Abstract
Spinal cord injury (SCI) is a major cause of disability, its clinical outcome depending mostly on the extent of damage in which proapoptotic cytokines have a crucial function. In particular, the inducers of apoptosis belonging to TNF receptor superfamily and their respective ligands are upregulated after SCI. In this study, the function of the proapoptotic cytokine tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in SCI-induced damage was investigated in the mouse. SCI resulted in severe trauma, characterized by prominent inflammation-related damage and apoptosis. Immunostaining for TRAIL and its receptor DR5 was found in the white and gray matter of the perilesional area, as also confirmed by western blotting experiments. Immunoneutralization of TRAIL resulted in improved functional recovery, reduced apoptotic cell number, modulation of molecules involved in the inflammatory response (FasL, TNF-α, IL-1β, and MPO), and the corresponding signaling (caspase-8 and -3 activation, JNK phosphorylation, Bax, and Bcl-2 expression). As glucocorticoid-induced TNF receptor superfamily-related protein (GITR) activated by its ligand (GITRL) contributes to SCI-related inflammation, interactions between TRAIL and GITRL were investigated. SCI was associated with upregulated GITR and GITRL expression, a phenomenon prevented by anti-TRAIL treatment. Moreover, the expression of both TRAIL and DR5 was reduced in tissues from mice lacking the GITR gene (GITR−/−) in comparison with wild-type mice suggesting that TRAIL- and GITRL-activated pathways synergise in the development of SCI-related inflammatory damage. Characterization of new targets within such molecular systems may constitute a platform for innovative treatment of SCI.
Keywords: neuronal death, cytokines, GITR/GITRL, neuroinflammation, immunoneutralization, pharmacological treatment
INTRODUCTION
Spinal cord injury (SCI) is a major cause of disability. The functional decline after SCI is contributed by both mechanical injury and mechanism factors set into motion by trauma (Profyris et al, 2004). The mechanical forces imparted to the spinal cord cause tissue disruption, with axonal injury, inducing death of neurons that is very unlikely to be regenerated (DeVivo et al, 1987; Tator, 1995). Moreover, neuronal death continues for hours after SCI, because of multiple mechanisms including excitotoxicity, vascular abnormalities, and inflammation (Liu et al, 1999; Tator and Koyanagi, 1997; Hausmann, 2003). The clinical outcome of SCI depends, in part, on the extent of secondary damage, which evolves with contribution of apoptosis (Lu et al, 2000).
Cytokines, with special regard to the TNF superfamily, have been suggested to be responsible for increased apoptotic rate in the central nervous system (CNS), involving both neurons (Robertson et al, 2001) and glia (Satoh and Kuroda, 2001). In this line, Plunkett et al (2001) described upregulation of CD95L and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mRNA after excitotoxic SCI in the rat.
TRAIL is a member of the TNF superfamily, which binds to five receptors (Wiley et al, 1995; Pitti et al, 1996). Among these, DR4 and DR5 are type I membrane proteins (Pan et al, 1997a, 1997b; Sheridan et al, 1997) that contain a cytoplasmic death domain and transduce a death signal (Walczak et al, 1997). Both DR4 and DR5 are present in the CNS of mammalians (Dörr et al, 2002) and mediate the detrimental effects of TRAIL in brain ischemia (Martin-Villalba et al, 1999) and in β-amyloid-dependent neurotoxicity (Cantarella et al, 2003). Although it has been shown that after SCI, expression of TNF, CD95, and CD95L is increased at the lesion site (Zurita et al, 2001; Xu et al, 1998; Li et al, 2000), the in vivo function of the receptors belonging to TNFR superfamily and the respective ligands in SCI is still controversial. Neutralization of TNF (Lee et al, 2000) and CD95L (Demjen et al, 2004) reduces the number of apoptotic cells after SCI. Demjen et al (2004), showed that neutralization of CD95L, but not of TNF, promotes functional recovery after SCI. Moreover, recent evidence showed a relevant function of GITR in the regulation of the inflammatory response in SCI (Nocentini et al, 2008). GITR is a protein, originally cloned in a glucocorticoid-treated hybridoma T cell line (Nocentini et al, 2007). It is expressed in several cells and tissues, including T cells, in which it acts as a co-stimulatory molecule (Ronchetti et al, 2007; Ronchetti et al, 2004; Tone et al, 2003) after activation by its ligand (GITRL), mainly expressed on antigen-presenting cells and endothelial cells (Krausz et al, 2007).
Here, we show for the first time the involvement of TRAIL in the pathophysiology of SCI and that neutralization of TRAIL reduces apoptotic cell death and improves the functional and histopathological outcome after SCI. Besides, we show the function of GITR in the regulation of TRAIL proinflammatory property.
MATERIALS AND METHODS
Animals
Male CD1 adult mice (25–30 g, Harlan Nossan) were housed in a controlled environment and provided with standard rodent chow and water. Animal care was in compliance with Italian regulations on protection of animals used for experimental and other scientific purposes (D.M. 116192) as well as with the EEC regulations (O.J. of E.C. L 358/1 12/18/1986).
Spinal Cord Injury
Mice were anesthetized using chloral hydrate (400 mg/kg body weight). We used the clip compression model described by Rivlin and Tator (1978) and produced SCI by extradural compression of a section of the SCI exposed through a four-level T5–T8 laminectomy, in which the prominent spinous process of T5 was used as a surgical guide. A six-level laminectomy was chosen to expedite timely harvest and to obtain enough SCI tissue for biochemical examination. With the aneurysm clip applicator oriented in the bilateral direction, an aneurysm clip with a closing force of 24 g was applied extradurally at T5–T8 level. The clip was then rapidly released with the clip applicator, which caused SC compression. In the injured groups, the cord was compressed for 1 min. After surgery, 1.0 ml of saline was administered subcutaneously to replace the blood volume lost during the surgery. During recovery from anesthesia, the mice were placed on a warm heating pad and covered with a warm towel. The mice were singly housed in a temperature-controlled room at 27°C for a survival period of 20 days. Food and water were provided to the mice ad libitum. During this time period, the animals' bladders were manually voided twice a day until the mice were able to regain normal bladder function. Sham-injured animals were only subjected to laminectomy.
Experimental Design
Mice were randomized into four groups (N=40 animals/group, randomized 20 (10 to histology and 10 to western and myeloperoxidase assay) at 24 h and 20 for motor score). Sham animals were subjected to the surgical procedure except that the aneurysm clip was not applied and they were treated intraperitoneally (i.p.) with vehicle (saline) or anti-TRAIL (Alexis Biochemicals) (50 μg/kg) 30 min before surgical procedure. The remaining mice were subjected to SCI (as described above) and treated with an i.p. bolus of vehicle or anti-TRAIL (50 μg/kg) 30 min before SCI.
Experimental Groups Study GITR−/− Mice
Mice were randomly allocated into the following groups—(1) SCI-GITR+/+ group: mice were subjected to SCI; (2) SCI-GITR−/− group: mice were subjected to SCI; (3) control GITR+/+ group (sham): GITR+/+ mice were subjected to the surgical procedures as the above except that the aneurysm clip was not applied; and (4) control GITR−/− group (sham): GITR−/− mice were subjected to the surgical procedures as the above groups except that the aneurysm clip was not applied.
Mice from each group were killed 24 h after SCI to collect samples for the evaluation of the parameters as described below.
Grading of Motor Disturbance
The motor function of mice subjected to compression trauma was assessed in a blinded manner once a day for 10 days after injury. Recovery from motor disturbance was graded using the modified murine Basso, Beattie, and Bresnahan (BBB) (Basso et al, 1995) hind limb locomotor rating scale (Joshi and Fehlings, 2002a, 2002b). The following criteria were considered: 0=no hind limb movement; 1=slight (<50% range of motion) movement of one to two joints; 2=extensive (>50% range of motion) movement of one joint and slight movement of one other joint; 3=extensive movement of two joints; 4=slight movement in all three joints; 5=slight movement of two joints and extensive movement of one joint; 6=extensive movement of two joints and slight movement of one joint; 7=extensive movement of all three joints; 8=sweeping without weight support or plantar placement and no weight support; 9=plantar placement with weight support in stance only or dorsal stepping with weight support; 10=occasional (0–50% of the time) weight-supported plantar steps and no coordination (front/hind limb coordination); 11=frequent (50–94% of the time) to consistent (95–100% of the time) weight-supported plantar steps and no coordination; 12=frequent to consistent weight-supported plantar steps and occasional coordination; 13=frequent to consistent weight-supported plantar steps and frequent coordination; 14=consistent weight-supported plantar steps, consistent coordination, and predominant paw position is rotated during locomotion (lift off and contact) or frequent plantar stepping, consistent coordination, and occasional dorsal stepping; 15=consistent plantar stepping and coordination, no/occasional toe clearance, and paw position is parallel at initial contact; 16=consistent plantar stepping and coordination (front/hind limb coordination), frequent toe clearance, and predominant paw position is parallel at initial contact and rotated at lift off; 17=consistent plantar stepping and coordination and frequent toe clearance and predominant paw position is parallel at initial contact and lift off; 18=consistent plantar stepping and coordination and consistent toe clearance and predominant paw position is parallel at initial contact and rotated at lift off; 19=consistent plantar stepping and coordination, consistent toe clearance, and predominant paw position is parallel at initial contact and lift off; 20=consistent plantar stepping, coordinated gait, consistent toe clearance, predominant paw position is parallel at initial contact and lift off, and trunk instability; 21=consistent plantar stepping, coordinated gait, consistent toe clearance, predominant paw position is parallel at initial contact and lift off, and trunk stability.
Immunohistochemistry
Twenty-four hours after SCI, the tissues were fixed in 10% (w/v) PBS-buffered formaldehyde and 8 μm sections were prepared from paraffin-embedded tissues. After deparaffinization, endogenous peroxidase was quenched with 0.3% (v/v) hydrogen peroxide in 60% (v/v) methanol for 30 min. The sections were permeabilized with 0.1% (w/v) Triton X-100 in PBS for 20 min. Non-specific adsorption was minimized by incubating the section in 2% (v/v) normal goat serum in PBS for 20 min. Endogenous biotin or avidin binding sites were blocked with biotin and avidin (DBA), respectively. Sections were incubated overnight with anti-Fas-L, anti-Bax, anti-Bcl-2, anti-GITR, anti-GITRL, anti-DR5, or anti-TRAIL antibodies (1 : 100). Sections were washed with PBS and incubated with secondary antibody. Specific labeling was detected with a biotin-conjugated goat anti-rabbit IgG and avidin-biotin peroxidase complex (DBA). To verify binding specificity, some sections were incubated only with the secondary antibody. Immunohistochemical photographs (n=5 slices from each sample collected from each mouse in each experimental group; N per group=10) were assessed by densitometry as described earlier (Shea, 1994) by using Optilab Graftek software.
Terminal Deoxynucleotidyltransferase-Mediated UTP End Labeling Assay
Terminal deoxynucleotidyltransferase-mediated UTP end labeling (TUNEL) assay was conducted by using a TUNEL detection kit according to the manufacturer's instructions (Apotag, HRP kit DBA, Milano, Italy). Briefly, sections were incubated with 15 μg/ml proteinase K for 15 min at room temperature and then washed with PBS. Endogenous peroxidase was inactivated by 3% H2O2 for 5 min at room temperature and then washed with PBS. Sections were dipped into terminal deoxynucleotidyltransferase buffer, incubated in a humid atmosphere at 37°C for 90 min, and then washed with PBS. The sections were incubated at room temperature for 30 min with anti-horseradish peroxidase-conjugated antibody, and the signals were visualized with diaminobenzidine. The number of TUNEL positive cells/high-power field was counted in 5–10 fields for each coded slide.
Light Microscopy
Spinal cord tissues were taken from all the mice in each experimental groups at 24 h after trauma. Tissue segments containing the lesion (1 cm on each side of the lesion) were paraffin embedded and cut into 5-μm-thick sections. Tissue sections were deparaffinized with xylene, stained with hematoxylin/eosin, and studied using light microscopy (Dialux 22 Leitz). The segments of each spinal cord were evaluated by an experienced histopathologist. Damaged neurons were counted and the histopathologic changes of the gray matter were scored on a 6-point scale: 0, no lesion observed; 1, gray matter contained 1–5 eosinophilic neurons; 2, gray matter contained 5–10 eosinophilic neurons; 3, gray matter contained more than 10 eosinophilic neurons; 4, small infarction (less than one-third of the gray matter area); 5, moderate infarction (one-third to one-half of the gray matter area); 6, large infarction (more than half of the gray matter area). The scores from all the sections from each spinal cord were averaged to give a final score for individual mice. All the histological studies were performed in a blinded manner.
Protein Extraction
Spinal cord tissue were homogenized with a Polytron homogenizer in a lysis buffer containing 150 mM NaCl, 50 mM Tris–HCl [pH 7.5], 5 mM ethylenediaminetetraacetic acid, 1 mM Na3VO4, 30 mM Na pyrophosphate, 50 mM NaF, 1 mM acid phenyl-methyl-sulfonyl-fluoride, 5 μg/ml aprotinin, 2 μg/ml leupeptin, 1 μg/ml pepstatin, 10% glycerol, and 0.2% Triton X-100. The homogenates were then centrifuged at 14 000 r.p.m. for 10 min at 4°C. The protein concentration of the supernatant was determined by the Bradford method (Bradford, 1976).
Western Blot Analysis
Cellular protein (30 μg) were electrophoresed on 8 and 12% polyacrylamide gel and transferred to a nitrocellulose membranes (Amersham). The membranes were incubated at room temperature overnight with an anti-DR5 antibody (Alexis Biochemicals) (1 : 1000), or an anti-TRAIL antibody (BD Transductions Laboratories), or anti-caspase-8 antibody (Cell Signaling Technology), or anti-caspase-3 antibody (Cell Signaling Technology), or anti-JNK1 antibody (Santa Cruz Biotechnology), or anti-p-JNK1 antibody (Santa Cruz Biotechnology), or anti-GITR antibody (R&D System), or anti-GITRL antibody (Santa Cruz Biotechnology), or anti-β-tubulin antibody (Santa Cruz Biotechnology). The secondary HRP-conjugated antibody (Amersham) and a chemiluminescence blotting substrate kit assay (Amersham) were used for immunodetection.
All the experiments were repeated at least three times; β-tubulin (Santa Cruz Biotechnology) was used as an internal control to validate the right amount of protein loaded on the gels. One mouse was used for each quantification.
Measurement of TNF-α and IL-1β
Portions of spinal cord tissues, collected at 24 h after SCI, were homogenized as described earlier in PBS containing 2 mM of phenyl-methyl-sulfonyl-fluoride to evaluate TNF-α and IL-1β tissue levels. The assay was carried out by using a colorimetric, commercial kit (Calbiochem-Novabiochem Corporation) according to the manufacturer instructions. All TNF-α and IL-1β determinations were performed in duplicate serial dilutions.
Myeloperoxidase Activity
MPO activity, an indicator of polymorphonuclear leukocyte accumulation, was determined in the spinal cord tissues as described earlier (Mullane, 1989) at 24 h after SCI. The time of 24 h after SCI was chosen in agreement with other studies (Genovese et al, 2005). MPO activity was defined as the quantity of enzyme degrading 1 μmol of peroxide/min at 37°C and was expressed in milliunits/g of wet tissue.
Materials
All compounds were obtained from Sigma-Aldrich Company Ltd. All other chemicals were of the highest commercial grade available. All stock solutions were prepared in non-pyrogenic saline (0.9% NaCl; Baxter).
Statistical Evaluation
All values in the figures and text are expressed as mean±SD of N observations. For the in vivo studies, N represents the number of animals studied. For all the experiments, the respective figures are representative of a single experiment out of three comparable experiments, performed on different days on the sample collected from all the animals of each group. Data, pooled from all the appropriate experiments, were analyzed by one-way ANOVA followed by a Bonferroni post hoc test for multiple comparisons. A p-value of <0.05 was considered significant.
BBB scale data were analyzed by the Mann–Whitney test and considered significant when p-value was <0.05.
RESULTS
Expression of TRAIL and Its Receptors Is Induced by SCI in the Mouse
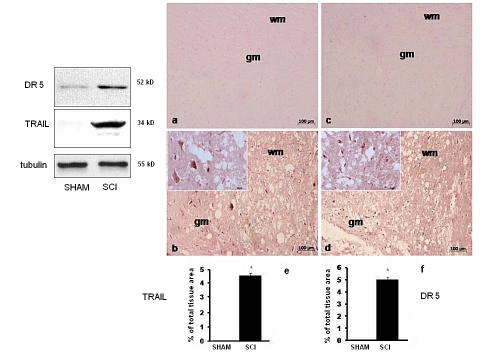
We examined the expression of TRAIL and its receptor DR5 in mice 24 h after SCI. Western blot analysis of spinal cord proteins extracts from sham-operated mice groups did not show TRAIL expression and very little DR5, whereas spinal cord sections obtained from mice subjected to SCI exhibited an increased expression of TRAIL and DR5 (Figure 1, blots). Similar information was obtained through immunostaining. No specific immunostaining for either TRAIL (Figure 1a and densitometry graph e) or DR5 (Figure 1c and densitometry graph f) was observed in spinal cords obtained from sham-operated mice, and specific immunostaining for TRAIL (Figure 1b and densitometry graph e) and DR5 (Figure 1d and densitometry graph f) was found in both white matter and gray matter of the spinal cord tissues of SCI.
Figure 1.
Expression of TRAIL and its DR5 receptor in mice undergone SCI. Blots: expression of DR5 (upper) or TRAIL (middle) in either sham-operated or SCI mice. Lower blot: constitutive β-tubulin expression. Representative images from immunohistochemical analysis of TRAIL (photographs a and b) or DR5 (photographs c and d) performed on sections from the spinal cord or sham-operated (a, c) or SCI (b, d) mice. Histograms: densitometric analysis of the expression of TRAIL (e) and its DR5 (f) receptor in sham-operated or SCI mice was assessed. Data are expressed as % of total tissue area. This figure is representative of at least three experiments performed on different experimental days. Data are means±SD of 10 mice for each group. *p<0.01 vs sham. Insets: higher magnification.
TRAIL-Neutralizing Antibody Prevents SCI-Induced Apoptosis, and Improves Motor Function and Related Tissue Scores in the Mouse
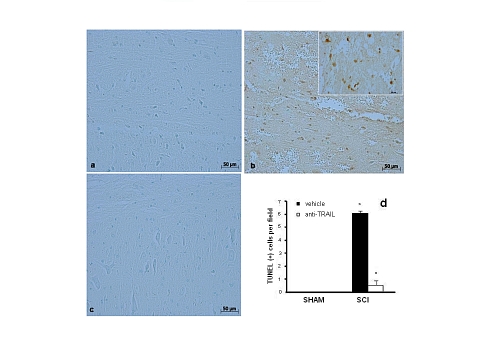
Subsequently, animals were treated with a TRAIL-neutralizing antibody, and perilesional spinal cord tissues underwent TUNEL staining for evaluation of apoptosis. Virtually, no apoptotic cells were detectable in the spinal cord tissue from sham-operated mice (Figure 2a). Twenty-four hours after the trauma, tissues obtained from SCI mice showed a marked appearance of dark brown apoptotic fragments (Figure 2b, see number of TUNEL positive cells panel d) in the gray matter of the spinal cord tissues. In contrast, tissues obtained from TRAIL-neutralizing antibody-treated mice displayed a lesser number of apoptotic fragments (Figure 2c, see number of TUNEL positive cells panel d).
Figure 2.
TRAIL-neutralizing antibody injection prevents SCI-induced apoptosis in the gray matter. TUNEL staining for evaluation of apoptosis in sham-operated (photograph a), SCI (photograph b), or SCI mice treated with a TRAIL-neutralizing antibody (photograph c). The number of apoptotic nuclei in the three groups is compared in the histogram in (d). Figure is representative of at least three experiments performed on different experimental days. Inset: higher magnification. Data are means±SD. *p<0.01 vs SHAM; °p<0.01 vs SCI+vehicle.
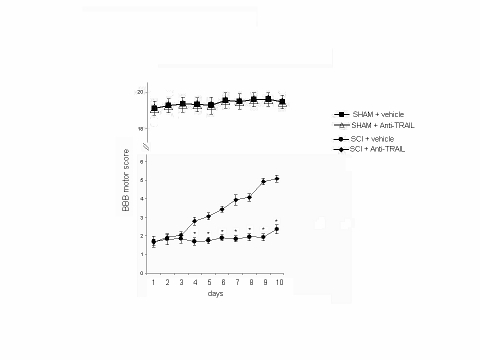
Concomitantly, animals were evaluated for motor activity score by using the modified BBB hind limb locomotor rating scale (Basso et al, 1995). Animals that underwent SCI showed significant decrease of motor activity. On the other hand, motor activity was significantly improved in animals treated with TRAIL-neutralizing antibody (Figure 3). Sham-operated animals did not show any change in motor activity score (data not shown).
Figure 3.
TRAIL-neutralizing antibody injection improves hind limb motor disturbance after SCI in the mouse. BBB motor score (expressed as arbitrary units) in mice that underwent SCI after treatment with a TRAIL-neutralizing monoclonal antibody. The degree of motor disturbance was assessed every day until 10 days after SCI by Basso, Beattie, and Bresnahan (BBB) criteria. Treatment with anti-TRAIL reduces the motor disturbance after SCI. Values shown are mean±SD of 10 mice for each group. *p<0.01 vs SCI+anti-TRAIL.
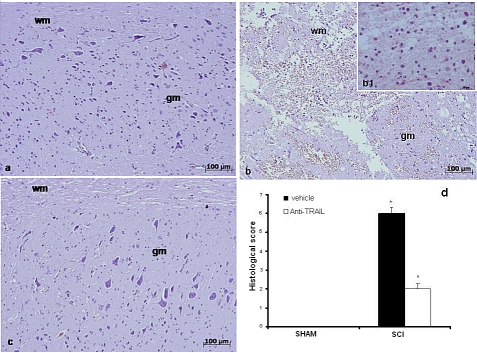
In addition, the severity of the trauma at the level of the perilesional area, assessed by alteration of the white matter (Figure 4b and densitometry graph d), was evaluated at 24 h after injury. Significant damage to the spinal cord was observed in tissues from SCI mice when compared with sham-operated mice (Figure 4a). Notably, significant protection against SCI was observed in mice treated with TRAIL-neutralizing antibody (Figure 4c and densitometry graph d).
Figure 4.
Histological alteration score in the SCI in the mouse after treatment with TRAIL-neutralizing antibody. Edema and alterations of the white matter in tissues from sham-operated (a), untreated (b), and TRAIL-neutralizing antibody treated (c) SCI mice. (d) Histological score (arbitrary units) for tissue damage in sham-operated (SHAM), TRAIL-neutralizing antibody (ANTI-TRAIL, empty bars), and untreated (vehicle, filled bars) SCI mice. wm: white matter; gm: gray matter. This figure is representative of at least three experiments performed on different experimental days. Values shown are mean±SD of 10 mice for each group. *p<0.01 vs SHAM; °p<0.01 vs SCI+vehicle. Inset: higher magnification.
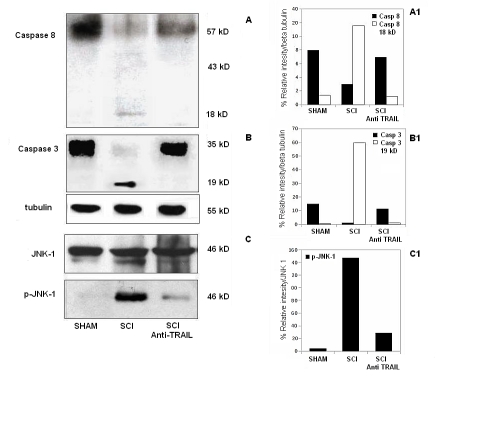
TRAIL-Neutralizing Antibody Inhibits Apoptosis-Associated Activation of Caspases and JNK in Mice that Underwent SCI
The increased expression of TRAIL and DR5 was paralleled by the activation of caspase-8 in SCI (Figure 5a). Treatment of mice subjected to SCI with TRAIL-neutralizing antibody significantly reduced the activation of caspase-8 in the spinal cord extracts. To test whether the effect of the TRAIL-neutralizing antibody resulted in inhibition of SCI-induced apoptotic cell death, we examined the expression of active caspase-3, an enzyme that is known to be expressed after SCI and that is critically involved in the execution of the mammalian apoptotic cell death program (Springer et al, 1999; Ekshyyan and Aw, 2004). Indeed, western blot analysis revealed that caspase-3 was cleaved in the spinal cord of SCI 24 h after injury. Treatment of mice subjected to SCI with TRAIL-neutralizing antibody significantly prevented such effect (Figure 5b).
Figure 5.
Expression of caspase-8 and -3, and of the stress kinase JNK-1 in the tissues from SCI mice treated with a TRAIL-neutralizing antibody. (Blot a) Western blot analyses of caspase-8. (Blot b) Caspase-3 and the house keeping β-tubulin in the tissues from sham-operated (SHAM), SCI (SCI), and SCI pretreated with a TRAIL-neutralizing antibody (SCI anti-TRAIL). (Blot c) Western blot analyses of the stress kinase JNK-1 (upper right blot), and its phosphorylated form (p-JNK-1, lower right blot) in the tissues from sham-operated (SHAM), SCI (SCI), and SCI pretreated with a TRAIL-neutralizing antibody (SCI anti-TRAIL). Densitometric analysis of the expression of caspase-8 (a1), caspase-3 (b1), and p-JNK-1 (c1) Data are expressed as percentage of relative intensity vs control.
Twenty-four hours after SCI, the expression of phospho-JNK in spinal cord was also investigated. Western blot analysis showed a significant increase of phospho-JNK in spinal cords from mice that underwent SCI, whereas TRAIL-neutralizing antibody treatment prevented the SCI-induced (Figure 5c) expression of this kinase. The expression of phospho-JNK was compared with the basal expression of JNK-1 in all groups.
Protein Expression of Bax and Bcl-2 Correlates with Biochemical and Morpho-functional Data in Mice that Underwent SCI and Treated with TRAIL-Neutralizing Antibody
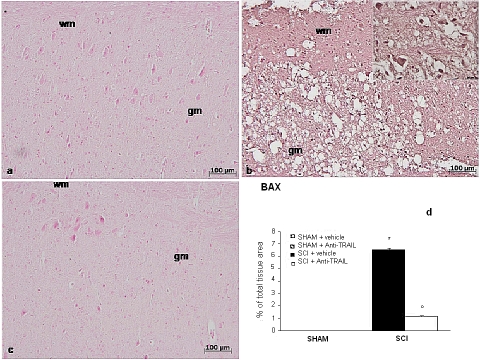
To confirm morphological and biochemical data on apoptosis, the apoptosis-related family of protein Bcl-2 was studied by means of immunohistochemistry. Spinal cord sections from sham-operated mice did not show specific immunostaining for Bax (Figure 6a), whereas SCI-operated mice exhibited positive staining for Bax (Figure 6b and densitometry graph d). Anti-TRAIL treatment reduced the degree of positive staining for Bax in spinal cord sections from SCI-treated mice (Figure 6c and densitometry graph d).
Figure 6.
Bax expression in tissue sections from sham-operated mice (a), SCI (24 h) (b), and anti-TRAIL-treated mice (c). Densitometry analysis of immunocytochemistry photographs (n=5 photos from each sample collected from all mice in each experimental group) for Bax (d) from spinal cord tissues was assessed. This figure is representative of at least three experiments performed on different experimental days. *p<0.01 vs sham. °p<0.01 vs SC I+ vehicle. Data are means±SD. Inset: higher magnification.
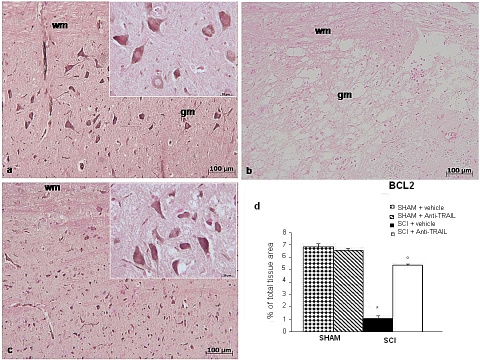
In addition, spinal cord from sham-operated mice showed positive staining for Bcl-2 (Figure 7a and densitometry graph d). Spinal cord sections obtained from SCI-operated mice exhibited significantly less staining for Bcl-2 (Figure 7b and densitometry graph d). Anti-TRAIL treatment upregulated protein expression of anti-apoptotic Bcl-2 (Figure 7c and densitometry graph d).
Figure 7.
Immunohistochemical expression of Bcl-2 in tissue sections from sham-operated mice (a), SCI (24 h) (b), and anti-TRAIL-treated mice (c). Densitometry analysis of immunocytochemistry photographs for Bcl-2 (d) from spinal cord tissues was assessed. Data are expressed as % of total tissue area. This figure is representative of at least three experiments performed on different experimental days. *p<0.01 vs sham. °p<0.01 vs SC I+ vehicle. Data are means±SD. Insets: higher magnification.
TRAIL-Neutralizing Antibody Blunts TRAIL-Dependent Increase of Tissue Proapoptotic Proinflammatory Molecules in Animals with SCI
Our data show that damage occurring in SCI tissues is depending, at least in part, on the effect of the proapoptotic cytokine TRAIL. It is reported that tissue damage is unlikely to be determined by a unique factor, but rather by the concerted action of several molecules of the same or related families, eventually recruited by an ignition factor (Fleming et al, 2006) and, thus, we investigated whether the functional inactivation of TRAIL modulates the expression of other cytokines, such as FasL, TNF-α, IL-1β, and/or activation of MPO, participating in the inflammation after SCI.
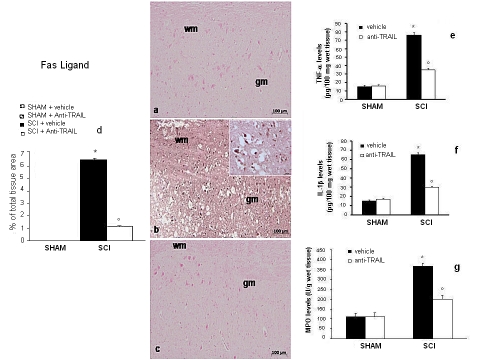
First of all, we studied the expression of the cytokine FasL, which is already known to have a function in SCI in the mouse, 24 h after injury (Demjen et al, 2004). Immunohistochemistry data showed that, whereas specific FasL immunoreactivity is absent in sections from the spinal cord of sham-operated rats (Figure 8a and densitometry graph d), it is on the other hand well detectable in sections from mice that underwent SCI (Figure 8b and densitometry graph d). The positive staining was localized in various cells in the gray matter. Treatment of mice with TRAIL-neutralizing antibody implied a significant decrease of FasL immunoreactive staining in SCI spinal cord sections (Figure 8c and densitometry graph d).
Figure 8.
Immunohistochemical and immunoenzymatic evidence of different cytokines in the spinal cord of SCI mice. Specific immunostaining for Fas-ligand (a) in spinal cord sections from sham-operated, SCI (24 h) (b), and anti-TRAIL-treated mice (c). Densitometry analysis of immunocytochemistry photographs for Fas-ligand (d) from spinal cord tissues was assessed. Data are expressed as % of total tissue area. Tissue levels of TNF-α (e) and IL-1β (f) in spinal cords from SCI (24 h) mice. (g) MPO activity in spinal cord from SCI mice. This figure is representative of at least three experiments performed on different experimental days. *p<0.01 vs sham. °p<0.01 vs SCI+vehicle. Data are means±SD. Inset: higher magnification.
A similar pattern was observed for TNF-α, IL-1β, and MPO, as assessed by colorimentric assay, showing that changes occur in sham-operated animals, in contrast with the dramatic increase scored in animals that underwent SCI. Interestingly, treatment with TRAIL-neutralizing antibody produced a significant reduction of these molecules in the tissues from SCI animals (Figure 8e–g).
Reciprocal Regulatory Interplay Between TRAIL and GITR in Mice with SCI
A recently characterized cytokine, GITRL, seems to have a function in the tissue damage occurring in the SCI model (Nocentini et al, 2008). However, in spite of the wide literature reporting relationships between TRAIL and other cytokines, there are no data relating TRAIL and GITRL. For this reason, and with the aim to unravel novel molecular targets for therapy, we addressed the question whether the two molecules could have reciprocal interplay within the SCI model.
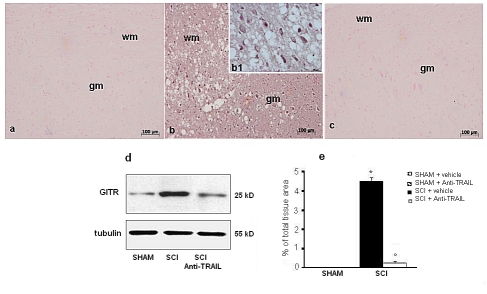
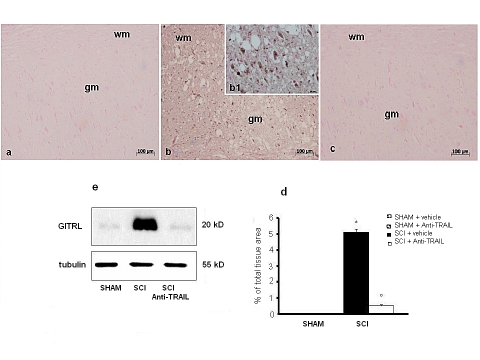
To do so, the expression of GITR and GITRL in spinal cord was investigated by immunohistochemistry at 24 h after SCI. A significant increase in GITR (Figure 9b, d and densitometry graph e) and GITRL (Figure 10b, c and densitometry graph e) expression was observed in the spinal cords from mice subjected to SCI. On the contrary, anti-TRAIL treatment prevented the SCI-induced GITR (Figure 9c and densitometry graph e) and GITRL (Figure 10c and densitometry graph e) expression. The specific immunostaining for GITR (Figure 9a and densitometry graph e) and GITRL (Figure 10a and densitometry graph e) seems dot shaped in the white matter of sham-operated mice. Western blot analysis confirmed the results obtained with immunohistochemistry.
Figure 9.
Immunoreactivity for GITR in tissue sections from sham-operated (a), SCI (b), and anti-TRAIL-treated (c) SCI mice. (e) Densitometry analysis of immunocytochemistry photographs for GITR from spinal cord tissues. Data are expressed as % of total tissue area. This figure is representative of at least three experiments performed on different experimental days. *p<0.01 vs sham. °p<0.01 vs SCI+vehicle. Data are means±SD. (d) Western blot analysis of GITR protein in spinal cords from sham-operated (SHAM), SCI, or anti-TRAIL-treated SCI mice (SCI+anti-TRAIL). Inset: higher magnification.
Figure 10.
Immunoreactivity for GITRL in tissue sections from sham-operated (a), SCI (b), and anti-TRAIL-treated (c) SCI mice. (d) Densitometry analysis of immunocytochemistry photographs for GITRL from spinal cord tissues. Data are expressed as % of total tissue area. This figure is representative of at least three experiments performed on different experimental days. *p<0.01 vs Sham. °p<0.01 vs SCI+vehicle. Data are means±SD. (e) Western blot analysis of GITRL protein in spinal cords from sham-operated (SHAM), SCI, or anti-TRAIL-treated SCI mice (SCI+anti-TRAIL). Inset: higher magnification.
To verify whether GITR modulates SCI-induced TRAIL activity, we evaluated both TRAIL and DR5 expression in GITR lacking mice (GITR−/−) undergoing SCI. Levels of TRAIL and DR5 immunoreactivity were significantly attenuated in GITR−/− mice subjected to SCI as compared with SCI wild-type (WT) mice (data not shown).
DISCUSSION
It is known that proapoptotic cytokines have a relevant function as contributors to neuronal damage and functional impairment associated with SCI (Harrington et al, 2005; Lee et al, 2000). In this line, increased FasL and TNF-α expression have also been reported after SCI, although their neutralization is not always correlated to functional outcome (Demjen et al, 2004; Genovese et al, 2008; Yu et al, 2009). In this paper, we show the prominent function of TRAIL and the related molecules in the cell death phenomena related to SCI, as well as how the molecular phenomena underlying can be partially prevented by neutralization of TRAIL, resulting in significant improvement of histopathological and functional outcome.
Indeed, the expression of TRAIL and its DR5 death receptor were significantly increased in mice that underwent SCI. These data, in line with those showing lack of expression of TRAIL and relatively scarce expression of DR5 in the spinal cord of intact mice, are well in accordance with a number of literature reports suggesting that TRAIL is not expressed in the normal CNS of mammalian species, which rather display weak expression of the DR5 death receptor (Cantarella et al, 2003; Aktas et al, 2007). On the other hand, expression of TRAIL increases dramatically after various types of nervous tissue injuries, as those occurring after vascular accidents (Martin-Ventura et al, 2007), challenge of neurons with toxic stimuli, including amyloid-β (Cantarella et al, 2003). In the same line, increased expression of TRAIL has been detected by immunohistochemistry in the post-mortem brains of Alzheimer's patients, localized in the close vicinity of amyloid plaques (Uberti et al, 2004).
Interestingly, post-SCI impairment of motor activity was significantly improved in animals treated with TRAIL-neutralizing antibody. It has been reported that, although SCI is associated with significant elevation of various cytokines in the spinal cord tissue, frequently this does not correspond to amelioration of their functional performances (Profyris et al, 2004). These data suggest that, although involved, each single cytokine may have a contribution function, rather than a primary function, in maintaining neuroinflammatory parameters associated with SCI and other CNS injury models. However, it is plausible to hypothesize that some molecules, such as, for example, TRAIL, may have a major coordinating function in the cytokine orchestra of neuroinflammation, and thus its neutralization implies improvement of neuronal function and, therefore, of motor performances.
In line with the TRAIL-related signaling, the increased expression of the cytokine and its receptors in the SCI is associated with increased activation of the initiator caspase-8 as well as the effector caspase-3, suggesting that TRAIL is setting into motion the cell death machinery (Ashkenazi and Dixit, 1998; Almasan and Ashkenazi, 2003). Moreover, systemic administration of a TRAIL-neutralizing antibody resulted in reduced expression of TRAIL in SCI lysates, as well as in reduced activation of caspase-8 and -3. The relevant result that the perilesional edema and alteration of the white matter present in SCI mice was significantly reduced strongly suggests that TRAIL is directly involved in SCI-related tissue damage and, thus, its neutralization can bring about relevant improvement of pathophysiological parameters altered in SCI. In fact, this is in line with the observations made for other proapoptotic cytokines, the expression of which is increased in the brain in course of neuronal damage (Martin-Villalba et al, 1999). Moreover, SCI is not the only model of nervous system damage in which neutralization of TRAIL results in improvement of neuronal function. For example, it has been reported that TRAIL antagonism results in decreased amyloid-β-induced toxicity in human neuronal cells in vitro (Cantarella et al, 2003; Uberti et al, 2007).
To confirm the specificity of detrimental effects of TRAIL in SCI, we found that the expression of the cell death-related phosphorylated form of kinase JNK was increased 24 h after SCI in spinal cords. Increased phosphorylation of JNK has been reported after treatment with TRAIL in other in vivo and also in in vitro models (Corazza et al, 2006; Jurewicz et al, 2006; Cantarella et al, 2007) and represents a step of the canonical transduction pathway set into motion after binding of TRAIL to its DR5 death receptor (Jaganathan et al, 2002). Thus, it seemed obvious that prevention of TRAIL detrimental effects in SCI by means of TRAIL-neutralizing antibody was associated with decrease activation of the death-related kinase JNK.
Evidence showed that proinflammatory and proapoptotic cytokines, including TNF-α, IL-1β, and FasL regulate cellular events after SCI (Streit et al, 1998, Martin-Villalba et al, 1999). In this study, we have shown by immunohistochemistry a significant increase in positive staining for TNF-α in SCI mice group compared with sham-operated animal groups as well as FasL. Besides, Taoka et al (1997) showed that activated neutrophils are involved in SCI-induced trauma in rats. We showed that the administration of TRAIL-neutralizing antibody in SCI reduced the expression of both TNF-α and FasL as well as the inflammatory cell infiltration as assessed by the specific granulocyte enzyme MPO after SCI. The reduced neutrophil recruitment represents an important additional mechanism for the protective effects of the anti-TRAIL treatment. Neutrophils recruited into the tissue can, in fact, contribute to tissue destruction by the production of reactive oxygen metabolites (Carlson et al, 1998; Tator, 1995), granule enzymes, and cytokines that further amplify the inflammatory response by their effects on macrophages and lymphocytes (Chatham et al, 1993).
It has been recently shown that the TNFR superfamily member GITR has a co-stimulatory function in T cells and a proinflammatory function in other cells of the immune system (Nocentini et al, 2007; Nocentini and Riccardi, 2009). Activation of GITR by its ligand seems to have a function also in the tissue damage occurring in the SCI model, as shown by the higher motor score and lower inflammatory mediators levels in GITR−/−, compared with WT mice (Nocentini et al, 2008). As a counterproof, it has been shown that GITR-Fc fusion protein inhibits GITR triggering and protects from the inflammatory response after SCI (Nocentini et al, 2008).
In the same line, Razmara et al (2009) showed that Fn14-TRAIL, a chimeric intercellular signal exchanger, attenuates experimental autoimmune encephalomyelitis inflammatory hallmarks. In this study, it is shown that neutralization of TRAIL by in vivo treatment with TRAIL-neutralizing antibody results in diminished expression of GITR and its ligand. Moreover, in GITR−/− mice, the expression of either TRAIL or its receptor DR5 is reduced after SCI, supporting the hypothesis that the interplay between the two systems is synergic and suggest that TRAIL and GITRL may have a leading function in the mechanisms of inflammation-driven neurodegeneration.
In summary, we have shown that the cytokine TRAIL has a primary function in the SCI model. TRAIL induces apoptotic neuronal death, tissue damage, and functional impairment in mice that underwent SCI. Neutralization of TRAIL implies a dramatic improvement of all these parameters. The effects of TRAIL seem mediated by activated caspases and involved the expression of apoptosis-related genes, such as Bcl-2 and Bax. In a similar line, neutralization of TRAIL abrogated the expression of various inflammatory molecules, including GITRL and its receptor GITR.
In conclusion, neutralization of TRAIL could be envisioned as a novel potential pharmacological tool contributing to prevention of extensive SCI-related damage.
Acknowledgments
We are indebted to Dr Vincenzo Guardabasso, Statistical Services, Policlinico ‘G. Rodolico', Catania, for his skilful and authorable advice. This work has been supported by a PRIN grant from MIUR, Italian Ministry for Research; PRA grant from the University of Catania; the PhD program in Preclinical and Clinical Pharmacology, University of Catania School of Medicine.
The authors declare no conflict of interest.
References
- Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signalling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003;14:337–348. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- Aktas O, Schulze-Topphoff U, Zipp F. The role of TRAIL/TRAIL receptors in central nervous system pathology. Front Biosci. 2007;12:2912–2921. doi: 10.2741/2281. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: signalling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor ratting scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cantarella G, Lempereur L, D'Alcamo MA, Risuglia N, Cardile V, Pennisi G, et al. Trail interacts redundantly with nitric oxide in rat astrocytes: potential contribution to neurodegenerative processes. J Neuroimmunol. 2007;182:41–47. doi: 10.1016/j.jneuroim.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Cantarella G, Uberti D, Carsana T, Lombardo G, Bernardini R, Memo M. Neutralization of TRAIL death pathway protects human neuronal cell line from beta-amyloid toxicity. Cell Death Differ. 2003;10:134–141. doi: 10.1038/sj.cdd.4401143. [DOI] [PubMed] [Google Scholar]
- Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L. Acute inflammatory response in spinal cord following impact injury. Exp Neurol. 1998;151:77–88. doi: 10.1006/exnr.1998.6785. [DOI] [PubMed] [Google Scholar]
- Chatham WW, Swaim R, Frohsin H, Jr, Heck LW, Miller EJ, Blackburn WD., Jr Degradation of human articular cartilage by neutrophils in synovial fluid. Arthritis Rheum. 1993;36:51–58. doi: 10.1002/art.1780360109. [DOI] [PubMed] [Google Scholar]
- Corazza N, Jakob S, Schaer C, Frese S, Keogh A, Stroka D, et al. TRAIL receptor-mediated JNK activation and Bim phosphorylation critically regulate Fas-mediated liver damage and lethality. J Clin Invest. 2006;116:2493–2499. doi: 10.1172/JCI27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demjen D, Klussmann S, Kleber S, Zuliani C, Stieltjes B, Metzger C, et al. Neutralization of CD95 ligand promotes regeneration and functional recovery after spinal cord injury. Nat Med. 2004;10:389–395. doi: 10.1038/nm1007. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Rutt RD, Stover SL, Fine PR. Employment after spinal cord injury. Arch Phys Med Rehabil. 1987;68:494–498. [PubMed] [Google Scholar]
- Dörr J, Bechmann I, Waiczies S, Aktas O, Walczak H, Krammer PH, et al. Lack of tumor necrosis factor-related apoptosis-inducing ligand but presence of its receptors in the human brain. J Neurosci. 2002;22:RC209. doi: 10.1523/JNEUROSCI.22-04-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekshyyan O, Aw TY. Apoptosis in acute and chronic neurological disorders. Front Biosci. 2004;9:1567–1576. doi: 10.2741/1357. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, et al. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muià C, Esposito E, et al. TNF-alpha blockage in a mouse model of SCI: evidence for improved outcome. Shock. 2008;29:32–41. doi: 10.1097/shk.0b013e318059053a. [DOI] [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Di Paola R, Cannavo G, Muia C, Bramanti P, et al. Role of endogenous ligands for the peroxisome proliferators activated receptors alpha in the secondary damage in experimental spinal cord trauma. Exp Neurol. 2005;194:267–278. doi: 10.1016/j.expneurol.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Harrington JF, Messier AA, Levine A, Szmydynger-Chodobska J, Chodobski A. Shedding of tumor necrosis factor type 1 receptor after experimental spinal cord injury. J Neurotrauma. 2005;22:919–928. doi: 10.1089/neu.2005.22.919. [DOI] [PubMed] [Google Scholar]
- Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369–378. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- Jaganathan J, Petit JH, Lazio BE, Singh SK, Chin LS. Tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in established and primary glioma cell lines. Neurosurg Focus. 2002;13:ecp1. doi: 10.3171/foc.2002.13.3.6. [DOI] [PubMed] [Google Scholar]
- Joshi M, Fehlings MG. Development and characterization of a novel, graded model of clip compressive spinal cord injury in the mouse: Part 1. Clip design, behavioral outcomes, and histopathology. J Neurotrauma. 2002a;19:175–190. doi: 10.1089/08977150252806947. [DOI] [PubMed] [Google Scholar]
- Joshi M, Fehlings MG. Development and characterization of a novel, graded model of clip compressive spinal cord injury in the mouse: Part 2. Quantitative neuroanatomical assessment and analysis of the relationships between axonal tracts, residual tissue, and locomotor recovery. J Neurotrauma. 2002b;19:191–203. doi: 10.1089/08977150252806956. [DOI] [PubMed] [Google Scholar]
- Jurewicz A, Matysiak M, Andrzejak S, Selmaj K. TRAIL-induced death of human adult oligodendrocytes is mediated by JNK pathway. Glia. 2006;53:158–166. doi: 10.1002/glia.20249. [DOI] [PubMed] [Google Scholar]
- Krausz LT, Bianchini R, Ronchetti S, Fettucciari K, Nocentini G, Riccardi C. GITR-GITRL system, a novel player in shock and inflammation. ScientificWorldJournal. 2007;7:533–566. doi: 10.1100/tsw.2007.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YB, Yune TY, Baik SY, Shin YH, Du S, Rhim H, et al. Role of tumor necrosis factor-alpha in neuronal and glial apoptosis after spinal cord injury. Exp Neurol. 2000;166:190–195. doi: 10.1006/exnr.2000.7494. [DOI] [PubMed] [Google Scholar]
- Li GL, Farooque M, Olsson Y. Changes of Fas and Fas ligand immunoreactivity after compression trauma to rat spinal cord. Acta Neuropathol. 2000;100:75–81. doi: 10.1007/s004010051195. [DOI] [PubMed] [Google Scholar]
- Liu D, Xu GY, Pan E, McAdoo DJ. Neurotoxicity of glutamate at the concentration released upon spinal cord injury. Neuroscience. 1999;93:1383–1389. doi: 10.1016/s0306-4522(99)00278-x. [DOI] [PubMed] [Google Scholar]
- Lu J, Ashwell KW, Waite P. Advances in secondary spinal cord injury: role of apoptosis. Spine. 2000;25:1859–1866. doi: 10.1097/00007632-200007150-00022. [DOI] [PubMed] [Google Scholar]
- Martin-Ventura JL, Munoz-Garcia B, Egido J, Blanco-Colio LM. TRAIL and vascular injury. Front Biosci. 2007;12:3656–3667. doi: 10.2741/2342. [DOI] [PubMed] [Google Scholar]
- Martin-Villalba A, Herr I, Jeremias I, Hahne M, Brandt R, Vogel J, et al. CD95 ligand (Fas-L/APO-1L) and tumor necrosis factor-related apoptosis-inducing ligand mediate ischemia-induced apoptosis in neurons. J Neurosci. 1999;19:3809–3817. doi: 10.1523/JNEUROSCI.19-10-03809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullane K. Neutrophil-platelet interactions and post-ischemic myocardial injury. Prog Clin Biol Res. 1989;301:39–51. [PubMed] [Google Scholar]
- Nocentini G, Riccardi C. GITR: a modulator of immune response and inflammation. Adv Exp Med Biol. 2009;647:156–173. doi: 10.1007/978-0-387-89520-8_11. [DOI] [PubMed] [Google Scholar]
- Nocentini G, Cuzzocrea S, Genovese T, Bianchini R, Mazzon E, Ronchetti S, et al. Glucocorticoid-induced tumor necrosis factor receptor-related (GITR)-Fc fusion protein inhibits GITR triggering and protects from the inflammatory response after spinal cord injury. Mol Pharmacol. 2008;73:1610–1621. doi: 10.1124/mol.107.044354. [DOI] [PubMed] [Google Scholar]
- Nocentini G, Ronchetti S, Cuzzocrea S, Riccardi C. GITR/GITRL: more than an effector T cell co-stimulatory system. Eur J Immunol. 2007;37:1165–1169. doi: 10.1002/eji.200636933. [DOI] [PubMed] [Google Scholar]
- Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997a;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, et al. The receptor for the cytotoxic ligand TRAIL. Science. 1997b;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- Plunkett JA, Yu CG, Easton JM, Bethea JR, Yezierski RP. Effects of interleukin-10 (IL-10) on pain behavior and gene expression following excitotoxic spinal cord injury in the rat. Exp Neurol. 2001;168:144–154. doi: 10.1006/exnr.2000.7604. [DOI] [PubMed] [Google Scholar]
- Profyris C, Cheema SS, Zang D, Azari MF, Boyle K, Petratos S. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis. 2004;15:415–436. doi: 10.1016/j.nbd.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Razmara M, Hilliard B, Ziarani AK, Murali R, Yellayi S, Ghazanfar M, et al. Fn14-TRAIL, a chimeric intercellular signal exchanger, attenuates experimental autoimmune encephalomyelitis. Am J Pathol. 2009;174:460–474. doi: 10.2353/ajpath.2009.080462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivlin AS, Tator CH. Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surg Neurol. 1978;10:38–43. [PubMed] [Google Scholar]
- Robertson J, Beaulieu JM, Doroudchi MM, Durham HD, Julien JP, Mushynski WE. Apoptotic death of neurons exhibiting peripherin aggregates is mediated by the proinflammatory cytokine tumor necrosis factor-alpha. J Cell Biol. 2001;155:217–226. doi: 10.1083/jcb.200107058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchetti S, Nocentini G, Bianchini R, Krausz LT, Migliorati G, Riccardi C. Glucocorticoid-induced TNFR-related protein lowers the threshold of CD28 costimulation in CD8+ T cells. J Immunol. 2007;179:5916–5926. doi: 10.4049/jimmunol.179.9.5916. [DOI] [PubMed] [Google Scholar]
- Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G, et al. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34:613–622. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- Satoh JI, Kuroda Y. Alpha-synuclein expression is up-regulated in NTera2 cells during neuronal differentiation but unaffected by exposure to cytokines and neurotrophic factors. Parkinsonism Relat Disord. 2001;8:7–17. doi: 10.1016/s1353-8020(00)00075-4. [DOI] [PubMed] [Google Scholar]
- Shea TB. Technical report. An inexpensive densitometric analysis system using a Macintosh computer and a desktop scanner. Biotechniques. 1994;16:1126–1128. [PubMed] [Google Scholar]
- Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- Springer JE, Azbill RD, Knapp PE. Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nat Med. 1999;5:943–946. doi: 10.1038/11387. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Semple-Rowland SL, Hurley SD, Miller RC, Popovich PG, Stokes BT. Cytokine mRNA profiles in contused spinal cord and axotomized facial nucleus suggest a beneficial role for inflammation and gliosis. Exp Neurol. 1998;152:74–87. doi: 10.1006/exnr.1998.6835. [DOI] [PubMed] [Google Scholar]
- Taoka Y, Okajima K, Uchiba M, Murakami K, Kushimoto S, Johno M, et al. Role of neutrophils in spinal cord injury in the rat. Neuroscience. 1997;79:1177–1182. doi: 10.1016/s0306-4522(97)00011-0. [DOI] [PubMed] [Google Scholar]
- Tator CH. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995;5:407–413. doi: 10.1111/j.1750-3639.1995.tb00619.x. [DOI] [PubMed] [Google Scholar]
- Tator CH, Koyanagi I. Vascular mechanisms in the pathophysiology of human spinal cord injury. J Neurosurg. 1997;86:483–492. doi: 10.3171/jns.1997.86.3.0483. [DOI] [PubMed] [Google Scholar]
- Tone M, Tone Y, Adams E, Yates SF, Frewin MR, Cobbold SP, et al. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc Natl Acad Sci USA. 2003;100:15059–15064. doi: 10.1073/pnas.2334901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uberti D, Cantarella G, Facchetti F, Cafici A, Grasso G, Bernardini R, et al. TRAIL is expressed in the brain cells of Alzheimer's disease patients. Neuroreport. 2004;15:579–581. doi: 10.1097/00001756-200403220-00002. [DOI] [PubMed] [Google Scholar]
- Uberti D, Ferrari-Toninelli G, Bonini SA, Sarnico I, Benarese M, Pizzi M, et al. Blockade of the tumor necrosis factor-related apoptosis inducing ligand death receptor DR5 prevents beta-amyloid neurotoxicity. Neuropsychopharmacology. 2007;32:872–880. doi: 10.1038/sj.npp.1301185. [DOI] [PubMed] [Google Scholar]
- Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, et al. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997;16:5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- Xu J, Fan G, Chen S, Wu Y, Xu XM, Hsu CY. Methylprednisolone inhibition of TNF-alpha expression and NF-kB activation after spinal cord injury in rats. Brain Res Mol Brain Res. 1998;59:135–142. doi: 10.1016/s0169-328x(98)00142-9. [DOI] [PubMed] [Google Scholar]
- Yu WR, Liu T, Fehlings TK, Fehlings MG. Involvement of mitochondrial signaling pathways in the mechanism of Fas-mediated apoptosis after spinal cord injury. Eur J Neurosci. 2009;29:114–131. doi: 10.1111/j.1460-9568.2008.06555.x. [DOI] [PubMed] [Google Scholar]
- Zurita M, Vaquero J, Zurita I. Presence and significance of CD-95 (Fas/APO1) expression after spinal cord injury. J Neurosurg. 2001;94:257–264. doi: 10.3171/spi.2001.94.2.0257. [DOI] [PubMed] [Google Scholar]