Abstract
Background.
Vitamin D deficiency is common among older adults and is associated with poor physical performance; however, studies examining longitudinal changes in 25-hydroxyvitamin D (25[OH]D) and physical performance are lacking. We examined the association between 25(OH)D and physical performance over 12 months in older adults participating in the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P), a multicenter physical activity intervention trial.
Methods.
Plasma 25(OH)D and physical performance, assessed by the short physical performance battery (SPPB) and 400-m walk test, were measured at baseline, 6-month, and 12-month follow-up in community-dwelling adults aged 70–89 years at risk for disability (n = 368). Mixed models were used to examine the association between 25(OH)D and physical performance adjusting for demographics, intervention group, season, body mass index, and physical activity.
Results.
One half of the participants were vitamin D deficient (25[OH]D < 20 ng/mL) at baseline. In cross-sectional analyses, vitamin D deficiency was associated with lower SPPB scores and slower 400-m walk speeds (mean difference [SE]: 0.35 [0.16], p = .03 and 0.04 [0.02] m/s, p = .01, respectively). Although baseline 25(OH)D status was not significantly associated with change in physical performance over 12 months, individuals who were vitamin D deficient at baseline but no longer deficient at follow-up had significant improvements in SPPB scores (mean difference [SE]: 0.55 [0.22], p = .01) compared with those whose 25(OH)D status remained the same.
Conclusion.
Increases in 25(OH)D to greater than or equal to 20 ng/mL were associated with clinically significant improvements in physical performance among older adults.
Keywords: Vitamin D, Physical performance, Aging
THE proportion of older adults in the United States is growing and expected to double to approximately 20% by the year 2030 (1). With the growth of the older population comes a parallel expectation of growth in the burden of age-related physical disability. Limitations in physical function as assessed by objective physical performance tests strongly predict future physical disability, nursing home admission, and mortality in older adults (2–4). Identifying risk factors that prevent or delay the onset of physical disability is thus a high public health priority.
Low 25-hydroxyvitamin D (25[OH]D) is common in older adults with wide variability in prevalence depending on geographic location, season, and the cut-points used to define deficiency (5). In the National Health and Nutrition Examination Survey 2000–2004, approximately 30% of adults aged 70 years and older were vitamin D deficient (25[OH]D <20 ng/mL; to convert to nmol/L, multiply by 2.496) (6). Older adults are at risk for low 25(OH)D because of reduced exposure to ultraviolet B radiation, reduced efficiency of pre-vitamin D synthesis in the skin, and inadequate dietary intake of vitamin D (5,7). In the past two decades, it has become evident that the role of vitamin D extends beyond calcium homeostasis and musculoskeletal health. Low 25(OH)D has been associated with many comorbid conditions, including cardiovascular disease, diabetes, hypertension, and osteoarthritis (8), conditions that are also directly related to the development of limitations in physical function (9).
The evidence from cross-sectional studies indicating that 25(OH)D is associated with physical performance is relatively consistent (10–15). However, the few longitudinal studies are inconsistent, showing either no association or a greater decline in physical performance among those with low 25(OH)D at baseline (16–20). Furthermore, how change in 25(OH)D over time affects physical performance in older adults has not been previously reported. Low 25(OH)D may also indirectly affect physical performance via hyperparathyroidism secondary to vitamin D deficiency (5). However, parathyroid hormone (PTH) may be important for maintaining muscle integrity and physical function independent of 25(OH)D (13,15,21).
The primary objective of these analyses was to examine the association between 25(OH)D, as well as change in 25(OH)D, and physical performance using data from the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P). The association between PTH and physical performance and the role of PTH as a potential mediator in the association between 25(OH)D and physical performance was also examined.
METHODS
Study Population
Data for this analysis are from LIFE-P, a single-blind, multicenter, randomized controlled trial in which older adults at risk for disability were assigned to either a walking-based physical activity program or a successful aging health information and education program for 12 months (ClinicalTrials.gov Identifier: NCT00116194). LIFE-P enrolled 424 community-dwelling men and women aged 70–89 years between May 2004 and February 2005. Participants were recruited from four communities: Pittsburgh, PA; Winston-Salem, NC; Dallas, TX; and Palo Alto, CA. Participants were eligible if they were at risk for disability (defined as having a short physical performance battery [SPPB] score of <10), were sedentary (<20 minutes of exercise each week for the past month), and able to complete a 400-m walk within 15 minutes. Complete details on the study design and inclusion and exclusion criteria can be found elsewhere (22,23). All participants provided written informed consent and all protocols were approved by the institutional review boards at all field centers. These analyses were conducted in 368 participants with available blood samples at study baseline. Participants were evaluated at baseline and at 6 and 12 months postrandomization.
Physical Performance
The SPPB was administered to summarize lower extremity physical performance (2). The SPPB consists of progressively more challenging standing balance tasks held for 10 seconds each, time to complete five repeated chair stands, and the faster of two 4-m walks to assess usual gait speed. Each of the three performance measures was assigned a score ranging from 0 to 4, with 0 representing inability to do the test and 4 representing the highest level of performance. The three measures were summed to create an SPPB summary score ranging from 0 (worst) to 12 (best). The SPPB was completed by 368 participants at baseline, 351 at 6-month, and 346 at 12-month follow-up. Because of eligibility criteria, the maximum SPPB score at baseline was 9.
Walking endurance was measured by a 400-m walk test. The course was 20 m long marked by cones at each end. Participants were instructed to walk 10 laps at their usual pace, and the time to complete the 400-m walk was recorded. Participants were allowed to stop and rest if necessary but were not allowed to sit or use an assistive device (including a cane) or the help of another person. The test was stopped if the participant was unable to complete the walk in 15 minutes. The 400-m walk test was completed by 368 participants at baseline, 325 at 6-month, and 311 at 12-month follow-up.
25-Hydroxyvitamin D and PTH
Fasting blood samples were collected in the morning after a 12-hour fast, centrifuged, and stored at −80°C. Plasma 25(OH)D was measured at baseline (n = 368), 6-month (n = 315), and 12-month follow-up (n = 307) by radioimmunoassay (RIA kit; DiaSorin, Stillwater, MN). Intact PTH was measured at baseline (n = 368) in EDTA plasma with a two-site immunoradiometric assay kit (N-tact PTHSP; DiaSorin, Stillwater, MN). The interassay coefficient of variation for 25(OH)D was 5.0 ± 3.6% and for PTH was 4.1 ± 3.0%.
Potential Confounders
Covariates included sociodemographic variables (age, gender, race, field center, and education), smoking, body mass index (weight in kilograms/height in meters squared), physical activity (moderate physical activity calculated from the Community Healthy Activities Model Program for Seniors questionnaire), cognition (Mini-Mental State Examination score), and depression (Center for Epidemiologic Studies Depression scale). Adjudicated disease diagnoses were based on self-reported history and included the following conditions: cardiovascular disease (myocardial infarction, congestive heart failure, and stroke), hypertension, diabetes, and arthritis. Season of the year was included as a categorical variable to account for seasonal effects on 25(OH)D and PTH. Randomization arm (physical activity vs successful aging), study visit, and a randomization arm by study visit interaction term were included to account for the effects of the intervention on physical performance over time.
Statistical Analyses
All analyses were performed using SAS software, version 9.2 (SAS Institute, Cary, NC) with a two-sided alpha level of .05 to indicate statistical significance. 25(OH)D status was categorized as 25(OH)D less than 20 ng/mL and greater than or equal to 20 ng/mL, a common cut-point used to define vitamin D deficiency (8). PTH was examined both as a continuous variable (transformed using the natural logarithm) and categorized into tertiles with the lower two tertiles combined (<42.6 vs ≥42.6 pg/mL). Differences in participant characteristics at baseline by 25(OH)D status were compared by chi-square analysis for categorical variables and by two-sample t tests for continuous variables. Mixed models were used to calculate the intraclass correlation for continuous log 25(OH)D using data from all three visits. A statistical method for simultaneously modeling cross-sectional and longitudinal effects was used to model 25(OH)D status and physical performance (24). Mixed models with a random subject (intercept) effect were used to examine the associations between baseline 25(OH)D status, PTH, and their interaction and physical performance. All models were adjusted for age, gender, race, field center, season, education, study visit, randomization arm, randomization arm by study visit interaction, body mass index, and physical activity. Season by field center interactions and 25(OH)D status by randomization arm interactions were tested but were not significant.
RESULTS
The mean age of the study sample was 76.7 years, 68% were women, and 76% were white. Participants who were excluded from this analysis due to a lack of blood samples at study baseline (n = 56) were similar to the study sample with regard to age, gender, and race and had similar baseline SPPB scores but slower 400-m walk speed (0.79 vs 0.86 m/s, p = .003). The mean (SD) 25(OH)D was 20.9 (10.2) ng/mL at baseline. The prevalence of vitamin D deficiency (25[OH]D < 20 ng/mL) was 50%, whereas only 10% had sufficient 25(OH)D (25[OH]D ≥ 30 ng/mL). Given the low prevalence of 25(OH)D greater than or equal to 30 ng/mL, participants with 25(OH)D of 20 to less than 30 ng/mL and greater than or equal to 30 ng/mL were combined (nondeficient). 25(OH)D at baseline, 6-month, and 12-month follow-up were highly correlated (intraclass correlation = .78). The mean (SD) PTH was 39.7 (22.8) pg/mL and was inversely correlated with 25(OH)D (r = −.36, p < .001). The descriptive characteristics of the study population by 25(OH)D status are shown in Table 1. The prevalence of vitamin D deficiency was higher among blacks (78.3%) than non-blacks (43.5%). Vitamin D–deficient participants also had a higher body mass index.
Table 1.
Participant Characteristics by Baseline 25-Hydroxyvitamin D (25[OH]D): Lifestyle Interventions and Independence for Elders Pilot*
| 25(OH)D |
p Value | ||
| <20 ng/mL (n = 184) | ≥ 20 ng/mL (n = 184) | ||
| Age (y) | 76.6 ± 4.2 | 76.8 ± 4.3 | .61 |
| Female gender (%) | 69.0 | 67.4 | .74 |
| Race (%) | <.001 | ||
| Black | 29.4 | 8.2 | |
| White | 64.7 | 88.0 | |
| Other | 6.0 | 3.8 | |
| Field center (%) | .09 | ||
| Dallas, TX | 27.7 | 26.1 | |
| Palo Alto, CA | 19.6 | 19.6 | |
| Pittsburgh, PA | 32.6 | 23.9 | |
| Winston-Salem, NC | 20.1 | 30.4 | |
| High school education or less (%) | 34.2 | 32.2 | .61 |
| Smoking status (%) | .38 | ||
| Former smoker | 16.8 | 12.0 | |
| Current smoker | 3.3 | 2.7 | |
| Never smoker | 79.9 | 85.3 | |
| Current drinker (%) | 33.2 | 39.7 | .18 |
| BMI (kg/m2) | 31.7 ± 6.1 | 28.7 ± 5.1 | <.001 |
| Moderate physical activity (kcal/wk) | 572.7 ± 892 | 674.7 ± 107 | .33 |
| Season (%) | .13 | ||
| Winter | 16.8 | 12.5 | |
| Spring | 3.2 | 8.2 | |
| Summer | 47.8 | 44.0 | |
| Fall | 32.1 | 35.3 | |
| Depression (CES-D score) | 7.1 ± 6.9 | 6.9 ± 6.5 | .79 |
| Cognition (MMSE score) | 27.1 ± 2.3 | 27.4 ± 2.1 | .17 |
| Prevalent disease (%) | |||
| Cardiovascular disease | 18.5 | 18.5 | 1.00 |
| Diabetes | 23.9 | 18.5 | .20 |
| Hypertension | 72.3 | 65.2 | .16 |
| Arthritis | 19.0 | 25.0 | .17 |
| PTH (pg/mL) | 45.6 ± 26.1 | 33.7 ± 16.9 | <.001 |
| Randomized to physical activity arm (%) | 50.5 | 48.4 | .68 |
Notes: BMI = body mass index; CES-D = Center for Epidemiologic Studies Depression Scale; MMSE = Mini-Mental State Examination; PTH = parathyroid hormone.
Means ± SD or frequencies with chi-square or t test to evaluate the distribution.
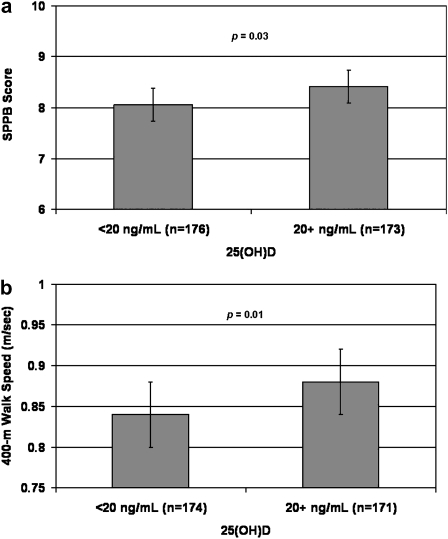
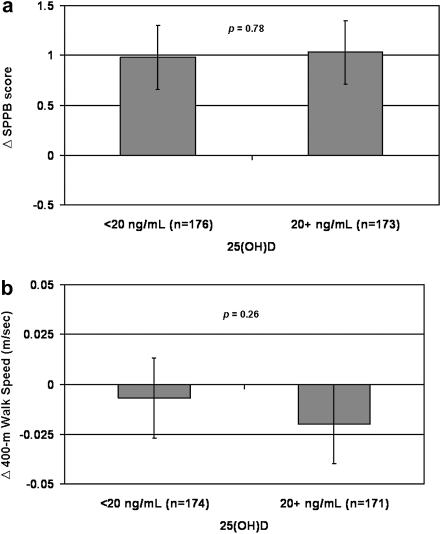
Figure 1 shows the cross-sectional associations between 25(OH)D status and physical performance at baseline. Participants who were vitamin D deficient (25[OH]D < 20 ng/mL) had significantly lower SPPB scores (mean difference [SE]: 0.35 [0.16], p = .03) and slower 400-m walk speed (mean difference [SE]: 0.04 [0.02] m/s, p = .01). Figure 2 shows the associations between baseline 25(OH)D status and change in physical performance over 12 months. The change in SPPB score and 400-m walk speed over 12 months did not differ by baseline 25(OH)D status (mean difference [SE]: 0.27 [0.15], p = .08 and 0.01 [0.01] m/s, p = .15, respectively). Addition of baseline PTH to the models did not substantially change the association between 25(OH)D status and physical performance.
Figure 1.
Cross-sectional associations between 25(OH)D status and physical function: Lifestyle Interventions and Independence for Elders Pilot. (a) Short physical performance battery (SPPB). (b) Four hundred meter walk speed. Least squared means with 95% confidence intervals. Models adjusted for age, gender, race, field center, season, education, visit, randomization arm, visit by randomization arm interaction, body mass index, and physical activity.
Figure 2.
Baseline 25(OH)D status and change in physical function over 12 months: Lifestyle Interventions and Independence for Elders Pilot. (a) Short physical performance battery (SPPB). (b) Four hundred meter walk speed. Least squared means with 95% confidence intervals. Models adjusted for age, gender, race, field center, season, education, visit, randomization arm, visit by randomization arm interaction, body mass index, and physical activity.
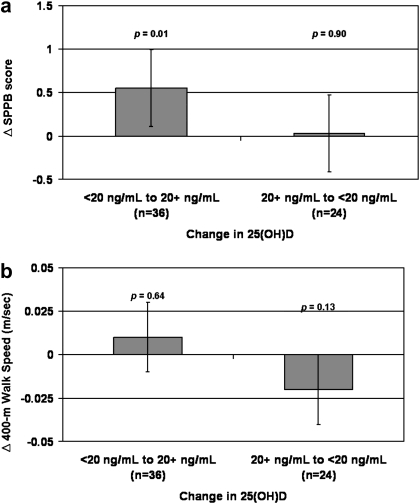
Change in 25(OH)D status and change in physical performance is shown in Figure 3. Over 12 months of follow-up, 25(OH)D improved from deficient (25[OH]D < 20 ng/mL) to nondeficient (25[OH]D ≥ 20 ng/mL) in 12.0% (n = 37, mean [SE] change: +6.3 [0.6] ng/mL), declined from nondeficient to deficient in 8.1% (n = 25, mean [SE] change: −5.6 [0.8] ng/mL), and remained either nondeficient (n = 126) or deficient (n = 119) in 79.8% (mean [SE] change: −0.2 [0.6] and +0.5 [0.3] ng/mL, respectively). An interaction term of baseline 25(OH)D status and change in 25(OH)D status was first tested to determine if the effect of change in 25(OH)D status on physical performance differed depending on baseline 25(OH)D status, and a significant interaction for SPPB score was found (p = .04). Among participants who were vitamin D deficient at baseline but not at follow-up, SPPB scores improved significantly compared with those whose 25(OH)D status remained the same (mean difference [SE]: +0.55 [0.22], p = .01). Results were similar when participants who were vitamin D deficient at baseline but not at follow-up were compared only with those who remained deficient at follow-up (mean SPPB difference [SE]: +0.63 [0.22], p = .005) and when participants who were vitamin D nondeficient at baseline but not at follow-up were compared only with those who remained nondeficient (mean SPPB difference [SE]: 0.01 [0.23], p = .98). The SPPB change in individuals whose 25(OH)D levels remained nondeficient did not differ significantly from those whose 25(OH)D levels remained deficient (mean difference [SE]: +0.16 [0.19], p = .40). Change in 25(OH)D status was not associated with improvements in 400-m walk speed (mean difference [SE]: 0.01 [0.01] m/s, p = .64). The association between change in 25(OH)D status and change in physical performance did not change substantially when baseline PTH was added to the models.
Figure 3.
Associations between change in 25(OH)D status and change in physical function over 12 months: Lifestyle Interventions and Independence for Elders Pilot. (a) Short physical performance battery (SPPB). (b) Four hundredmeter walk speed. Least squared means with 95% confidence intervals. Reference group: no change in 25(OH)D status. Models adjusted for age, gender, race, field center, season, education, visit, randomization arm, visit by randomization arm interaction, body mass index, and physical activity.
Baseline PTH was significantly associated with 400-m walk speed at baseline (β coefficient [SE] per unit of the natural logarithm of PTH: −0.04 [0.02] m/s, p = 0.03). There were no significant associations between baseline PTH and baseline SPPB scores (β coefficient [SE] per unit of the natural logarithm of PTH: −0.24 [0.16], p = 0.14). When baseline PTH was examined comparing the lower two tertiles (<42.6 pg/mL) versus the upper tertile (≥42.6 pg/mL), there were no significant associations with baseline SPPB scores (Least squared means [SE]: 7.61 [0.15] vs 7.40 [0.18], respectively, p = .20) or 400-m walk speed (0.88 [0.02] vs 0.85 [0.02] m/s, respectively, p = 0.13). Baseline PTH (either per unit of the natural logarithm or comparing the lower two tertiles vs. the upper tertile) was not significantly associated with change in SPPB scores or 400-m walk speed.
DISCUSSION
This study shows that vitamin D deficiency (25[OH]D < 20 ng/mL) is common and is associated with worse physical performance, a strong predictor of future disability, in older community-dwelling men and women at risk for disability. Vitamin D deficiency was associated with significantly lower SPPB scores and slower 400-m walk speed after adjusting for demographics, intervention group, season, body mass index, and physical activity. Furthermore, individuals who were vitamin D deficient at baseline but not at follow-up had a significant and clinically meaningful improvement in SPPB scores (>0.5 points) at follow-up.
The association between 25(OH)D and physical performance is relatively consistent in cross-sectional studies (10–15). However, data from the few longitudinal studies which examined baseline 25(OH)D and change in physical performance are inconsistent, showing either no association between 25(OH)D and physical performance (18–20) or a greater decline in physical performance among those with low 25(OH)D (16,17). The discrepancies among these studies may stem from substantial variation in the measurement of 25(OH)D because of different assay methodology (25,26), as well as differences in the study population characteristics and 25(OH)D cut-points used. In the current study, we found that vitamin D deficiency was associated with poor physical performance in cross-sectional analyses but was not associated with greater declines in physical performance over 12 months. However, improvements in 25(OH)D from vitamin D deficient at baseline to nondeficient at follow-up were associated with significant improvements in physical performance over 12 months of follow-up.
Interventions to improve physical function in older adults have primarily focused on resistance exercise and increasing physical activity. Randomized controlled trials show that physical activity interventions, both resistance and endurance exercise, improve a variety of physical performance measures in older adults (27,28). Whether or not improving 25(OH)D, which can be done inexpensively and safely with vitamin D supplementation, will improve physical performance and delay the onset of disability in older adults has not been determined definitively. However, we found that improvement in 25(OH)D from vitamin D deficient at baseline to nondeficient at follow-up was associated with significant improvements in SPPB scores; an improvement that was similar to that of the LIFE-P physical activity intervention at 12-month follow-up (0.6 points) (28). Based on work by Perera and colleagues (29) demonstrating that a change of 0.5 points on the SPPB represents a clinically meaningful change, the improvement in SPPB among those whose 25(OH)D increased from deficient to nondeficient represents a clinically significant improvement.
Randomized controlled trials of vitamin D supplementation have shown mixed effects on physical performance among older adults (30,31). Possible reasons for the inconsistencies include inclusion of participants with sufficient baseline 25(OH)D, inadequate vitamin D supplement dose, lack of sufficient 25(OH)D at follow-up, length of follow-up, and sample selection (eg, institutionalized, home-bound and falls clinic participants vs community-dwelling older adults). Vitamin D intakes of 800–1,000 IU/d have been suggested to prevent falls and fractures as well as other adverse health outcomes in older adults (8,32,33). Although we are unable to determine why 25(OH)D improved in the current study, those who were vitamin D deficient at baseline but no longer deficient at follow-up had significant improvements in physical performance. Based on vitamin D supplementation dosing studies, which report an increase in serum 25(OH)D of 0.24–0.48 ng/mL per 1 μg of vitamin D/d (1 μg = 40 IU) (34), the increase in 25(OH)D observed in the current study would be expected with an increase in vitamin D intake of 532–1,068 IU/d.
25(OH)D plays an important role in muscle function through its regulation of calcium transport, uptake of inorganic phosphate for the production of energy-rich phosphate compounds, and protein synthesis in the muscle (35). Vitamin D deficiency is also a recognized cause of secondary hyperparathyroidism. In animal models, administration of PTH increases protein catabolism and decreases the number of type 2 muscle fibers, intracellular energy-rich phosphate compounds, and mitochondrial oxygen uptake (5,35). Previous observational studies have shown an association between PTH and physical performance (13,15,21). However, in the current study, PTH was associated with 400-m walk speed at baseline only.
There are important characteristics of LIFE-P that limit the generalization of these findings. First, participants were recruited to be at risk of disability; thus, these results may not be generalizable to the general population. Second, the analyses were conducted in the context of a randomized controlled trial of a walking-based physical activity intervention. However, all analyses were adjusted for randomization arm and a randomization arm by study visit interaction term. Furthermore, there was not a significant interaction between 25(OH)D status and randomization arm, and the associations between 25(OH)D status and physical performance were similar when the analyses were restricted to the successful aging arm. Among those participants whose 25(OH)D improved, we do not have information on why 25(OH)D improved, such as changes in dietary or supplemental intake of vitamin D or sun exposure. A decline in 25(OH)D from nondeficient (25[OH]D ≥ 20 ng/mL) to deficient (25[OH]D < 20 ng/mL), however, was not associated with change in physical performance, possibly because of the small number of participants whose 25(OH)D status declined over the 12-month period. Because participants were not recruited throughout the year, we cannot rule out residual confounding by season; however, results were similar when only baseline and 12-month 25(OH)D status were used. Finally, the observational design of our study does not allow us to evaluate a causal association between 25(OH)D and physical performance. Although it is biologically plausible that low 25(OH)D may result in poor physical performance, it is also possible that those with poor physical performance had less exposure to ultraviolet B rays resulting in low 25(OH)D. A major strength of the current study is that both 25(OH)D and physical performance were collected at baseline, 6-month, and 12-month follow-up allowing an examination of the association between change in 25(OH)D and change in physical performance.
In conclusion, vitamin D deficiency was common and was associated with poor physical performance among community-dwelling older men and women at risk for disability. Physical performance also improved significantly at follow-up among individuals who were vitamin D deficient at baseline but no longer deficient at follow-up. Thus, attaining 25(OH)D levels of 20 ng/mL or greater may slow the decline in physical performance in older adults and, ultimately, delay the onset of disability. These results along with other recent findings showing the importance of 25(OH)D on multiple health outcomes underscore the need for definitive trials of vitamin D supplementation on physical performance and disability as well as other health outcomes among older adults.
FUNDING
This work was supported by grants from the National Institute on Aging (U01 AG22376; P30 AG21332; K01 AG030506 to D.K.H.) and supported in part by the Intramural Research Program, National Institute on Aging at the National Institutes of Health, and the Paul and Ferne Sticht Foundation.
References
- 1.Federal Interagency Forum on Aging-Related Statistics. Older Americans 2004: Key Indicators of Well-Being. Washington, DC: U.S. Government Printing Office; 2004. [Google Scholar]
- 2.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 3.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 5.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22(4):477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 6.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr. 2008;88(6):1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey RL, Dodd KW, Goldman JA, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140(4):817–822. doi: 10.3945/jn.109.118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 9.Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45(1):92–100. doi: 10.1111/j.1532-5415.1997.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 10.Mowe M, Haug E, Bohmer T. Low serum calcidiol concentration in older adults with reduced muscular function. J Am Geriatr Soc. 1999;47(2):220–226. doi: 10.1111/j.1532-5415.1999.tb04581.x. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff HA, Stahelin HB, Urscheler N, et al. Muscle strength in the elderly: its relation to vitamin D metabolites. Arch Phys Med Rehabil. 1999;80(1):54–58. doi: 10.1016/s0003-9993(99)90307-6. [DOI] [PubMed] [Google Scholar]
- 12.Zamboni M, Zoico E, Tosoni P, et al. Relation between vitamin D, physical performance, and disability in elderly persons. J Gerontol A Biol Sci Med Sci. 2002;57(1):M7–M11. doi: 10.1093/gerona/57.1.m7. [DOI] [PubMed] [Google Scholar]
- 13.Dhesi JK, Bearne LM, Moniz C, et al. Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J Bone Miner Res. 2002;17(5):891–897. doi: 10.1359/jbmr.2002.17.5.891. [DOI] [PubMed] [Google Scholar]
- 14.Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004;80(3):752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 15.Houston DK, Cesari M, Ferrucci L, et al. Association between vitamin D status and physical performance: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2007;62(4):440–446. doi: 10.1093/gerona/62.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wicherts IS, van Schoor NM, Boeke AJ, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92(6):2058–2065. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 17.Dam TT, von MD, Barrett-Connor EL. Sex-specific association of serum vitamin D levels with physical function in older adults. Osteoporos Int. 2009;20(5):751–760. doi: 10.1007/s00198-008-0749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verreault R, Semba RD, Volpato S, Ferrucci L, Fried LP, Guralnik JM. Low serum vitamin D does not predict new disability or loss of muscle strength in older women. J Am Geriatr Soc. 2002;50(5):912–917. doi: 10.1046/j.1532-5415.2002.50219.x. [DOI] [PubMed] [Google Scholar]
- 19.Faulkner KA, Cauley JA, Zmuda JM, et al. Higher 1,25-dihydroxyvitamin D3 concentrations associated with lower fall rates in older community-dwelling women. Osteoporos Int. 2006;17(9):1318–1328. doi: 10.1007/s00198-006-0071-8. [DOI] [PubMed] [Google Scholar]
- 20.Bartali B, Frongillo EA, Guralnik JM, et al. Serum micronutrient concentrations and decline in physical function among older persons. JAMA. 2008;299(3):308–315. doi: 10.1001/jama.299.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88(12):5766–5772. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 22.Rejeski WJ, Fielding RA, Blair SN, et al. The lifestyle interventions and independence for elders (LIFE) pilot study: design and methods. Contemp Clin Trials. 2005;26(2):141–154. doi: 10.1016/j.cct.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Katula JA, Kritchevsky SB, Guralnik JM, et al. Lifestyle Interventions and Independence for Elders pilot study: recruitment and baseline characteristics. J Am Geriatr Soc. 2007;55(5):674–683. doi: 10.1111/j.1532-5415.2007.01136.x. [DOI] [PubMed] [Google Scholar]
- 24.Fitzmaurice GM, Laird NM, Ware JH. Hoboken, NJ: Wiley-Interscience; 2004. Applied Longitudinal Analysis; pp. 418–423. [Google Scholar]
- 25.Binkley N, Krueger D, Cowgill CS, et al. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab. 2004;89(7):3152–3157. doi: 10.1210/jc.2003-031979. [DOI] [PubMed] [Google Scholar]
- 26.Carter GD, Carter R, Jones J, Berry J. How accurate are assays for 25-hydroxyvitamin D? Data from the international vitamin D external quality assessment scheme. Clin Chem. 2004;50(11):2195–2197. doi: 10.1373/clinchem.2004.040683. [DOI] [PubMed] [Google Scholar]
- 27.Keysor JJ. Does late-life physical activity or exercise prevent or minimize disablement? A critical review of the scientific evidence. Am J Prev Med. 2003;25:129–136. doi: 10.1016/s0749-3797(03)00176-4. (3 suppl 2) [DOI] [PubMed] [Google Scholar]
- 28.Pahor M, Blair SN, Espeland M, et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61(11):1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 29.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 30.Latham NK, Anderson CS, Reid IR. Effects of vitamin D supplementation on strength, physical performance, and falls in older persons: a systematic review. J Am Geriatr Soc. 2003;51(9):1219–1226. doi: 10.1046/j.1532-5415.2003.51405.x. [DOI] [PubMed] [Google Scholar]
- 31.Annweiler C, Schott AM, Berrut G, Fantino B, Beauchet O. Vitamin D-related changes in physical performance: a systematic review. J Nutr Health Aging. 2009;13(10):893–898. doi: 10.1007/s12603-009-0248-x. [DOI] [PubMed] [Google Scholar]
- 32.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of Vitamin D on falls: a meta-analysis. JAMA. 2004;291(16):1999–2006. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- 33.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293(18):2257–2264. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 34.Heaney RP. The case for improving vitamin D status. J Steroid Biochem Mol Biol. 2007;103(3–5):635–641. doi: 10.1016/j.jsbmb.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Pfeifer M, Begerow B, Minne HW. Vitamin D and muscle function. Osteoporos Int. 2002;13(3):187–194. doi: 10.1007/s001980200012. [DOI] [PubMed] [Google Scholar]