
| Young | Old | |
| n | 11 | 12 |
| k1 (s-1) | 0.61 ± 0.38 | 0.34 ± 0.10* |
| k2 (s-1) | 0.11 ± 0.06 | 0.10 ± 0.03 |
| k3 (s-1) | 0.003 ± 0.003 | 0.004 ± 0.002 |
| PSInflux (mL/s/g) | 0.27 ± 0.09 | 0.18 ± 0.06** |
| PSEfflux (mL/s/g) | 0.10 ± 0.04 | 0.09 ± 0.02 |
p < .05 young versus old,
p < .01 young versus old.
Crone–Renkin PS Product for Acetaminophen
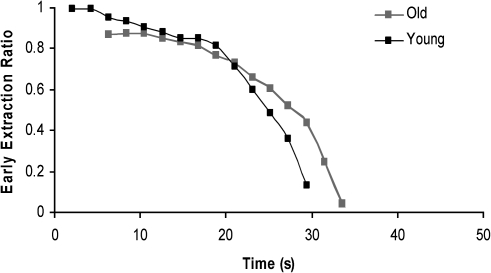
Figure 2 shows a representative curve for a young and an old rat illustrating the relationship between time and the early extraction of acetaminophen. The early extraction of acetaminophen was reduced in old rats, and the plateau time occurred later (Table 4). The permeability-surface area (PS) product was reduced significantly in old rats compared with young rats. This is consistent with reduced extraction due to a barrier to uptake at the level of the LSECs.
Figure 2.
Representative curve demonstrating the relationship between time and the early extraction of acetaminophen for a young and an old rat. The reference marker is Evans Blue.
Table 4.
Early Extraction and Permeability-Surface Area (PS) Product for Acetaminophen Calculated Using the Early Extraction Method of Crone–Renkin. Data Are Presented as Mean ± SD
| Young | Old | |
| n | 11 | 12 |
| PS product (mL/s/g) | 0.048 ± 0.014 | 0.034 ± 0.006* |
| PS product (mL/s) | 0.61 ± 0.17 | 0.42 ± 0.08** |
| Early extraction (%) | 92 ± 3 | 85 ± 4** |
| Plateau time (s) | 8.75 ± 2.34 | 9.93 ± 2.83 |
p < .01 young versus old,
p < .005 young versus old.
Electron Microscopy
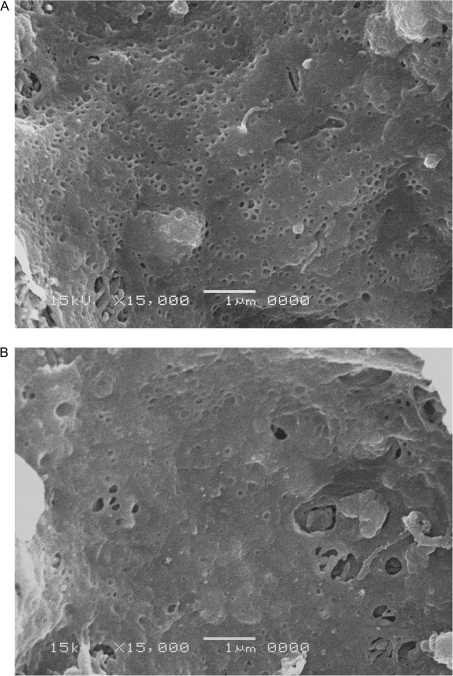
Representative scanning electron micrographs are shown in Figure 3 for a young (Figure 3A) and an old (Figure 3B) rat liver sinusoid. The sinusoidal endothelium of the old rat liver shows reduced numbers of fenestrations consistent with age-related pseudocapillarization.
Figure 3.
Representative scanning electron micrographs of hepatic sinusoids. The sinusoidal endothelium of the young animal (A) is perforated with fenestrations in clusters (“sieve plates”). The sinusoidal endothelium of the old animal (B) has a reduced number of fenestrations.
DISCUSSION
In this study, we investigated the effect of age-related pseudocapillarization of the LSECs on the hepatic disposition of acetaminophen. We found that the hepatic extraction and volume of distribution of acetaminophen was reduced in old rats compared with young rats and that defenestration and thickening of the sinusoidal endothelium impaired the transfer of acetaminophen from the sinusoidal lumen to the hepatocytes. Scanning electron microscopy confirmed the reduced porosity of the liver sieve in old rats compared with young rats, consistent with aging changes reported in rats (5), mice (32), nonhuman primates (33), and humans (6), as well as those reported with the poloxamer model of the aging sinusoidal endothelium (15). We therefore propose that the age-related change in the hepatic extraction of acetaminophen can be attributed to pseudocapillarization of the hepatic sinusoid.
Using the multiple indicator dilution method, we observed the hepatic extraction of acetaminophen and its ratio to that of the nonextracted marker sucrose was significantly lower in old rats compared with young rats. The hepatic extraction ratio of 0.41 for young rats and 0.36 for old rats was consistent with the ratios reported by other authors (EH = 0.08–0.65) (34,35). Interestingly, the hepatic extraction of acetaminophen can vary depending on the time of day (0.21–0.41) (16). However, as our liver perfusions were performed between 10 AM and 2 PM with old and young rats being perfused within 1 hour of each other, this is unlikely to be a major factor for our study. Therefore, the results indicate that in our study of rat livers perfused with albumin but no erythrocytes, the extraction ratio falls midway between reported values and is consistent with those that we reported previously in the perfused livers of young rats with poloxamer-mediated defenestration (EH = 0.33) (14). Furthermore, this mid-range extraction in rats is consistent with values reported for humans (36). Importantly, the reduction in hepatic extraction in old rats parallels the reduction in acetaminophen clearance in aging humans (10).
In old rats, acetaminophen had restricted access to both the extracellular sucrose space and the Evans Blue space indicated by a reduction in the ratio of apparent volume of distribution of acetaminophen to sucrose and to Evans Blue. Evans Blue is usually considered to be a vascular reference; however, it is best considered to be a marker of the albumin space (18). Our results indicate that acetaminophen has access to the entire Evans Blue space in young but not old rat livers. The small but significant increase in the Evans Blue volume of distribution may reflect the small increase in extracellular (sucrose) volume with age reported previously (37) and also observed in our study. This indicates that the pseudocapillarized aging endothelium does not impede the distribution of sucrose into the extracellular space (8). Similarly, capillarization in cirrhotic livers does not impede the distribution of sucrose (38).
The sequestration/barrier limited model of Goresky using Evans Blue as a reference marker was applied to the data. The permeability-surface area (PS) product for the influx (PSinflux) and efflux (PSefflux) of acetaminophen across the liver sinusoidal endothelium were calculated (25). There was a highly significant reduction in the PSinflux product for acetaminophen in old rats compared with young rats. The PSefflux product was smaller than PSinflux but was unchanged between old and young rats, indicating no age-related difference in acetaminophen efflux. Importantly, k3, which reflects enzyme activity, was unchanged with age. Pseduocapillarization of the hepatic sinusoid in aging resulting in an age-specific reduction in the hepatic extraction of acetaminophen may provide a novel explanation of the age-related reduction in acetaminophen clearance in older adults (10,39).
The Crone–Renkin early extraction model was applied to the data, and a significant reduction with age in the permeability-surface (PS) area product of acetaminophen was observed. This reduction is consistent with what we have previously observed with our poloxamer model with the PS product for acetaminophen being reduced by approximately 8% in rats with defenestration (14). In rats with carbon tetrachloride–induced cirrhosis, the PS product for drugs with minimal protein binding was reduced by 10% (3). Despite the authors investigating the permeability layer at the level of the hepatocyte membrane and not at the LSECs as in our study, the authors did acknowledge that in cirrhotic livers collagenization of the space of Disse results in impaired uptake of solute across the thickened and defenestrated endothelium (3).
Defenestration of the LSEC influences the hepatic clearance of medications due to impaired transfer of protein-bound substrates or dissolved substrates through the fenestrations (1). Poloxamer-mediated defenestration has been associated with increased recovery of small chylomicrons (15) and acetaminophen in the isolated perfused liver (14). Similarly, defenestration associated with cirrhosis resulted in exclusion of albumin-bound indocyanine green from the space of Disse in humans (40), and with impaired transfer and clearance of albumin-bound lignocaine (4) and propranolol (41) in rats. In this study, old age was associated with increased recovery of acetaminophen following a single pass through the isolated perfused rat liver. Given that acetaminophen is minimally protein bound, this indicates that fenestrations facilitate the passage of water-soluble substrates, which is reduced with pseudocapillarization of the hepatic sinusoid in old age.
We acknowledge the limitations of this study. There may have been contamination of the outflow curves by the metabolites of acetaminophen, which we were unable to quantify. Following a single pass through the liver at the tracer concentrations used in this study, the main metabolite is the sulfate metabolite (42). However, this metabolite is much lower in concentration and is quite delayed compared with acetaminophen and contributes only a small amount to total outflow radioactivity (42); therefore, this is unlikely to have affected our result. The pH is an important parameter for the state of ionization of the solutes and is a key regulator of trafficking of solutes across cell membranes. However, pH is unlikely to be an important variable in this study because acetaminophen has a pKa of 9.9 and is therefore unionized at physiological pH (43), pH partitioning is across membranes, and is therefore not directly relevant for fenestrations. Additionally there is no effect of old age on intracellular pH (44).
The risk of hepatotoxicity from acetaminophen may be reduced in old age, despite reduced clearance (10,12,13). The mechanism is poorly understood. The expression of CYP2E1, which metabolizes acetaminophen into the toxic metabolite, NAPQI, appears to be increased in old age (45). However, the activity has been reported to be decreased in old rats (45) or unchanged in humans (46). Our study supports the hypothesis that it is pseudocapillarization of the sinusoidal endothelium that may impede the transfer of acetaminophen to the liver in old age, reducing the risk of hepatotoxicity. Given that the intercompartmental rate constant k3, an indicator of enzyme activity, was unchanged with age, it is likely that most of the age-related reduction in hepatic clearance of acetaminophen (10) is due to psuedocapillarization. Furthermore, as transfer across the LSEC from the sinusoidal lumen to space of Disse is the first step for hepatic uptake of a drug, the effects of any changes in subsequent parts of the pathway (such as in conjugation or cytochrome P450 enzymes) will be minimized.
CONCLUSION
This study has shown that age-related pseudocapillarization of the sinusoidal endothelium resulted in a small but significant reduction in the extraction and apparent volume of distribution of acetaminophen in the isolated perfused rat liver. These results confirm those reported previously using the poloxamer 407–induced defenestration model (14). Furthermore, these results support the role of the fenestrations in the hepatic disposition of pharmaceutical substrates and confirm that defenestration will reduce hepatic extraction of water-soluble medications with low protein binding by restricting access to the extracellular space of Disse (1). These results have important implications for medication efficacy and safety in old age.
FUNDING
This study was funded by the Geoff and Elaine Penney Aging Research Unit, Royal North Shore Hospital, by the National Health and Medical Research Council of Australia project grants #570968 and #570937, the Ageing and Alzheimers Research Foundation and was supported in part by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health, USA.
Acknowledgments
We gratefully acknowledge Dr. Laurent Rivory who created the software, which was used for the Goresky analysis. Dr. Rafael de Cabo is supported by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health, USA.
References
- 1.Le Couteur DG, Fraser R, Hilmer SN, Rivory LP, McLean AJ. The hepatic sinusoid in aging and cirrhosis: effects on hepatic substrate disposition and drug clearance. Clin Pharmacokinet. 2005;44(2):187–200. doi: 10.2165/00003088-200544020-00004. [DOI] [PubMed] [Google Scholar]
- 2.Fraser R, Dobbs BR, Rogers GT. Lipoproteins and the liver sieve: the role of the fenestrated sinusoidal endothelium in lipoprotein metabolism, atherosclerosis, and cirrhosis. Hepatology. 1995;21(3):863–874. [PubMed] [Google Scholar]
- 3.Hung DY, Chang P, Cheung K, et al. Cationic drug pharmacokinetics in diseased livers determined by fibrosis index, hepatic protein content, microsomal activity, and nature of drug. J Pharmacol Exp Ther. 2002;301(3):1079–1087. doi: 10.1124/jpet.301.3.1079. [DOI] [PubMed] [Google Scholar]
- 4.Varin F, Huet PM. Hepatic microcirculation in the perfused cirrhotic rat liver. J Clin Invest. 1985;76(5):1904–1912. doi: 10.1172/JCI112186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Couteur DG, Cogger VC, Markus AMA, et al. Pseudocapillarization and associated energy limitation in the aged rat liver. Hepatology. 2001;33(3):537–543. doi: 10.1053/jhep.2001.22754. [DOI] [PubMed] [Google Scholar]
- 6.McLean AJ, Cogger VC, Chong GC, et al. Age-related pseudocapillarization of the human liver. J Pathol. 2003;200(1):112–117. doi: 10.1002/path.1328. [DOI] [PubMed] [Google Scholar]
- 7.Reichen J, Egger B, Ohara N, Zeltner TB, Zysset T, Zimmermann A. Determinants of hepatic-function in liver-cirrhosis in the rat—multivariate analysis. J Clin Invest. 1988;82(6):2069–2076. doi: 10.1172/JCI113828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilmer SN, Cogger VC, Fraser R, McLean AJ, Sullivan D, Le Couteur DG. Age-related changes in the hepatic sinusoidal endothelium impede lipoprotein transfer in the rat. Hepatology. 2005;42(6):1349–1354. doi: 10.1002/hep.20937. [DOI] [PubMed] [Google Scholar]
- 9.Le Couteur DG, McLean AJ. The aging liver. Drug clearance and an oxygen diffusion barrier hypothesis. Clin Pharmacokinet. 1998;34(5):359–373. doi: 10.2165/00003088-199834050-00003. [DOI] [PubMed] [Google Scholar]
- 10.Butler JM, Begg EJ. Free drug metabolic clearance in elderly people. Clin Pharmacokinet. 2008;47(5):297–321. doi: 10.2165/00003088-200847050-00002. [DOI] [PubMed] [Google Scholar]
- 11.Ito Y, Abril ER, Bethea NW, McCuskey MK, McCuskey RS. Dietary steatotic liver attenuates acetaminophen hepatotoxicity in mice. Microcirculation. 2006;13(1):19–27. doi: 10.1080/10739680500383423. [DOI] [PubMed] [Google Scholar]
- 12.Rikans LE, Moore DR. Acetaminophen hepatotoxicity in aging rats. Drug Chem Toxicol. 1988;11(3):237–247. doi: 10.3109/01480548809017880. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell SJ, Hilmer SN, Murnion BP, Matthews S. Hepatotoxicity of therapeutic short-course paracetamol in hospital inpatients: impact of ageing and frailty. J Clin Pharm Ther. 2010 doi: 10.1111/j.1365-2710.2010.01193.x. In press. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell SJ, Huizer-Pajkos A, Cogger VC, McLachlan AJ, Couteur DGL, Hilmer SN. Poloxamer 407 increases the recovery of paracetamol in the isolated perfused rat liver. J Pharm Sci. 2011;100(1):344–340. doi: 10.1002/jps.22235. [DOI] [PubMed] [Google Scholar]
- 15.Cogger VC, Hilmer SN, Sullivan D, Muller M, Fraser R, Le Couteur DG. Hyperlipidemia and surfactants: the liver sieve is a link. Atherosclerosis. 2006;189(2):273–281. doi: 10.1016/j.atherosclerosis.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 16.Bélanger PM, Lalande M, Doré F, Labrecque G. Time-dependant variations in the organ extraction ratios of acetaminophen in rats. J Pharmacokinet Biopharm. 1987;15(2):133–143. doi: 10.1007/BF01062340. [DOI] [PubMed] [Google Scholar]
- 17.Goresky CA. A linear method for determining liver sinusoidal and extravascular volumes. Am J Physiol. 1963;204:626–640. doi: 10.1152/ajplegacy.1963.204.4.626. [DOI] [PubMed] [Google Scholar]
- 18.Goresky CA. Kinetic interpretation of hepatic multiple-indicator dilution studies. Am J Physiol. 1983;245(1):G1–G12. doi: 10.1152/ajpgi.1983.245.1.G1. [DOI] [PubMed] [Google Scholar]
- 19.Hilmer SN, Cogger VC, Muller M, Le Couteur DG. The hepatic pharmacokinetics of doxorubicin and liposomal doxorubicin. Drug Metab Dispos. 2004;32(8):794–799. doi: 10.1124/dmd.32.8.794. [DOI] [PubMed] [Google Scholar]
- 20.Goresky CA, Silverman M. Effect of correction of catheter distortion on calculated liver sinusoidal volumes. Am J Physiol. 1964;207:883–892. doi: 10.1152/ajplegacy.1964.207.4.883. [DOI] [PubMed] [Google Scholar]
- 21.Le Couteur DG, Rivory LP, Pond SM. Glucose transport and hypoxia-reoxygenation injury in the perfused rat liver. J Gastroenterol Hepatol. 1994;9(4):385–390. doi: 10.1111/j.1440-1746.1994.tb01260.x. [DOI] [PubMed] [Google Scholar]
- 22.Goresky CA, Nadeau BE. Uptake of materials by the intact liver. The exchange of glucose across the cell membranes. J Clin Invest. 1974;53:634–646. doi: 10.1172/JCI107598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pang KS, Lee W-F, Cherry WF, et al. Effects of perfusate flow on measured blood volume, Disse space, intracellular water space and drug extraction in the perfused rat liver preparation: characterisation by the multiple indicator dilution technique. J Pharmacokinet Biopharm. 1988;16(6):595–632. doi: 10.1007/BF01062014. [DOI] [PubMed] [Google Scholar]
- 24.Rivory LP. Brisbane: University of Queensland; 1990. Probing hepatic structure and function with multiple indicator dilution technique (PhD thesis) [Google Scholar]
- 25.Le Couteur DG, Rivory LP, Pond SM. Hepatic intracellular pH during the prereplicative period following partial hepatectomy. Am J Physiol. 1993;264(4 Pt 1):G767–G773. doi: 10.1152/ajpgi.1993.264.4.G767. [DOI] [PubMed] [Google Scholar]
- 26.Cho CS, McLean AJ, Rivory LP, Gatenby PA, Hardman DT, Le Couteur DG. Carbon monoxide wash-in method to determine gas transfer in vascular beds: application to rat hindlimb. Am J Physiol Heart Circ Physiol. 2001;280(4):H1802–H1826. doi: 10.1152/ajpheart.2001.280.4.H1802. [DOI] [PubMed] [Google Scholar]
- 27.Crone C. The permeability of capillaries in various organs as determined by use of the indicator diffusion method. Acta Physiol Scand. 1963;58:292–305. doi: 10.1111/j.1748-1716.1963.tb02652.x. [DOI] [PubMed] [Google Scholar]
- 28.Crone C, Levitt DG. Handbook of Physiology The Cardiovascular System. Bethesda, MD: American Physiology Society; 1984. Capillary Permeability to Small Solutes; pp. 411–466. [Google Scholar]
- 29.Renkin EM. Transport of potassium-42 from blood to tissue in isolated mammalian skeletal muscles. Am J Physiol. 1959;197:1205–1210. doi: 10.1152/ajplegacy.1959.197.6.1205. [DOI] [PubMed] [Google Scholar]
- 30.Le Couteur DG, Yin ZL, McLean AJ, Rivory LP. Wash-in methodology and modeling to determine hepatocellular D-glucose transport in the perfused rat liver. Jnp J Physiol. 2004;54(4):421–429. doi: 10.2170/jjphysiol.54.421. [DOI] [PubMed] [Google Scholar]
- 31.Hilmer SN, Warren A, Cogger VC, et al. The effect of aging on the immunohistochemistry of apolipoprotein E in the liver. Exp Gerontol. 2004;39(1):53–57. doi: 10.1016/j.exger.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 32.Warren A, Bertolino P, Cogger VC, McLean AJ, Fraser R, Le Couteur DG. Hepatic pseudocapillarization in aged mice. Exp Gerontol. 2005;40(10):807–812. doi: 10.1016/j.exger.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Cogger VC, Warren A, Fraser R, Ngu M, McLean AJ, Le Couteur DG. Hepatic sinusoidal pseudocapillarization with aging in the non-human primate. Exp Gerontol. 2003;38(10):1101–1107. doi: 10.1016/j.exger.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Goresky CA, Pang KS, Schwab AJ, Barker F, III, Cherry WF, Bach GG. Uptake of a protein-bound polar compound, acetaminophen sulfate, by perfused rat liver. Hepatology. 1992;16:173–90. doi: 10.1002/hep.1840160129. [DOI] [PubMed] [Google Scholar]
- 35.Hirate J, Zhu CY, Horikoshi I, Bhargava VO. 1st-Pass metabolism of acetaminophen in rats after low and high-doses. Biopharm Drug Dispos. 1990;11(3):245–252. doi: 10.1002/bdd.2510110309. [DOI] [PubMed] [Google Scholar]
- 36.Rawlins MD, Henderson DB, Hijab AR. Pharmacokinetics of paracetamol after intravenous and oral administration. Eur J Clin Pharmacol. 1977;11(4):283–286. doi: 10.1007/BF00607678. [DOI] [PubMed] [Google Scholar]
- 37.Le Couteur DG, Rivory LP, Yi C, Pond SM. Aging, acute oxidative injury and hepatocellular glucose transport in the rat. Int Hepatol Commun. 1995;3:244–253. [Google Scholar]
- 38.Reichen J, Le M. Verapamil favorably influences hepatic microvascular exchange and function in rats with cirrhosis of the liver. J Clin Invest. 1986;78(2):448–455. doi: 10.1172/JCI112596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wynne HA, Cope LH, Herd B, Rawlins MD, James OF, Woodhouse KW. The association of age and frailty with paracetamol conjugation in man. Age Ageing. 1990;19(6):419–424. doi: 10.1093/ageing/19.6.419. [DOI] [PubMed] [Google Scholar]
- 40.Huet PM, Goresky CA, Villeneuve JP, Marleau D, Lough JO. Assessment of liver microcirculation in human cirrhosis. J Clin Invest. 1982;70(6):1234–1244. doi: 10.1172/JCI110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fenyves D, Gariepy L, Villeneuve JP. Clearance by the liver in cirrhosis. I. Relationship between propranolol metabolism in vitro and its extraction by the perfused liver in the rat. Hepatology. 1993;17(2):301–306. [PubMed] [Google Scholar]
- 42.Pang KS, Simard A, Schwab AJ, Goresky CA. Sulfation of acetaminophen by the perfused rat liver: the effect of red blood cell carriage. Hepatology. 1995;22(1):267–282. [PubMed] [Google Scholar]
- 43.Bainbridge CA, Kelly EL, Walkling WD. In vitro adsorption of acetaminophen onto activated charcoal. J Pharm Sci. 1977;66(4):480–483. doi: 10.1002/jps.2600660405. [DOI] [PubMed] [Google Scholar]
- 44.Le Couteur DG, Rivory LP, Pond SM. The effects of aging and nutritional state on hypoxia-reoxygenation injury in the perfused rat liver. Transplantation. 1994;58(5):531–536. doi: 10.1097/00007890-199409150-00001. [DOI] [PubMed] [Google Scholar]
- 45.Wauthier V, Schenten V, Verbeeck RK, Calderon PB. Ageing is associated with increased expression but decreased activity of CYP2E1 in male Wistar rats. Life Sci. 2006;79(20):1913–1920. doi: 10.1016/j.lfs.2006.06.046. [DOI] [PubMed] [Google Scholar]
- 46.Hunt CM, Strater S, Stave GM. Effect of normal aging on the activity of human hepatic cytochrome P450IIE1. Biochem Pharmacol. 1990;40(7):1666–1669. doi: 10.1016/0006-2952(90)90470-6. [DOI] [PubMed] [Google Scholar]