Abstract
Background.
Advanced glycation end products (AGEs) are thought to cause inflammation through interaction with the receptor for AGEs (RAGE), therefore contributing to adverse aging-related processes. The relationship between AGEs, RAGE, and inflammation has not been well characterized.
Methods.
We examined the relationship of plasma endogenous secretory RAGE (esRAGE); carboxymethyl-lysine (CML), a circulating AGE; and inflammatory mediators in 1,298 adults, 20–97 years, who participated in the InCHIANTI study in Tuscany, Italy. Blood levels of esRAGE, CML, interleukin-1 receptor antagonist (IL-1RA), IL-1β, tumor necrosis factor-α (TNF-α), IL-6, IL-6 receptor (IL-6R), IL-18, C-reactive protein (CRP), transforming growth factor-β (TGF-β), and fibrinogen were measured.
Results.
Log plasma esRAGE was associated with log IL-1RA (β = −0.069, SE = 0.036, p = .05) and log IL-6 (β = 0.077, SE = 0.035, p = .03), respectively, in separate multivariable linear regression models, adjusting for potential confounders. Log plasma esRAGE was also negatively associated with log TGF-β but did not reach statistical significance (β = −0.091, SE = 0.053, p = .09). Log plasma esRAGE was not significantly associated with log IL-1β, log TNF-α, IL-6R, log IL-18, or CRP. Log plasma CML was not associated with any of the inflammatory mediators except for IL-6R (β = −14.10, SE = 5.94, p = .02) and fibrinogen (β = 13.95, SE = 7.21, p = .05) in separate multivariable models, adjusting for potential confounders.
Conclusions.
Plasma esRAGE is correlated with higher IL-6 and lower IL-1RA. These findings suggest that plasma esRAGE plays a role in modulating inflammation, although the exact mechanisms remain to be elucidated.
Keywords: Advanced glycation end products, C-reactive protein, Endogenous secretory receptor for advanced glycation end products, Interleukin-1 receptor antagonist, Interleukin-6
INTRODUCTION
A low-grade proflammatory state characterized by elevated inflammatory mediators is common among older adults and is associated with pathological processes in multiple systems such as endothelial dysfunction, atherosclerosis, and sarcopenia that are characteristic of declining health (1). The underlying causes of this inflammatory state are not well understood. Recent studies suggest that advanced glycation end products (AGEs) and the receptor for AGEs (RAGE) play a role in a wide spectrum of pathological processes related to aging and perhaps, part of this effect is due to upregulated inflammation (2). AGEs are bioactive compounds formed by the nonenzymatic glycation of proteins and other molecules. Humans are exposed to exogenous AGEs in foods that are absorbed in digestion and endogenous AGEs generated by abnormal glucose metabolism and oxidative stress (2).
AGEs increase reactive oxygen species and inflammation through binding with RAGE (3). RAGE is a member of the immunoglobulin superfamily of cell surface molecules and contains one V-type and two C-type immunoglobulin domains (3). The binding of ligands with RAGE results in the upregulation of proinflammatory cytokines such as IL-6 via the NF-κB pathway and increases the expression of cell surface RAGE (3). RAGE exists in the circulation in two main forms, RAGE, which has been cleaved off the cell surface by matrix metalloproteinases, and endogenous secretory RAGE (esRAGE), a novel splice variant that is expressed by cells and is capable of binding AGEs (4). The function of esRAGE is not well understood. Whether elevated esRAGE is related to the common proinflammatory state of aging is not clear.
The proinflammatory state in older adults is characterized by elevations in inflammatory mediators, such as interleukin-1 receptor antagonist (IL-1RA), IL-1β, tumor necrosis factor-α (TNF-α), IL-6, IL-6 receptor (IL-6R), IL-18, C-reactive protein (CRP), transforming growth factor-β (TGF-β), and fibrinogen (1). These inflammatory mediators are commonly elevated in older adults, and many of these mediators are predictive of adverse clinical outcomes (5).
RAGE signaling plays a role in inflammation and oxidative stress (2,3), but the relationship of circulating esRAGE and AGEs with inflammation has not been well characterized in older adults. We hypothesized that elevated esRAGE and carboxymethyl-lysine (CML), a major circulating AGE, were associated with elevated inflammatory mediators. To address this hypothesis, we examined the relationships between plasma esRAGE and CML and inflammation in a population-based study of community-dwelling adults.
METHODS
The study participants consisted of men and women, aged 20–97 years, who participated in the Invecchiare in Chianti, “Aging in the Chianti Area” (InCHIANTI) study, conducted in two small towns in Tuscany, Italy. The rationale, design, and data collection have been described elsewhere, and the main outcome of this longitudinal study is mobility disability (6). Briefly, in August 1998, 1,299 subjects aged 65 or older years and 431 subjects from age strata 20–29, 30–39, 40–49, 50–59, and 60–64 years were randomly selected from a population registry of two sites in the region of Florence, Greve in Chianti (population 11,709) and Bagno a Ripoli (population 4,704). Of those subjects, 1,453 agreed to participate. Participants received an extensive description of the study and participated after written informed consent. The study protocol complied with the Declaration of Helsinki and was approved by the Italian National Institute of Research and Care on Aging Ethical Committee. The plan for additional laboratory and data analyses was approved by the Institutional Review Board of the Johns Hopkins University School of Medicine.
Of the 1,453 participants seen at enrollment, 1,298 (89.3%) participated in the blood drawing and had laboratory measurements conducted. The participants who did not complete the blood drawing were generally older and had greater comorbidity than those who participated in the blood drawing, as reported elsewhere (7). A home interview and a medical evaluation provided information on age, sex, education, smoking history, aspirin use, body mass index, Mini-Mental State Examination, and chronic diseases as previously described (6).
Demographic information and information on smoking and medication use were collected using standardized questionnaires. Education was recorded as years of school. All participants were examined by a trained geriatrician, and diseases were ascertained according to standard preestablished criteria and algorithms based upon those used in the Women’s Health and Aging Study for coronary heart disease, chronic heart failure, stroke, and cancer (8). Fasting plasma glucose was defined as normal, impaired, or diabetic based upon a fasting plasma glucose of less than or equal to 99, 100–125, and more than 125 mg/dL, respectively (9). The diagnostic algorithm for the diagnosis of diabetes was based upon the use of insulin, oral hypoglycemic agents, and a questionnaire administered to the primary care physician of the study participant (6). Weight was measured using a high-precision mechanical scale. Standing height was measured to the nearest 0.1 cm. Body mass index was calculated as weight/height2 (kilograms per square meter). A Mini-Mental State Examination score less than 24 was considered consistent with cognitive impairment (10). Estimated glomerular filtration rate (eGFR; mL/min/1.73 m2) was calculated using the four-variable Modification of Diet in Renal Disease Study equation of Levey and colleagues (11).
Blood samples were collected in the morning after a 12-hour fast. Aliquots of serum and plasma were immediately obtained and stored at −80°C. Plasma esRAGE was measured using a sandwich enzyme-linked immunosorbent assay (ELISA) (12) with a detection limit of 0.05 ng/mL and a reference range of 0.05–3.2 ng/mL (ELISA) according to the manufacturer (esRAGE ELISA Kit; B-Bridge International, Mountain View, CA). The interassay and intra-assay coefficients of variation for esRAGE in the present laboratory analysis were 9.3% and 4.4%, respectively. Plasma CML was measured using a competitive ELISA with a detection limit of 5 ng/mL and a reference range of 5–125 ng/mL according to the manufacturer (AGE-CML ELISA; Microcoat, Penzberg, Germany) (13). The interassay and intra-assay coefficients of variation for CML in the present laboratory analysis were 12.1% and 3.0%, respectively.
Serum IL-1RA, IL-1β, TNF-α, IL-6, and IL-6R were measured in duplicate using different ELISA with detection limits of 4, 0.01, 0.9, 0.1, and 8 pg/mL and reference ranges of 30–1,700, 0.31–20, 0.5–32.0, 0.16–10, and 62.5–4,000 pg/mL respectively, as determined by the manufacturer (all BIOSOURCE International, Camarillo, CA). IL-18 and TGF-β were measured in duplicate by ELISA method, using two commercial kits with detection limits of 0.7 and 7 pg/mL and reference ranges of 40–1,000 and 31.2–2,000 pg/mL respectively, according to the manufacturer (Quantikine; R&D Systems, Minneapolis, MN). CRP was measured in duplicate using a particle-enhanced immunonephelometric assay and the Dade Behring BNII nephelometer (Dade Behring Inc., Deerfield, IL). Plasma fibrinogen was determined through an automated Sta-Stago Analyzer system using a commercially available STA fibrinogen kit according to the Clauss method (Diagnostica Stago; Roche Diagnostics GmbH, Mannheim, Germany). The interassay and intra-assay coefficients of variation for IL-1RA, IL-1β, TNF-α, IL-6, IL-6R, IL-18, TFG-β, CRP, and fibrinogen were all less than 7% for all analytes in the present laboratory analysis. Total cholesterol, triglycerides, uric acid, and blood glucose levels were determined by enzymatic colorimetric methods (Roche Diagnostic GmbH). Low-density lipoprotein was calculated using the Friedewald formula. High-density lipoprotein cholesterol was measured by a direct method using a detergent for the selective solubilization of high-density lipoprotein cholesterol that was then detected by an enzymatic colorimetric assay and a Roche analyzer (Roche Diagnostics GmbH). Polyunsaturated fatty acids (PUFA) were measured using gas chromatography as described in detail elsewhere (14). Carotenoids were measured using high-performance liquid chromatography.
Variables with a skewed distribution (all inflammatory mediators except fibrinogen and IL-6R) were log transformed to normalize the distribution. Variables are reported as means (SD) or as percentages. Univariable linear regression models were used to examine the relationship between esRAGE and demographic and other potential risk factors. Separate multivariate linear regression models were used to examine the relationship between esRAGE and individual inflammatory mediators, where the individual inflammatory mediator was the dependent variable. Additional multivariate linear regression models were used to examine the relationship between CML and individual inflammatory mediators. All models were examined for interactions between esRAGE and age and between CML and age, respectively. Because the inflammatory biomarkers in this study represent a complex network of interacting cytokines, receptors, and other mediators, adjustment for multiple comparisons would inappropriately reduce the power of our analyses. All analyses were performed using SAS (v. 9.1.3; SAS Institute, Inc., Cary, NC) with a Type I error of 0.05.
RESULTS
The demographic characteristics, anthropometry, lipid profiles, antioxidants, inflammatory mediators, and chronic diseases of the study participants are shown in Table 1. The prevalence of chronic diseases was fairly low, as would be expected in this population-based sample. In Table 2, univariable linear regression models were used to explore the relationship between demographic, laboratory, and other variables with plasma esRAGE. Gender, body mass index, estimated glomerular filtration rate, triglycerides, CML, and congestive heart failure were significantly associated with plasma esRAGE concentrations. Age, education, Mini-Mental State Examination score, alcohol intake, smoking status, aspirin use, fasting blood glucose, total cholesterol, high-density lipoprotein and low-density lipoprotein cholesterol, polyunsaturated fatty acid, total carotenoids, α-tocopherol, uric acid, hypertension, angina, stroke, diabetes, peripheral artery disease, and cancer were not significantly associated with plasma esRAGE concentrations.
Table 1.
Demographic and Other Characteristics of Adults, 20–97 Years, in the InCHIANTI Study
| Characteristic | N | M (SD) or % |
| Age (years) | 1,297 | 68.4 (15.6) |
| Gender | ||
| Male | 573 | 44.2 |
| Female | 724 | 55.8 |
| Education (y) | 1,296 | 6.5 (4.2) |
| Body mass index (kg/m2) | 1,221 | 27.2 (4.1) |
| Mini-Mental State Exam score <24 (%) | 308 | 23.8 |
| Alcohol intake (g/d) | 1,063 | 21.2 (20.4) |
| Aspirin use (%) | 367 | 28.3 |
| Current smoking (%) | 236 | 18.2 |
| Estimated glomerular filtration rate (mL/min/1.732 m2) | 1,292 | 78.2 (18.3) |
| Fasting plasma glucose (mg/dL) | 1,292 | 93.9 (25.3) |
| Triglycerides (mg/dL) (normal range: <150 mg/dL) | 1,292 | 125.7 (76.1) |
| Total cholesterol (mg/dL) (normal range: <200 mg/dL) | 1,292 | 214.8 (40.6) |
| High-density lipoprotein cholesterol (mg/dL) (normal range: 40–60 mg/dL) | 1,292 | 58.7 (14.9) |
| Low-density lipoprotein cholesterol (mg/dL) (normal range: <100 mg/dL) | 1,292 | 134.0 (35.5) |
| Total plasma polyunsaturated fatty acids | 1,154 | 38.3 (5.1) |
| Total carotenoids (μmol/L) | 1,296 | 1.8 (1.0) |
| α-Tocopherol (μmol/L) | 1,296 | 33.4 (7.5) |
| Uric acid (mg/dL) | 1,291 | 5.1 (1.4) |
| Log endogenous secretory RAGE (ng/mL) | 1298 | −0.91 (0.46) |
| Log carboxymethyl-lysine (ng/mL) | 1,262 | 5.85 (0.29) |
| Log interleukin-1 receptor antagonist (pg/mL) | 1,296 | 4.88 (0.58) |
| Log interleukin-1β (pg/mL) | 1,296 | −2.15 (1.23) |
| Log tumor necrosis factor-α (pg/mL) | 1,284 | 0.85 (0.74) |
| Log interleukin-6 (pg/mL) | 1,290 | 1.03 (0.59) |
| Interleukin-6 receptor (pg/mL) | 1,290 | 103.0 (56.2) |
| Log interleukin-18 (pg/mL) | 1,296 | 5.92 (0.37) |
| Log C-reactive protein (μg/mL) | 1,297 | 0.91 (1.13) |
| Log transforming growth factor-β (pg/mL) | 1,287 | 9.15 (0.8) |
| Fibrinogen (mg/dL) | 1,281 | 348 (75) |
| Hypertension (%) | 555 | 42.8 |
| Angina (%) | 46 | 3.6 |
| Stroke (%) | 57 | 4.4 |
| Diabetes (%) | 145 | 11.2 |
| Congestive heart failure (%) | 55 | 4.2 |
| Peripheral artery disease (%) | 62 | 4.8 |
| Cancer (%) | 67 | 5.2 |
Table 2.
Univariable Linear Regression Models of the Relationship of Demographic, Inflammatory, and Other Factors With Plasma esRAGE Levels in Adults, 20–97 Years, in the InCHIANTI Study
| Characteristic | β | SE | p |
| Age (y) | 0.001 | 0.0008 | .19 |
| Gender | −0.076 | 0.030 | .003 |
| Education >12 (y) | −0.001 | 0.003 | .63 |
| Body mass index (kg/m2) | −0.021 | 0.003 | <.0001 |
| Mini-Mental State Exam score <24 | 0.054 | 0.03 | .07 |
| Alcohol intake (gm/d) | −0.0012 | 0.0007 | .12 |
| Current smoker | 0.035 | 0.033 | .30 |
| Aspirin use | 0.052 | 0.028 | .07 |
| Estimated glomerular filtration rate (mL/min/1.732) | −0.004 | 0.0007 | <.0001 |
| Total plasma polyunsaturated fatty acids (mg/L) | −0.00002 | 0.00002 | .27 |
| Fasting blood glucose (mg/dL) | −0.0004 | 0.0005 | .41 |
| Triglycerides (mg/dL) | −0.0003 | 0.0002 | .05 |
| Total cholesterol (mg/dL) | −0.0004 | 0.0003 | 0.23 |
| High-density lipoprotein cholesterol (mg/dL) | −0.0006 | 0.0009 | .50 |
| Low-density lipoprotein cholesterol (mg/dL) | −0.0001 | 0.0004 | .81 |
| Total carotenoids (μmol/L) | 0.023 | 0.019 | .22 |
| α-Tocopherol (μmol/L) | 0.0007 | 0.002 | .64 |
| Uric acid (mg/dL) | 0.008 | 0.009 | .37 |
| Log carboxymethyl-lysine (ng/mL) | 0.156 | 0.044 | .0004 |
| Hypertension | −0.043 | 0.026 | .10 |
| Angina | −0.020 | 0.069 | .77 |
| Stroke | −0.009 | 0.062 | .89 |
| Diabetes | −0.014 | 0.041 | .72 |
| Congestive heart failure | 0.15 | 0.063 | .02 |
| Peripheral artery disease | 0.09 | 0.060 | .13 |
| Cancer | 0.004 | 0.058 | .93 |
Note: esRAGE = endogenous secretory RAGE.
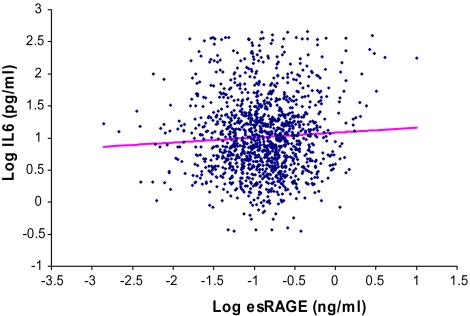
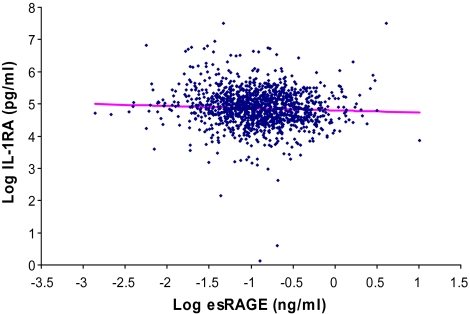
The relationship between plasma log esRAGE and different inflammatory mediators was analyzed in separate multivariable linear regression models where the specific inflammatory mediator was the dependent variable (Table 3). For each specific inflammatory mediator, Model 1 adjusted for age, sex, and body mass index; Model 2 included additional covariates for aspirin use, uric acid, estimated glomerular filtration rate, and congestive heart failure; and Model 3 was adjusted additionally for plasma CML. Log esRAGE was negatively associated with log IL-1RA in a multivariable linear regression model adjusting for all the covariates in Model 3. Log esRAGE was positively associated with log IL-6 in respective multivariable linear regression models adjusting for all the covariates in Model 3. There was no significant relationship between log esRAGE and log TNF-α, IL-6R, log IL-18, log CRP, or fibrinogen. The relationship between log esRAGE and log TGF-β in Model 3 was of borderline significance (p = .09). No significant interactions were found between esRAGE and age in relation to any of the inflammatory mediators. Scatterplots of log IL-6 versus log esRAGE and log IL-1RA and log esRAGE are shown in Figures 1 and 2, respectively.
Table 3.
Separate Multivariable Linear Regression Models for the Relationship of Plasma Log esRAGE With Respective Inflammatory Mediators in Adults, 20-97 Years, in the InCHIANTI Study *
| Model 1, Adjusting for Age, Sex, and BMI |
Model 2, Adjusting for Age, Sex, BMI, Aspirin, Uric Acid, eGFR, and Congestive Heart Failure |
Model 3, Adjusting for Age, Sex, BMI, Aspirin, Uric Acid, eGFR, Congestive Heart Failure, and CML |
|||||||
| β | SE | p | β | SE | p | β | SE | p | |
| Log interleukin-1 receptor antagonist | −0.101 | 0.036 | .005 | −0.106 | 0.035 | .003 | −0.069 | 0.036 | .05 |
| Log interleukin-1β | 0.121 | 0.080 | .13 | 0.111 | 0.080 | .17 | 0.133 | 0.082 | .11 |
| Log tumor necrosis factor-α | 0.031 | 0.046 | .50 | 0.001 | 0.047 | .99 | −0.004 | 0.048 | .93 |
| Log interleukin-6 | 0.072 | 0.034 | .03 | 0.061 | 0.035 | .07 | 0.077 | 0.0035 | .03 |
| Interleukin-6 receptor | 1.730 | 3.661 | .64 | −1.272 | 3.689 | .73 | 0.114 | 3.76 | .98 |
| Log interleukin-18 | −0.016 | 0.022 | .47 | −0.030 | 0.022 | .17 | −0.024 | 0.022 | .27 |
| Log C-reactive protein | −0.070 | 0.066 | .29 | −0.107 | 0.066 | .102 | −0.072 | 0.068 | .29 |
| Log transforming growth factor-β | −0.077 | 0.051 | .13 | −0.083 | 0.052 | .107 | −0.091 | 0.05 | .09 |
| Fibrinogen | 2.233 | 4.457 | .62 | 1.102 | 4.522 | .81 | −0.715 | 4.578 | .88 |
Notes: BMI = body mass index; eGFR = estimated glomerular filtration rate; esRAGE = endogenous secretory RAGE.
The individual inflammatory mediators are the dependent variable in the separate multivariable models shown.
Figure 1.
Scatterplot of log IL-6 versus log endogenous secretory RAGE (esRAGE), adjusted for age, sex, body mass index, aspirin, uric acid, estimated glomerular filtration rate, congestive heart failure, and carboxymethyl-lysine (p = 0.03).
Figure 2.
Scatterplot of log interleukin-1 receptor antagonist (IL-1RA) versus log endogenous secretory RAGE (esRAGE), adjusted for age, sex, body mass index, aspirin, uric acid, estimated glomerular filtration rate, congestive heart failure, and carboxymethyl-lysine (p = .05).
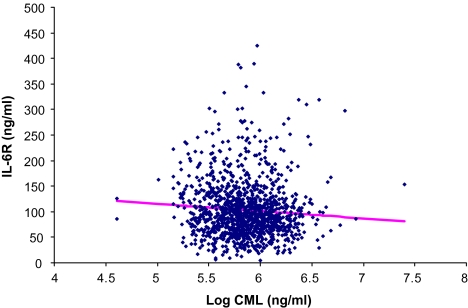
Log plasma CML was not significantly associated with any of the inflammatory mediators in separate multivariable linear regression models, as shown in Table 4, except for IL-6R (β = −14.10, SE = 5.94, p = .02) and fibrinogen (β = −13.95, SE = 7.21, p = .05). No significant interaction terms were found between log esRAGE and log CML for the respective multivariable linear regression models shown in Table 4. Significant interactions were found between CML and age with log IL-18 (p = .05) and with log CRP (p = .01). Scatterplots of IL-6R and log CML and of fibrinogen versus log CML are shown in Figures 3 and 4, respectively.
Table 4.
Separate Multivariable Linear Regression Models for the Relationship of Plasma Log CML With Respective Inflammatory Mediators in Adults, 20–97 Years, in the InCHIANTI Study*
| Model 1, Adjusting for Age, Sex, and BMI |
Model 2, Adjusting for Age, Sex, BMI, Aspirin, Uric Acid, eGFR, and Congestive Heart Failure |
Model 3, Adjusting for Age, Sex, BMI, Aspirin, Uric Acid, eGFR, Congestive Heart Failure, and esRAGE |
|||||||
| β | SE | p | β | SE | p | β | SE | p | |
| Log interleukin-1 receptor antagonist | −0.029 | 0.056 | .61 | −0.054 | 0.056 | .34 | −0.051 | 0.056 | .37 |
| Log interleukin-1β | −0.141 | 0.128 | .27 | −0.19 | 0.13 | .14 | −0.197 | 0.129 | .127 |
| Log tumor necrosis factor-α | 0.035 | 0.752 | .64 | −0.023 | 0.076 | .76 | −0.023 | 0.076 | .76 |
| Log interleukin-6 | 0.05 | 0.054 | .36 | 0.035 | 0.055 | .53 | 0.032 | 0.055 | .56 |
| Interleukin-6 receptor | −10.143 | 5.9 | .09 | −14.098 | 5.93 | .02 | −14.102 | 5.939 | .02 |
| Log interleukin-18 | 0.05 | 0.034 | .15 | 0.025 | 0.034 | .46 | 0.026 | 0.034 | .45 |
| Log C-reactive protein | 0.006 | 0.105 | .95 | −0.052 | 0.106 | .62 | −0.049 | 0.106 | .64 |
| Log transforming growth factor-β | −0.123 | 0.082 | .13 | −0.124 | 0.083 | .14 | −0.119 | 0.083 | .15 |
| Fibrinogen | 15.231 | 7.097 | .03 | 13.982 | 7.205 | .05 | 13.951 | 7.211 | .05 |
Notes: BMI = body mass index; eGFR = estimated glomerular filtration rate; esRAGE = endogenous secretory RAGE.
The individual inflammatory mediators are the dependent variable in the separate multivariable models shown.
Figure 3.
Scatterplot of interleukin-1 receptor (IL-6R) versus log carboxymethyl-lysine, adjusted for age, sex, body mass index, aspirin, uric acid, estimated glomerular filtration rate, congestive heart failure, and endogenous secretory RAGE (p = .02).
Figure 4.
.Scatterplot of fibrinogen versus log carboxymethyl-lysine (CML), adjusted for age, sex, body mass index, aspirin, uric acid, estimated glomerular filtration rate, congestive heart failure, and endogenous secretory RAGE (p = .05).
DISCUSSION
The present study shows that plasma esRAGE is negatively associated with IL-1RA and positively associated with IL-6 in community-dwelling adults. Plasma esRAGE was also negatively associated with TGF-β, a finding that was of borderline significance. IL-1RA is produced by the liver as an acute-phase protein and inhibits the effects of IL-1 on target cells (15). TGF-β inhibits monocyte/macrophage MHC class II expression, suppresses the proliferation and differentiation of T and B cells, and limits the synthesis of proinflammatory cytokines, such as TNF-α (15). IL-1RA and TGF-β are thus considered anti-inflammatory mediators and are strong markers for inflammation (15,16). IL-6 is produced by macrophages and T lymphocytes and has both pro- and anti-inflammatory roles. TNF-α and IL-1β stimulate the release of IL-6, which induces the production of fibrinogen and CRP and also limits the extent of an inflammatory response by downregulating the release of TNF-α and IL-1β and promoting the release of IL-1RA (17). These findings suggest that elevated plasma esRAGE is associated with lower concentrations of circulating IL-1RA and TGF-β and higher concentrations of circulating IL-6. All three of these inflammatory mediators are involved in the low-grade proinflammatory state that affects primarily older adults (1).
The strengths of this study were the large sample size, the population-based sampling for selecting the study subjects, the large number of inflammatory mediators that were measured, and the epidemiological approach that adjusted for demographic factors and other potential confounders. The results of the present study contrast with a previous study involving 76 patients with type 2 diabetes and 78 age- and sex-matched controls without diabetes (18). Contrary to our findings, in the latter study, when the cases and controls were pooled together, a significant negative correlation was found between esRAGE and IL-6 in crude unadjusted analyses (18).
AGE–RAGE binding is known to upregulate TNF-α, IL-6, CRP, and cell surface RAGE via the NF-κB pathway (3). In the present study, CML, which is a ligand for RAGE, was not significantly associated with any of the proinflammatory cytokines. Although AGE–RAGE binding is known to increase inflammation, at least in vitro and in animal studies, a possible explanation for the lack of an association of CML with inflammation is that the inflammation from AGE–RAGE binding may occur in specific tissues that are predisposed to the effect of AGEs. The localized nature of this process may not be reflected by significant changes in circulating levels of inflammatory markers.
Interestingly, increased levels of circulating esRAGE, which are likely subsequent to AGE–RAGE binding, were more strongly associated with inflammation than CML. The inflammatory mediators associated with esRAGE in the present study are also known to predict adverse outcomes in older adults. High circulating IL-6 has been associated with cognitive decline (19), loss of muscle mass and strength (20), and greater mortality (21). Elevated plasma IL-1RA and IL-6 were predictive of poor skeletal muscle strength (22) and greater mortality in nonagenarians (23). In a population-based study of older women, elevated esRAGE concentrations were also shown to predict adverse outcomes, such as decline in renal function (24), anemia (25), and cardiovascular disease mortality (26). In contrast, in patients with end-stage renal disease, low esRAGE concentrations were associated with cardiovascular mortality (27). In patients with type 1 diabetes, low esRAGE concentrations were associated with increased diabetic complications (28). The differences between these results may be due to differences in the study populations and chronic disease. However, when comparing the InCHIANTI cohort esRAGE median of 0.41 ng/mL to those in the aforementioned studies, the median esRAGE level for patients with end-stage renal disease was 0.56 ng/mL, with a slightly younger population (mean age = 55.6 years) (27). For nondiabetic participants in a study that examined the association between esRAGE and diabetes and coronary plaque progression, the mean was 0.37 ng/mL as compared with 0.46 ng/mL in the InCHIANTI population (29).
A limitation of the study is its cross-sectional nature, as the associations described cannot necessarily be seen as causal. Further studies could be done to examine the relationships between esRAGE and inflammation using a prospective design, but such investigations may be problematic given the close temporal relationship of the AGE–RAGE pathway with inflammation. The results from this study cannot necessarily be generalized to other populations.
In conclusion, elevated plasma esRAGE concentrations are associated with elevated IL-6 and decreased IL-1RA. Elevated plasma esRAGE appears to play a role in the low-grade proinflammatory state that is common in older adults.
FUNDING
This work was supported by National Institute on Aging Grants R01 AG027012, R01 AG029148, R01 HL094507; the Italian Ministry of Health (ICS110.1/RF97.71), NIA contracts 263 MD 9164, 263 MD 821336, N.1-AG-1-1, N.1-AG-1-2111, and N01-AG-5-0002; and the Intramural Research Program, National Institute on Aging, National Institutes of Health.
References
- 1.Ferrucci L, Corsi A, Lauretani F, et al. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol A Biol Sci Med Sci. 2010;65:963–75. doi: 10.1093/gerona/glq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basta G. Receptor for advanced glycation endproducts and atherosclerosis: from basic mechanisms to clinical implications. Atherosclerosis. 2008;196:9–21. doi: 10.1016/j.atherosclerosis.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 4.Sakurai S, Yamamoto Y, Tamei H, et al. Development of an ELISA for esRAGE and its application to type 1 diabetic patients. Diabetes Res Clin Pract. 2006;73:158–165. doi: 10.1016/j.diabres.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Morley JE, Baumgartner RN. Cytokine-related aging process. J Gerontol A Biol Sci Med Sci. 2004;59:M924–M929. doi: 10.1093/gerona/59.9.m924. [DOI] [PubMed] [Google Scholar]
- 6.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 7.Schrager MA, Metter EJ, Simonsick E, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol. 2007;102:919–925. doi: 10.1152/japplphysiol.00627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME. The Women's Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD: National Institute on Aging; 1995. NIH Publication 95-4009. [Google Scholar]
- 9.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 10.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.Sakurai S, Yamamoto Y, Tamei H, et al. Development of an ELISA for esRAGE and its application to type 1 diabetic patients. Diabetes Res Clin Pract. 2006;73(2):158–165. doi: 10.1016/j.diabres.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Boehm BO, Schilling S, Rosinger S, et al. Elevated serum levels of Nϵ-carboxymethyl-lysine, an advanced glycation end product, are associated with proliferative diabetic retinopathy and macular oedema. Diabetologia. 2004;47:1376–1379. doi: 10.1007/s00125-004-1455-y. [DOI] [PubMed] [Google Scholar]
- 14.Ferrucci L, Cherubini A, Bandinelli S, et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91:439–446. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 15.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 16.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 17.Hurst SM, Wilkinson TS, McLoughlin RM, et al. IL-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–714. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- 18.Choi KM, Yoo HJ, Kim HY, et al. Association between endogenous secretory RAGE, inflammatory markers and arterial stiffness. Int J Cardiol. 2009;132:96–101. doi: 10.1016/j.ijcard.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 19.Rafnsson SB, Deary IJ, Smith FB, et al. Cognitive decline and markers of inflammation and hemostasis: the Edinburgh Artery Study. J Am Geriatr Soc. 2007;55:700–707. doi: 10.1111/j.1532-5415.2007.01158.x. [DOI] [PubMed] [Google Scholar]
- 20.Schaap LA, Pluijm SM, Deeg DJ, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64:1183–1189. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walston J, Xue Q, Semba RD, et al. Serum antioxidants, inflammation, and total mortality in older women. Am J Epidemiol. 2006;163:18–26. doi: 10.1093/aje/kwj007. [DOI] [PubMed] [Google Scholar]
- 22.Tiainen K, Hurme MM, Hervonen A, Luukkaala T, Jylhä M. Inflammatory markers and physical performance among nonagenarians. J Gerontol A Biol Sci Med Sci. 2010;65:658–63. doi: 10.1093/gerona/glq056. [DOI] [PubMed] [Google Scholar]
- 23.Jylhä M, Pavilainen P, Lehtimäki T, et al. Interleukin-1 receptor antagonist, interleukin-6, and C-reactive protein as predictors of mortality nonagenarians: the Vitality 90+ study. J Gerontol A Biol Sci Med Sci. 2007;62:1016–1021. doi: 10.1093/gerona/62.9.1016. [DOI] [PubMed] [Google Scholar]
- 24.Semba RD, Ferrucci L, Fink JC, et al. Advanced glycation end products and their circulating receptors and level of kidney function in older community-dwelling women. Am J Kidney Dis. 2009;53:51–58. doi: 10.1053/j.ajkd.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Semba RD, Ferrucci L, Sun K, et al. Elevated serum advanced glycation end products and their circulating receptors are associated with anaemia in older community-dwelling women. Age Ageing. 2009;38:383–389. doi: 10.1093/ageing/afp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Semba RD, Ferrucci L, Sun K, et al. Advanced glycation end products and their circulating receptors predict cardiovascular disease mortality in older community-dwelling women. Aging Clin Exp Res. 2009;21(2):182–90. doi: 10.1007/bf03325227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koyama H, Shoji T, Fukumoto S, et al. Low circulating endogenous secretory receptor for AGEs predicts cardiovascular mortality in patients with end-stage renal disease. Arterioscler Thromb Vasc Biol. 2007;27:147–153. doi: 10.1161/01.ATV.0000251502.88818.4b. [DOI] [PubMed] [Google Scholar]
- 28.Katakami N, Matsuhisa M, Kaneto H, et al. Decreased endogenous secretory advanced glycation end product receptor in type 1 diabetic patients: its possible association with diabetic vascular complications. Diabetes Care. 2005;28:2716–2721. doi: 10.2337/diacare.28.11.2716. [DOI] [PubMed] [Google Scholar]
- 29.Peng WH, Lu L, Hu J, et al. Decreased serum esRAGE level is associated with angiographically determined coronary plaque progression in diabetic patients. Clin Biochem. 2009;42(12):1252–1259. doi: 10.1016/j.clinbiochem.2009.04.017. [DOI] [PubMed] [Google Scholar]